Abstract
Previous studies have demonstrated that EGF and bFGF maintain the stem cell properties of proliferating human adipose-derived stromal/stem cells (hASCs) in vitro. While the expansion and cryogenic preservation of isolated hASCs are routine, these manipulations can impact their proliferative and differentiation potential. This study examined cryogenically preserved hASCs (n = 4 donors), with respect to these functions, after culture with basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) at varying concentrations (0–10 ng/ml). Relative to the control, cells supplemented with EGF and bFGF significantly increased proliferation by up to three-fold over 7–8 days. Furthermore, cryopreserved hASCs expanded in the presence of EGF and bFGF displayed increased oil red O staining following adipogenic induction. This was accompanied by significantly increased levels of several adipogenesis-related mRNAs: aP2, C/EBPα, lipoprotein lipase (LPL), PPARγ and PPARγ co-activator-1 (PGC1). Adipocytes derived from EGF- and bFGF-cultured hASCs exhibited more robust functionality based on insulin-stimulated glucose uptake and atrial natriuretic peptide (ANP)-stimulated lipolysis. These findings indicate that bFGF and EGF can be used as culture supplements to optimize the proliferative capacity of cryopreserved human ASCs and their adipogenic differentiation potential.
Keywords: adipogenesis, adipose-derived stem cells, edipermal growth factor, basic fibroblast growth factor, cryopreservation, differentiation
1. Introduction
There is a growing interest in adult human adipose-derived stromal/stem cells (hASCs), which are abundant, plastic and useful for exploring the mechanisms of adipose tissue differentiation. The ASCs have been confirmed to differentiate along multiple lineage pathways (adipocyte, chondrocyte, neuronal-like and osteoblast) in vitro at the clonal level, consistent with the definition of a ‘stem cell’ (Guilak et al., 2006; Zuk et al., 2001, 2002; Halvorsen et al., 2000, 2001; Gronthos et al., 2001). It is of interest that the adipogenic potential was most difficult to maintain with extensive expansion of the clonal populations (Guilak et al., 2006). Since the number of human pre-adipocyte cell lines are limited, our laboratory and others utilize primary cultures of hASCs. Consequently, it is necessary that isolated hASCs be cryopreserved for future experiments; however, current techniques for expanding, preserving and inducing such cells leave room for improvement. While it has been reported that bone marrow-derived mesenchymal stem cells (BMSCs) can be cryopreserved without loss of viability or osteogenic potential (Kotobuki et al., 2004), it has been reported that cryopreservation can reduce the viability, proliferation and differentiation ability of CD34+ haematopoietic stem cells (de Boer et al., 2002; Keung et al., 1996; Stylianou et al., 2006). Furthermore, while cryopreservation of intact human adipose tissue has been achieved (Shoshani et al., 2001), studies suggest that optimal viability requires the presence of cryoprotectant agents and controlled rate freezing (Moscatello et al., 2005). We have noted a decreased viability in the cryopreserved isolated hASCs as a function of cell concentration, although differentiation function was generally maintained (Goh et al., 2007). During our efforts to optimize hASC cryopreservation (Devireddy et al., 2005; Goh et al., 2007; Thirumala et al., 2005a, 2005b), we have noted a loss of adipogenic differentiation potentiality in some lots of cryopreserved hASCs post-thaw.
To date, a standard expansion procedure for hASCs after cryopreservation has not been recognized. Multiple laboratories, including our own, maintain the hASCs in a stromal medium consisting of Dulbecco’s modified Eagle’s medium (DMEM)/F-12 Ham’s, supplemented only with 10% fetal bovine serum (FBS) and 1% antibiotics/antimycotics (Mitchell et al., 2006]. Some laboratories have supplemented their expansion medium with epidermal growth factor (EGF) and/or basic fibroblast growth factor (bFGF) to accelerate hASC proliferation (Hauner et al., 1995; Iwashima et al., 2008; Skurk et al., 2007), while others have employed commercial media optimized for endothelial cell growth (Miranville et al., 2004; Rehman et al., 2004; Suga et al., 2007). These latter compositions are supplemented with polypeptide growth factors, including EGF and bFGF, that have the potential to increase cell proliferation and modulate differentiation of hASCs. Previous studies have speculated that a synergy exists between EGF and bFGF (Hauner et al., 1995; Suga et al., 2007) but the dose-dependent effects of these two factors in combination have not been examined closely.
The effects of EGF and bFGF vary greatly among species; however, numerous studies demonstrate that EGF and bFGF increase proliferation and modulate the differentiation potential of hASCs (Butterwith et al., 1993; Hauner et al., 1995; Tamama et al., 2006; Zaragosi et al., 2006). EGF is a single-chain polypeptide noted for having proliferative and differentiating effects on many mammalian tissues (Hauner et al., 1995; Kurachi et al., 1993). EGF is known to enhance migration and cell proliferation of bone marrow-derived mesenchymal stem cells while maintaining differentiation potential (Tamama et al., 2006), and the involvement of EGF in adipose tissue development has been postulated based on the correlation between increased levels of EGF and obesity onset in ovariectomized mice (Kurachi et al., 1993). Also, EGF has significant effects on in vitro adipocyte development and function at concentrations comparable to those found in human serum (Hauner et al., 1995). Likewise, bFGF has been found to act as a mitogen and chemoattractant that enhances angiogenesis, migration and adipogenic differentiation of both ASCs and BMSCs (Hauner et al., 1995; Locklin et al., 1999; Schmidt et al., 2006; Vashi et al., 2006). Zarogosi et al. (2006), by inhibiting bFGF receptor signalling, have demonstrated that hASCs express bFGF as an autocrine factor. In similar studies, Rider et al. (2008) found that inhibition of endogenous bFGF reduced the rate of hASC proliferation. Thus, bFGF appears to be necessary for stem cell self-renewal, proliferation and the maintenance of hASC pluripotency. In contrast, the presence of bFGF in osteogenic medium has been noted to inhibit murine ASC extracellular matrix mineralization and alkaline phosphatase enzyme activity (Quarto and Longaker, 2006). The addition of bFGF inhibited the murine ASC expression of the BMP 1B receptor in response to retinoic acid; however, this mechanism appears to be species-specific, since bFGF addition was not observed to inhibit human ASC osteogenesis in vitro (Quarto et al., 2008).
Recently, we observed that EGF and bFGF induced hASC secretion of the hepatic growth factor (HGF) (Kilroy et al., 2007). This cytokine has the potential to function in an autocrine manner, since hASCs express its receptor (c-Met) mRNA (Kilroy et al., 2007). A related study has determined that HGF expression by human umbilical cord blood-derived MSCs correlated with both increased proliferative capacity and adipogenic potential in vitro (Markov et al., 2007). Based on these observations, we hypothesized that the presence of EGF and bFGF would enhance the proliferation and differentiation potential of cryopreserved hASCs. The current study examines the effect of EGF and bFGF with respect to hASC cell proliferation and adipogenesis.
2. Materials and methods
2.1. Human subjects
Adult human lipoaspirates were obtained with consent from nine female patients undergoing elective liposuction procedures [mean age 36.9 (range 27–62) years; mean BMI 25.6 (range 21.6–29.4); demographic data for one of the nine subjects was not obtained and is missing from these mean values; Table 1]. The hASCs were isolated from the stromal vascular fraction of adipose tissue and cryopreserved in liquid nitrogen after the initial passage at a concentration of 0.5 × 106 cells/ml in 10% dimethylsulphoxide, 10% DMEM/F-12 Ham’s (DMEM/F-12), and 80% FBS according to published methods (Goh et al., 2007). All protocols were reviewed and approved by the Pennington Biomedical Research Center Institutional Review Board.
Table 1.
Donor demographics
| Donor | Race | Gender | Age | BMI | Use |
|---|---|---|---|---|---|
| 1 | Caucasian | Female | 32 | 26.17 | Cell count |
| 2 | Caucasian | Female | 36 | 28.17 | Cell count |
| 3 | Caucasian | Female | 32 | 28.72 | Cell count, PCR, GPDH |
| 4 | Caucasian | Female | 40 | 23.32 | Cell count, PCR, GPDH |
| 5 | Caucasian | Female | 62 | 24.23 | PCR, GPDH, lipolysis |
| 6 | Caucasian | Female | 33 | 21.63 | PCR, GPDH, lipolysis, glucose uptake |
| 7 | Caucasian | Female | 33 | 29.4 | Cell count, oil red O staining |
| 8 | ND | Female | ND | ND | Cell count, oil red O staining |
| 9 | Caucasian | Female | 27 | 23.19 | Cell count, oil red O staining |
| Mean ± SD | 36.9 ± 10.8 | 25.6 ± 2.9 |
ND, not determined.
2.2. Cell culture
All cells were cryopreserved for a minimum period of >1 month in liquid nitrogen prior to plating (Goh et al., 2007). Vials of hASCs were thawed rapidly with vigorous agitation in a 37 °C waterbath washed once with stromal medium (DMEM/F-12, 10% FBS (Hyclone, Logan, UT, USA), 1% antibiotic/antimycotic (MP Biomedicals, Solon, OH, USA) and cultured at 37 °C, 5% CO2. Following a 48 h expansion, the hASCs were harvested by trypsin digestion and replated at a density of 5000 cells/cm2 in stromal medium. After 24 h to allow for adherence, the stromal medium was converted to DMEM/F-12 containing 3% FBS and 1% antibiotic/antimycotic and supplemented with EGF (0, 0.1, 1.0 or 10 ng/ml) and/or bFGF (0, 0.1, 1.0 or 10 ng/ml). Cells used in proliferation assays were maintained under these conditions for 7–8 days.
2.2.1. Adipogenic conditions
Cells for GPDH assays, oil red O staining and qRT–PCR were induced for adipogenesis at day 8 of culture with adipogenic medium (DMEM/F-12, 3% FBS, 1% antibiotic/antimycotic, 33 μM biotin, 17 μM pantothenate, 1 μM insulin, 1 μM dexamethasone, 0.5 mM IBMX, 5 μM Rosiglitazone (AK Scientific, Mountain View, CA, USA). After 3 days media was converted to adipogenesis maintenance medium (DMEM/F-12, 3% FBS, 1% antibiotic/antimycotic, 33 μM biotin, 17 μM pantothenate, 1 μM insulin, 1 μM dexamethasone) and cells were fed three times/week (Halvorsen et al., 2001; Mitchell et al., 2006).
2.3. Cell proliferation assay (n = 5 donors)
Cell proliferation was determined on passage 1 hASCs after 7–8 days of conditioning with varying concentrations of EGF and bFGF. Cells from individual wells of a 24-well plate were harvested using 0.05% trypsin in EDTA. An aliquot of cells was stained with Trypan blue, and total number cells per well was determined using a haematocytometer.
2.4. Oil red O staining (n = 5 donors)
Cells grown in a 24-well plate for 7 days of preconditioning with varying concentrations of EGF- and bFGF-supplemented conditions were induced for adipogenesis and maintained for up to 9 days. The cells were then washed three times with PBS, fixed in 10% formalin (1 h, 4°C) and stained using oil red O (Halvorsen et al., 2001). The plates were rinsed three times with distilled water and photographs were taken of a representative field for each condition at ×100 magnification. In a subset of studies performed on three independent donors, the oil red O stain retained by the hASCs following adipogenesis was eluted by incubation of the well in the presence of isopropanol. Parallel wells incubated under identical conditions were left unstained, harvested and used to determine total cell counts per well. The optical density at 510 nm (OD510) was determined for the eluted oil red O stain per well using a SpectraMax Plux 384 plate reader from Molecular Devices and the values were normalized relative to the total number of cells per well under each culture condition.
2.5. RT–PCR (n = 4 donors)
Total RNA was extracted from cells using TRI-Reagent according to the manufacturer’s instructions (Molecular Research Center, Cincinnati, OH, USA). Cells cultured in adipogenic medium were harvested 8 days after induction. Real-time PCR was performed in a final reaction volume of 10 μl, including forward and reverse primers (0.1 mM), 1.5 μg reverse-transcribed RNA and 5 μl SYBR green master mix (Applied Biosystems, Warrington, UK), using an ABI Prism 7900 instrument (Applied Biosystems, Foster City, CA, USA). The following forward (F) and reverse (R) primer pairs (Accession Nos given) were employed: aP2 (NM 001 442), (F) AAAGAAGTAGGAGTGGGCTTTGC; (R) CCCCATTCACACTGATGATCAT; C/EBPα (NM 004 364.2), (F) GGGTCTGAGACTCCCTTTCCTT; (R) CTCATTGGTCCCCCAGGAT; Cyclophilin B (M60857), (F) GGAGATGGCACAGGAGGAAA; (R) CGTAGTGCTTCAGTTTGAAGTTCTCA; LPL (NM 000 237.1), (F) CAGATGCCCTACAAAGTCTTCCA; (R) TGATTGGTATGGGTTTCACTCTCA; PGC1a (NM 013 261.2), (F) CCCAAGGGTTCCCCATTT; (R) TTAGGCCTGCAGTTCCAGAGA; PPARγ 2 (NM 015 869), (F) AGGCGAGGGCGATCTTG; (R) CCCATCATTAAGGAATTCATGTCATA. The expression levels of each mRNA were normalized to cyclophilin B, which has been used successfully as a housekeeping gene for comparative purposes in both in vitro and in vivo studies by our laboratory (Wu et al., 2007, 2008; Zvonic et al., 2006).
2.6. Lipolysis assay (n = 2 donors)
The hASCs were seeded in 24-well plates and equal number of wells were preconditioned in the absence or presence of EGF (10 ng/ml) and bFGF (10 ng/ml) for a period of 6 days. At that time, all hASCs were induced with adipogenic medium for 3 days without any EGF or bFGF supplementation and then fed with adipogenic maintenance medium 3 times/week. Twelve days following adipogenic induction, the hASCs were washed with DMEM/F-12 and left overnight in DMEM/F-12 supplemented with 0.1% bovine serum albumin (BSA). The following day, the adipocyte-differentiated hASCs were washed with phosphate-buffered saline, the medium in each well was replaced with 150 μl freshly prepared DMEM/F-12 containing 2% BSA and supplemented with increasing concentrations of isoproterenol (10−9–10−5 M; Sigma Chemical Co., St. Louis, MO, USA) or human atrial natriuretic peptide 1–28 (10−10–10−6 M; Bachem, King of Prussia, PA, USA) (Moro et al., 2004, 2005). Following a 3 h incubation at 37 °C and 5% CO2, the medium was removed and stored at −20 °C for spectrophotometric assay of glycerol release (Catalogue No. F6428, Sigma-Aldrich, St. Louis, MO, USA) (Moro et al., 2005), while the adherent cells were harvested in lysis buffer (150 mM NaCl, 20 mM Tris–HCl, pH 8.0, 1 mM EDTA, 2% SDS, 1% Igepal CA-630) for protein determination (BCA Protein Assay Kit, Pierce, Rockford, IL, USA). Glycerol release was normalized as μM/mg protein/3 h.
2.7. Glucose uptake assay (n = 1 donor)
Glucose uptake in hASCs was determined as described by Klip et al(1984). Briefly, hASCs were differentiated in 24-well plates in a manner identical to that used for the lipolysis assay. Twelve days following the induction of adipocyte differentiation, the hASCs were incubated overnight in serum-free low-glucose (1000 mg/l) DMEM medium (Catalogue No. SH30021.01, Hyclone, Logan, UT, USA) containing 1% BSA. The following day, the cultures were fed with serum-free medium with or without 100 nM insulin for 10 min at 37 °C, 5% CO2. The cells were then rinsed twice in KRPH buffer (5 mM Na2HPO4, 20 mM HEPES, pH 7.4, 1 mM MgSO4, 1 mM CaCl2, 137 mM NaCl and 4.7 mM KCl), and glucose uptake was assessed with 100 μM 2-deoxy-D-glucose [5 μCi/ml (1,2-3H)-2-deoxy-D-glucose from Perkin-Elmer Life Sciences, Boston, MA, USA) in KRPH for 7 min at 37 °C, 5% CO2. In control wells to monitor for non-specific uptake, 10 μM cytochalasin B was added at the same time as the glucose; subsequently, the non-specific CPM values were subtracted from the experimental points. The cells were then washed three times with PBS and lysed in 500 μl 0.2 N NaOH/well. Aliquots of 400 μl cell lysate were transferred to scintillation vials and radioactivity was counted. An aliquot of 50 μl cell lysate was used to determine protein concentration. The results were normalized by protein concentration and glucose uptake was expressed as ng/mg protein/min. All assays were performed in triplicate.
2.8. Statistics
Data are reported as the mean ± standard deviation (SD). Comparison between culture conditions was determined using Student’s t-test, where p < 0.05 was considered statistically significant.
3. Results
3.1. Effects of EGF and bFGF on cell proliferation
The addition of EGF and bFGF to the cell culture medium significantly increased the proliferation of cryopreserved hASCs in a dose-dependent manner (Table 2). The two growth factors acted in an additive manner. While hASCs grown in 10 ng/ml bFGF or 10 ng/ml EGF alone increased proliferation by 31% and 195%, respectively, relative to controls without growth factors, hASCs preconditioned in the presence of both 1 ng/ml EGF and 1 ng/ml bFGF increased by a factor of 242% relative to controls. In the absence of growth factors, the cultures achieved near confluency, with a mean of 33 281 hASCs/cm2, while in the presence of 10 ng/ml bFGF and EGF the cultures reached a mean density of 75 192 hASCs/cm2.
Table 2.
hASC proliferation in response to EGF and bFGF
| bFGF\EGF (ng/ml) | 0 ng/ml | 0.1 ng/ml | 1.0 ng/ml | 10 ng/ml |
|---|---|---|---|---|
| 0 | 1.00 ± 0.72 | 1.92 ± 0.81 | 2.39 ± 1.06 | 2.95 ± 0.77 |
| 0.1 | 0.97 ± 0.56 | 1.97 ± 0.50 | 2.94 ± 1.14 | 3.06 ± 0.64* |
| 1.0 | 1.29 ± 0.65* | 2.22 ± 0.36** | 3.42 ± 1.20** | 3.07 ± 0.82* |
| 10 | 1.31 ± 0.72* | 2.49 ± 0.92** | 3.40 ± 0.92** | 3.40 ± 1.04** |
Mean values of fold increase ± SD from control of mean number of cells per well are shown (n = 4 donors).
p < 0.05 or
p < 0.01 relative to 0 ng/ml bFGF and 0 ng/ml EGF based on paired, 1-tailed t-test.
3.2. Effects of EGF and bFGF on adipogenesis
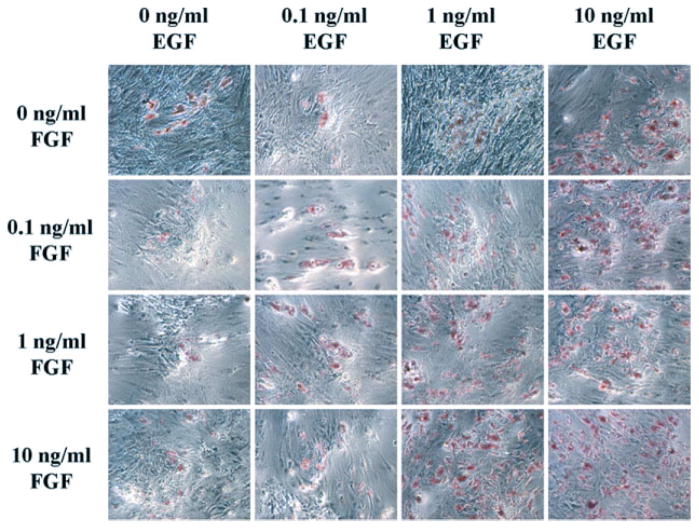
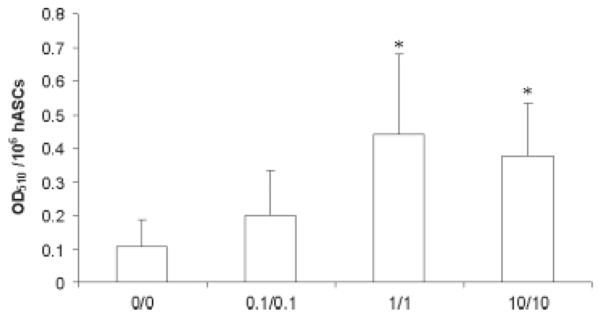
Preconditioning of hASCs with EGF and bFGF led to subsequent dose-dependent increases in oil red O staining of neutral lipids with adipogenic differentiation (Figure 1). The addition of EGF alone was more effective than equal concentrations of bFGF alone with respect to lipid accumulation (Figure 1, representative of n = 4 donors). The effects of the two growth factors were additive, as hASCs preconditioned in 10 ng/ml EGF or bFGF alone had less oil red O staining than hASCs preconditioned with 10 ng/ml of both growth factors. The combined EGF and bFGF induction of oil red O staining was independent of the effect of the growth factors on cell proliferation (Figure 2). When normalized for cell number, the extracted oil red O following adipogenesis increased by a mean factor of 2.4-, 5.6- and 5.3-fold in cells pre-conditioned in the presence of 0.1, 1.0 or 10 ng/ml of both growth factors relative to unconditioned cells, respectively (n = 3 donors; Figure 2).
Figure 1.
Effects of growth factors on adipogenesis. Photomicrographs of cells from one representative donor grown in 16 different EGF- and bFGF-supplemented conditions (EGF concentrations on x axis, bFGF concentrations on y axis) prior to induction of adipogenesis. Similar photomicrographs taken for three other donors are not shown. The cells are stained with oil red O. Magnification, ×100
Figure 2.
Effects of growth factors on oil red O staining. Human ASCs from n = 3 donors were preconditioned for 8 days in the absence or presence of EGF and bFGF at concentrations of 0.1, 1.0 or 10 ng/ml of both reagents. The hASCs under the different conditions were then induced to undergo adipogenesis. Following adipogenic differentiation for 9 days, the hASCs were stained with oil red O. The retained dye was eluted from the adherent cells and quantified by absorbance reading at OD510. Unfixed and unstained representative wells maintained under each of the EGF/bFGF preconditions and under the subsequent adipogenic culture condition were trypsinized and the total number of cells per well determined. The mean ± SD (n = 3 donors) oil red O absorbance was normalized relative to 106 hASCs, as indicated on the y axis. The OD510 values are displayed relative to the preconditioning EGF/bFGF concentrations (x axis)
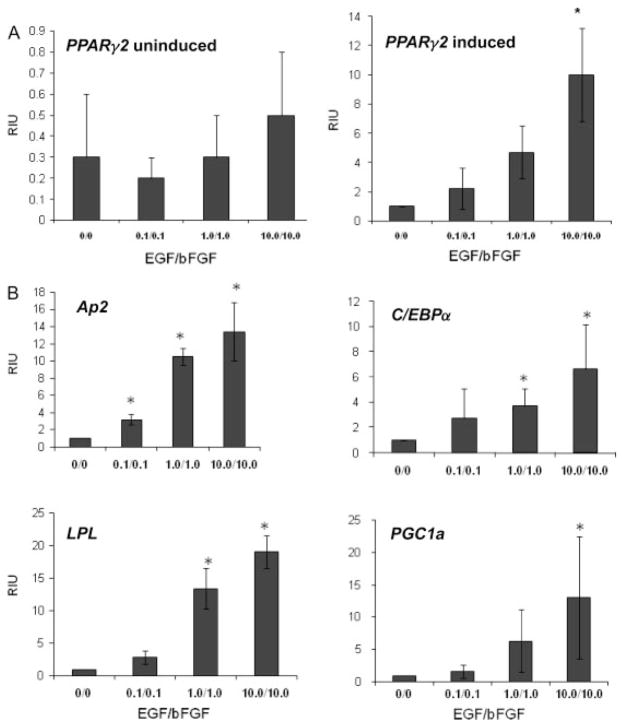
Preconditioning with EGF and bFGF induced dose-dependent increases in adipogenesis-related mRNAs (Figure 3). Significant increases occurred in the expression of mRNAs encoding aP2, C/EBPα, lipoprotein lipase (LPL), PPARγ and PPARγ co-activator-1 (PGC1) as a function of preconditioning and adipogenic induction. In the presence of 10 ng/ml EGF and bFGF, the maximum fold increase in mRNA levels relative to hASCs preconditioned in the absence of EGF and bFGF ranged from a low of ~6.6-fold (C/EBPα) to a high of 19-fold (LPL) (Figure 3). In the absence of adipogenic induction, the presence or absence of EGF and bFGF did not significantly increase any of the mRNA expression levels. The mRNA levels of aP2, C/EBPα, LPL and PGC1 in undifferentiated hASCs were expressed at levels three or more orders of magnitude less than that of the adipogenic-induced hASCs, independent of the EGF and bFGF preconditioning (data not shown). While the mRNA levels of PPARγ in undifferentiated hASCs showed a similar lack of response to EGF and bFGF preconditioning, the baseline level of the mRNA was higher than that of other adipogenic biomarkers. The PPARγ mRNA levels in undifferentiated hASCs were in the range 0.2–0.5-fold of that observed in the adipogenic induced hASCs preconditioned in the absence of EGF and bFGF. Together, these data document that EGF and bFGF preconditioning significantly increased adipogenesis following cryopreservation.
Figure 3.
Effects of growth factors on adipogenic mRNA levels. Results of quantitative PCR analysis for cells grown in EGF- and bFGF-supplemented conditions prior to the induction of adipogenesis. Quantities were normalized to cyclophilin B. Values are the mean ± SD fold increase for n = 3 donors. (A) Values for PPARγ2 mRNA from hASCs preconditioned with the indicated levels of EGF/bFGF in the absence (left panel) and presence (right panel) of adipogenic induction. All levels are expressed as fold increase relative to the level of hASCs preconditioned in the absence of EGF/bFGF and induced for adipogenesis; this value is defined as ‘1’ relative inductive unit (RIU) on the y axis. (B) Relative mRNA values for the adipogenic biomarkers aP2 (fatty acid binding protein 4), C/EBPα (CAAT/enhancer binding protein α), LPL (lipoprotein lipase), and PGC1 (PPARγ co-activator 1) in hASCs induced to undergo adipogenesis. All mRNA values for these same biomarkers in hASCs without adipogenic induction were two or three orders of magnitude lower (data not shown). Values on the x axis refer to concentrations of EGF/bFGF in ng/ml. Statistical significance relative to the hASC preconditioned in the absence of EGF and bFGF and induced to undergo adipogenesis is indicated by *(p < 0.05)
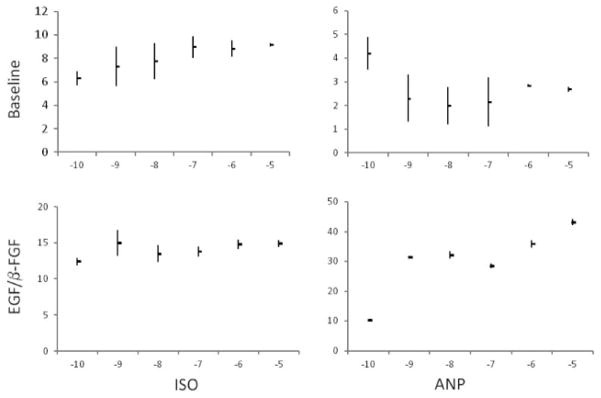
The mature adipocytes generated from hASCs preconditioned with maximal concentrations of EGF and bFGF (10 ng/ml) exhibited functionality based on lipolytic and glucose uptake assays. Atrial natriuretic peptide (ANP) increased lipolysis in a dose-dependent manner, based on glycerol release assay, by a factor of ca. four-fold from hASC adipocytes preconditioned with EGF and bFGF; however, ANP had little inductive effect on lipolysis in hASC adipocytes differentiated without EGF and bFGF preconditioning (Figure 4 Right Panel). In contrast, both populations of adipocytes increased lipolysis in response to the β-adrenergic agonist isoproterenol (Figure 4 Left Panel). Mature hASC adipocytes preconditioned with or without EGF and bFGF displayed comparable baseline levels of 2-deoxyglucose uptake (0.290 ± 0.031 and 0.299 ± 0.022 ng/mg/min, respectively); however, the hASCs preconditioned with EGF and bFGF exhibited a significant induction of glucose uptake (0.415 ± 0.077 ng/mg/min, +43%, p = 0.006) in the presence of insulin, while the unconditioned hASCs did not (0.315 ± 0.055, +5%, p = 0.275).
Figure 4.
Effects of EGF and FGF on lipolysis. Lipolytic response of adipocytes preconditioned prior to adipogenesis in the absence (top panel) or presence (bottom panel) of 10 ng/ml EGF and 10 ng/ml FGF were compared in response to increasing concentrations of ANP (right panels) and isoproterenol (left panels). Average lipolysis (with maximum and minimum values) for n = 2 donors is shown
4. Discussion
This study demonstrates that the use of EGF and FGF supplementation during the expansion culture of undifferentiated cryopreserved hASCs improves their proliferation rate and subsequent adipogenic differentiation and functionality. These actions may be inter-related. The improved proliferative capacity of the cryopreserved hASCs may allow them to achieve a greater density, thereby improving their subsequent adipogenic differentiation upon exposure to inductive agents. The additive effects of EGF and bFGF permit their use in combination at low concentrations to achieve significant results, as opposed to the more costly use of high concentrations of a single growth factor alone. Our results support the original studies by Hauner et al. (1995), which found that EGF had notable effects on the growth and development of hASCs at physiological concentrations (0.5–2 ng/ml). Moreover, our findings confirm and extend a recent report by Suga et al. (2007), which demonstrated that the individual components of a commercial endothelial growth medium (EGF and bFGF) elicited less robust hASC proliferation than the endothelial growth medium itself. Likewise, studies in the murine 3T3-L1 preadipocyte model have demonstrated that EGF supplementation, when added subsequent to induction, enhanced adipogenesis (Adachi et al., 1994). Finally, these studies extend those of Shoshani et al. (2001) and Moscatello et al. (2005), which demonstrated the feasibility of maintaining viable intact human adipose tissue by cryopreservation. Observations made using the human bone marrow MSC model are comparable. Tamama et al. (2006) found that EGF supplementation increased the expansion of BMSCs for in vivo transplantation. Martin et al. (1997) observed that bFGF increased the size of individual BMSC clones by 2.5-fold in vitro, consistent with enhanced proliferation. Furthermore, they found that bFGF preconditioning increased the ability of BMSCs to mineralize their extracellular matrix in vitro and to form bone in vivo, suggesting that bFGF preconditioning enhanced the differentiation potential of the BMSCs (Martin et al., 1997). Recently, Stewart et al. (2007) have shown that chondrogenesis was enhanced by bFGF in equine BMSCs. Thus, these growth factors have been found to modulate ASC and BMSC proliferation and/or differentiation in multiple species.
Stromal medium supplementation with EGF and bFGF maintained the differentiation potential of cryopreserved hASCs following expansion and enhanced their subsequent adipogenic response. A dose-dependent increase in qualitative oil red O staining was observed in treated hASCs when compared to their control. PCR analysis in these same experiments demonstrated EGF- and bFGF-dependent increases in mRNA levels encoding adipogenic transcription factors (C/EBPα, PPARγ, PGC1) as well as their downstream targets (aP2, LPL). In addition to providing improved adipogenesis, EGF and bFGF supplementation produced adipocytes with equivalent or improved functionality relative to controls. The hASC-differentiated adipocytes preconditioned with EGF and bFGF displayed enhanced insulin sensitivity based on glucose uptake. While the lipolytic response to a β-adrenergic agent was equivalent in the treated and untreated hASCs, the treated hASCs displayed an enhanced lipolytic response to ANP. Recent studies have demonstrated that ANP, like isoproterenol, is a potent lipolytic stimulus (Moro et al., 2004, 2005). It remains to be determined whether EGF and bFGF act at the level of the ANP receptor or of downstream elements in its signal transduction pathway. Nevertheless, these findings demonstrate that the inclusion of EGF and bFGF during hASCs expansion improve their adipogenic response and the potential functionality of the differentiated adipocytes in vitro.
In conclusion, EGF/bFGF preconditioning at concentrations in the range 1–10 ng/ml prior to, but not during, induction of differentiation led to a rapid expansion of hASCs and improved their subsequent adipogenic differentiation. Further studies will be necessary to fully characterize the in vitro and in vivo effects of EGF and bFGF prior to using the two growth factors in clinical applications, but the above lines of evidence support the use of EGF and bFGF as media supplements to improve cell proliferation and adipogenesis of thawed, cryopreserved hASCs.
Acknowledgments
This work was supported in part by support (to J.M.G. and X.W.) from the Pennington Biomedical Research Center, Pennington Biomedical Research Foundation, and a CNRU Center Grant (No. 1P30 DK072476, to J.M.G and G.Y.) entitled ‘Nutritional Programming: Environmental and Molecular Interactions’, sponsored by NIDDK. The authors wish to thank Ms Laura Dallam for excellent editorial assistance.
Footnotes
Conflict of interest statements
Dr Gimble consults or collaborates with the following tissue engineering-related companies: Toucan Capital, Cognate Bioservicese, Vesta Therapeutics and Zen-Bio.
References
- Adachi H, Kurachi H, Homma H, et al. Epidermal growth factor promotes adipogenesis of 3T3-L1 cell in vitro. Endocrinology. 1994;135:1824–1830. doi: 10.1210/endo.135.5.7956906. [DOI] [PubMed] [Google Scholar]
- Butterwith SC, Peddie CD, Goddard C. Regulation of adipocyte precursor DNA synthesis by acidic and basic fibroblast growth factors: interaction with heparin and other growth factors. J Endocrinol. 1993;137:369–374. doi: 10.1677/joe.0.1370369. [DOI] [PubMed] [Google Scholar]
- de Boer F, Drager AM, Pinedo HM, et al. Extensive early apoptosis in frozen-thawed CD34-positive stem cells decreases threshold doses for haematological recovery after autologous peripheral blood progenitor cell transplantation. Bone Marrow Transpl. 2002;29:249–255. doi: 10.1038/sj.bmt.1703357. [DOI] [PubMed] [Google Scholar]
- Devireddy RV, Thirumala S, Gimble JM. Cellular response of adipose-derived passage-4 adult stem cells to freezing stress. J Biomech Eng. 2005;127:1081–1086. doi: 10.1115/1.2073673. [DOI] [PubMed] [Google Scholar]
- Goh BC, Thirumala S, Kilroy G, et al. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1:322–324. doi: 10.1002/term.35. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- Guilak F, Lott KE, Awad HA, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- Halvorsen YC, Wilkison WO, Gimble JM. Adipose-derived stromal cells – their utility and potential in bone formation. Int J Obesity Relat Metab Disord. 2000;24(suppl 4):S41–44. doi: 10.1038/sj.ijo.0801503. [DOI] [PubMed] [Google Scholar]
- Halvorsen YD, Bond A, Sen A, et al. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: biochemical, cellular, and molecular analysis. Metabolism. 2001;50:407–413. doi: 10.1053/meta.2001.21690. [DOI] [PubMed] [Google Scholar]
- Hauner H, Rohrig K, Petruschke T. Effects of epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) on human adipocyte development and function. Eur J Clin Invest. 1995;25:90–96. doi: 10.1111/j.1365-2362.1995.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Iwashima S, Ozaki T, Maruyama S, et al. Novel culture system of mesenchymal stromal cells from human subcutaneous adipose tissue. Stem Cells Dev. 2008;18:533–543. doi: 10.1089/scd.2008.0358. [DOI] [PubMed] [Google Scholar]
- Keung YK, Cobos E, Morgan D, et al. High cellular concentration of peripheral blood progenitor cells during cryopreservation adversely affects CFU-GM but not hematopoietic recovery. J Hematother. 1996;5:73–77. doi: 10.1089/scd.1.1996.5.73. [DOI] [PubMed] [Google Scholar]
- Kilroy GE, Foster S, Wu X, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- Klip A, Li G, Logan WJ. Induction of sugar uptake response to insulin by serum depletion in fusing L6 myoblasts. Am J Physiol. 1984;247:E291–296. doi: 10.1152/ajpendo.1984.247.3.E291. [DOI] [PubMed] [Google Scholar]
- Kotobuki N, Hirose M, Takakura Y, et al. Cultured autologous human cells for hard tissue regeneration: preparation and characterization of mesenchymal stem cells from bone marrow. Artif Organs. 2004;28:33–39. doi: 10.1111/j.1525-1594.2004.07320.x. [DOI] [PubMed] [Google Scholar]
- Kurachi H, Adachi H, Ohtsuka S, et al. Involvement of epidermal growth factor in inducing obesity in ovariectomized mice. Am J Physiol. 1993;265:E323–331. doi: 10.1152/ajpendo.1993.265.2.E323. [DOI] [PubMed] [Google Scholar]
- Locklin RM, Oreffo RO, Triffitt JT. Effects of TGFβ and bFGF on the differentiation of human bone marrow stromal fibroblasts. Cell Biol Int. 1999;23:185–194. doi: 10.1006/cbir.1998.0338. [DOI] [PubMed] [Google Scholar]
- Markov V, Kusumi K, Tadesse MG, et al. Identification of cord blood-derived mesenchymal stem/stromal cell populations with distinct growth kinetics, differentiation potentials, and gene expression profiles. Stem Cells Dev. 2007;16:53–73. doi: 10.1089/scd.2006.0660. [DOI] [PubMed] [Google Scholar]
- Martin I, Muraglia A, Campanile G, et al. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138:4456–4462. doi: 10.1210/endo.138.10.5425. [DOI] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, et al. The immunophenotype of human adipose derived cells: temporal changes in stromal- and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Moro C, Galitzky J, Sengenes C, et al. Functional and pharmacological characterization of the natriuretic peptide-dependent lipolytic pathway in human fat cells. J Pharmacol Exp Ther. 2004;308:984–992. doi: 10.1124/jpet.103.060913. [DOI] [PubMed] [Google Scholar]
- Moro C, Polak J, Richterova B, et al. Differential regulation of atrial natriuretic peptide- and adrenergic receptor-dependent lipolytic pathways in human adipose tissue. Metabolism. 2005;54:122–131. doi: 10.1016/j.metabol.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Moscatello DK, Dougherty M, Narins RS, et al. Cryopreservation of human fat for soft tissue augmentation: viability requires use of cryoprotectant and controlled freezing and storage. Dermatol Surg. 2005;31:1506–1510. doi: 10.2310/6350.2005.31235. [DOI] [PubMed] [Google Scholar]
- Quarto N, Longaker MT. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 2006;12:1405–1418. doi: 10.1089/ten.2006.12.1405. [DOI] [PubMed] [Google Scholar]
- Quarto N, Wan DC, Longaker MT. Molecular mechanisms of FGF-2 inhibitory activity in the osteogenic context of mouse adipose-derived stem cells (mASCs) Bone. 2008;42:1040–1052. doi: 10.1016/j.bone.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- Rider DA, Dombrowski C, Sawyer AA, et al. Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells. 2008;26:1598–1608. doi: 10.1634/stemcells.2007-0480. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Ladage D, Schinkothe T, et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24:1750–1758. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- Shoshani O, Ullmann Y, Shupak A, et al. The role of frozen storage in preserving adipose tissue obtained by suction-assisted lipectomy for repeated fat injection procedures. Dermatol Surg. 2001;27:645–647. doi: 10.1046/j.1524-4725.2001.00146.x. [DOI] [PubMed] [Google Scholar]
- Skurk T, Ecklebe S, Hauner H. A novel technique to propagate primary human preadipocytes without loss of differentiation capacity. Obesity (Silver Spring) 2007;15:2925–2931. doi: 10.1038/oby.2007.349. [DOI] [PubMed] [Google Scholar]
- Stewart AA, Byron CR, Pondenis H, et al. Effect of fibroblast growth factor-2 on equine mesenchymal stem cell monolayer expansion and chondrogenesis. Am J Vet Res. 2007;68:941–945. doi: 10.2460/ajvr.68.9.941. [DOI] [PubMed] [Google Scholar]
- Stylianou J, Vowels M, Hadfield K. Novel cryoprotectant significantly improves the post-thaw recovery and quality of HSC from CB. Cytotherapy. 2006;8:57–61. doi: 10.1080/14653240500501021. [DOI] [PubMed] [Google Scholar]
- Suga H, Shigeura T, Matsumoto D, et al. Rapid expansion of human adipose-derived stromal cells preserving multipotency. Cytotherapy. 2007;9:738–745. doi: 10.1080/14653240701679873. [DOI] [PubMed] [Google Scholar]
- Tamama K, Fan VH, Griffith LG, et al. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:686–695. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- Thirumala S, Zvonic S, Floyd E, et al. Effect of various freezing parameters on the immediate post-thaw membrane integrity of adipose tissue derived adult stem cells. Biotechnol Prog. 2005a;21:1511–1524. doi: 10.1021/bp050007q. [DOI] [PubMed] [Google Scholar]
- Thirumala SG, Gimble JM, Devireddy RV. Transport phenomena during freezing of adipose tissue-derived adult stem cells. Biotechnol Bioeng. 2005b;92:372–383. doi: 10.1002/bit.20615. [DOI] [PubMed] [Google Scholar]
- Vashi AV, Abberton KM, Thomas GP, et al. Adipose tissue engineering-based on the controlled release of fibroblast growth factor-2 in a collagen matrix. Tissue Eng. 2006;12:3035–3043. doi: 10.1089/ten.2006.12.3035. [DOI] [PubMed] [Google Scholar]
- Wu X, Yu G, Parks H, et al. Circadian mechanisms in murine and human bone marrow mesenchymal stem cells following dexamethasone exposure. Bone. 2008;42:861–870. doi: 10.1016/j.bone.2007.12.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zvonic S, Floyd ZE, et al. Induction of circadian gene expression in human subcutaneous adipose-derived stem cells. Obesity (Silver Spring) 2007;15:2560–2570. doi: 10.1038/oby.2007.308. [DOI] [PubMed] [Google Scholar]
- Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006;24:2412–2419. doi: 10.1634/stemcells.2006-0006. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Conrad SA, et al. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]