Abstract
While saliva is a source of easily accessible bodily fluids, there has been little effort to study its value in cancer diagnosis. We hypothesized that certain proteins would be elevated in the saliva of patients with breast cancer. Our study included 49 healthy individuals and 49 breast cancer patients. The levels of vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and carcinoembryonic antigen (CEA) in the saliva were measured with enzyme-linked immunosorbent assay (ELISA). We observed that salivary fluid protein levels were significantly elevated in cancer patients as follows: i) VEGF, 3.7±1.6 in cancer versus 2.1±1.2 ng/ml in control (p<0.0001); ii) EGF, 3.7±1.7 versus 2.1±1.3 ng/ml (p<0.0001); and iii) CEA, 83±31 versus 66.1±27.1 ng/ml (p=0.0106). The areas under the receiver operating characteristic (ROC) curve (AUC) were 80, 77 and 65%, respectively. The best prediction was from the combination of salivary VEGF and EGF with a sensitivity of 83%, specificity of 74% and AUC of 84%. We conclude that saliva is a novel avenue for tumor marker research and deserves further studies. Saliva may potentially be useful in supplementing current methods of breast cancer detection.
Keywords: saliva, breast cancer, tumor marker, vascular endothelial growth factor, epidermal growth factor, carcinoembryonic antigen
Introduction
Breast cancer is the most common form of cancer and the second leading cause of cancer deaths in women in the US. In 2007, approximately 180,510 patients were estimated to have been diagnosed with invasive breast cancer, and an estimated 40,910 will die of this disease. Furthermore, approximately 62,030 females will be newly diagnosed with in situ breast carcinoma (1). Early detection has been credited for a small decrease in age-adjusted breast cancer mortality. However, the current standard diagnostic/screening tests for breast cancer, including physical exams and mammograms, are not perfect. Thus, there is much active research in developing novel methods to improve early detection.
Serum tumor markers, such as carcinoembryonic antigen (CEA) and CA15-3 or CA27–29, are used in current clinical practice to assess widespread disease or to detect recurrent breast cancer, but not to detect new breast cancer (2). Many researchers are using a number of new technologies, such as proteomics or DNA/RNA arrays, to discover novel markers in the blood (3,4). While saliva is a source of easily accessible bodily fluids, there has been very little effort to study salivary fluid. We hypothesized that a profile of angiogenic and tumor markers in saliva could be complementary to the current methods used for breast cancer diagnosis. In this pilot study, we set out to determine whether the levels of certain growth/tumor marker(s) is/are correlated with breast cancer. We studied vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) because they are potent angiogenic factors with successful targeted therapeutic agents already approved by the FDA, such as Avastin and Tarceva (5,6). We also measured CEA, a well-established serum tumor marker for breast cancer. We observed that the levels of the above proteins in the saliva are elevated in breast cancer patients in comparison to normal controls. Thus, it is possible that saliva may serve as a novel avenue in the search for breast tumor markers.
Patients and methods
Subjects
Subject recruitment and sample collection were within the guidelines of the Institutional Review Board at the University of California Los Angeles Medical Center (UCLA). The inclusion criteria for the cancer group were as follows: i) capable of giving informed consent; ii) not pregnant or lactating; iii) no active oral/dental disease; iv) no prior (within 2 years) or concurrent non-breast malignancies, except for non-melanomatous skin cancers, carcinoma in situ of the cervix, or benign tumors such as adenomas; and v) a diagnosis of breast cancer. These patients were enrolled prior to definitive surgery for the excision of the tumor. The control subjects were healthy volunteers recruited from both the dental and medical centers at UCLA.
Saliva collection
Unstimulated whole saliva samples were collected according to previously established protocols (7,8). Subjects were asked to refrain from eating, drinking, smoking or oral hygiene procedures for ~30 min prior to collection. The lip area was cleaned, and each subject rinsed her mouth once with plain water. Typically, patients donated ~5–10 ml of saliva. Samples were then centrifuged at 2,600 × g for 15 min at 4°C. The supernatant was then stored at −80°C until use. Of note, a protease inhibitor cocktail containing 1 μl aprotinin, 10 μl PMSF (phenylmethanesulfonyl fluoride) and 3 μl sodium orthovanadate (all from Sigma, St. Louis, MO) was added to each 1-ml saliva sample.
ELISA analysis
Measurement of protein factors was performed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. ELISAs for VEGF and EGF were purchased from R&D (Minneapolis, MN), and from Biomeda Corp. (Foster City, CA) for CEA. The minimum detectable levels were as follows: 9 pg/ml for VEGF, 0.7 pg/ml for EGF and 1.5 ng/ml for CEA. The dilutions were as follows: 1:8 for VEGF, 1:25 for EGF and 1:4 for CEA, using the sample diluents provided. The signals were read on a Biotek microplate reader (Winooski, VT).
Data analysis
To assess the association of the level of protein expression with breast cancer, the means and standard deviations of salivary factors were calculated separately for each group of subjects, and the groups were compared using the Wilcoxon rank sum test. Multiple regression analysis was also performed to consider the potential effect of age and ethnicity (results not shown). For example, the regression model for each of the three proteins was constructed with age, ethnicity and cancer/normal group as independent variables.
To evaluate the predictive power of each of the peptides, receiver operating characteristic (ROC) curve analysis was conducted on the simple logistic models with protein expression as an independent variable and the cancer/control group as a dependent variable. We used forward stepwise logistic regression to construct a model for disease status prediction using combinations of markers. The best model was then evaluated using ROC analysis. Subsequently, the area under the curve (AUC) was computed via numerical integration of the ROC curves. The factor or combination of factors that had the largest AUC was identified as having the strongest predictive power for the detection of breast cancer.
Results
Subject characteristics
All subjects were female. Table I summarizes the characteristics of the 49 control subjects and 49 breast cancer patients. There was no significant difference between the healthy controls and cancer patients in regards to number, tobacco use, diabetes, hepatitis or HIV status. The mean age of the controls was lower than that of the breast cancer patients (41.4±12.4 versus 54.8±11.2 years, p<0.0001). Race was also a significant factor (p=0.0116). In the cancer group, 6 patients had stage 0 (DCIS-ductal carcinoma in situ). Of the 43 invasive cancer cases, all except one had final pathologic staging as follows: 1 patient had a local recurrence, 14 patients had stage 1, 16 stage 2, 8 stage 3 (one of whom had only residual DCIS at the time of saliva collection after neoadjuvant chemotherapy), and 3 had stage 4.
Table I.
Subject characteristics.
| Healthy controls | Cancer patients | p-value | |
|---|---|---|---|
| Number | 49 | 49 | NS |
| Age, mean ± SD (years) | 41.4±12.4 | 54.8±11.2 | <0.0001a |
| Race | 0.0116b | ||
| Caucasian (including Hispanic) | 25 (51%) | 39 (80%) | |
| Black | 11 (22%) | 4 (8%) | |
| Asian | 13 (27%) | 6 (12%) | |
| Tobacco use | 2 | 3 | NS |
| Diabetes | 2 | 2 | NS |
| Hepatitis | 0 | 2 | NS |
| HIV | 0 | 0 | NS |
Wilcoxon test,
χ2 test; NS, not significant.
Salivary protein levels
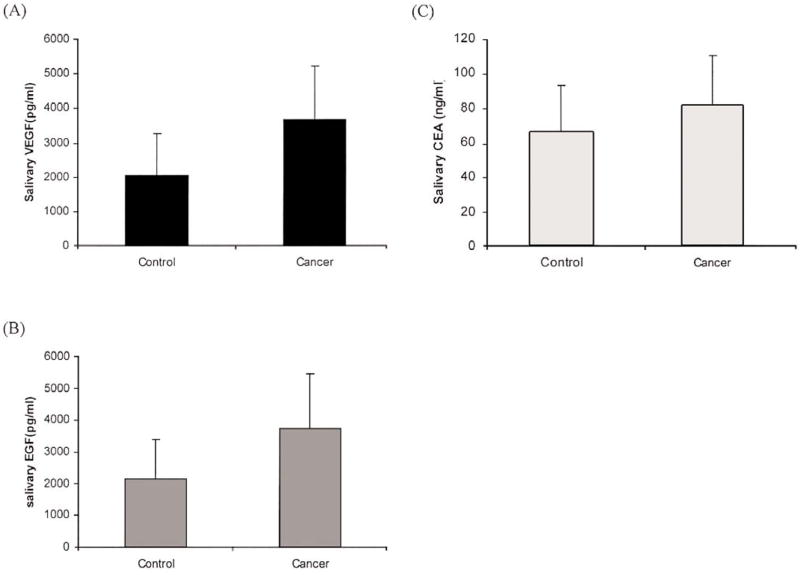
Salivary fluid protein levels for the cancer and control groups are listed in Table IIA. The Wilcoxon test showed that there was a significant difference between breast cancer patients and the control group in terms of protein expression levels. To consider the potential effect of age and ethnicity, multiple logistic model analysis was also performed (results not shown) on the log-transformed expression score. Our results were consistent with previous findings which showed that, after including age and ethnicity in the model, significant positive associations between each protein and cancer remained. The breast cancer patients had higher levels of the three proteins compared to those in the control group (Fig. 1).
Table II.
Salivary fluid protein levels and the predictive power of each protein individually and in combination.
| A, Wilcoxon test for each salivary protein (mean ± SD) (ng/ml). | |||
|---|---|---|---|
| Healthy controls | Cancer patients | p-value | |
| VEGF | 2.1±1.2 | 3.7±1.6 | <0.0001 |
| EGF | 2.1±1.3 | 3.7±1.7 | <0.0001 |
| CEA | 66.1±27.1 | 83.0±31.0 | 0.0106 |
| B, ROC curve analysis on the logistic regression models. | |||
|---|---|---|---|
| AUC (%) | Sensitivity (%) | Specificity (%) | |
| VEGF | 80 | 74 | 73 |
| EGF | 77 | 78 | 68 |
| CEA | 65 | 70 | 56 |
| VEGF + EGF | 84 | 83 | 74 |
AUC, area under the curve.
Figure 1.
The levels of (A) VEGF, (B) EGF and (C) CEA in control subjects and breast cancer patients (mean ± SD).
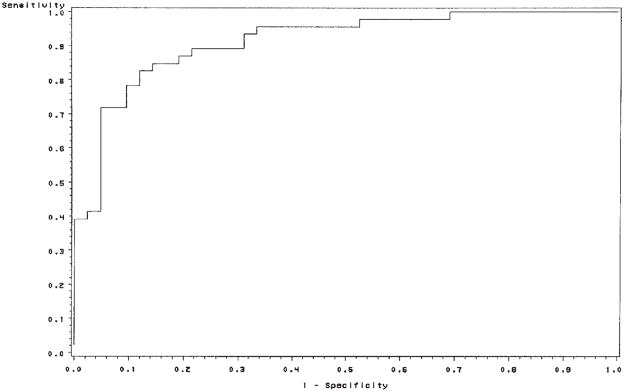
To evaluate the predictive power of each protein individually and in combination, we first found the best logistic regression model by the stepwise model selection method. This method revealed that the logistic model, including the VEGF and EGF proteins together, fit the data best. Then ROC analysis was performed on four logistic models with each protein separately along with the best combination in Table IIB. The AUCs for VEGF, EGF and CEA were 80, 77 and 65%, respectively. The sensitivity and specificity were as follows: 74 and 73% for VEGF, 78 and 68% for EGF, and 70 and 56% for CEA. The best combination was salivary VEGF plus EGF with 83% sensitivity, 74% specificity and AUC 84%. The corresponding ROC curve is shown in Fig. 2.
Figure 2.
ROC curve of salivary VEGF and EGF values in specimens from control subjects and breast cancer patients. Area under the ROC curve, 84%.
Discussion
We report here for the first time that VEGF and CEA levels are significantly increased in the saliva of breast cancer patients in comparison with healthy control subjects. The most potent angiogenic factor VEGF was previously detected in the saliva of healthy individuals (9,10). We also observed elevated EGF levels, which is consistent with a study conducted by the Navarro group in Spain (11). In the US, the Streckfus group reported that Her-2 and CA15-3 levels are elevated in cancer versus control subject saliva (12,13). Streckfus and Bigler showed that salivary Her-2 exhibits a significant difference between pre- and post-therapy values (14). This group has recently proteomically profiled additional salivary breast cancer candidate biomarkers (15).
The finding of elevated angiogenic factors in saliva is consistent with the fact that the process of angiogenesis, i.e., the formation of new blood vessels, plays a critical role in breast tumor growth and metastasis (16). Since many angiogenic factors have been identified and sequenced, we were among the first to ask whether the level of any of these factors could be detected in bodily fluids, and whether their levels would have any clinical relevance in cancer diagnostics and monitoring (17–19). These angiogenic molecules are either released by the tumor cells themselves (20), mobilized from the extracellular matrix and/or released by host cells, such as macrophages recruited into the tumor. Studies by our laboratory and by other institutions showed that angiogenic factors can be significantly elevated in the serum and urine of breast cancer patients. The levels of certain angiogenic factors have been shown to correlate with the disease stage of the tumor (21).
This pilot study constitutes a phase II validation study within the guidelines set forth by the NCI Early Detection Research Network (EDRN) (22). The next step is to conduct a phase III blinded detection trial with a large number of new cases of breast cancer and control subjects to determine the robustness of VEGF, EGF and CEA in predicting and discriminating saliva from controls versus breast cancer patients.
In summary, we conclude that saliva is a novel avenue for tumor marker research in breast cancer and deserves further study. Saliva can be obtained non-invasively, sparing the patient unpleasant needles from blood drawing procedures. We do not expect a fluid-based tumor marker test to replace the standard screening physical exam and mammogram. However, we can envision at least one possible scenario where a new salivary test may potentially enhance our ability to detect breast cancer early, when it is still curable with existing treatment methods. The salivary avenue of research may prove to be just as useful as studies searching for biomarkers in the blood.
Acknowledgments
Research was performed at UCLA, Los Angeles, CA and supported by PHS grants to D.T. Wong (R01 DE15970, U01 DE16275).
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 3.Posadas EM, Simpkins F, Liotta LA, MacDonald C, Kohn EC. Proteomic analysis for the early detection and rational treatment of cancer - realistic hope? Ann Oncol. 2005;16:16–22. doi: 10.1093/annonc/mdi004. [DOI] [PubMed] [Google Scholar]
- 4.Anker P, Mulcahy H, Stroun M. Circulating nucleic acids in plasma and serum as a noninvasive investigation for cancer: time for large-scale clinical studies? Int J Cancer. 2003;103:149–152. doi: 10.1002/ijc.10791. [DOI] [PubMed] [Google Scholar]
- 5.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 6.Byrne BJ, Garst J. Epidermal growth factor receptor inhibitors and their role in non-small-cell lung cancer. Curr Oncol Rep. 2005;7:241–247. doi: 10.1007/s11912-005-0045-6. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, St John MAR, Zhou X, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10:8442–8450. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 8.Navazesh M. Methods for collecting saliva. Ann NY Acad Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 9.Pammer J, Weninger W, Mildner M, Burian M, Wojta J, Tschachler E. Vascular endothelial growth factor is constitutively expressed in normal human salivary glands and is secreted in the saliva of healthy individuals. J Pathol. 1998;186:186–191. doi: 10.1002/(SICI)1096-9896(1998100)186:2<186::AID-PATH148>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Taichman NS, Cruchley AT, Fletcher LM, et al. Vascular endothelial growth factor in normal human salivary glands and saliva: a possible role in the maintenance of mucosal homeostasis. Lab Invest. 1998;78:869–875. [PubMed] [Google Scholar]
- 11.Navarro MA, Mesia R, Diez-Gilbert O, Rueda AN, Ojeda B, Alonso MC. Epidermal growth factor in plasma and saliva of patients with active breast cancer and breast cancer patients in follow-up compared with healthy women. Breast Cancer Res Treat. 1997;42:83–86. doi: 10.1023/a:1005755928831. [DOI] [PubMed] [Google Scholar]
- 12.Streckfus C, Bigler L, Tucci M, Thigpen JT. A preliminary study of CA15-3, c-erbB-2, epidermal growth factor receptor, cathepsin-D, and p53 in saliva among women with breast carcinoma. Cancer Invest. 2000;18:101–109. doi: 10.3109/07357900009038240. [DOI] [PubMed] [Google Scholar]
- 13.Streckfus C, Bigler L, Dellinger T, Dai X, Kingman A, Thigpen JT. The presence of soluble c-erbB-2 in saliva and serum among women with breast carcinoma: a preliminary study. Clin Cancer Res. 2000;6:1363–1370. [PubMed] [Google Scholar]
- 14.Streckfus C, Bigler L. The use of soluble, salivary c-erbB-2 for the detection and post-operative follow-up of breast cancer in women: the results of a five-year translational research study. Adv Dent Res. 2005;18:17–24. doi: 10.1177/154407370501800105. [DOI] [PubMed] [Google Scholar]
- 15.Streckfus CF, Mayorga-Wark O, Arreola D, Edwards C, Bigler L, Dubinsky WP. Breast cancer related proteins are present in saliva and are modulated secondary to ductal carcinoma in situ of the breast. Cancer Invest. 2008;26:159–167. doi: 10.1080/07357900701783883. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen M, Watanabe H, Budson A, Richie J, Hayes D, Folkman J. Elevated levels of an angiogenic peptide, basic fibroblast growth factor, in the urine of patients with a wide spectrum of cancers. J Natl Cancer Inst. 1994;86:356–361. doi: 10.1093/jnci/86.5.356. [DOI] [PubMed] [Google Scholar]
- 18.Sartippour MR, Zhang L, Lu M, Wang HJ, Brooks MN. Nipple fluid basic fibroblast growth factor in patients with breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2995–2998. doi: 10.1158/1055-9965.EPI-05-0412. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Wang JL, Chang H, Barsky SH, Nguyen M. Breast cancer diagnosis with nipple fluid bFGF. Lancet. 2000;356:567. doi: 10.1016/S0140-6736(00)02587-3. [DOI] [PubMed] [Google Scholar]
- 20.Soutter A, Nguyen M, Watanabe H, Folkman J. Basic fibro-blast growth factor secreted by an animal tumor is detectable in urine. Cancer Res. 1993;53:5297–5299. [PubMed] [Google Scholar]
- 21.Nguyen M. Angiogenic factors as tumor markers. Invest New Drugs. 1997;15:29–37. doi: 10.1023/a:1005766511385. [DOI] [PubMed] [Google Scholar]
- 22.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]