Abstract
Accumulating data have implicated the selenium-containing cytosolic glutathione peroxidase, GPx-1 as a determinant of cancer risk and a mediator of the chemopreventive properties of selenium. Genetic variants of GPx-1 have been shown to be associated with cancer risk for several types of malignancies. To investigate the relationship between GPx-1 enzyme activity and genotype, we measured GPx-1 enzyme activity and protein levels in human lymphocytes as a function of the presence of two common variations, a leucine/proline polymorphism at codon 198 and a variable number of alanine repeat codons. Differences in GPx activity among these cell lines, as well as in the response to the low-level supplementation of the media with selenium, indicated that factors other than just genotype are significant in determining activity. To restrict the study to genotypic effects, human MCF-7 cells were engineered to exclusively express allelic variants representing a combination of either a codon 198 leucine or proline and either 5 or 7 alanine repeat codons following transfection of GPx-1 expression constructs. Transfectants were selected and analyzed for GPx-1 enzyme activity and protein levels. GPx-1 with 5 alanines and a leucine at codon 198 showed a significantly higher induction when cells were incubated with selenium and showed a distinct pattern of thermal denaturation as compared to GPx-1 encoded by the other examined alleles. The collective data obtained using both lymphocytes and MCF-7 indicate both intrinsic and extrinsic factors cooperate to ultimately determine the levels of this enzyme available to protect cells against DNA damage and mutagenesis.
Keywords: selenium, selenoproteins, glutathione peroxidase, polymorphisms
Introduction
There is considerable interest in developing strategies that prevent cancer that involve minimal risk or toxicity. Selenium is an essential trace element for which there are many years of animal studies showing that low, non-toxic supplementation with either organic and inorganic forms could reduce cancer incidence following exposure to a wide variety of carcinogens (1). In humans, several studies have shown that there is an inverse association between nutritional selenium intake and cancer risk, although not all reports have indicated this relationship (2). The promise of selenium supplementation above that obtained in the diet as a means of reducing cancer risk was stimulated by the results of the study conducted by the National Prevention of Cancer (NPC) Study Group that reported that supplementation with 200 μg/day of selenium as selenized yeast reduced cancer incidence (3, 4). Follow-up analyses to this study have indicated that those with the lowest base-line levels of selenium exhibited the greatest benefit (4). This study, along with the wealth of in vitro and animal data supporting a potential chemopreventive role for selenium, has led to the large cancer prevention trial (SELECT) investigating the efficacy of selenium, both alone and in combination with vitamin E, in reducing prostate cancer incidence (5). However, this trial was recently terminated early due to the apparent lack of protection by selenium (6).
Selenium exists within the cell as both non-protein metabolites and selenoproteins containing the UGA-encoded amino acid selenocysteine, often located at the protein's active site (7, 8). Non-protein forms of selenium have been shown to have anti-tumor activity in animal models and to influence the expression of signaling pathways involved in the carcinogenic process (9). Alternatively, selenium may influence cancer incidence by its effects on selenoprotein levels or function (10). Studies to investigate the role of selenoproteins independent of selenium status were greatly facilitated by the development of the i6A− transgenic mouse model (11). These animals express reduced selenoprotein levels due to the ectopic expression of a mutant selenocysteine tRNA gene, but do not exhibit overt signs of pathology unless further challenged. However, i6A− mice demonstrated accelerated development of prostate pathology indicative of carcinogenesis as compared to control mice (12) and are more susceptible to the development of aberrant crypt foci, a colonic pre-neoplastic lesion, following the administration of carcinogen (13).
Of the 25 selenoproteins shown to be encoded by the human genome (14), genetic data has implicated the selenium-dependent, cytosolic form of glutathione peroxidase (GPx-1) in cancer etiology. The gene for GPx-1 is frequently deleted during cancer development, with loss of heterozygosity being demonstrated in breast, colon and lung cancers as well as cancers of the head and neck (10). In addition, common polymorphisms in the GPx-1 gene have been shown to be associated with cancer risk. A codon 198 variation resulting in either a leucine (leu) or proline (pro) at this position has been described (15). The risk of cancer of the bladder, lung, breast and liver has been positively associated with the leu allele (16) and lung cancer patients harboring the leu allele were shown to excrete higher levels of the DNA oxidation product 8-OH-dG in their urine (17). Not all studies have shown the relationship between the codon 198 polymorphism and cancer risk and it is notable that the association between the leu allele and cancer risk was reported for liver and breast cancer, but only when present in conjunction with an allele for another at-risk polymorphism in the gene for MnSOD (18, 19). Another GPx-1 variation, a variable number of alanine-repeat codons in the amino terminus of the protein has been associated with higher risk of cancer as well (20-23).
The data summarized above indicate a possible role of the GPx-1 selenium-containing protein in cancer etiology. Since the activity of this protein is responsive to selenium availability in the diet, it is possible that the chemopreventive activity of selenium may be dependent, at least in part, on allelic-specific effects on enzyme activity. Here, we examine the levels and activity of GPx-1, as well the response of the protein to selenium levels, in human lymphocytes as well as MCF-7 breast carcinoma cells engineered to express different GPx-1 allelic variants.
Materials and Methods
Cell Culture
Immortalized human lymphoblast cell lines were obtained from the cell repository of the Coriell Institute for Medical Research and incubated in RPMI-1640 containing 10% fetal bovine serum in a humidified atmosphere at 37°C with 5% CO2. The MCF-7 human breast carcinoma cell line obtained from the American Type Culture Collection and derivative lines were maintained in Modified Eagle's Medium (MEM, Invitrogen) supplemented with 10% fetal bovine serum at 37°C with 5% CO2. Transfectants were generated using the Lipofectin Transfection Reagent (Invitrogen) following the manufacturer's protocol. Colonies were selected with 500 μg/ml G418 (Sigma), expanded and screened for GPx-1 activity.
Genotyping at the GPx-1 locus
The identity of the GPx-1 codon 198 polymorphism in the lymphocyte cell lines used in this study were previously reported (24). Genotyping the trinucleotide repeat (GCG) variation in exon 1, which results in 5-, 6-, or 7-Ala repeats in the N-terminus of the protein, was preformed. Sequences were amplified using the forward primer (ALAREPF: 5’-pCCTGCACTGCCGGTAACAT-3’) and reverse primer (ALAREPR: 5’-pCGCCGAGAAGGCATACA-3’) and the products sequenced using the sequence primer ALAREPSEQ2: 5'-GCACTCTCCAGCCTTTTCC-3' by the Research Resource Center at the University of Illinois at Chicago.
Generation of derivative GPx-1 Expression Constructs
GPx-1 expression constructs containing 5 alanine repeats and differing only by a single nucleotide resulting in either a leucine or proline at GPx-1 codon 198 (designated hGPX198pro) and (hGPX198leu) were previously generated (25). Using these constructs as templates, derivative hGPx-1 plasmids with 7 alanine repeats were generated using the Quick Change Site Directed Mutagenesis Kit (Invitrogen) using the forward (5’-pCTAGCGGCGGCGGCGGCGGCGGCCCAG-3’) and reverse primers (5’-pCTGGGCCGCCGCCGCCGCGCGCGCTAG -3’) for mutagenesis. Successful mutagenesis was confirmed by sequencing using services provided by the Research Resource Center at the University of Illinois at Chicago.
A derivative GPx-1 mutant designated as A5/Leu-Y96F was generated by in vitro mutagenesis, resulting in a change of codon 96 from tyrosine (Y) to phenylalanine (F) using the QuickChange II Site-Directed Mutagenesis Kit (Stratagene). Template DNA was amplified using two overlapping primers (forward primer: 5’-pGAATTCCCTCAAGTTCGTCCGGCCTGGTG-3’; reverse primer: 5’-pCACCAGGCCGGACGAACTTGAGGGAATTC-3’) and digested by Dpn I to cleave the original unaltered template. Successful mutagenesis was confirmed by Rsa 1 diagnostic digestion.
GPx-1 activity and levels
GPx activity was determined by coupled spectrophotometric assay using hydrogen peroxide or cumene hydroperoxide as substrate (25). Assays were minimally done in triplicate, using three independently generated lysates and activity was reported as nmoles NADPH oxidized/minute/mg protein. GPx-1 protein levels were determined by western blotting by electrophoresis of extracts on 14% SDS-Polyacrylamide (PAGE) gels, transfer to polyvinylidine fluoride membranes (Immobilon-P; Millipore) and detected using an affinity purified anti-hGPx-1 or mouse anti-human GPx-1 monoclonal antibodies (MBL) as previously described (12). Filters were also probed with anti-ß–actin antibodies to control for equal loading of protein on the gel. GPx-1 or the ß–actin band density was measured with a Fluor-S MAX MutiImager (Bio-Rad) using three independently generated lysates from each MCF-7 transfectant. The same lysates used for western blot analysis were used to measure GPx-1 enzyme activity.
GPx-1 isolation by immunoprecipitation and mass spectrometry
Harvested cells were lysed, debris removed by centrifugation and incubated with mouse monoclonal antibody to human GPx-1 (Abcam) overnight at 4°C and then bound to Protein G beads (rec-Protein G-Sepharose 4B Conjugate; Invitrogen) Immunoprecipitated samples electrophoresed were on a 14% Tris-Glycine gel, and stained overnight with coomassie G-250 stain. GPx-1 protein was excised, digested, and fragments were analyzed by mass spectrometry at the Research Resources Center at UIC by LCMS-MS using a Thermo Finnigan LTQFT mass spectrometer interfaced to a Dionex Ultimate 3000 HPLC system. Peptides were loaded to an Agilent Zorbax 300SB-C18 trapping cartridge, eluted onto an Agilent Zorbax 300SB-C18 capillary column and separated at 200 nL/min. The gradient is as following: 0-5 min 100% A, 7 min 10%A, 47 min 60%A, 52 min 90%A, 57 min 90%A. The column was equilibrated with 100% A for 5 min before the next injection. Solvent A was 95% water, 5 % acetonitrile, 0.1 % formic acid, and solvent B was 5% water, 95% acetonitrile, 0.1 % formic acid. Data dependent MS/MS analysis was set up as following: A high resolution FT scan was performed at 100,000 resolution, followed by collisionally induced dissociation MS/MS analysis of the 5 most intense ions with signal count >1,000.
Statistical Analysis
The differences in the fold induction of GPx-1 activity in response to selenium supplementation were assessed by the two tailed Student's t-test. Differences were considered significant at P < 0.05. Two-way analyses of variance were 2-sided and were performed using SAS 9.1.
Results
Variations in GPx enzyme activity and response to selenium among human lymphoblast cell lines
GPx activity was determined in a collection of 30 human lymphoblast cell lines established by Epstein Barr virus immortalization of peripheral blood mononuclear cells and obtained from the Coriell Repository. The partial GPx-1 gene sequence from each of these cell lines has been reported (24) and the identity of the nucleotide polymorphism resulting in a leucine or proline at codon 198 was confirmed by RFLP analysis (data not shown). To extend these data to include another genetic variation in GPx-1, that being the presence of 4, 5 or 6 GCG triplets in the amino terminus coding portion of the gene (20), the DNA from these lines was genotyped to determine the number of alanine repeat codons by direct sequencing following PCR amplification. This additional genetic information expanded the previously reported number of common GPx-1 haplotypes, and the genotypes of each of these cell lines is presented in Table 1.
Table 1.
Genotype of human B lymphocyte cell lines obtained from the NIGMS Human Genetic Cell Repository of the Coriell Institute
| Catalog ID |
Haplotype |
GPx-1 polymorphisms |
|
|---|---|---|---|
| Coriell Insititute |
Pro198Leu | Ala repeats | |
| NIGMS collection | |||
| GM11524 | NCP081 (4,4) | Leu/Leu | 6 Ala |
| GM07057 |
NCP119 (13,13) |
Leu/Leu |
6 Ala |
| GM11993 | NCP083 (12,12) | Pro/Pro | 5 Ala |
| GM12863 | NCP088 (12,12) | Pro/Pro | 5 Ala |
| GM12749 | NCP085 (1,12) | Pro/Pro | 5 Ala |
| GM11523 |
NCP080 (12,1) |
Pro/Pro |
5 Ala |
| GM10849 | NCP077 (10,3) | Pro/Pro | 7 Ala |
| GM07038 | NCP073 (3,3) | Pro/Pro | 7 Ala |
| GM10861 | NCP123 (10,3) | Pro/Pro | 7 Ala |
| GM10832 | NCP124 (10,10) | Pro/Pro | 7 Ala |
| GM12273 | NCP084 (10,3) | Pro/Pro | 7 Ala |
| GM13618 |
NCP093 (10,10) |
Pro/Pro |
7 Ala |
| GM06990 | NCP118 (10,1) | Pro/Pro | 5 Ala/7 Ala |
| GM06987 | NCP072 (1,3) | Pro/Pro | 5 Ala/7 Ala |
| GM07348 | NCP074 (1,10) | Pro/Pro | 5 Ala/7 Ala |
| GM07349 | NCP075 (10,12) | Pro/Pro | 5 Ala/7 Ala |
| GM10860 | NCP122 (12,10) | Pro/Pro | 5 Ala/7 Ala |
| GM10831 | NCP076 (10,1) | Pro/Pro | 5 Ala/7 Ala |
| GM12909 | NCP089 (3,1) | Pro/Pro | 5 Ala/7 Ala |
| GM13617 | NCP092 (12,10) | Pro/Pro | 5 Ala/7 Ala |
| GM11521 | NCP078 (10,12) | Pro/Pro | 5 Ala/7 Ala |
| GM11525 |
NCP082 (12,10) |
Pro/Pro |
5 Ala/7 Ala |
| GM10858 |
NCP120 (10,1) |
Pro/Pro |
6 Ala/7 Ala |
| GM10833 | NCP125 (12,4) | Pro/Leu | 5 Ala/6 Ala |
| GM12911 | NCP090 (12,4) | Pro/Leu | 5 Ala/6 Ala |
| GM12912 |
NCP091 (4,1) |
Pro/Leu |
5 Ala/6 Ala |
| GM10859 | NCP121 (10,14) | Pro/Leu | 6 Ala/7 Ala |
| GM12813 | NCP086 (4,3) | Pro/Leu | 6 Ala/7 Ala |
| GM12841 | NCP087 (4,10) | Pro/Leu | 6 Ala/7 Ala |
| GM11522 | NCP079 (10,4) | Pro/Leu | 6 Ala/7 Ala |
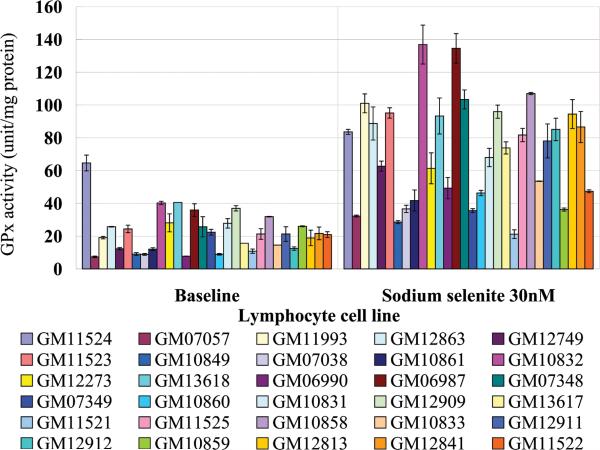
GPx enzyme activity was determined for each of the lymphoblast cell lines whose genotype is presented in Table 1. To determine whether GPx activity would be stimulated in the lymphoblastoid cells by selenium addition, cultures were incubated in media supplemented with 30 nM selenium in the form of sodium selenite for 3 days. The dosage and form of selenium used in the studies here allowed direct comparisons with our previous published studies (25-28). The data presented in Figure 1 indicate that lymphocyte cell lines expressed significant variation in GPx-1 enzyme activity when cultured under standard conditions (7.3-64.7 units/mg protein). The degree of induction of GPx-1 activity following selenium supplementation was different among those lines, ranging from as low as 1.3-fold (GM11524) and as high as 6.9-fold (GM12912).
Figure 1. Human lymphocytes cell lines exhibit different baseline and selenium-induced GPx enzyme activity.
The genotype of the 30 selected lymphocyte cell lines at the alanine-repeat and codon 198 polymorphisms are presented in Table 1. The GPx activity obtained with or without 30 nM sodium selenite (Se) for 3 days is presented in the graph. Each bar represents the mean of three independent experiments, and error bars indicate the SD.
The degree of variation observed among these cell lines was unexpected given the fact that the lines were derived from the same cell type and this observation was even extended to cell lines with the same GPx-1 haplotype. For example, examining the data for several cell lines with the most common GPx-1 haplotype (A5,7/Pro, homozygous for Pro198 and heterozygoous for 5- and 7-Ala repeats), the observed baseline GPx-1 activity ranged from 7.7 units/mg protein (GM06990) to 37.0 units/mg protein (GM12909). The degree of induction of enzyme activity observed when the media was supplemented with selenium ranged from 1.6-fold (GM07349) to 6.4-fold (GM06990). Thus, neither genotype nor haplotype alone was the only determinant for either baseline activity or the degree of induction, and this observation also extended to the other genotypes presented in Table 1.
Selenium supplementation increases GPx-1 protein levels in lymphocytes
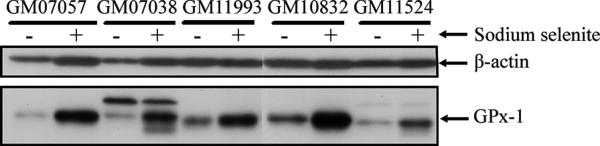
We have previously observed that induction of GPx-1 activity achieved with selenium supplementation is due to enhanced translation and not transcription (25). This was shown to be the case for the lymphocytes as well. GPx-1 protein levels were analyzed in extracts obtained from 5 different lymphocyte cell lines that were all homozygous for both the codon 198 and alanine-repeat polymorphisms by western blot analysis, and the intensity of the GPx-1 band observed approximated the levels of activity determined by enzyme assay (Figure 2).
Figure 2. Relative induction of GPx-1 protein and enzyme activity in homozygote lymphocytes in response to increasing doses of supplemental selenium.
Total cell extracts were analyzed for GPx-1 protein levels by western blotting with a anti-human GPx-1 monoclonal antibody and the filter was re-probed with anti-ß-actin antibodies as an indication of equal loading of protein on the gel.
GPx-1 allele-specific response to selenium supplementation in MCF-7 breast carcinoma cells
Each of the lymphocyte cell lines described above were obtained from different individuals and therefore represent distinct cellular environments that are likely to influence GPx-1 activity in addition to any consequences of GPx-1 genotype alone. In order to focus on genotype effects, allele-specific GPx-1 expression constructs were generated by in vitro mutagenesis. GPx-1 expression constructs representing combinations of either a leucine or proline triplet at codon 198 and 5 or 7 alanine repeats at the protein's amino terminus were generated. Human MCF-7 breast carcinoma cells are a useful tool to study the impact of GPx-1 polymorphisms in a clonal cell population. These cells express negligible GPx-1 activity and mRNA (25, 29). Thus, MCF-7 cells transfected with GPx-1 expression constructs allow for the essentially exclusive expression of transfected GPx-1 alleles in mammalian cells. These constructs were transfected into MCF-7 cells and G418 resistant colonies were expanded for further analysis.
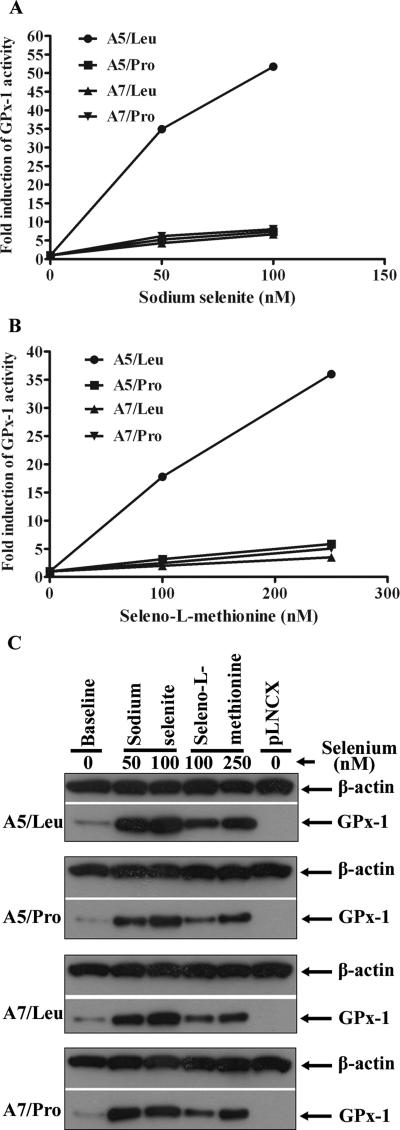
Sodium selenite is commonly used for cell culture and animal studies, and selenomethionine is the most common form of selenium obtained from the diet. To test whether GPx-1 alleles respond differently to these sources of selenium, the culture medium of MCF-7 transfectants was supplemented with increasing doses of either sodium selenite (Figure 3A) or selenomethionine (Figure 3B) for 3 days. Both forms of selenium supplementation significantly increased GPx-1 activity, with the A5/Pro, A7/Pro and A7/Pro transfectants exhibiting an approximately 4- to 8-fold (sodium selenite) or 3- to 6-fold (selenomethionine) increase in activity (Figure 3). As is typically observed, more selenomethionine was required to achieve a similar induction in activity as was obtained with the more bioavailable form of selenium, selenite. In contrast, there was a greater induction in GPx-1 activity in cells expressing the A5/Leu, either when exposed to sodium selenite (234.6- and 347.1 units/mg protein at 50 nM and 100 nM, respectively) or by selenomethionine (119.3-to 241.6 units/mg protein at 100 nM and 250 nM, respectively). The induction of GPx-1 activity observed for A5/Leu was 35.0- to 51.7-fold with sodium selenite (Figure 3A) or 17.8- to 36.0-fold with selenomethionine (Figure 3B).
Figure 3. Relative induction of GPx-1 levels in MCF-7 transfectants in response to increasing doses of selenium supplementation.
The growth media of MCF-7 transfectants was supplemented with increasing doses of selenium, either in the form of A, sodium selenite at 50 nM, and 100 nM or B, seleno-L-methionine at 100 nM, and 250 nM and cells were harvested for GPx enzyme activity following three days of incubation. Each value in the graphs represents the mean fold induction of GPx-1 activity, expressed as mean GPx-1 activity after selenium treatment relative to mean GPx-1 activity at baseline (n=3). The mean fold induction observed for A5/Pro, A7/Leu and A7/Pro following treatment with either sodium selenite or selenomethionine were not statistically different from each other, but in both cases were significantly different for the mean fold induction observed for A5/Leu (P<0.0001). C, induction of GPx-1 protein levels coincides with the induction of enzyme activity. The same extracts prepared for enzyme assay (Figure 3A, B) were analyzed for GPx-1 protein levels by western blotting with anti-human GPx-1 and filters were reprobed with ß-actin antibodies as a control for equal loading of protein on the gel. pLNCX; vector without insert.
Selenium increases GPx-1 protein levels in MCF-7 cells
In order to quantify GPx-1 protein levels in transfectants at both baseline culture conditions and following selenium supplementation, GPx-1 protein in the same cellular extracts used to measure activity was assessed by western blotting with anti-GPx-1 specific antibodies (Figure 3C). Surprisingly, the amount of GPx-1 detected in the western blots was similar among the MCF-7 transfectants, despite differences in the GPx-1 activity observed in the extracts. Both selenite and selenomethionine supplementation significantly increased GPx-1 protein levels with similar patterns.
Impact of genetic variations and selenium on GPx-1 structure
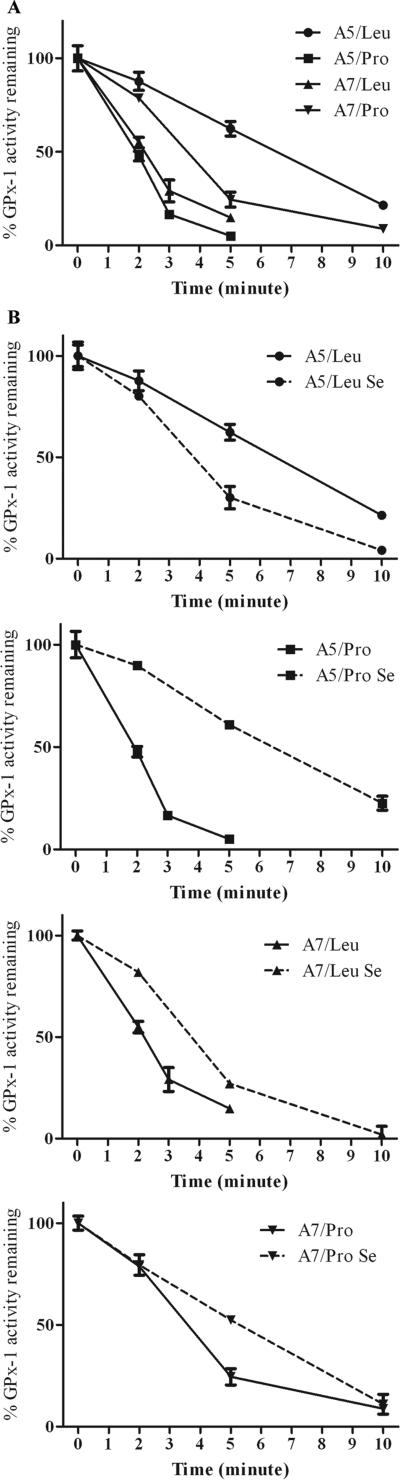
There is an expectation that polymorphisms that affect an enzyme's activity may also cause detectable changes in that protein's structure As an initial attempt to investigate the degree with which the selected variations in the GPx-1 protein impact the molecule's structure, the thermal stability of the proteins was investigated. Enzyme activity over 10 minutes at 55°C was measured and compared to the activity obtained when the cell extract was maintained at 4°C. Differences in thermostability were evident with the greatest stability observed for A5/Leu (Figure 4A).
Figure 4. GPx-1 allelic variation influences thermostability of the protein.
A, The GPx enzyme activity remaining after incubation of cellular extracts of the indicated transfectant at 55°C was expressed as a percentage of the control activity obtained when the same extract was maintained at 4°C . B, Solid lines indicate the amount of remaining GPx activity without supplemental selenium and the dashed lines indicate the parallel study in which cells were preincubated with 30 nM sodium selenite for 3 days prior to harvesting. Each data point represents the mean ±SD of three independent experiments. The mean activity levels for all isoforms were different from each other (P<0.01).
Since selenium supplementation of the culture media was also associated with increased activity, it was examined whether incubation of cells with 30 nM sodium selenite also influenced GPx-1 thermostability. These data are presented in Figure 4B and demonstrated that the selenium supplementation resulted in a significant increase in stability at 55°C over the time course examined for A5/Pro, A7/Leu and A7/Pro alleles and in contrast, selenium supplementation resulted in a decrease in stability for A5/Leu (Figure 4B). These data indicated that the naturally occurring amino acid differences among GPx-1 alleles had a profound effect on protein structure and that there were additional temperature-sensitive structural changes in these proteins as a function of selenium availability.
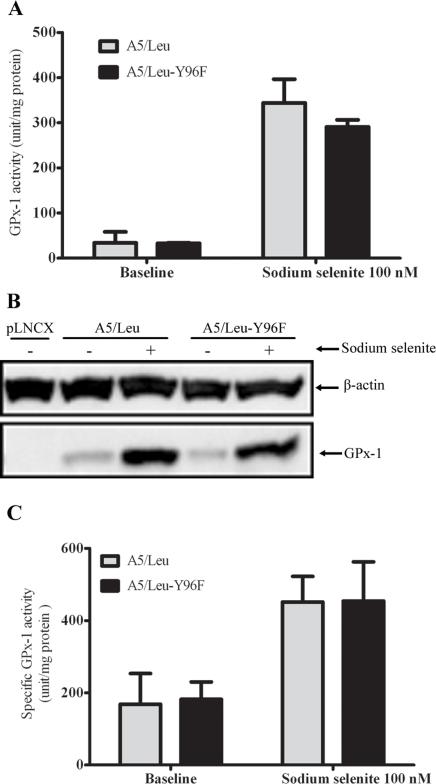
Mutation of Tyr-96 from a tyrosine to a phenylalanine does not change GPx-1 activity in MCF-7 cells
One possible means by which polymorphisms or selenium availability might affect GPx-1 activity is by post-translational modification. A tyrosine at GPx-1 codon 96 has been reported to be phosphorylated by the abl and arg tyrosine kinases, resulting in the activation of that enzyme (30). Analysis of each of the four GPx-1 alleles immunoprecipitated from total protein extracts failed to reveal evidence for phosphorylation either by using 1) anti-phosphotyrosine antibodies, 2) the Pro-Q Diamond phosphoprotein detection system or by 3) assessing changes in GPx-1 mobility by gel electrophoresis following phosphatase treatment (data not shown). In addition, GPx-1 proteins immunoprecipitated from the allele-specific transfectants were analyzed by mass spectrometry. The sequence of the peptide fragments were confirmed by comparison with the known human GPx-1 sequence. Tyrosine phosphorylation of peptides may be identified by mass shifts of 80 kDa, however, no phosphorylated peptides were detected (data not shown).
In order to investigate a possible role of phosphorylation of Tyr-96 of GPx-1, a derivative expression construct in which Tyr-96 was converted to phenylalanine by in vitro mutagenesis was generated and transfected into MCF-7 cells. Lysates containing both the Tyr-96 GPx-1 and Phe-96 GPx-1 were assayed for GPx-1 enzyme activity (Figure 5A) and normalized to the amount of protein observed by western blotting of the same extracts (Figure 5B). Mutation of Tyr-96 to a phenylalanine did not affect that enzymes induction with 100 nM selenium, nor the specific activity determined by normalizing the enzymatic activity by the amount of protein obtained from the western blot (Figure 5C).
Figure 5. Mutation of Tyr at codon 96 of GPx-1 does not affect GPx-1 activity.
A GPx-1 mutant (A5/Leu-96F) lacking tyrosine codon 198 was compared to a MCF-7 transfected with the unmodified A5/leu expression construct. The media of both transfectants was supplemented with 100 nM sodium selenite for 3 days as indicated and GPx-1 activity was determined. A. GPx1 activity obtained after 3 days in 100 nM sodium selenite. B. GPx-1 was quantified by band density on the western blot film. Data were expressed as GPx-1 band density relative to ß-actin band density of the same sample. C. Specific GPx-1 activity was measured by normalizing enzyme activity to GPx-1 protein levels determined by western blot analysis. Each bar represents the mean ± SD (n = 3). pLNCX; vector without insert.
Discussion
The studies described herein investigated the genetic and molecular determinants of GPx-1 enzyme activity. Over-expression of GPx-1 is associated with a wide range of effects, including the prevention of apoptosis, the protection against toxicity and the reduction of DNA damage (31-36). Too much GPx-1 activity may also have adverse effects, as exemplified by a recent report indicating that its over-expression can disrupt mitochondrial function (37). Given human epidemiological data indicating significant associations between polymorphisms in GPx-1 and the risk of several cancer types, the levels of GPx-1 activity in a single cell type, lymphocytes, was examined at standard culturing conditions and following supplementation of the culture media with selenium in a collection of human lymphocyte cell lines. Previous sequence analysis of the DNA from these lines revealed multiple polymorphisms within the GPx-1 gene (24), and with the additional data indicating the number of alanine-repeats, there was a lack of discernable pattern between genotype and GPx-1 activity, both in the presence or absence of supplemented selenium. This variation could be due a wide range of possible differences among the cellular environments within the cell lines examined, including direct and indirect effects on transcriptional/translational regulation or potential protein binding partners; changes that may have developed during the passage of the lines. To minimize the number of variables involved in GPx-1 regulation, expression of allelic variants from a transfected construct was achieved in the MCF-7 cell line that expresses marginal endogenous GPx-1 activity.
The MCF-7 transfectants, expressing combinations of the Pro198Leu and alanine-repeat variations, showed increased GPx activity with increasing selenium supplementation to the culture media, as is generally seen for cells grown in culture (26, 28, 38-41) as well as the lymphocyte lines examined here. And while the amount of selenomethione achieving the same induction as with selenite was higher, the patterns of induction for each individual haplotype were very similar. Selenomethionine is converted to H2Se through transulfuration and β-lyase cleavage, whereas selenite interacts with glutathione (GSH) to form GSSeSG which is subsequently reduced to H2Se. H2Se derived via both pathways can be converted to selenophosphate which is then used in the synthesis of selenoproteins. This difference in selenium metabolism is likely to account for the greater efficiency of induction of selenite over selenomethionine, as has been reported for a variety of cell types (42-44). In all cases, the increase in enzyme activity was paralleled by an increase in the amount of GPx-1 protein as determined by western blotting. Using MCF-7 cells transfected with a GPx-1 expression construct, it was previously reported that the increase in protein obtained by selenium supplementation occurred without an increase in steady state GPx-1 mRNA levels (25), and neither form of selenium used (Figure 3) resulted in an increase in GPx-1 mRNA as determined by RT-PCR (data not shown).
After addition of selenium either in the form of selenite or selenomethionine, GPx-1 enzyme activity increased to a similar degree for the A5/Pro, A7/Leu and A7/Pro alleles, but the induction obtained was much greater for the A5/Leu GPx-1 variant. While the studies reported herein were conducted using H2O2 as the GPx-1 substrate, similar patterns of induction were obtained using a different substrate, cumene hydroperoxide (data not shown). It has been suggested that the Pro198Leu polymorphism may have a profound effect on the conformation of GPx-1 (17) and the variable number of alanine repeats may also affect protein structure and stability (23). Both the N-terminus of the protein containing the alanine repeats and the carboxy terminus including the Pro198Leu polymorphism reside on the outer surface of each of the four subunits that comprise the functional protein (45, 46). The distinct response to selenium of the A5/Leu variant as compared to the others investigated here indicates that there is an interaction between these two positions that ultimately influences activity. While such an interaction could affect the protein's conformation, stability or perhaps it subcellular localization (i.e to the mitochondria), it is also possible that the identity of the amino acids at these positions affects the interaction of GPx-1 with other cellular proteins given that assays were conducted using whole cell extracts. Future studies to investigate the impact of allelic differences in protein structure on GPx-1 enzyme function will be performed on purified protein to determine any contributions of the identity of the amino acid at codon 198 to enzyme kinetics and/or interaction with other cellular proteins.
Previous work has shown that phosphorylation of GPx-1 at Tyr-96 by the c-arg or c-abl tyrosine kinases results in the protein's activation (30). However, using GPx-1 obtained from MCF-7 transfectants, no evidence of phosphorylation could be found. In addition, changing the tyrosine at position 96 to phenylalanine neither altered the protein's response to selenium or its specific enzyme activity. One possible explanation for the differences observed is that the previous work demonstrating GPx-1 phosphorylation was conducted using a derivative GPx-1 in which the active site selenocysteine was converted to a cysteine, and these previous studies were performed in a human embryonic kidney epithelium cell line (30) as compared to the breast carcinoma cells used here.
The surprising interaction between the GPx-1 Pro198Leu and alanine-repeat variations raises some interesting possibilities with regard to cancer risk in humans. There have been several reports showing an association between the leu allele of GPx-1 and the risk of cancer at several different sites, although not all studies have seen this relationship. One particular study reported associations between the leu allele and both lower GPx-1 enzyme activity and the risk of breast cancer (47). The in vitro studies presented here used doses in the nM range to distinguish the allelic responses to selenium supplementation, only a few fold higher than the total selenium in the culture media provided in the FBS. In contrast, human serum typically contains approximately 1-2 μM selenium, but much of this is in the form of selenoproteins such as the plasma GPx (GPx-3) and Selenoprotein P, and the selenium concentration in specific organs can be considerably less, as has been reported for prostate tissue, for example (48). Therefore, individuals with the A5/Leu genotype may experience a more dramatic decline in GPx-1 activity under conditions of moderate selenium deficiency than individuals with the other GPx-1 genotypes. Although few studies have examined the interaction between both the codon 198 polymorphism and the number of alanine repeats with regard to cancer risk, it is noteworthy that Knight et al. reported that premenopausal women with the A5/Leu genotype were at a higher risk of breast cancer as compared to women whose DNA encoded a GPx-1 with 6 or 7 alanine codons (23).
Recently, the large prostate cancer prevention trial, SELECT, was terminated early in part due to a lack of efficacy of selenium provided in the form of selenomethionine (6). These results were in contrast to results from an earlier supplementation study using the same dose of selenium (200 μg/day), but in a different form (selenized yeast) that indicated that men in the lowest tertile of selenium status saw a significant decline in prostate cancer incidence (3, 49). In addition to the difference in forms of selenium used in these trials, the baseline plasma selenium status of participants in both these trials was considerably different, averaging 114 ng/ml (1.44 mM) for the NPC trial (3) and 135.0 ng/ml (1.71 mM) for SELECT (6). These contrasting results, when taken in concert with the in vitro data presented here showing allelic differences in the activity of GPx-1, an enzyme implicated in cancer etiology and in its response to selenium availability, indicate that it is possible that the benefits of selenium supplementation in the reduction of prostate and perhaps other cancers might become apparent when cohorts are stratified by both GPx-1 genotype and selenium status.
Acknowledgements
The authors would like to thank Dr. Tricia Y. Li at Brigham and Women's Hospital for assistance in statistical analyses. This work was supported by a Penny Severns Breast, Cervical and Ovarian Cancer Research Grant No. 96180115 and NCI/NIH grants 5R21CA129590-02 to AMD and RO1 CA120582-01 to UP.
Literature Cited
- 1.El-Bayoumy K. The role of selenium in cancer prevention. In: De Vita VT, Hellman S, Rosenberg SA, editors. Cancer prevention. J.B. Lippincott Co.; Philadelphia: 1991. pp. 1–15. [Google Scholar]
- 2.Gromadzinska J, Reszka E, Bruzelius K, Wasowicz W, Akesson B. Selenium and cancer: biomarkers of selenium status and molecular action of selenium supplements. Eur J Nutr. 2008;47(Suppl 2):29–50. doi: 10.1007/s00394-008-2005-z. [DOI] [PubMed] [Google Scholar]
- 3.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- 4.Duffield-Lillico AJ, Dalkin BL, Reid ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–12. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein EA, Thompson IM, Lippman SM, et al. SELECT: the next prostate cancer prevention trial. Selenum and Vitamin E Cancer Prevention Trial. J Urol. 2001;166:1311–5. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- 6.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gladyshev VN, Hatfield DL. Selenocysteine-containing proteins in mammals. J Biomed Sci. 1999;6:151–60. doi: 10.1007/BF02255899. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Holmgren A. Selenoproteins. J Biol Chem. 2009;284:723–7. doi: 10.1074/jbc.R800045200. [DOI] [PubMed] [Google Scholar]
- 9.El-Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutat Res. 2005;591:224–36. doi: 10.1016/j.mrfmmm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Diwadkar-Navsariwala V, Diamond AM. The link between selenium and chemoprevention: a case for selenoproteins. J Nutr. 2004;134:2899–902. doi: 10.1093/jn/134.11.2899. [DOI] [PubMed] [Google Scholar]
- 11.Moustafa ME, Carlson BA, El-Saadani MA, et al. Selective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing isopentenyladenosine-deficient selenocysteine tRNA. Mol Cell Biol. 2001;21:3840–52. doi: 10.1128/MCB.21.11.3840-3852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diwadkar-Navsariwala V, Prins GS, Swanson SM, et al. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci U S A. 2006;103:8179–84. doi: 10.1073/pnas.0508218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irons R, Carlson BA, Hatfield DL, Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136:1311–7. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- 14.Kryukov GV, Castellano S, Novoselov SV, et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–43. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 15.Moscow JA, Schmidt L, Ingram DT, Gnarra J, Johnson B, Cowan KH. Loss of heterozygosity of the human cytosolic glutathione peroxidase I gene in lung cancer. Carcinogenesis. 1994;15:2769–73. doi: 10.1093/carcin/15.12.2769. [DOI] [PubMed] [Google Scholar]
- 16.Zhuo P, Diamond AM. Molecular mechanisms by which selenoproteins affect cancer risk and progression. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratnasinghe D, Tangrea JA, Andersen MR, et al. Glutathione peroxidase codon 198 polymorphism variant increases lung cancer risk. Cancer Res. 2000;60:6381–3. [PubMed] [Google Scholar]
- 18.Cox DG, Tamimi RM, Hunter DJ. Gene x Gene interaction between MnSOD and GPX-1 and breast cancer risk: a nested case-control study. BMC Cancer. 2006;6:217. doi: 10.1186/1471-2407-6-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton A, Nahon P, Pessayre D, et al. Genetic polymorphisms in antioxidant enzymes modulate hepatic iron accumulation and hepatocellular carcinoma development in patients with alcohol-induced cirrhosis. Cancer Res. 2006;66:2844–52. doi: 10.1158/0008-5472.CAN-05-2566. [DOI] [PubMed] [Google Scholar]
- 20.Shen Q, Townes PL, Padden C, Newburger PE. An in-frame trinucleotide repeat in the coding region of the human cellular glutathione peroxidase (GPX1) gene: in vivo polymorphism and in vitro instability. Genomics. 1994;23:292–4. doi: 10.1006/geno.1994.1499. [DOI] [PubMed] [Google Scholar]
- 21.Kote-Jarai Z, Durocher F, Edwards SM, et al. Association between the GCG polymorphism of the selenium dependent GPX1 gene and the risk of young onset prostate cancer. Prostate Cancer Prostatic Dis. 2002;5:189–92. doi: 10.1038/sj.pcan.4500569. [DOI] [PubMed] [Google Scholar]
- 22.Jefferies S, Kote-Jarai Z, Goldgar D, et al. Association between polymorphisms of the GPX1 gene and second primary tumours after index squamous cell cancer of the head and neck. Oral Oncol. 2005;41:455–61. doi: 10.1016/j.oraloncology.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Knight JA, Onay UV, Wells S, et al. Genetic variants of GPX1 and SOD2 and breast cancer risk at the Ontario site of the Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2004;13:146–9. doi: 10.1158/1055-9965.epi-03-0164. [DOI] [PubMed] [Google Scholar]
- 24.Foster CB, Aswath K, Chanock SJ, McKay HF, Peters U. Polymorphism analysis of six selenoprotein genes: support for a selective sweep at the glutathione peroxidase 1 locus (3p21) in Asian populations. BMC Genet. 2006;7:56. doi: 10.1186/1471-2156-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu YJ, Diamond AM. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63:3347–51. [PubMed] [Google Scholar]
- 26.Diamond AM, Dale P, Murray JL, Grdina DJ. The inhibition of radiation-induced mutagenesis by the combined effects of selenium and the aminothiol WR-1065. Mutat Res. 1996;356:147–54. doi: 10.1016/0027-5107(96)00016-4. [DOI] [PubMed] [Google Scholar]
- 27.Nasr MA, Fedele MJ, Esser K, Diamond AM. GPx-1 modulates Akt and P70S6K phosphorylation and Gadd45 levels in MCF-7 cells. Free Radic Biol Med. 2004;37:187–95. doi: 10.1016/j.freeradbiomed.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Mansur DB, Hao H, Gladyshev VN, et al. Multiple levels of regulation of selenoprotein biosynthesis revealed from the analysis of human glioma cell lines. Biochem Pharmacol. 2000;60:489–97. doi: 10.1016/s0006-2952(00)00366-x. [DOI] [PubMed] [Google Scholar]
- 29.Esworthy RS, Baker MA, Chu FF. Expression of selenium-dependent glutathione peroxidase in human breast tumor cell lines. Cancer Res. 1995;55:957–62. [PubMed] [Google Scholar]
- 30.Cao C, Leng Y, Huang W, Liu X, Kufe D. Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. J Biol Chem. 2003;278:39609–14. doi: 10.1074/jbc.M305770200. [DOI] [PubMed] [Google Scholar]
- 31.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 32.Baliga MS, Wang H, Zhuo P, Schwartz JL, Diamond AM. Selenium and GPx-1 overexpression protect mammalian cells against UV-induced DNA damage. Biol Trace Elem Res. 2007;115:227–42. doi: 10.1007/BF02685998. [DOI] [PubMed] [Google Scholar]
- 33.Diamond AM, Hu YJ, Mansur DB. Glutathione peroxidase and viral replication: implications for viral evolution and chemoprevention. Biofactors. 2001;14:205–10. doi: 10.1002/biof.5520140126. [DOI] [PubMed] [Google Scholar]
- 34.Chu FF, Esworthy RS, Akman S, Doroshow JH. Modulation of glutathione peroxidase expression by selenium: effect on human MCF-7 breast cancer cell transfectants expressing a cellular glutathione peroxidase cDNA and doxorubicin-resistant MCF-7 cells. Nucleic Acids Res. 1990;18:1531–9. doi: 10.1093/nar/18.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gouaze V, Andrieu-Abadie N, Cuvillier O, et al. Glutathione peroxidase-1 protects from CD95-induced apoptosis. J Biol Chem. 2002;277:42867–74. doi: 10.1074/jbc.M203067200. [DOI] [PubMed] [Google Scholar]
- 36.Liebman J, Fisher J, Lipshultz C, Kuno R, Kaufman DC. Enhanced glutathione peroxidase expression protects cells from hydroperoxides but not from radiation or doxorubicin. Cancer Res. 1995;55:4465–70. [PubMed] [Google Scholar]
- 37.Handy DE, Lubos E, Yang Y, et al. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 2009;284:11913–21. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chada S, Whitney C, Newburger PE. Post-transcriptional regulation of glutathione peroxidase gene expression by selenium in the HL-60 human myeloid cell line. Blood. 1989;74:2535–41. [PubMed] [Google Scholar]
- 39.Sandstrom PA, Murray J, Folks TM, Diamond AM. Antioxidant defenses influence HIV-1 replication and associated cytopathic effects. Free Radic Biol Med. 1998;24:1485–91. doi: 10.1016/s0891-5849(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 40.Romanowska M, Kikawa KD, Fields JR, et al. Effects of selenium supplementation on expression of glutathione peroxidase isoforms in cultured human lung adenocarcinoma cell lines. Lung Cancer. 2007;55:35–42. doi: 10.1016/j.lungcan.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Rebsch CM, Penna FJ, 3rd, Copeland PR. Selenoprotein expression is regulated at multiple levels in prostate cells. Cell Res. 2006;16:940–8. doi: 10.1038/sj.cr.7310117. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Huang K, Wei C, Chen F, Pan C. Regulation of cellular glutathione peroxidase by different forms and concentrations of selenium in primary cultured bovine hepatocytes. J Nutr Biochem. 2009 doi: 10.1016/j.jnutbio.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Leist M, Maurer S, Schultz M, Elsner A, Gawlik D, Brigelius-Flohé R. Cytoprotection against lipid hydroperoxides correlates with increased glutathione peroxidase activities, but not selenium uptake from different selenocompounds. Biol Trace Elem Res. 1999;68:159–74. doi: 10.1007/BF02784404. [DOI] [PubMed] [Google Scholar]
- 44.Brigelius-Flohé R, Lötzer K, Maurer S, Schultz M, Leist M. Utilization of selenium from different chemical entities for selenoprotein biosynthesis by mammalian cell lines. Biofactors. 19951996;5:125–31. [PubMed] [Google Scholar]
- 45.Epp O, Ladenstein R, Wendel A. The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution. Eur J Biochem. 1983;133:51–69. doi: 10.1111/j.1432-1033.1983.tb07429.x. [DOI] [PubMed] [Google Scholar]
- 46.Ladenstein R, Epp O, Bartels K, Jones A, Huber R, Wendel A. Structure analysis and molecular model of the selenoenzyme glutathione peroxidase at 2.8 A resolution. J Mol Biol. 1979;134:199–218. doi: 10.1016/0022-2836(79)90032-9. [DOI] [PubMed] [Google Scholar]
- 47.Ravn-Haren G, Olsen A, Tjonneland A, et al. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis. 2006;27:820–5. doi: 10.1093/carcin/bgi267. [DOI] [PubMed] [Google Scholar]
- 48.Arnold WN, Thrasher JB. Selenium concentration in the prostate. Biol Trace Elem Res. 2003;91:277–80. doi: 10.1385/BTER:91:3:277. [DOI] [PubMed] [Google Scholar]
- 49.Duffield-Lillicoe A, Reid M, Turnbull J, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–9. [PubMed] [Google Scholar]