Abstract
Cathelicidins are mammalian defense peptides with direct antimicrobial activity and the potential to exert other immunomodulatory effects during the innate immune response. One such function of human cathelicidin is direct binding and inhibition of bacterially derived lipopolysaccharide (LPS), a ligand of toll-like receptor 4 (TLR4) . Here, we show that physiological concentrations of exogenous murine cathelicidin blunt activation of p38 and ERK mitogen-activated protein kinases (MAPKs) and decrease tumor necrosis factor-α (TNFα) release in murine macrophages exposed to LPS, but also other TLR agonists such as lipoteichoic acid and flagellin. In this context, CRAMP is capable of aborting MyD88 synthesis and MyD88/IRAK (interleukin-1 receptor-associated kinase)-4 association in the stimulated macrophages. Exogenous CRAMP can reverse diminished MAPK activation associated with LPS tolerance. By analyzing macrophages from CRAMP−/− mice, we find their endogenous production of cathelicidin does not inhibit LPS MAPK and cytokine activation, rather CRAMP−/− cells show slightly diminished responses. CRAMP deficiency does not render mice more susceptible to lethal LPS challenge. These studies indicate the immunomodulatory effects of cathelicidin on macrophage TLR response may vary both on the exogenous vs endogenous origin of peptide and the prior activation state of the cell.
Keywords: cathelicidin, antimicrobial peptide, macrophage, toll-like receptor, lipopolysaccharide, innate immunity
Antimicrobial peptides (AMPs) are widely distributed among living organisms with ancient and prominent roles in innate immune defense.1,2 Most AMPs are cationic, which facilitates their interaction with and insertion into anionic bacterial cell walls and membranes,3 causing functional disturbances that promote microbial death. In mammals, cathelicidins are a major AMP family, produced on epithelial cell surfaces and by leukocytes. Studies of the mouse cathelicidin, CRAMP, were the first to show an essential function for AMPs in mammalian innate immune defense.4 Like mice, humans express a single cathelicidin, LL-37, which closely resembles the murine CRAMP in size, encoding gene architecture, proteolytic processing from its propeptide to mature form, amphipathic α-helical structure, tissue distribution of expression and antimicrobial spectrum.
Considerable evidence has emerged in recent years that the contribution of cathelicidin peptides to mammalian innate immune responses extends far beyond direct bacterial killing.5–7 Examples of such cathelicidin-mediated immunomodulatory effects include chemoattraction and activation of phagocytic cells,8 stimulation of mast cell histamine and prostaglandin release,9 promotion of angiogenesis and wound healing,10 delay of neutrophil apoptosis11 and synergistic activation of IL-1β-mediated cytokine release.12 With cathelicidins strongly expressed in epithelium in response to injury or infection that threatens barrier integrity, it appears that the mammalian host has further evolved to utilize them as alarms and signaling molecules for mobilization of a more comprehensive antimicrobial response.
One long recognized property of cathelicidin relevant to host defense is the ability to bind lipopolysaccharide (LPS), a constituent of Gram-negative bacterial cell walls, with high affinity.13 LPS is a natural ligand for the membrane-spanning pattern recognition receptor toll-like receptor 4 (TLR4), which activates gene expression pathways important for the innate immune response. Consequently, human cathelicidin LL-37 is reported to suppress LPS-induced macrophage and dendritic inflammatory responses, including the release of nitric oxide and tumor necrosis factor-α (TNFα).13–15 The mechanisms underlying such interference with LPS responses are still incompletely understood. LL-37 significantly inhibited LPS-induced nuclear factor-κB translocation into the nucleus,14 but is also known to be capable of activating mitogen-activated protein kinase (MAPK) cascades, in particular p38 and extracellular signal-related kinase (ERK), in the absence of other costimulatory stimuli.16 Perhaps reflecting these complexities, studies in a rodent model of LPS-induced sepsis showed that exogenous administration of low doses of LL-37-reduced mortality, whereas higher doses of the human cathelicidin were associated with increased mortality.17
The aim of this study was to probe cathelicidin effects on the macrophage response to LPS employing both primary cells and peptide of the mouse.
RESULTS AND DISCUSSION
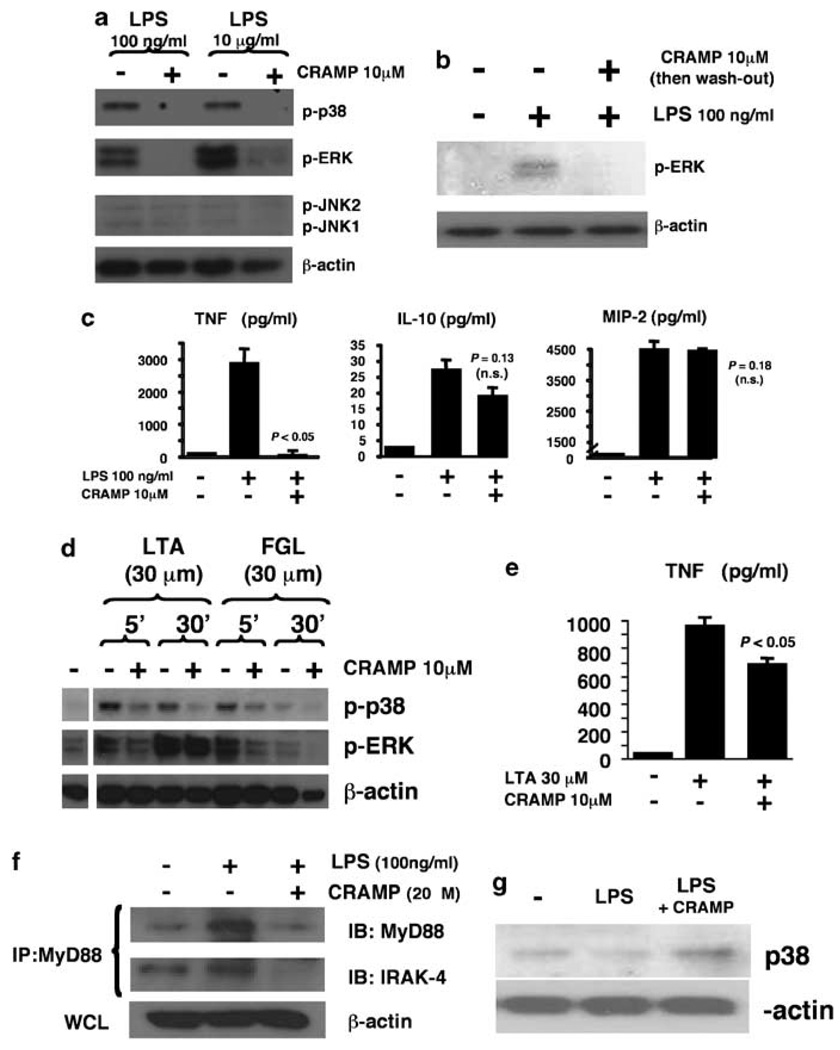
We first sought to determine the effect of the murine cathelicidin CRAMP on the responsiveness of primary mouse macrophages to LPS. Cells were stimulated with LPS in the presence or absence of supplemental CRAMP in the cell media and activation of MAPKs was analyzed. We found CRAMP was able to exert a strong inhibition of LPS-induced p38 and ERK phosphorylation (Figure 1a), whereas c-Jun N-terminal kinase (JNK) pathway activation was similar in both conditions. A washout experiment in which macrophages were stimulated with CRAMP, then washed extensively with excess phosphate-buffered saline (three times), followed by subsequent stimulation of LPS, produced a similar reduction in phosphorylated ERK (pERK) activation (Figure 1b), indicating that potential direct binding of ligand by CRAMP is not alone sufficient to explain the inhibitory phenotype. The presence of CRAMP dramatically reduced the amount of the proinflammatory cytokine TNFα released by murine macrophages following LPS stimulation, but did not affect the production of the anti-inflammatory cytokine interleukin (IL)-10 or the C-X-C family chemokine macrophage inflammatory protein-2 (MIP-2) (Figure 1c). CRAMP exposure also did not effect the levels of release of an additional macrophage-derived chemokine, MCP-1 (603±35 vs 556±60 pgml−1, P=0.31).
Figure 1.
Exogenous cathelicidin inhibits macrophage mitogen-activated protein kinase (MAPK) activation in response to lipopolysaccharide (LPS) and other toll-like receptor ligands. (a) Wild-type (WT) mouse bone marrow-derived macrophages (BMDM) were stimulated with LPS in the presence or absence of murine cathelicidin CRAMP; phosphorylation of MAPKs was assessed by western immunoblot. (b) WT BMDM stimulation experiment as mentioned above, but CRAMP removed by extensive washing before LPS stimulation and assessment of pERK stimulation. (c) WT mouse BMDM was stimulated with LPS for 3h in the presence or absence of CRAMP; tumor necrosis factor-α (TNFα), interleukin (IL)-12 and macrophage inflammatory protein-2 (MIP-2) release in supernatant determined by enzyme-einked emmunosorbent assay (ELISA), triplicate wells ± s.d. (d) WT mouse BMDM stimulated with lipoteichoic acid or flagellin in the presence or absence of CRAMP; phosphorylation of MAPK assessed by western immunoblot. (e) WT mouse BMDM stimulated with lipoteichoic acid in the presence or absence of CRAMP; TNFα levels measured by ELISA, mean of triplicate wells+s.d. All experiments were carried out three times with similar results; representative experiment are shown. (f) WT mouse BMDM were stimulated with LPS in the presence or absence of CRAMP. Cell lysates were immunoprecipitated with anti-MyD88 antibody and MyD88 and IL-1 receptor-associated kinase (IRAK)-4 expression were detected by western immunoblot. (g) WT mouse BMDM were primed overnight with LPS (100ng ml−1). On the second day, cells were restimulated with LPS for 3 h in the presence or absence of 10 µM CRAMP; p38 phosphorylation was detected by western immunoblot.
Earlier, LL-37 was shown to reduce human blood leukocyte cytokine release in response to bacterial cell wall-derived lipoteichoic acid (LTA) in addition to LPS.14,15 As LTA is a known agonist of TLR2, we considered whether CRAMP could affect murine macrophage signaling responses to ligands of additional TLR receptors. We found the addition of CRAMP inhibited both p38 and ERK phosphorylation in response to LTA as well as bacterial flagellin, a pattern recognition molecule for TLR5 (Figure 1d). The presence of CRAMP also produced a significant reduction of macrophage TNFα secretion in response to LTA (Figure 1e), though not as pronounced as that observed with LPS treatment. Although a degree of direct ligand binding of LTA and flagellin by CRAMP cannot be excluded, our data and the results of the earlier washout experiment suggested that additional effects on the macrophage signaling response are likely required to fully explain CRAMP inhibition of MAPK phosphorylation following exposure to a variety of TLR agonists.
To corroborate CRAMP inhibition of multiple macrophage TLR responses, we examined MyD88, a universal adaptor protein that binds the intracytoplasmic TIR domain of TLRs, recruits members of the IL-1 receptor-associated kinase (IRAK) family, and initiates signaling through MAPK pathways.18 We carried out immunoprecipitation studies to investigate MyD88 and IRAK-4 in murine macrophages stimulated with LPS in the presence or absence of CRAMP. Following stimulation with LPS, the total amount of MyD88 increased, but this effect was aborted in the presence of exogenous CRAMP (Figure 1f). Furthermore, IRAK-4 could be coimmunoprecipitated with MyD88 from macrophages after LPS stimulation alone, but not when CRAMP was added simultaneously (Figure 1f). Prior exposure of macrophages to LPS induces a hyporesponsive state to a subsequent challenge with LPS that has been termed LPS tolerance. 19,20 We hypothesized that CRAMP’s inhibitory effects on LPS-induced TLR signaling could influence the tolerance phenotype. To induce tolerance, we exposed macrophages to LPS for 18 h, and then stimulated those cells with a second dose of LPS in the presence or absence of CRAMP (Figure 1g). In this setting, treatment with CRAMP was associated with increased p38 phosphorylation, indicating the influence of exogenous cathelicidin may be inhibitory or activatory dependent on the baseline activation state of the macrophage.
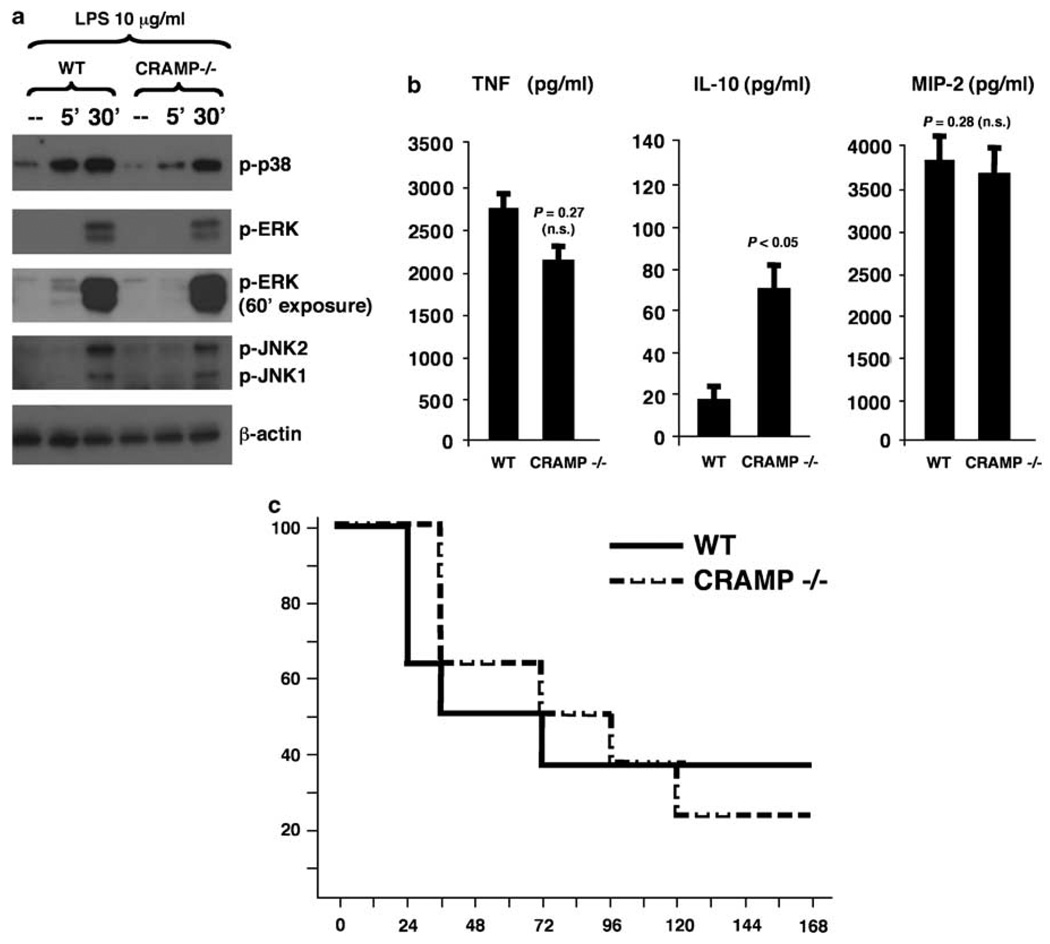
Murine macrophages express CRAMP constitutively and increase their expression upon exposure to microbial stimuli.21 The availability of CRAMP-deficient mice allowed us to analyze whether this autogenous expression of CRAMP by the macrophage was itself sufficient to reduce TLR signaling. When wild-type (WT) and CRAMP−/− macrophages were stimulated LPS, the predicted inhibitory effect of CRAMP MAPK phosphorylation was not observed (Figure 2a), indicating that this effect may be restricted to the presence of high concentrations of exogenous CRAMP. Indeed, a stronger phosphorylation of p38 and a more rapid kinetic of phosphorylation of ERK was observed in the WT cells (Figure 2a). Furthermore, WT and CRAMP−/− macrophages produced similar amounts of TNFα and MIP-2, whereas the mutant cells had an increased production of the anti-inflammatory cytokine IL-10 (Figure 2b). Finally, using a whole animal model, CRAMP−/− mice and WT showed similar mortality curves when challenged with a lethal systemic dose of LPS (Figure 2c). Thus, although the therapeutic administration of supraphysiologic cathelicidin administration may blunt LPS-induced toxicity, the inability to express cathelicidin in the CRAMP−/− mice did not significantly change cell susceptibility to LPS mortality, TNFα production, or alter final p38 phosphorylation at 30min, although it retarded this phosphorylation at 5min and increased IL-10 secretion levels.
Figure 2.
Endogenous cathelicidin production by macrophages does not block lipopolysaccharide (LPS)-induced inflammatory responses and CRAMP−/− mice are not hypersusceptible to to LPS-induced mortality. (a) Wild-type (WT) and CRAMP−/− bone-marrow-derived macrophages (BMDM) were stimulated with LPS and phosphorylation of mitogen-activated protein kinase (MAPKs) was assessed by western immunoblot. (b) WT and CRAMP−/− BMDM were stimulated with LPS for 3 h and tumor necrosis factor-α (TNFα), interleukin (IL)-10 and macrophage inflammatory protein-2 (MIP-2) levels determined in the supernatant by enzyme-einked emmunosorbent assay (ELISA); experiments were carried out three times with similar results; representative result is shown. IL-10 could not be detected in non-stimulated cells. (c) WT and CRAMP−/− mice received intraperitoneal injection of LPS (250 µg per mouse), and mortality was recorded in the following 7 days (n=8 mice per group).
In summary, we observe that exogenous application of cathelicidin is able to blunt not only the TNF production and MAPK activation in response to LPS, but also in response to the molecular agonists of other TLRs, suggesting more complex mechanism(s) than simple binding and depletion of LPS are at play. We provide evidence that exogenous CRAMP is capable of aborting MyD88 synthesis and MyD88/IRAK-4 association. As CRAMP is known to perturb host cell membrane function and structure,22 we hypothesize that the peptides can alter the generation of ionic fluxes essential for TLR activation.23 However, by employing CRAMP-deficient animals, we reveal for the first time that endogenous production of cathelidicin by macrophages is neither sufficient to interfere with LPS-induced MAPK and cytokine activation nor to impact sepsis mortality. These studies provide novel insight into the modulation of TLR responses by cathelicidin and suggest the particular conditions, for example, high exogenous concentrations at tissue foci of infection,12 under which these biological effects may occur during the innate immune response.
METHODS
Mice
CRAMP−/− mice backcrossed on the Balb/c background were generated in our laboratory as described previously.4 WT Balb/c mice were purchased from Charles Rivers Laboratories (Wilmington, MA, USA). All animals were studied at 8–10 weeks old, and experimental protocols were approved by the Veterans Administration San Diego Committee on Animal Use and the University of California, San Diego Animal Subjects Program (San Diego, CA, USA).
Reagents
Lipopolysaccharide was obtained from Alexis Biochemicals (Plymouth Meeting, PA, USA). LTA and flagellin were purchased from Invivogen (San Diego, CA, USA). Anti-phospho-p38, anti-phospho-JNK and anti-phospho-ERK antibodies were obtained from Cell Signaling Technology (Santa Cruz, CA, USA). Antiactin antibody was obtained from Sigma (St Louis, MO, USA).
Cells
Bone marrow cells were collected from mice femurs and tibias and cultured for 7 days in media containing Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, penicillin–streptomycin, and macrophage colony-stimulating factor (M-CSF) (Peprotech Inc., Rocky Hill, NJ, USA) to induce macrophage differentiation.
Cytokine detection
Macrophages were distributed in 6-well plates 106 cells per well and stimulated 3 h later. Cytokine levels were measured in the supernatants by ELISA (enzymeeinked emmunosorbent assay), following the manufacturer’s protocol (BD OptEIA, BD Biosciences, San Jose, CA, USA).
Western immunoblotting
After stimulation, cells were lysed in buffer containing 50mM Tris-HCl ph 7.4, 150mM NaCl, 0.25% deoxycholic acid, 1mM EDTA and 0.5% Triton-X, centrifugated at 14 000 g for 15 min at 4 °C, and subjected to SDS-polyacrylamide gel electrophoresis or immunoprecipitated with 2 µg of the indicated antibody and 20 µl of protein G-Sepharose 4 Fast Flow beads (Amersham Biosciences, Pittsburgh, PA, USA) for 2 h at 4 °C. Immunoprecipitates were separated by SDS- polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane, and developed with the indicated blotting antibody plus a relevant secondary relevant antibody coupled to horseradish peroxidase.
Statistical analysis
Values were compared using the Mann–Whitney U-test and reported as median ± s.d. Differences were considered significant when P<0.05.
ACKNOWLEDGEMENTS
This study was supported by NIH Grants AI48176 and AR052728 (RLG with VN); FPS has also received support from CAPES (Ministry of Sciences, Brazil).
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Bulet P, Stocklin R, Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev. 2004;198:169–184. doi: 10.1111/j.0105-2896.2004.0124.x. [DOI] [PubMed] [Google Scholar]
- 3.Oren Z, Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998;47:451–463. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 6.Kai-Larsen Y, Agerberth B. The role of the multifunctional peptide LL-37 in host defense. Front Biosci. 2008;13:3760–3767. doi: 10.2741/2964. [DOI] [PubMed] [Google Scholar]
- 7.Gallo RL. Sounding the alarm: multiple functions of host defense peptides. J Invest Dermatol. 2008;128:5–6. doi: 10.1038/sj.jid.5701073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 9.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlow PG, Li Y, Wilkinson TS, Bowdish DM, Lau YE, Cosseau C, et al. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol. 2006;80:509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1β, augments immune responses by multiple pathways. J Immunol. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 13.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 15.Kandler K, Shaykhiev R, Kleemann P, Klescz F, Lohoff M, Vogelmeier C, et al. The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol. 2006;18:1729–1736. doi: 10.1093/intimm/dxl107. [DOI] [PubMed] [Google Scholar]
- 16.Bowdish DM, Davidson DJ, Speert DP, Hancock RE. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol. 2004;172:3758–3765. doi: 10.4049/jimmunol.172.6.3758. [DOI] [PubMed] [Google Scholar]
- 17.Fukumoto K, Nagaoka I, Yamataka A, Kobayashi H, Yanai T, Kato Y, et al. Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr Surg Int. 2005;21:20–24. doi: 10.1007/s00383-004-1256-x. [DOI] [PubMed] [Google Scholar]
- 18.Kim TW, Staschke K, Bulek K, Yao J, Peters K, Oh KH, et al. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med. 2007;204:1025–1036. doi: 10.1084/jem.20061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 20.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci USA. 2004;101:2422–2427. doi: 10.1073/pnas.0304455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J Immunol. 2007;178:1829–1834. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 23.Scheel O, Papavlassopoulos M, Blunck R, Gebert A, Hartung T, Zahringer U, et al. Cell activation by ligands of the toll-like receptor and interleukin-1 receptor family depends on the function of the large-conductance potassium channel MaxiK in human macrophages. Infect Immun. 2006;74:4354–4356. doi: 10.1128/IAI.01783-05. [DOI] [PMC free article] [PubMed] [Google Scholar]