Abstract
Showing an arousing central stimulus in a scene often leads to enhanced memory for the arousing central information and impaired memory for peripheral details. However, it is not clear from previous work whether arousing stimuli impair memory for all non-arousing nearby information or just background information. In several experiments, we tested how emotionally arousing pictures affect memory for nearby pictures and for background information. We found that when two pictures were presented together, having one of the pictures be arousing did not affect item and location memory for the other picture. In contrast, an arousing picture impaired memory for a background pattern. These findings suggest that arousal impairs memory for information that is the target of perceptual suppression, such as background information when there is a figure-ground distinction, but does not impair memory for other foreground information.
Arousing stimuli often create memory trade-offs, in which memory is enhanced for the arousing stimuli but impaired for other nearby stimuli. For instance, laboratory studies typically find worse memory for the visually peripheral details of the scenes when there is a central arousing item than when there is not (e.g., Burke et al., 1992; Mitchell et al., 1998; Kensinger et al., 2005). What exactly counts as a peripheral detail varies across studies, but there is general agreement that emotion impairs “information that is irrelevant or spatially peripheral to the source of the emotional arousal” (Christianson, 1992) or background visual details not part of the gist of an event (Reisberg & Heuer, 2004).
In addition to impairing memory for peripheral information shown at the same time, arousing items also tend to impair memory for other items shown just before or afterwards (for a review see Mather, 2007). Memory impairment for preceding and following information has been shown most often when there is one arousing item in a sequence of neutral items (e.g., Strange et al., 2003). Retrograde and anterograde impairments can occur even with up to a 3 to 6 s interval between the emotional oddball and the preceding and following neutral items (Detterman & Ellis, 1972; Hurlemann et al., 2007; Runcie & O'Bannon, 1977; Schmidt, 2002).
Thus, from an array of studies, we have evidence that arousing items impair memory for peripheral background details and also for separate items presented shortly before or afterwards. However, one issue that is not clear from previous research is whether emotionally arousing visual items impair memory for distinct non-arousing items shown at the same time that are not peripheral items. In other words, if someone were presented with two items at the same time, would having one of the items be emotionally arousing lead to worse memory for the other item? Given the research reviewed above on the arousal-induced impairments for nearby peripheral information and temporally adjacent items, the natural prediction seems to be yes. Furthermore, we know that when presented with an arousing and a neutral picture next to each other, people tend to look first at the arousing picture and spend more time looking at it than at the neutral picture (e.g., Knight et al., 2007). Thus, competing for attentional resources with an arousing item seems likely to impair memory for a neutral bystander item, even if that bystander item is not a peripheral or background detail.
However, we could find no direct evidence for this type of impairment in the literature. The closest approximation comes from studies examining whether people engage in mental rubbernecking when emotional items are shown with neutral items (Johnson et al., 2005). On each trial, participants read three words listed in a column. In some cases one of the three words (in any of the three positions) was emotional but two were neutral (ENN) and in others all three were neutral (NNN). After a brief interval, participants either saw one of the words again to be read or a black dot appeared in one of the three word locations, signaling participants to say the word that had been in that location (a refresh trial).
Participants were slower to refresh neutral words than emotional words. However, one critical aspect of the experimental design was that participants were tested half the time on emotional words and half the time on neutral words from ENN trials, which meant that over time, they had the opportunity to learn that they were more likely to be tested on the one emotional word from a trial than on either of the two neutral words. The possibility that the emotional refresh advantage was due to participants working harder to maintain items that were more likely to be tested is supported by a follow-up experiment in which only neutral items were tested (Johnson et al., 2006). Unlike in a previous experiment, there was no significant disadvantage for refreshing neutral items from ENN trials compared with NNN trials. This line of studies suggests that, even if there is mental rubbernecking when neutral items are shown with emotional items, it is weak enough to be overcome by only testing the neutral items. Furthermore, these refresh studies did not examine what the effect of being shown together with an emotional item is for later memory of a neutral item.
We recently found that at least one type of memory is unaffected by the arousal level of an adjacent picture (Mather & Nesmith, 2008, Experiment 3). In the first phase of this study, participants were asked to indicate the color of dots shown one at time on the screen in different locations. At regular intervals, instead of a dot, two pictures appeared in different locations on the screen simultaneously for 2000 ms. Pictures required no response. After the dot-color task phase, there was a surprise memory test, in which participants were asked to indicate the previous location of each picture.
Participants were better at remembering the locations of arousing pictures than non-arousing pictures (both for positive and negative arousing pictures), consistent with other studies showing that people are better at remembering features that are an integral part of arousing items (such as color or location) than features of non-arousing items (for a review see Mather, 2007). But despite this arousal-enhanced location memory, the arousal level of one picture had no significant impact on location memory for the adjacent picture. This lack of a bystander effect is surprising given the previous findings reviewed earlier that arousing items impair memory for peripheral background details and also for separate items presented shortly before or afterwards.
However, this lack of a bystander effect for remembering the location of pictures seen with arousing pictures may be specific to location memory. Even if location memory is not affected, arousing pictures may impair recognition and recall of bystander pictures. Mather and Nesmith's Experiment 3 cannot address this issue, because it did not include item memory tests. Consistent with the possibility that arousal might have different effects on location memory binding and item memory, in another experiment, Mather and Nesmith (2008) found that when participants looked at individual pictures for longer, they were more likely to recognize those pictures later, but were not more likely to remember those picture-location conjunctions (see also Malmberg & Shiffrin, 2005). Thus, competing for attentional resources during encoding with arousing stimuli may not impair memory for contextual features such as location while impairing item memory.
To examine how arousing items affect memory for nearby information, we conducted three experiments. In Experiment 1, we examined how item memory is affected by nearby arousing items. In Experiment 2, we examined how memory binding of contextual information is affected by nearby arousing items. Finally, in Experiment 3 we examined the effect of arousing foreground items on memory for neutral background information. In all of these experiments, the encoding task was to indicate the color of dots that appeared between presentations of the pictures.
In Experiments 1 and 2, we tested memory either immediately after the dot task or two days later to examine whether the effects of emotional arousal would increase over time. Many studies with animals indicate that stress hormones and the amygdala enhance long-term memory consolidation for emotional events (for a review see McGaugh, 2004). These findings suggest that memories of stimuli that evoked emotional arousal should last longer than memories of neutral stimuli. Although many studies with humans have found better memory for emotional stimuli compared with neutral stimuli at both short and long-term test delays, surprisingly few studies have compared memory for emotional and neutral stimuli at different time points (but for exceptions see LaBar & Phelps, 1998; Mather & Knight, 2005; Sharot & Phelps, 2004; Sharot & Yonelinas, 2008). Likewise, neuroimaging studies have shown that increased amygdala activity while viewing particular pictures or short film clips predicts that those stimuli will be better remembered about 45 minutes after learning (Dolcos et al., 2004) or weeks later (Cahill et al., 1996; Canli et al., 2000; Hamann et al., 1999), but have not examined whether amygdala activation at encoding is increasingly influential as the time between encoding and retrieval increases.1 By including a delay manipulation in Experiments 1 and 2, we could also test whether the effects of competition with arousing stimuli at encoding increase or decrease as time passes.
Experiment 1
In this experiment, participants viewed pairs of pictures that alternated with dots. Their task was to indicate whether the dots were yellow or green (see Figure 1). To examine how memory for bystander pictures was affected by nearby arousing pictures, each presentation pair had one designated bystander picture that remained the same across participants. The other picture switched between arousing and non-arousing matched versions across participants (e.g., the matched picture in the first trial of Figure 1 would either be a man holding a gun to his head or a man holding a hairdryer to his head). In addition, we compared an immediate test condition to a 2-day delay condition to see if there were any effects of retention interval on memory for bystander items.
Figure 1.
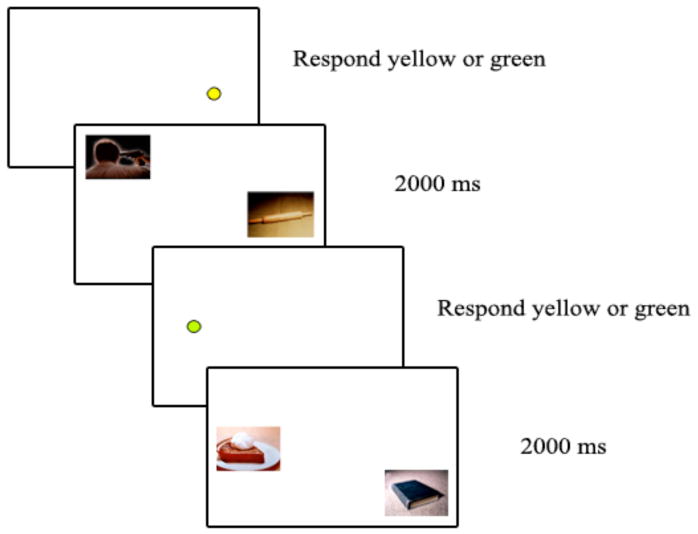
Trial sequence for the encoding phase in Experiment 1
Method
Participants
Thirty-six undergraduates participated for course credit (M age = 19.25, SD = 1.2, range = 18-22, 25 female) and were randomly assigned to either an immediate or a 2-day delay condition for the memory tests.
Materials
In order to equate the arousing and non-arousing pictures as much as possible on all dimensions other than arousal, we matched each arousing picture to a less arousing picture that was similar in appearance, complexity, content, and focus of interest. The 72 picture pairs (144 pictures) were grouped into three sets of 24 pairs based on the type of arousing picture in the pair: medium arousal negative, medium arousal positive and high arousal negative. We used this set of matched pictures in three previous experiments involving 104 participants altogether who rated each picture arousal and valence at the end of their session (Mather & Nesmith, 2008). Before using this picture set for the current experiments, we edited a few of the pictures to increase the visual similarity within the pairs.
Each participant was shown just one of the pictures from a matched pair; whether it was the arousing or the non-arousing version was counterbalanced across participants. During the dot-task phase, participants were shown 36 matched pictures (6 arousing and 6 non-arousing pictures from each of the three pair types) and 36 bystander non-arousing pictures, with one bystander picture seen together with each matched picture (see Figure 1 for an example trial sequence). Across participants, each arousing picture had a yoked non-arousing control picture shown in the same configuration, allowing all stimuli features to remain constant except for whether there was an arousing picture or a non-arousing picture in each presentation pair. At test, participants were shown 72 pictures from the matched pairs set and 72 bystander pictures. Which 36 pictures were old or new within each of these sets was counterbalanced across participants. The order of both study and test trials was randomized.
We presented stimuli using Psyscope (Cohen et al., 1993). The screen (on a 17” monitor) was divided into a three by three grid (without visible lines) and the outer eight cells served as locations for the pictures. Pictures were presented as large as possible within the boundaries of the cells (each one 7.6 mm wide and 6.5 mm high, with 2 mm separation between cells) without distorting the picture dimensions.
Procedure
For the dot-task phase of the experiment, the instructions informed participants that they should indicate whether each dot they saw was yellow or green; in addition, they would see pictures, but no response was needed when they saw the pictures and they should just view them as though they were pictures in a slide show. During the dot-task phase, either a yellow or green dot appeared in one of the eight locations on the screen (see Figure 1 for trial sequence). The dot remained on the screen until the participant indicated which color it was by pressing one of two response keys, at which point two pictures appeared together for 2000 ms. One picture appeared in the location the dot had been in and the other appeared in a different location; which picture in the presentation pair was cued by the dot was counterbalanced across participants. Then the next trial began with a dot.
After the dot-color session was complete (36 trials), participants in the immediate test condition were given a sheet of paper and asked to list descriptions of as many of the pictures as they could recall. This recall task was self-paced. Next, they were given a old/new recognition test in which they were shown one picture at a time on the screen and asked to indicate whether it was a picture in the dot-color task (old) or not (new).
After the memory tests, participants completed arousal ratings for each of the 144 pictures, followed by valence ratings for each picture. For the arousal ratings, participants were asked to rate each picture's “emotional intensity” on a scale of 1 to 9 with 9 equaling “very emotionally intense.” A picture rated high in emotional intensity was defined for participants as evoking strong emotions such as being excited, disgusted, amazed, or fearful, whereas, a picture low in emotional intensity would evoke calm or bored feelings. The valence ratings were also made on a scale of 1 to 9, with 1 equal to ‘very negative,’ 5 equal to ‘neutral’ and 9 equal to ‘very positive.’
Results
Dot-color accuracy
In what follows, we report means and 95% confidence intervals. Participants were quite accurate at indicating which color the dots were, and performance was equivalent for the two delay groups (M = .97 ± .02 for both groups).
Recall coding
Two coders judged which picture from the set each recalled description matched most closely, or if there was no acceptable match. Inter-rater reliability for which pictures were recalled was 73%.2 After discussion, the two coders resolved their disagreements. Further categorization of the pictures into one of the six matched-pictures categories (e.g., non-arousing positive match) or a bystander picture revealed that the coders initially disagreed about the category of an item in their initial scoring on only 4% of the items recalled, thus initial mismatches in coding some items should not affect the analyses below which are based on categories.
Recall of matched pictures
In these analyses, we included both pair type and arousal as factors. Pair type (e.g., medium arousal negative) refers to the three sets of 24 pairs of matched arousing and neutral pictures and so does not distinguish neutral and arousing picture versions. The arousal factor compares the arousing and neutral versions. A 2 (arousing vs. non-arousing version) × 3 (high arousal negative, medium arousal negative, or medium arousal positive pair type) × 2 (immediate vs. delayed test) ANOVA revealed a powerful effect of version, with more arousing pictures recalled (M = 4.00 ± .29) than non-arousing matched pictures (M = .92 ± .15), F(1,33) = 87.93, p<.001, ηp2 = .73. In addition, as expected, participants recalled more pictures when tested immediately (M = 6.10 ± .44) than two days later (M = 3.73 ± .50), F(1,33) = 12.70, p = .001, ηp2 = .28. However, there was not a significant interaction of delay and version, F(1,33) = 1.61, p = .21, ηp2 = .05, indicating that forgetting of arousing pictures did not differ significantly from forgetting of non-arousing matches (see left side of Figure 2).
Figure 2.
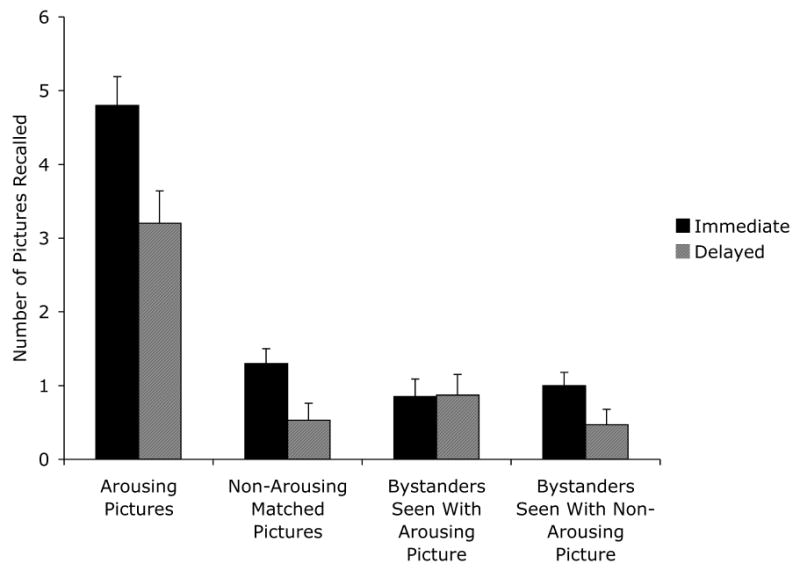
Number of pictures recalled in the immediate and delayed recall conditions of Experiment 1.
There also was a main effect of pair type, F(2,66) = 21.76, p<.001, ηp2=.40, that was qualified by an interaction of whether the picture version was arousing or not and pair type, F(2,66) = 18.90, p<.001, ηp2=.36. As can be seen in Table 1, the non-arousing versions of matched pictures yielded low recall levels that did not differ much by pair type, but the arousing versions yielded higher recall of the high arousal negative pictures than of the medium arousal positive or negative pictures. There were no other significant effects for recall of the matched pictures.
Table 1.
Average number of arousing and non-arousing matched pictures recalled in Experiment 1.
| From High Arousal Negative Pair | From Med. Arousal Negative Pair | From Med. Arousal Positive Pair | |
|---|---|---|---|
| Immediate Test | |||
| Arousing Picture | 2.55 (.20) | 1.05 (.19) | 1.20 (.21) |
| Non-arousing Match | 0.35 (.13) | 0.40 (.10) | 0.55 (.10) |
| Delayed Test | |||
| Arousing Picture | 1.87 (.23) | 0.60 (.22) | 0.73 (.25) |
| Non-arousing Match | 0.27 (.15) | 0.13 (.12) | 0.13 (.12) |
Note: Standard errors are in parentheses.
Recall of bystander neutral pictures
A 2 (seen with an arousing vs. non-arousing picture version) × 3 (seen with a high arousal negative, medium arousal negative, or medium arousal positive pair type) × 2 (immediate vs. delayed test) ANOVA revealed no significant effects. Of particular interest, the number of bystander pictures recalled was not lower when the other pictures shown at the same time were arousing (M = .86 ± .18) than when they were non-arousing (M = .73 ± .14), F(1,33) = .32, p>.5, ηp2 = .01. Thus, the dramatic boost in memory for arousing pictures occurred with no cost in recall of pictures shown at the same time. In addition, there was not a significant interaction of delay and version, F(1,33) = 1.52, p>.2, ηp2 = .04 (see right side of Figure 2).
Recognition of matched pictures
We compared d′ for the arousing and non-arousing pictures using a 2 (arousing vs. non-arousing version) × 3 (from a high arousal negative, medium arousal negative, or medium arousal positive pair type) × 2 (immediate vs. delayed test) ANOVA (see Table 3 for d′ scores, raw hits and false alarms). Participants remembered the arousing versions of the pictures much better (M = 2.14 ± .29) than the non-arousing versions (M = 1.15 ± .26), F(1,34)=79.03, p<.001, ηp2=.70. D′ scores were higher when tested immediately (M = 1.94 ± .34) than when tested after two days (M = 1.36 ± .38), F(1,34)=5.52, p<.05, ηp2=.14. However, there was not a significant arousal by delay interaction, F(1,34)=.58, p=.45, ηp2=.017, indicating similar rates of forgetting for arousing and non-arousing pictures (see Figure 3).3
Table 3.
Recognition memory for arousing and non-arousing matched pictures in Experiment 1.
| Immediate Test | Delayed Test | |||||
|---|---|---|---|---|---|---|
| From High Arousal Negative Pair | From Med. Arousal Negative Pair | From Med. Arousal Positive Pair | From High Arousal Negative Pair | From Med. Arousal Negative Pair | From Med. Arousal Positive Pair | |
| d′ | ||||||
| Ars | 3.06 (.30) | 2.25 (.26) | 2.13 (.26) | 2.40 (.34) | 1.61 (.29) | 1.42 (.29) |
| NA | 1.39 (.20) | 1.44 (.29) | 1.37 (.23) | 1.30 (.23) | 0.50 (.32) | 0.90 (.26) |
| Hits | ||||||
| Ars | 0.76 (.05) | 0.57 (.06) | 0.58 (.05) | 0.74 (.05) | 0.53 (.07) | 0.44 (.06) |
| NA | 0.43 (.05) | 0.37 (.04) | 0.30 (.05) | 0.32 (.06) | 0.27 (.05) | 0.29 (.06) |
| False Alarms | ||||||
| Ars | 0.07 (.04) | 0.05 (.03) | 0.08 (.04) | 0.15 (.03) | 0.15 (.04) | 0.15 (.04) |
| NA | 0.10 (.03) | 0.09 (.05) | 0.06 (.04) | 0.09 (.03) | 0.19 (.05) | 0.14 (.04) |
Notes: Ars = Arousing pictures; NA = Non-arousing pictures. Standard errors are in parentheses.
Figure 3.
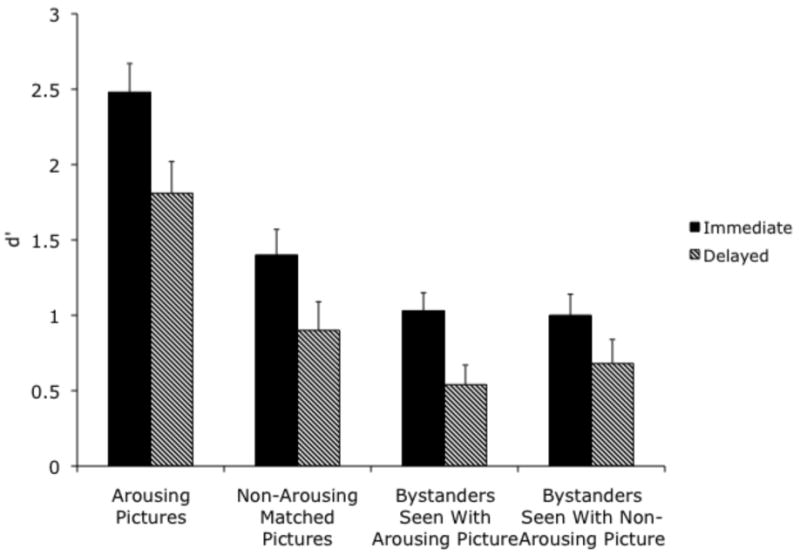
Recognition of pictures (d') in the immediate and delayed memory test conditions of Experiment 1.
Recognition of neutral bystander pictures
Our main question was how being displayed with an arousing picture would influence memory for neutral bystander pictures (see Table 4 for means). We examined d′ scores for the neutral bystander pictures seen in each trial in a 2 (seen with an arousing vs. non-arousing version of a matched picture pair) × 3 (seen with a high arousal negative, medium arousal negative, or medium arousal positive pair type) × 2 (immediate vs. delayed test) ANOVA. Recognition accuracy was better on the immediate test (M = 1.02 ± .23) than on the delayed test (M = .61 ± .25), F(1,34)=5.91, p<.05, ηp2=.15, but there were no other significant effects. Of particular interest, there was no significant effect of whether the picture shown at the same time was arousing or not, F(1,34)=.25, p>.6, ηp2=.01 (see right side of Figure 3). Thus, despite the large effect of arousal on memory for the arousing pictures themselves, there was no significant spillover effect for the bystander pictures. Of course, as with any null effect, it is possible that there was an effect that would have been detected with a larger sample. However, unlike the substantial effect size for arousal on the matched pictures (ηp2=.70), the estimated non-significant effect of being seen with an arousing picture on the bystander pictures was so small (ηp2=.01), that Cohen (1988) estimates it would take more than 1000 subjects to detect the difference.
Table 4.
Recognition memory for bystander non-arousing pictures in Experiment 1.
| Immediate Test | Delayed Test | |||||
|---|---|---|---|---|---|---|
| From High Arousal Negative Pair | From Med. Arousal Negative Pair | From Med. Arousal Positive Pair | From High Arousal Negative Pair | From Med. Arousal Negative Pair | From Med. Arousal Positive Pair | |
| d′ | ||||||
| Ars | 0.78 (.26) | 1.01 (.19) | 1.23 (.19) | 0.55 (.29) | 0.98 (.21) | 0.52 (.21) |
| NA | 0.92 (.26) | 1.01 (.18) | 1.16 (.19) | 0.43 (.29) | 0.66 (.20) | 0.52 (.22) |
| Hits | ||||||
| Ars | 0.38 (.06) | 0.35 (.06) | 0.38 (.04) | 0.33 (.06) | 0.34 (.06) | 0.32 (.04) |
| NA | 0.33 (.06) | 0.33 (.05) | 0.38 (.04) | 0.40 (.06) | 0.39 (.06) | 0.32 (.04) |
| False Alarms | ||||||
| 0.11 (.04) | 0.09 (.02) | 0.11 (.03) | 0.25 (.04) | 0.15 (.03) | 0.19 (.03) | |
Notes: Ars = seen with arousing version of the matched picture pair; NA = seen with non-arousing version of the matched picture pair. Old vs. new bystander pictures were counterbalanced across arousing and non-arousing versions of the matched pictures, but always appeared with the same pair type, thus new items can be categorized by the pair type but not by arousal type. Standard errors are in parentheses.
Arousal and valence ratings
These ratings yielded the expected differences between categories (Mather & Nesmith, 2008), as did the next experiment. The combined ratings from Experiments 1 and 2 are reported in the Appendix.
Discussion
As expected, participants in this study remembered arousing pictures better than their visually matched non-arousing counterparts. However, memory enhancement for arousing pictures did not undermine memory for nearby pictures shown at the same time. Thus, when a pair of items is shown for a couple of seconds, the arousal level of one item has little impact on how well the other item will be remembered later. In addition, the effects of emotional arousal were consistent across the two different test delay conditions. The next experiment examines whether similar effects occur for memory binding.
Experiment 2
In Experiment 2 we examined how arousing pictures affect two types of memory binding: a within-object association (the location of the pictures) and a between-object association (which two pictures were shown together). We expected to replicate findings from Mather and Nesmith (2008) of arousal-enhanced location memory, but only for the arousing picture itself and not for the bystander picture. In addition, we expected that there would be no arousal benefit for between-object memory binding (Mather, 2007). We also included a delay manipulation to see if we might see larger effects of arousal on memory binding over time, as might be expected from some theoretical perspectives (e.g., McGaugh, 2000). To our knowledge, no previous studies have examined the effects of delay on how arousal modulates associative memory.
Method
Participants
Twenty-eight undergraduates participated for course credit (M age = 19.25, SD = 1.21, range = 18-22, 11 female) and were randomly assigned to either the immediate or the 2-day delay condition for the memory tests.
Materials and Procedure
The encoding phase had the same format as Experiment 1. However, instead of 36 trials at encoding, one picture from each of the 72 matched pair pictures was shown, since there was no need to reserve any to serve as new items on the memory tests. In addition, instead of presenting the trials in a random sequence as we did before, in this experiment we presented the arousing pictures in two blocks of 18 (pictures of different valences were randomly intermixed within this sequence) that alternated with two blocks of neutral pictures. There were no breaks in between the arousing and neutral picture blocks and which type came first was counterbalanced across participants. We blocked the arousing items to increase the impact of arousing items on bystander items. If the arousal evoked by a picture takes time to dissipate and so influences processing on subsequent trials, blocking the trials should increase the impact of arousal and potentially decrease the attentional resources available for neutral bystander items seen during the arousing blocks.
During the test phase, participants first completed a forced-choice memory test for picture-location conjunctions. Each test trial consisted of one of the pictures shown in the dot-task phase, displayed simultaneously in three different locations on the screen, each with a number printed next to it. One of the locations was the same one the picture had appeared in during the dot-task phase. Participants typed in the number that corresponded with the picture-location conjunction they thought they had seen before. Previously seen matched pictures and bystander pictures were tested in an intermixed random sequence. Next, participants completed a forced-choice memory test for the picture-picture conjunctions. On each trial, they were shown two pairs of pictures and were asked which pair had been shown together during the dot task. The two pairs were always from the same category. For example, if the correct pair in a trial consisted of a high arousal negative picture and its bystander picture, the mismatched pair would consist of a high arousal negative picture and the bystander picture seen with another high arousal negative picture. Test trials were in a random order.
After the memory tests, participants completed arousal and valence ratings for the pictures, as in Experiment 1, except that they rated only the 72 pictures they had seen, rather than all 144 pictures.
Results
Dot-color accuracy
As in the previous experiment, dot color accuracy was high (M = .96 ± .02) and did not differ significantly between the two groups (F<1).
Location memory for matched pictures
A 2 (arousing vs. non-arousing version) × 3 (high arousal negative, medium arousal negative, or medium arousal positive pair type) × 2 (immediate vs. delayed test) ANOVA revealed arousal-enhanced location memory, with greater accuracy for arousing pictures (M = .56 ± .06) than for matched non-arousing pictures (M = .40 ± .04), F(1,26) = 26.22, p<.001, ηp2=.50, replicating findings from Mather and Nesmith (2008). As expected, location memory was worse after a 2-day delay (M = .37 ± .06) than on an immediate test (M = .59 ± .06), F(1,26) = 28.84, p<.001, ηp2=.53. However, there was no significant interaction of arousal and test delay, F(1,26) = .04, ηp2=.00, indicating that the effect of arousal was similar across the two test delays (see left side of Figure 4). There were no other effects of arousal or delay (see Table 5 for means).4
Figure 4.
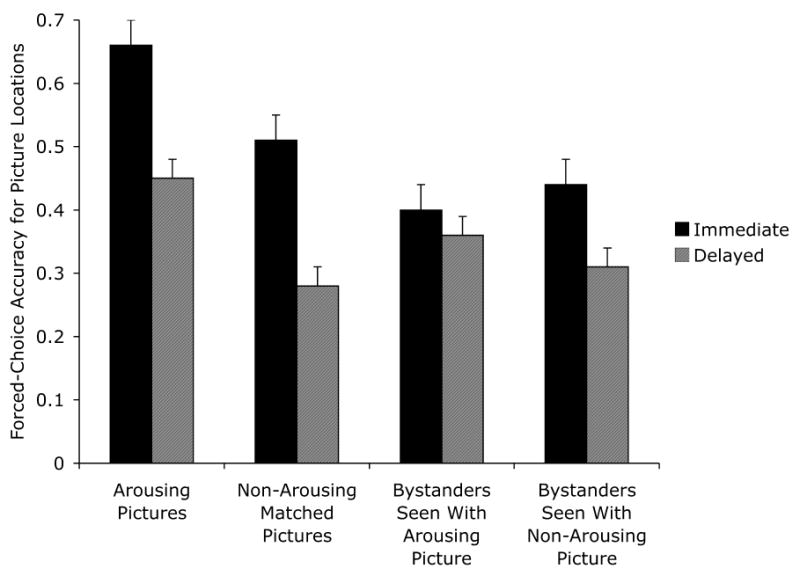
Forced-choice accuracy for location-picture conjunctions in the immediate and delayed memory test conditions of Experiment 2 (chance = 33%).
Table 5.
Three-alternative forced-choice location accuracy for arousing and nonarousing matched pictures in Experiment 2.
| From High Arousal Negative Pair | From Med. Arousal Negative Pair | From Med. Arousal Positive Pair | |
|---|---|---|---|
| Immediate Test | |||
| Arousing Picture | .81 (.06) | .56 (.07) | .62 (.05) |
| Non-arousing Match | .58 (.04) | .43 (.05) | .51 (.06) |
| Delayed Test | |||
| Arousing Picture | .49 (.06) | .33 (.07) | .52 (.05) |
| Non-arousing Match | .26 (.04) | .24 (.05) | .35 (.06) |
Note: Standard errors are in parentheses.
Location memory for bystander pictures
A 2 (seen with the arousing vs. non-arousing version of a matched picture pair) × 3 (seen with a high arousal negative, medium arousal negative, or medium arousal positive pair type) × 2 (immediate vs. delayed test) ANOVA revealed no significant impairment in location memory for pictures seen with an arousing picture (M = .38 ± .05) compared with those seen with a matched non-arousing picture (M = .38 ± .04), F(1,26) = .02, ηp2<.01. Thus, as with the item memory measure in Experiment 1, there was no significant bystander effect for memory binding, with a nearly non-existent effect size. Location accuracy was worse when tested two days later (M = .34 ± .05) than when tested immediately (M = .42 ± .05), F(1,26) = 5.56, p<.05, ηp2=.18.
Picture pair memory
A 2 (arousing vs. nonarousing version) × 3 (high arousal negative, medium arousal negative, or medium arousal positive pair type) × 2 (immediate vs. delayed test) ANOVA revealed no significant main effect of version, F(1,26) = .16, ηp2=.01, nor any interactions with version (see Figure 5). However, there was a marginally significant effect of delay, with worse performance on the 2-day test (M = .55 ± .07) than on the immediate test (M = .64 ± .06), F(1,26) = 3.50, p= .07, ηp2=.12.
Figure 5.
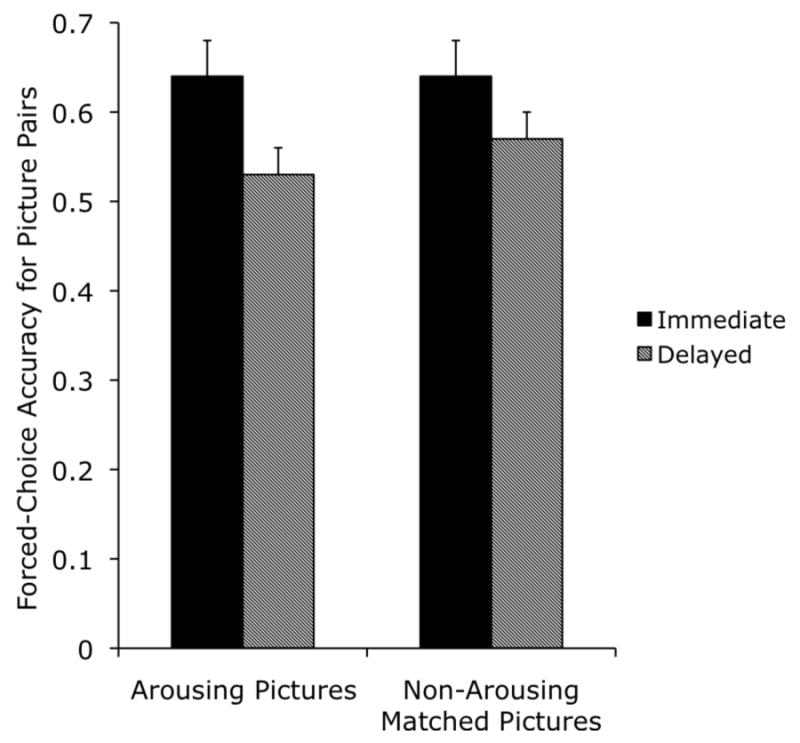
Forced-choice accuracy for the picture-picture pairs in the immediate and delayed memory test conditions of Experiment 2 (chance = 50%).
Arousal ratings
See Appendix.
Discussion
As in Mather and Nesmith's (2008) study, participants in this study remembered the location of arousing pictures better than the locations of their visually matched non-arousing counterparts. However, this arousal-enhanced picture-location memory binding did not impact picture-location binding for bystander pictures. In addition, memory for which two pictures were paired during the encoding phase was not enhanced when one of the pictures was arousing, suggesting that arousal associated with one item does not enhance memory for associations between that item and other items (Mather, 2007).
Experiment 3
In the previous two experiments, when participants were shown two pictures at the same time, memory for one picture was not affected by the arousal level of the other picture. This lack of an arousal effect for bystander pictures was seen for free recall, recognition and picture-location conjunction memory. These findings contrast with previous findings reviewed in the Introduction that reveal memory impairments for peripheral or background details. Thus, in Experiment 3, we wanted to see if we could demonstrate memory impairments for background information using the same emotional pictures and encoding task as in Experiments 1 and 2. Thus, in Experiment 3, we presented one picture at a time in various locations on the screen, each with a different background pattern behind it. Our prediction was that, unlike the lack of an arousal effect for bystander pictures, here we would see impaired memory for backgrounds seen behind arousing versions of pictures compared with the same backgrounds seen behind the non-arousing versions of the pictures. All participants in Experiment 3 were tested immediately after the dot-task session.
Method
Participants
Twenty-four undergraduates participated for course credit (M age = 19.38, SD = 1.61, range = 18-25, 21 female).
Materials and Procedure
We made several modifications to the procedures from the previous experiments. First, in the dot-task phase, instead of seeing two pictures between each dot presentation, participants were shown one picture at a time, presented in one of the eight locations in front of a background pattern that covered the rest of the screen (see Figure 6). There were 36 trials in the dot-task phase and the dot appeared in the location of the next picture half the time and in some other random location the rest of the time. Across participants, we counterbalanced whether the arousing or non-arousing version of each picture was shown and whether each picture/background was shown both on the dot-task and recognition memory tests (serving as old items) or just on the memory tests (serving as new items). After the dot-task phase, participants completed the memory tests. First were two recognition memory tests, one that showed 72 pictures and one that showed 72 backgrounds. In both recognition tests, there were 36 old and 36 new items and participants were asked to indicate whether they had seen the image in the previous phase or not. After the recognition tests, participants were shown two copies of each old picture in different locations on the screen and were asked to indicate which picture-location pairing they had seen in the dot-task phase.
Figure 6.
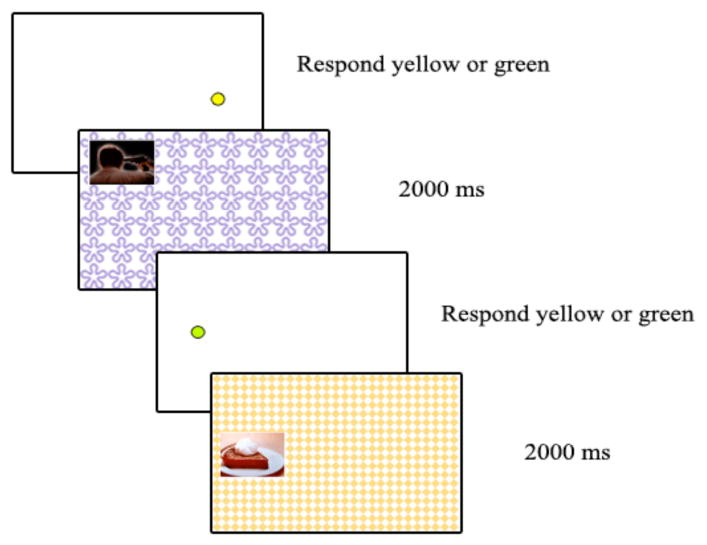
Trial sequence for the encoding phase in Experiment 3.
Results
Dot-color accuracy
As in the previous experiments, dot-color accuracy during the encoding task was high (M = .96 ± .02).
Recognition memory for pictures
Replicating Experiment 1, participants had higher d′ for the arousing versions of pictures (M = .80 ± .06) than for the non-arousing versions (M = .67 ± .08), F(1,23) = 16.23, p<.01, ηp2=.41, as indicated by a 2 (arousing vs. non-arousing version) × 3 (high arousal negative, medium arousal negative, or medium arousal positive pair type) ANOVA (see Figure 7). There also was a main effect of pair type, F(2,46) = 8.35, p<.01, ηp2=.27; pictures from high arousal negative pairs were remembered best (M = 2.74 ± .25), followed by those from medium arousal negative pairs (M = 2.45 ± .30) and positive pairs (M = 2.11 ± .36). There was not a significant interaction of the two factors.
Figure 7.
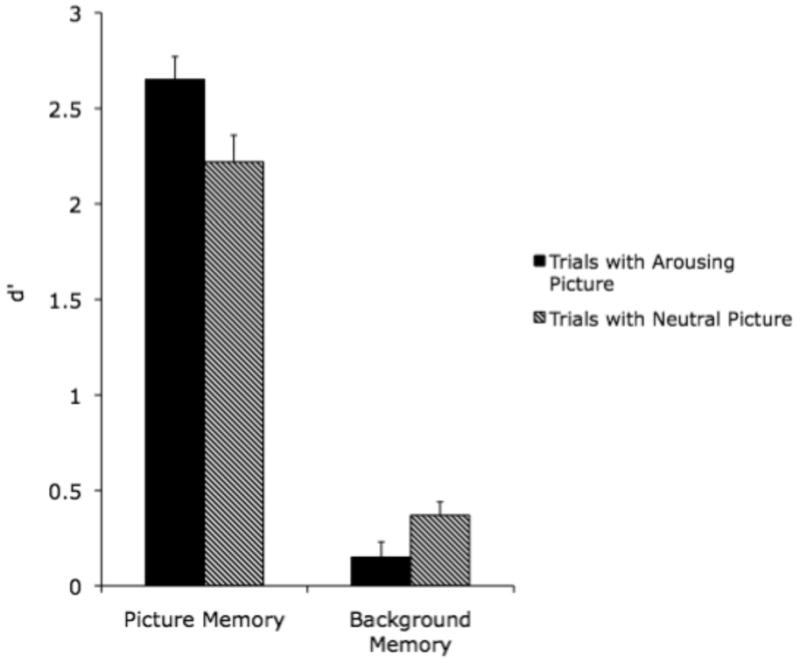
Recognition (d') for pictures and background patterns in Experiment 3.
Recognition memory for backgrounds
A 2 (seen with arousing vs. non-arousing version) × 3 (seen with high arousal negative, medium arousal negative, or medium arousal positive pair type) ANOVA for d′ for backgrounds paired with each type of picture revealed that participants were worse at identifying backgrounds previously shown with arousing pictures (M = .15 ± .17) than those previously shown with non-arousing pictures (M = .37 ± .14), F(1,23) = 7.83, p<.05, ηp2=.25 (see Figure 7). There were no other significant effects.
Location memory for matched pictures
A 2 (arousing vs. non-arousing version) × 3 (high arousal negative, medium arousal negative, or medium arousal positive pair type) ANOVA revealed arousal-enhanced location memory, with greater accuracy for arousing pictures (M = .73 ± .05) than for matched non-arousing pictures (M = .67 ± .05), F(1,23) = 5.68, p<.05, ηp2=.20, consistent with findings from Experiment 2 and Mather and Nesmith (2008). There were no other significant effects.
Discussion
Unlike in the previous two experiments, in Experiment 3 we found that the arousal level of a picture affected memory for nearby information. That is, seeing an arousing picture in the foreground decreased later recognition of a pattern shown in the background compared with seeing a non-arousing picture in the foreground. At the same time, participants were better at recognizing arousing pictures than non-arousing pictures and were also better at remembering the picture-location conjunction for arousing pictures than for non-arousing pictures (replicating our previous findings of enhanced item and location memory for arousing pictures).
General Discussion
In Experiments 1 and 2, we examined how an arousing picture affects item memory and associative memory for a bystander picture. Despite showing large effects of arousal on memory for the arousing item itself, neither experiment revealed significant effects of arousal on non-arousing bystander items. Indeed, the effect sizes for the bystander items were nearly zero. These findings are surprising given previous findings reviewed in the Introduction that arousing items impair memory for visually peripheral information surrounding an arousing item and impair memory for items appearing before or after an arousing item in a sequence.
One key aspect of many studies revealing that arousing items impair memory for nearby information is that the arousing item is perceptually dominant and that the other items are less prominent. Specifically, in comparison with unusual or distinctive central items, arousing central items in scenes are more likely to lead to impairment for background information (Brown, 2003; Christianson & Loftus, 1991; Christianson et al., 1991; Schmidt, 2002), although some studies have also shown that surprising central items can lead to similar impairments for peripheral information (Mitchell et al., 1998; Pickel, 1999, 1998). The arousal associated with the central item does seem to play a key role, as indicated by findings that spider phobics show greater impairments in memory for peripheral information presented with a spider than do non phobics (Wessel & Merckelbach, 1998, 1997).
Likewise, studies showing that emotional oddballs lead to impaired memory for preceding and subsequent items also have one dominant arousing item that stands out from all of the multiple non-arousing items on the lists. For instance, in some studies the oddball items are arousing or non-arousing photographs and the other list items are black-and-white line drawings (Detterman & Ellis, 1972; Erdelyi & Blumenthal, 1973; Hurlemann et al., 2007). In contrast, in the present Experiments 1 and 2 the emotional and bystander items were equally detailed and perceptually prominent. Together, this pattern of findings suggests that an emotional item is unlikely to impair memory for information that is equally perceptually prominent but is likely to impair memory for information that is less perceptually prominent than itself.
To test whether our emotional pictures could lead to memory impairment for perceptually subordinate information, in Experiment 3, we used our set of picture stimuli to see if arousing items would impair memory for background information. As expected given other researchers' previous findings, we found that seeing an arousing picture in the foreground impaired memory for the background pattern. In addition, consistent with Experiments 1 and 2, both recognition and picture-location conjunction memory were enhanced for arousing pictures over non-arousing pictures.
Importance of the perceptual configuration
Experiments 1 and 2 reveal that emotionally arousing pictures do not impair memory for nearby neutral pictures, indicating that emotionally arousing stimuli do not necessarily impair memory for all nearby information. In contrast, Experiment 3 demonstrates that the same emotionally arousing pictures can lead to memory impairment for background patterns. Unlike the bystander pictures in Experiments 1 and 2, the patterns in Experiment 3 were both less interesting and engaging and clearly behind the target pictures. Thus, these findings suggest a key factor: Whether memory for bystander information will be impaired by nearby arousing information depends on whether the arousing information is already perceptually dominant.
One way to make something perceptually dominant over other information is to place it in the foreground. Distinguishing an object from its background is a basic perceptual process that happens quickly and automatically. For instance, figure-ground segregation leads to significantly larger neuronal responses to elements belonging to the figure than to the ground even in primary visual cortex (Lamme, 1995). Thus, foreground objects dominate perceptual processing. In contrast, when two pictures are shown on the screen at the same time, they both have the basic perceptual properties of foreground objects and so start out on more equal footing in the competition for perceptual resources (Vecera, 2000). The arousal induced by a picture may only impair memory for nearby information if perceptual processing of that information is already being suppressed, as is the case for background information when figure-ground segmentation has occurred (e.g., Hupe et al., 1998).
The possibility that arousal does not impair memory for peripheral items that are perceived to be in the foreground is consistent with a couple of other studies. In one study, participants asked to attend to a central word and ignore peripheral words did not show any effect of the arousal of the central word on later memory for the peripheral word (Sharot & Phelps, 2004). In another study, participants who viewed a series of scenes that each had an unrelated line drawing of an object placed over one corner of the photograph remembered the objects equally well for arousing and non-arousing scenes (Touryan et al., 2007). However, further work is needed to test whether the foreground/background distinction or the reduced perceptual complexity in the background is more important in leading memory for the background information to suffer when it is shown behind an arousing picture rather than behind a non-arousing picture. Furthermore, the degree to which these effects reflect general distinctiveness should be examined. Recognition memory for the backgrounds in Experiment 3 was poorer than recognition memory for bystander pictures in Experiment 1, and so it may be the case that emotionally arousing stimuli are more likely to impair memory for any information that attracts less attention and is more poorly encoded.
More research is also needed to understand how the source of the arousal affects memory. As in our studies, most laboratory research has induced arousal using visual stimuli. However, in a study in which participants viewed a slide show while listening to a narration that either provided a neutral or an emotionally arousing interpretation of the slide show (Laney et al., 2004), thematically induced arousal enhanced memory for the gist of the slides and did not impair memory for visually peripheral details.
Arousing items may cause more interference in working memory than in perception
In these experiments, participants were asked to make judgments about intervening dots and did not know they would get a memory test. Thus, it is unlikely they were rehearsing the pictures. However, when participants see four pictures in a row in different locations and then try to maintain the four picture-location conjunctions, they are worse at remembering the locations when the four pictures are arousing than when they are all neutral (Mather et al., 2006; Mitchell et al., 2006). Maintaining multiple bound representations in working memory is challenging and requires frequent refreshing of each representation. Arousing pictures elicit more focused attention (for a review, see Mather, 2007). Thus, having multiple arousing pictures in the set may lead participants to forget the associations between other pictures and their locations while they focus on the representation of one arousing picture and its location. It may be easier to distribute attention across multiple representations when all the pictures are neutral than when they are each very attention demanding. In contrast, as seen in the present studies and in Mather and Nesmith (2008), arousing pictures do not appear to have disruptive effects on spatially or temporally adjacent pictures when there is no working memory load requiring distributed attention to multiple location-picture conjunctions.
Increasing the delay does not increase the advantage for arousing stimuli in memory
Inducing arousal through a stress manipulation or by showing an emotional film can enhance long-term memory for events that were experienced just before the arousal (Cahill et al., 2003; Nielson & Powless, 2007). Because the arousal occurs after the initial experience of the event, it appears to have its enhancing effects via modulation of consolidation processes (McGaugh, 2000). A further inference that can be made from these findings is that the benefits of arousal accrue as memories consolidate into more permanent memory traces and so the longer the test delay, the larger the advantage in memory for emotional stimuli should be.
However, few studies have actually included different test delays when examining memory for emotional stimuli, and these have mixed results. Perhaps the best known study showing that the memory advantage for arousing stimuli increases as time passes was done by Kleinsmith and Kaplan (1963), who found a dramatic crossover interaction. Word-digit pairs that elicited a higher galvanic skin response (GSR) during learning were initially less likely to be recalled, but as the delay interval increased, they were more likely to be recalled. However, as outlined in Mather (2007), these findings have an alternative explanation, which is that GSR is highest at the beginning and lowest at the end of a short list presentation and so the steep decline in recency effects together with enduring primacy effects as test delays increase can account for these findings. Indeed, studies that either had some buffer items to eliminate the primacy and recency effects (Schürer-Necker, 1990) or used items that were categorized by group norms rather than by each participant's skin-conductance responses (Kaplan & Kaplan, 1970; Maltzman et al., 1966) failed to find this arousal by delay interaction.
Despite the problems with the Kleinsmith and Kaplan findings, a few studies indicate that the memory advantage for emotional stimuli increases as the retention interval increases. In a couple of these studies, participants were exposed to arousing taboo words and neutral words and tested immediately or after a delay of 45 minutes (LaBar & Phelps, 1998) or 24 hours (Sharot & Phelps, 2004), resulting in a larger arousal advantage in the longer delay condition. In another study (Sharot & Yonelinas, 2008), participants saw 120 emotional and neutral photos in a session on one day and another 120 photos in a session 24 hours later, followed by a recognition memory test. Surprisingly, there was no emotional advantage for the pictures seen right before the memory test, but there was one for pictures seen 24 hours earlier.
In contrast, a study comparing the effects of stress on memory for a narrated slide show that depicts a surgeon dealing with patients from a car accident found that the enhancement in memory for emotional components of the slide show did not change in magnitude when comparing results for participants completing an immediate test to those completing a delayed test a week after learning (Payne et al., 2006). We also note that two other studies that did not report statistical comparisons of the emotional advantage over two delay conditions also found no emotion consolidation effects. One of these studies examined memory for pictures from a slide show and had one third positive, one third negative and one third neutral slides (Mather & Knight, 2005, Experiment 1). In this study, about 80% (rather than the 66% that would be expected by chance) of the pictures participants recalled consisted of emotional pictures both 20 minutes after the slide show and two days later, indicating a consistent emotional advantage over time. Likewise, a study that had participants recall pictures either 10 minutes after seeing them or 4 weeks later (Hamann et al., 1999) found that on both memory tests, 70% of the pictures recalled were emotional rather than the 50% that would be expected by chance. Thus, although overall recall declined, the emotional advantage remained constant.
Like these studies, Experiments 1 and 2 also revealed equivalent degrees of memory enhancement for arousing stimuli on immediate tests and tests two days later. This lack of support for arousal-enhanced memory consolidation is surprising given research with rodents suggesting that that enhanced long-term memory for emotional events results from the amygdala and stress hormones modulating long-term memory consolidation processes (LaBar & Cabeza, 2006; McGaugh, 2004). What might account for the discrepancy? One possibility is that we lacked sufficient power to detect the differential effects of delay on memory consolidation. However, the estimated partial eta squared effect sizes for the non-significant arousal by delay interactions ranged from .00 (location memory) to .01 (recognition memory) to .05 (recall), suggesting that if the passage of time does affect memory differently for arousing and non-arousing stimuli, the effect is not large.
Another possibility is that the timing of sleep may play an important role. Wagner et al. (2006) found that if participants slept immediately after reading neutral and emotionally arousing text passages, they had better memory four years later for the emotionally arousing passages than for the neutral passages. In contrast, if they spent the next few hours awake, they did not show enhanced memory for the arousing text passages compared with the neutral ones. Our participants were tested during the day and so were unlikely to go sleep immediately after the encoding session. More generally, the lack of emotional consolidation effects in our study suggests that the widespread assumption among memory researchers that the advantage in memory for emotional events increases over time merits a critical examination.
Limitations
One limitation of our studies is the lack of effect of arousing pictures on bystander pictures is a null effect and therefore may be the result of insufficient power. However, the estimated effect sizes for the effect of arousing pictures on memory for bystander pictures were nearly zero, in contrast with the large effect sizes for the difference in memory for arousing and non-arousing pictures themselves. Furthermore, Experiment 3 demonstrates that our emotional pictures and dot-color task can lead to arousal-induced impairments for background patterns.
Because we made the bystander information both less interesting and distinctive and also put it in the background in Experiment 3, further research is needed to examine what the key factors are that lead to arousal-induced impairments for bystander information. Based on our current findings and previous research in the literature, our prediction is that any factor that makes something less perceptually dominant than an arousing item will increase the likelihood that it will be subject to arousal-based memory impairments.
Conclusions
In these studies we found that presenting an arousing picture did not impair item or location memory for a nearby picture, but did impair item memory for a background pattern. These findings indicate that arousing information does not automatically diminish later memory for other information presented at the same time. Instead, arousal may be most likely to impair processing of nearby information if that information is already suffering from reduced mental processing because of perceptual competition processes. Thus, our findings suggest that arousal increases existing perceptual biases, such that representations of currently ignored or suppressed stimuli are diminished whereas representations of attended stimuli are enhanced.
Table 2.
Average number of bystander non-arousing pictures recalled in Experiment 1.
| Seen With High Arousal Negative Pair | Seen With Med. Arousal Negative Pair | Seen With Med. Arousal Positive Pair | |
|---|---|---|---|
| Immediate Test | |||
| With Arousing | 0.30 (.15) | 0.20 (.13) | 0.35 (.11) |
| With Non-arousing | 0.25 (.10) | 0.30 (.09) | 0.45 (.12) |
| Delayed Test | |||
| With Arousing | 0.40 (.17) | 0.33 (.15) | 0.13 (.13) |
| With Non-arousing | 0.33 (.12) | 0.07 (.10) | 0.07 (.14) |
Note: Standard errors are in parentheses.
Table 6.
Three-alternative forced-choice location memory for bystander pictures seen with arousing and non-arousing matched pictures in Experiment 2.
| Seen With Picture From High Arousal Negative Pair | Seen With Picture From Med. Arousal Negative Pair | Seen With Picture From Med. Arousal Positive Pair | |
|---|---|---|---|
| Immediate Test | |||
| Seen With Arousing | .43 (.06) | .26 (.06) | .51 (.06) |
| Seen With Non-Arousing | .45 (.05) | .43 (.06) | .45 (.06) |
| Delayed Test | |||
| Seen With Arousing | .41 (.06) | .42 (.06) | .26 (.06) |
| Seen With Non-Arousing | .31 (.05) | .24 (.06) | .38 (.06) |
Note: Standard errors are in parentheses.
Table 7.
Pair memory for which pictures were displayed together in Experiment 2.
| From High Arousal Negative Pair | From Med. Arousal Negative Pair | From Med. Arousal Positive Pair | |
|---|---|---|---|
| Immediate Test | |||
| Arousing | .76 (.08) | .64 (.09) | .52 (.08) |
| Non-Arousing | .55 (.09) | .74 (.08) | .62 (.08) |
| Delayed Test | |||
| Arousing | .57 (.08) | .43 (.09) | .60 (.08) |
| Non-Arousing | .60 (.09) | .55 (.08) | .57 (.08) |
Note: Standard errors are in parentheses.
Table 8.
Recognition memory for pictures and backgrounds and location memory for pictures in Experiment 3.
| From High Arousal Negative Pair | From Med. Arousal Negative Pair | From Med. Arousal Positive Pair | ||
|---|---|---|---|---|
| d′ - Pictures | ||||
| Arousing | 2.96 (.15) | 2.63 (.17) | 2.35 (.17) | |
| Non-arousing | 2.51 (.14) | 2.27 (.18) | 1.87 (.23) | |
| Hits - Pictures | ||||
| Arousing | 0.92 (.03) | 0.85 (.03) | 0.76 (.04) | |
| Non-arousing | 0.79 (.04) | 0.69 (.05) | 0.62 (.06) | |
| False Alarms - Pictures | ||||
| Arousing | 0.04 (02) | 0.06 (.02) | 0.04 (.02) | |
| Non-arousing | 0.02 (.01) | 0.01 (.01) | 0.06 (.02) | |
| d′– Backgrounds | ||||
| Arousing | 0.03 (.15) | 0.14 (.15) | 0.28 (.16) | |
| Non-arousing | 0.29 (.15) | 0.28 (.14) | 0.54 (.16) | |
| Hits – Backgrounds | ||||
| Arousing | 0.36 (.05) | 0.36 (.04) | 0.43 (.05) | |
| Non-arousing | 0.43 (.05) | 0.39 (.04) | 0.51 (.05) | |
| False Alarms – Backgrounds | ||||
| 0.32 (.03) | 0.29 (.03) | 0.34 (.04) | ||
| Location Memory – Pictures | ||||
| Arousing | 0.72 (.04) | 0.70 (.04) | 0.76 (.04) | |
| Non-arousing | 0.71 (.03) | 0.67 (.04) | 0.62 (.04) | |
Note: Standard errors are in parentheses. Old vs. new backgrounds were counterbalanced across arousing and non-arousing versions of the matched pictures, but always appeared with the same pair type, thus new backgrounds can be categorized by the pair type but not by arousal type.
Acknowledgments
This work was supported by a grant from the National Institute on Aging (AG025340).
Appendix
Experiments 1 and 2 Ratings of Pictures
Participants in the first two experiments completed the same ratings task (the only difference was that in Experiment 2 participants only rated the 72 pictures they had seen in the dot-color encoding task, rather than all 144 pictures). Separate analyses for these three experiments yielded the same pattern of results and significant findings, thus to simplify reporting we combined the data. We conducted 2 (version: arousing, non-arousing) × 3 (emotion type: high negative, medium negative, medium positive) ANOVAs for both the arousal and valence ratings (See Table A1 for means). For the arousal ratings, as expected, there was a main effect of version, with the arousing versions of pictures rated as more arousing (M = 6.09, SE = 0.15) than the non arousing versions (M = 2.49, SE = 0.12), F (1, 66) = 797.09, p< 0.001, ηp2= .92. In addition, there was a main effect of emotion type, F(2,132) = 80.85, p< 0.001, ηp2= .55, and an interaction of version and emotion type, F(2,132) = 96.25, p< 0.001, ηp2= .59. As can be seen in Table A1, arousal ratings varied more for the arousing versions of the three emotional pictures and did not vary much across the non-arousing versions. Negative high arousal pictures have the highest arousal followed by negative medium arousal pictures and then by positive medium arousal pictures.
As expected, the valence rating analysis yielded a main effect of emotion type, F(2,132) = 404.48, p< 0.001, ηp2=.86, and an interaction of emotion type and version, F(2,132) = 330.22, p< 0.001, ηp2=.83. As seen in Table A1, the non-arousing matched control pictures were all about the same valence, whereas the positive pictures were rated more positively and the negative pictures were rated more negatively than the matched control pictures.
Table A1.
Mean Arousal and Valence Ratings in Experiment 1 and 2.
| Picture Type | Arousal | Valence |
|---|---|---|
| Negative High Arousal | 7.48 (1.36) | 1.78 (0.80) |
| Negative Medium Arousal | 5.94 (1.45) | 2.78 (0.92) |
| Positive Medium Arousal | 4.83 (1.58) | 6.47 (1.32) |
| Non-arousing (Neg. High Arousal Match) | 2.50 (0.98) | 5.26 (0.93) |
| Non-arousing (Neg. Med. Arousal Match) | 2.52 (1.09) | 5.47 (0.90) |
| Non-arousing (Pos. Med. Arousal Match) | 2.46 (1.06) | 5.73 (0.96) |
| Non-arousing (Bystander) | 1.99 (0.69) | 5.02 (0.78) |
Notes: Ratings were made using 1-9 scales. Standard errors are listed in parentheses. Neg.= Negative, Pos. = Positive, Med. = Medium
Footnotes
Hamann et al. (1999) found a significant relationship between amygdala activation and emotional memory enhancement on a recognition test four weeks later, but not between amygdala activation and a recall test after 10 min. Because no recognition test was given at the short-term retention interval, it is not clear whether the influence of the amygdala depended on test delay or test type in their study.
Due to experimenter error, recall data from one participant was lost.
There was also a main effect of pair type, F(1,34)=10.50, p<.001, ηp2=.24, with pictures from high arousal negative pairs recognized more accurately (M = 2.04 ± .33) than pictures from medium arousal negative (M = 1.45 ± .33) or positive (M = 1.45 ± .25) pairs. Differences in how memorable the neutral items from the three sets were suggest that factors other than arousal may have distinguished how memorable high and low arousal negative items and low arousal positive items were. However, finding consistent arousal effects across the three sets of matched pictures with no interactions between arousal and pair type indicates that the arousal effects generalize across the various other dimensions (including valence) that the sets may differ on.
The only other significant effect was a main effect of pair type, with location memory worse for pictures from the medium arousal negative pairs (M = .39 ± .07) than for those from the medium arousal positive pairs (M = .50 ± .07) or high arousal negative pairs (M = .54 ± .06), F(2,52) = 6.07, p<.01, ηp2=.19.
Contributor Information
Mara Mather, University of Southern California.
Marissa Gorlick, University of Southern California.
Kathryn Nesmith, University of California, Berkeley.
References
- Brown JM. Eyewitness memory for arousing events: Putting things into context. Applied Cognitive Psychology. 2003;17:93–106. [Google Scholar]
- Burke A, Heuer F, Reisberg D. Remembering emotional events. Memory and Cognition. 1992;20:277–290. doi: 10.3758/bf03199665. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning and Memory. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer JB, Gabrieli JDE, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional response. Journal of Neuroscience. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson SÅ. Emotional stress and eyewitness memory: A critical review. Psychological Bulletin. 1992;112:284–309. doi: 10.1037/0033-2909.112.2.284. [DOI] [PubMed] [Google Scholar]
- Christianson SÅ, Loftus EF. Remembering emotional events: The fate of detailed information. Cognition and Emotion. 1991;5:81–108. [Google Scholar]
- Christianson SÅ, Loftus EF, Hoffman H, Loftus GR. Eye fixations and memory for emotional events. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17:693–701. doi: 10.1037//0278-7393.17.4.693. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt RR, Provost J. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Detterman DK, Ellis NR. Determinants of induced amnesia in short-term memory. Journal of Experimental Psychology. 1972;95:308–316. doi: 10.1037/h0033629. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Erdelyi MH, Blumenthal DG. Cognitive masking in rapid sequential processing: The effect of an emotional picture on preceding and succeeding pictures. Memory and Cognition. 1973;1:201–204. doi: 10.3758/BF03198095. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hupe JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J. Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature. 1998;394:784–787. doi: 10.1038/29537. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Matusch A, Hawellek B, Klingmuller D, Kolsch H, Maier W, et al. Emotion-induced retrograde amnesia varies as a function of noradrenergic-glucocorticoid activity. Psychopharmacology. 2007;194:261–269. doi: 10.1007/s00213-007-0836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Mitchell KJ, Raye CL, McGuire JT, Sanislow CA. Mental rubbernecking to negative information depends on task context. Psychonomic Bulletin & Review. 2006;13:614–618. doi: 10.3758/bf03193971. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: Prefrontal correlates of refreshing a just-activated representation. Cognitive Affective & Behavioral Neuroscience. 2005;5:339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Kaplan S, Kaplan R. The interaction of arousal and retention interval: Ipsative vs normal scoring. Psychonomic Science. 1970;19:115–117. [Google Scholar]
- Kensinger EA, Piguet O, Krendl AC, Corkin S. Memory for contextual details: Effects of emotion and aging. Psychology and Aging. 2005;20:241–250. doi: 10.1037/0882-7974.20.2.241. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. Journal of Experimental Psychology. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt J, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9:490–493. [Google Scholar]
- Lamme VAF. The neurophysiology of figure-ground segregation in primary visual cortex. Journal of Neuroscience. 1995;15:1605–1615. doi: 10.1523/JNEUROSCI.15-02-01605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laney C, Campbell HV, Heuer F, Reisberg D. Memory for thematically arousing events. Memory & Cognition. 2004;32:1149–1159. doi: 10.3758/bf03196888. [DOI] [PubMed] [Google Scholar]
- Malmberg KJ, Shiffrin RM. The “one-shot” hypothesis for context storage. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:322–336. doi: 10.1037/0278-7393.31.2.322. [DOI] [PubMed] [Google Scholar]
- Maltzman I, Kantor W, Langdon B. Immediate and delayed retention, arousal, and the orienting and defensive reflexes. Psychonomic Science. 1966;6:445–446. [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults' emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Mitchell KJ, Raye CL, Novak DL, Greene EJ, Johnson MK. Emotional arousal can impair feature binding in working memory. Journal of Cognitive Neuroscience. 2006;18:614–625. doi: 10.1162/jocn.2006.18.4.614. [DOI] [PubMed] [Google Scholar]
- Mather M, Nesmith K. Arousal-enhanced location memory for pictures. Journal of Memory and Language. 2008;58:449–464. doi: 10.1016/j.jml.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory: A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Livosky M, Mather M. The weapon focus effect revisited: The role of novelty. Legal and Criminological Psychology. 1998;3:287–303. [Google Scholar]
- Mitchell KJ, Mather M, Johnson MK, Raye CL, Greene EJ. A functional magnetic resonance imaging investigation of short-term source and item memory for negative pictures. Neuroreport. 2006;17:1543–1547. doi: 10.1097/01.wnr.0000234743.50442.e5. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Powless M. Positive and negative sources of emotional arousal enhance long-term word-list retention when induced as long as 30 min after learning. Neurobiology of Learning and Memory. 2007;88:40–47. doi: 10.1016/j.nlm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Ryan L, Hoscheidt S, Jacobs WJ, Nadel L. The impact of stress on neutral and emotional aspects of episodic memory. Memory. 2006;14:1–16. doi: 10.1080/09658210500139176. [DOI] [PubMed] [Google Scholar]
- Pickel KL. Unusualness and threat as possible causes of “weapon focus”. Memory. 1998;6:277–295. doi: 10.1080/741942361. [DOI] [PubMed] [Google Scholar]
- Pickel KL. The influence of context on the “weapon focus” effect. Law and Human Behavior. 1999;23:299–311. [Google Scholar]
- Reisberg D, Heuer F. Memory for emotional events. In: Reisberg D, Hertel P, editors. Memory and Emotion. N.Y.: Oxford University Press; 2004. pp. 3–41. [Google Scholar]
- Runcie D, O'Bannon RM. An independence of induced amnesia and emotional response. American Journal of Psychology. 1977;90:55–61. [PubMed] [Google Scholar]
- Schmidt SR. Outstanding memories: The positive and negative effects of nudes on memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:353–361. doi: 10.1037//0278-7393.28.2.353. [DOI] [PubMed] [Google Scholar]
- Schürer-Necker E. Arousal and paired-associate learning: Evidence refuting the action decrement theory of Walker and Tarte (1963) Pavlovian Journal of Biological Science. 1990;25:195–200. [PubMed] [Google Scholar]
- Sharot T, Phelps EA. How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Sharot T, Yonelinas AP. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2008;106:538–547. doi: 10.1016/j.cognition.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touryan SR, Marian DE, Shimamura AP. Effect of negative emotional pictures on associative memory for peripheral information. Memory. 2007;15:154–166. doi: 10.1080/09658210601151310. [DOI] [PubMed] [Google Scholar]
- Vecera SP. Toward a biased competition account of object-based segregation and attention. Brain and Mind. 2000;1:353–384. [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biological Psychiatry. 2006;60:788–790. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Wessel I, Merckelbach H. The impact of anxiety on memory for details in spider phobics. Applied Cognitive Psychology. 1997;11:223–231. [Google Scholar]
- Wessel I, Merckelbach H. Memory for threat-relevant and threat-irrelevant cues in spider phobics. Cognition & Emotion. 1998;12:93–104. [Google Scholar]


