Abstract
Purpose
Therapeutic strategies to enhance the efficacy of radioimmunotherapy have not been explored. Motexafin gadolinium (MGd) is a novel anti-cancer agent that targets redox-dependent pathways and enhances sensitivity of tumor cells to ionizing radiation.
Experimental Design
We performed pre-clinical studies examining MGd combined with rituximab and/or radiation in lymphoma cells. We subsequently completed a phase I clinical trial combining escalating doses of MGd concurrently with standard yttrium-90 (90Y)-ibritumomab tiuxetan for patients with relapsed/refractory non-Hodgkin lymphoma.
Results
In HF1 lymphoma cells, MGd and rituximab resulted in synergistic cytotoxicity (combination index 0.757) through a mitochondrial-mediated caspase-dependent pathway, while cell death in Ramos and SUDHL4 cells was additive. MGd/rituximab combined with radiation (1–3Gy) resulted in additive apoptosis. Twenty-eight of 30 patients were evaluable on the phase I clinical trial. Median age was 65 years (47–87), and histologies were: marginal-zone (n=1), mantle-cell (n=3), diffuse large-cell (n=6), and follicular lymphoma (n=18). 86% of all patients were rituximab-refractory. Therapy was well-tolerated and no dose limiting toxicity was seen. Overall response rate (ORR) was 57% (complete remission (CR) 43%) with median time-to-treatment failure (TTF) of 10 months (1–48+) and median duration-of-response of 17 months. Of note, all responses were documented at 4 weeks. Furthermore, in rituximab-refractory follicular lymphoma (n=14), ORR was 86% (CR 64%) with median TTF of 14 months (2–48+).
Conclusions
This represents the first report of a novel agent to be combined safely concurrently with radioimmunotherapy. Further, tumor responses with 90Y-ibritumomab tiuxetan/MGd were prompt with a high rate of CRs, especially in rituximab-refractory follicular lymphoma.
Keywords: lymphoma, radioimmunotherapy, rituximab-refractory, oxidative stress, MGd
Introduction
Clinical trials using single-agent yttrium-90 (90Y)-ibritumomab tiuxetan and iodine-131 tositumomab in relapsed and refractory NHL are associated with overall response rates (ORR) of 60% to 80% (complete remission (CR) rates of 20% to 50%) and median time to treatment failure (TTF) or progression-free survival (PFS) of 6 to 10 months.1–7 Two studies using 90Y-ibritumomab tiuxetan and iodine-131-tositumomab in rituximab-refractory follicular lymphoma showed ORRs of 62% and 74% with associated CR rates of 15% and 25%, respectively, and with associated median TTF of 7 and 9 months, respectively.5,7 One potential approach to increase the efficacy of radioimmunotherapy is through the addition of concurrent non-cross resistant therapy. No prior studies, however, have been reported combining concurrent therapy with radioimmunotherapy.
Motexafin gadolinium (MGd) (Xcytrin®, Pharmacyclics Inc, Sunnyvale, CA), an expanded porphyrin containing the lanthanide cation gadolinium, is a novel anti-cancer agent that targets the redox-dependent machinery of tumor cells.8–12 MGd is redox active as it catalyzes the intracellular oxidation of critical protein thiols and intracellular reducing metabolites that are protective to the tumor cell; depletion of these reducing metabolites leads to increased levels of reactive oxygen species (ROS) (Figure 1).8–11,13,14 Tipping the cellular redox balance within tumor cells through pharmacologic manipulation in favor of increasing intracellular ROS and/or depleting reducing metabolites leads to oxidative stress and resultant induction of apoptosis. Clinically, MGd had been studied initially in combination with whole brain radiation therapy (WBRT), to sensitize or increase the activity of ionizing radiation for patients with brain metastases from solid tumors.14–20
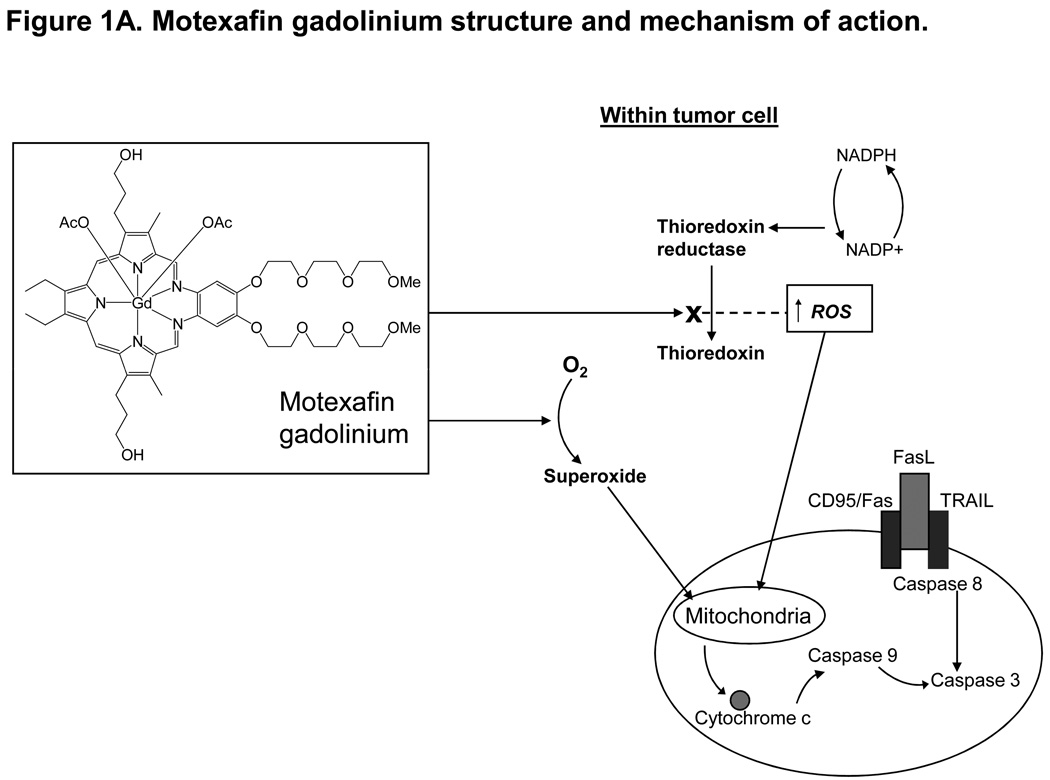
Figure 1. Motexafin gadolinium (MGd) mechanism of action and cell growth studies using MGd with rituximab in lymphoma cells.
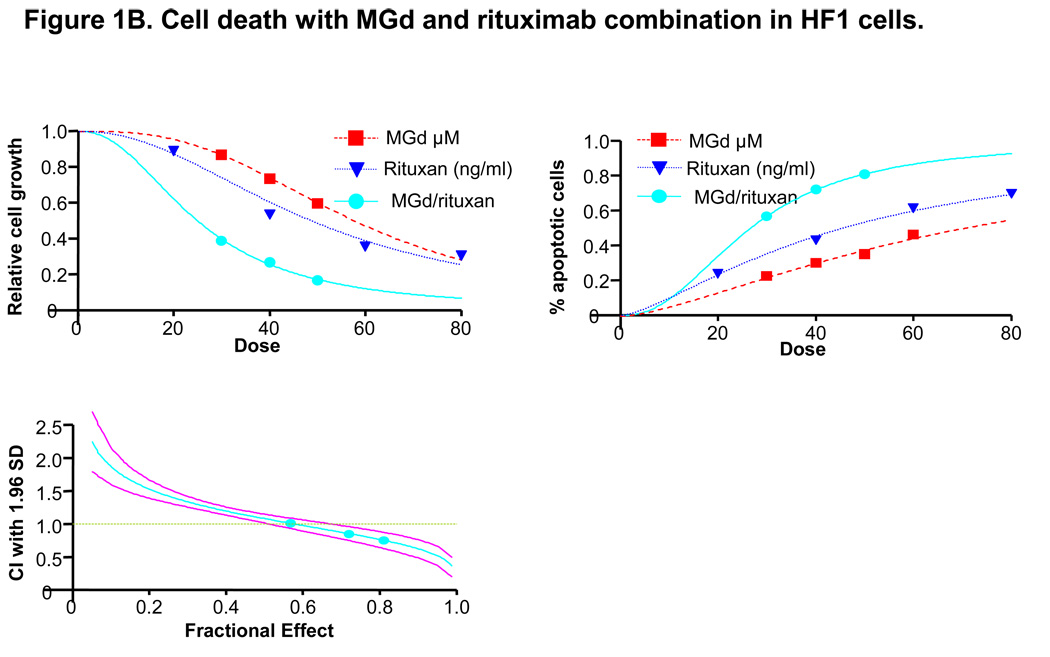
Figure 1A MGd is an aromatic macrocycle containing complexed gadolinium(III). In the presence of oxygen, MGd accepts electrons from cellular reducing metabolites and forms superoxide and other ROS by redox cycling. MGd further disrupts redox dependent pathways by targeting oxidative stress-related proteins such as thioredoxin reductase. MGd oxidizes these metabolites forming ROS, which leads to apoptosis. MGd-related apoptosis has been shown to occur through a caspase-dependent mechanism (mitochondrially-mediated). Figure 1B shows cell growth by Coulter counter analysis in HF1 cells with MGd and rituximab alone and combined. Induction of apoptosis, as measured by annexinV/PI, was increased with combined MGd/rituximab vs either agent alone. Clinically relevant concentrations of MGd and rituximab resulted in synergistic cell death (40µM and 48ng/mL, respectively, associated with combination index: 0.855; 50µM and 60ng/mL, respectively, with combination index: 0.757).
Our prior pre-clinical studies examined the effects of MGd in multiple myeloma cells and demonstrated dose-dependent apoptosis through redox-dependent pathways.8 Since MGd was known to enhance the sensitivity of tumor cells to ionizing radiation,14,18,21 we reasoned that MGd combined concurrently with the radioimmunoconjugate 90Y-ibritumomab tiuxetan would be additive or synergistic and provide higher response rates in relapsed/refractory lymphoma. We report here pre-clinical studies in lymphoma cell lines combining MGd with rituximab and/or radiation therapy. We subsequently completed a phase I trial using increasing doses of MGd, which was combined concurrently with fixed-dose 90Y-ibritumomab tiuxetan, for patients with relapsed or refractory NHL.
Methods
Cell lines and experimental conditions
The HF1, SUDHL4, and Ramos lymphoma cell lines were cultured in RPMI1640 (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum, L-glutamine, and penicillin/streptomycin. Cells were maintained at 37°C with 5% CO2. HF1, SUDHL4, and Ramos cells were treated with control vehicle solution (5% mannitol), increasing concentrations of MGd (30µM to 100µM), and/or increasing concentrations of rituximab (20ng/mL to 200ng/mL, San Francisco, CA). MGd was prepared as a 2mmol/L (2.3mg/mL) formulation in 5% aqueous mannitol. Cultures were irradiated using a 137Cs irradiator (Model 40 Gamma cell, J. L. Shepherd & Associates, San Fernando, CA) at a dose rate of 0.725 Gy/min and allowed to incubate for 72 hours. Cells were harvested and washed twice with a solution of 0.5% bovine serum albumin in phosphate buffered saline (PBS).
Cell viability and apoptosis studies
Cell numbers were determined using the Model Z2 Coulter counter (Beckman-Coulter, Miami, FL) and live cells were counted. Apoptosis was examined using FACS Calibur instrument (Becton-Dickinson, San Jose, CA) after staining the cells with annexin V-FITC and propidium iodide (PI) (Biosource-Invitrogen, Camarillo, CA). In brief, 1×106 cells were washed with phosphate buffered saline and then labeled with annexinV-FITC and PI in the binding buffer according to the annexinV-FITC apoptosis detection kit instruction provided by the manufacturer. Fluorescent signals of FITC and PI were detected at FL1 and FL3 channels, respectively. Percent apoptosis was determined by positivity for annexinV. Data are presented as the mean value ± the standard deviation. Measurement of mitochondrial membrane potential (MMP) was measured by flow cytometry using JC-1 staining. Cells were washed with Hank’s buffered salt solution (HBBS) and incubated with 4µg/ml JC-1 dye in HBSS for 15 minutes at 37°C in an incubator. Cells were washed with HBSS and immediately subjected to flow cytometric analysis. Caspase 3 activation was measured (EnzChek Caspase-3 Assay Kit #2, Molecular Probes, Eugene, OR), while Q-VD-OPh was used for pan-caspase inhibition (Calbiochem, San Diego CA). For further examination of apoptosis, the cleavage of poly(ADP-ribose) polymerase (PARP) was detected with Western blots. For Western blotting, cells were lysed in triple-detergent lysis buffer. Equal amount of protein was run on SDS-PAGE (Bio-rad, Hercules, CA), and then transferred to PVDF membrane. Membrane was blotted with anti-PARP antibody (Cell Signaling, Beverly, MA) and image quantitated using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE).
Clinical Trial
This was a phase I investigator-initiated clinical trial, which enrolled 30 relapsed/refractory NHL patients from 10/2003 through 10/2007. The protocol was approved by the Northwestern University Institutional Review Board. All patients had histologically confirmed relapsed or refractory B-cell NHL (all B-cell lymphoma histologies were allowed except Burkitt lymphoma). All nodal and bone marrow slides were reviewed by a single hematopathologist (DV). Patients had <25% bone marrow involvement with lymphoma and an ECOG performance status ≤3, absolute neutrophil count (ANC) ≥ 1,500 cells/mm3, hemoglobin ≥ 8.0 g/dL, platelet count ≥ 100,000 cells/mm3, serum creatinine and total bilirubin ≤ 2 mg/dL each, expected survival of 3 months, and no antineoplastic therapy for 4 weeks (6 weeks if treated with nitrosourea and/or mitomycin). One patient with diffuse large B-cell lymphoma (DLBCL) was removed from the study because of the development of acute renal failure due to a rapidly growing and obstructing perinephric mass that developed prior to (and precluded) administration of 90Y-ibritumomab tiuxetan. Therefore, 29 patients received MGd/90Y-ibritumomab tiuxetan therapy.
Trial design and treatment
Standard 90Y-ibritumomab tiuxetan was administered as described before.22 The 90Y-ibritumomab tiuxetan dose was 0.4 mCi/kg for patients with platelets ≥150,000 cells/mm3, while 0.3 mCi/Kg was given for 100,000cells/mm3 to 149,000cells/mm3. All patients regardless of dose calculation did not receive more than the maximum dose of 90Y ibritumomab tiuxetan of 32 mCi. Doses for rituximab, 111Indium-zevalin, and 90Y ibritumomab tiuxetan were based on adjusted ideal body weight. 90Y ibritumomab tiuxetan was not to be given if the predicted delivered dose of radiation to any non-tumor organ (liver, lung, kidneys, spleen or heart) was more than 20 Gy or if the dose to the bone marrow was more than 3 Gy; no patient met this criterion. Patients received 8 total doses of MGd (once daily): days 1 to 4 and 8 to 11 as shown in Supplemental Figure 1A. Doses for MGd were based on actual body weight. Prophylactic antiemetics were given 30 minutes prior to MGd (e.g., compazine 5mg). Day 1 and 8 rituximab infusions were given 1 hour following the MGd infusion. Gamma camera imaging with dosimetry was performed for all patients on days 1, 2, 4, and 7.
Patients were treated in the Northwestern University General Clinical Research Center (GCRC) and had daily history and physical examinations from day +1 through day +11 of treatment, then weekly for the 1st month, monthly for 6 months. Complete blood count with differential was performed weekly for the 1st three months, then monthly for one year. Serum chemistries were done every 3 days from day +1 through day +12, then monthly for 6 months. Response was assessed by International Workshop NHL response criteria.23 As a correlative analysis to study the timing of response, the initial restaging computerized tomograms (CTs) were performed 4 weeks following 90Y ibritumomab tiuxetan treatment. Subsequent CTs were completed every 3 months following or sooner if clinically indicated.
Phase I design
An abbreviated dose escalation of MGd was planned with three dose cohorts: 2.5 mg/kg, 3.5mg/kg, and 5.0 mg/kg as the phase I component of the trial. The standard dosing of MGd in previous clinical trials combined with WBRT was 5mg/kg/day given 2–5 hours before each fraction.19,20 Due to the known myelosuppressive toxicity of radioimmunotherapy based on the amount of lymphoma marrow infiltration,24 the initial cohort accrued only patients with ≤5% bone marrow involvement (Supplemental Figure 1B). Patients with 6% to 24% involvement began enrollment only after patients with ≤5% marrow involvement completed dose level 1. Patients among these two cohorts followed separate modified Fibonacci phase I schemas. At least 3 patients were treated at each dose level. All three patients at a given dose level must have completed had follow-up through day 90 without suffering dose-limiting toxicity (DLT) before patients were enrolled at the next dose level.
All toxicities were graded according to the NCI Common Toxicity Criteria, version 3.0. The occurrence of any of the following during treatment constituted DLT: grade 3 or 4 non-hematologic toxicity (other than grade 3 nausea or vomiting), grade 4 vomiting despite maximal antiemetic support, or the following hematologic parameters: grade 4 neutropenia and thrombocytopenia either lasting longer than 14 days (measured in days from the first date in grade 4 to last date in grade 4 after nadir (growth factor and transfusion independent, respectively). Rationale for the above hematologic parameters were that despite the modest cytopenias seen with 90Y ibritumomab tiuxetan, the range of grade 3 or 4 thrombocytopenia and neutropenia has been wide (ranges for “days from baseline to nadir” of: 28–63 days and 22–78 days, respectively; and ranges for “nadir to recovery” of: 7–53 days and 4–21 days, respectively).24
Statistical analysis
The pre-clinical synergy studies, with associated combination indices, between MGd and rituximab was tested using the isobologram analysis based on the method of Chou and Talay using the Calcusyn (Biosoft, Ferguson,MO) software program.25 The method is based on the equation: CI=(D)1/(Dx)1+(D)2/(Dx)2, where D1 and D2 are concentrations of drug 1 and drug 2 that have×effect when used in combination, and (Dx)1 and (Dx)2 are the concentrations of drug 1 and drug 2 that have the same x effect when used alone. As of April 2006, 14 patients with ≤5% bone marrow involvement had completed dose escalation without DLT, while only 1 patient had accrued to the 6–24% cohort. Thus an expansion (10 patients) of accrual at the MGd 5.0 mg/kg dose level was allowed for patients with ≤5% bone marrow involvement, while accrual continued to the 6–24% marrow group. For the expanded enrollment, a predicted “good” ORR of 60% and a “poor” ORR of 30% for all patients were used and for follicular lymphoma patients, a “good” ORR of 75% and a “poor” ORR of 50% were used. Using this information and type 1 error probability of 0.1, we calculated over 84% power to detect the difference for all patients and over 74% power to detect the above difference for relapsed follicular lymphoma patients, using an exact one sample test for response rate (probability).
TTF was calculated from day 1 of treatment to treatment failure (relapse, secondary malignancy, or death from any cause). Overall survival (OS) was calculated from day 1 of treatment to the date of death from any cause or until the date of last known follow-up. Duration of response was estimated from the day of response assessment until relapse, progression, or death from any cause. Survival analyses were performed using Kaplan and Meier curves.26 Prognostic factors were evaluated in univariate analyses using Cox proportional hazards regression27 for indicators of response or survival.
Results
Pre-clinical data
We and others previously reported single-agent cytotoxicity with MGd in B-cell lymphoma cell lines (Raji, SUDHL-4, and HF-1).28–30 We examined here the cytotoxicity of MGd with and without rituximab in the lymphoma cell lines, HF1, SUDHL4, and Ramos. In the follicular lymphoma cell line, HF1, modest in vitro concentrations of MGd and rituximab resulted in additive apoptosis (30µM and 36ng/mL, respectively, with a combination index of 1.013), while increasing concentrations resulted in synergistic cell death (40µM and 48ng/mL, respectively, associated with a combination index of 0.855; 50µM and 60ng/mL, respectively, with a combination index of 0.757) (Figure 1B).
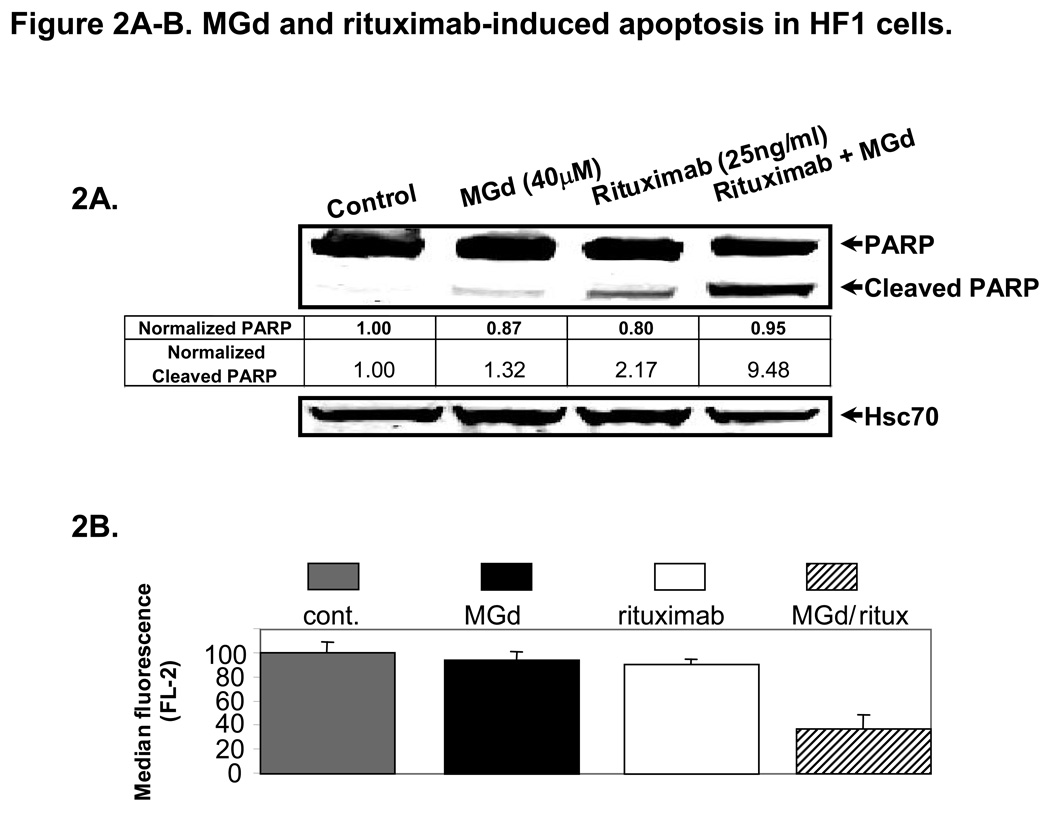
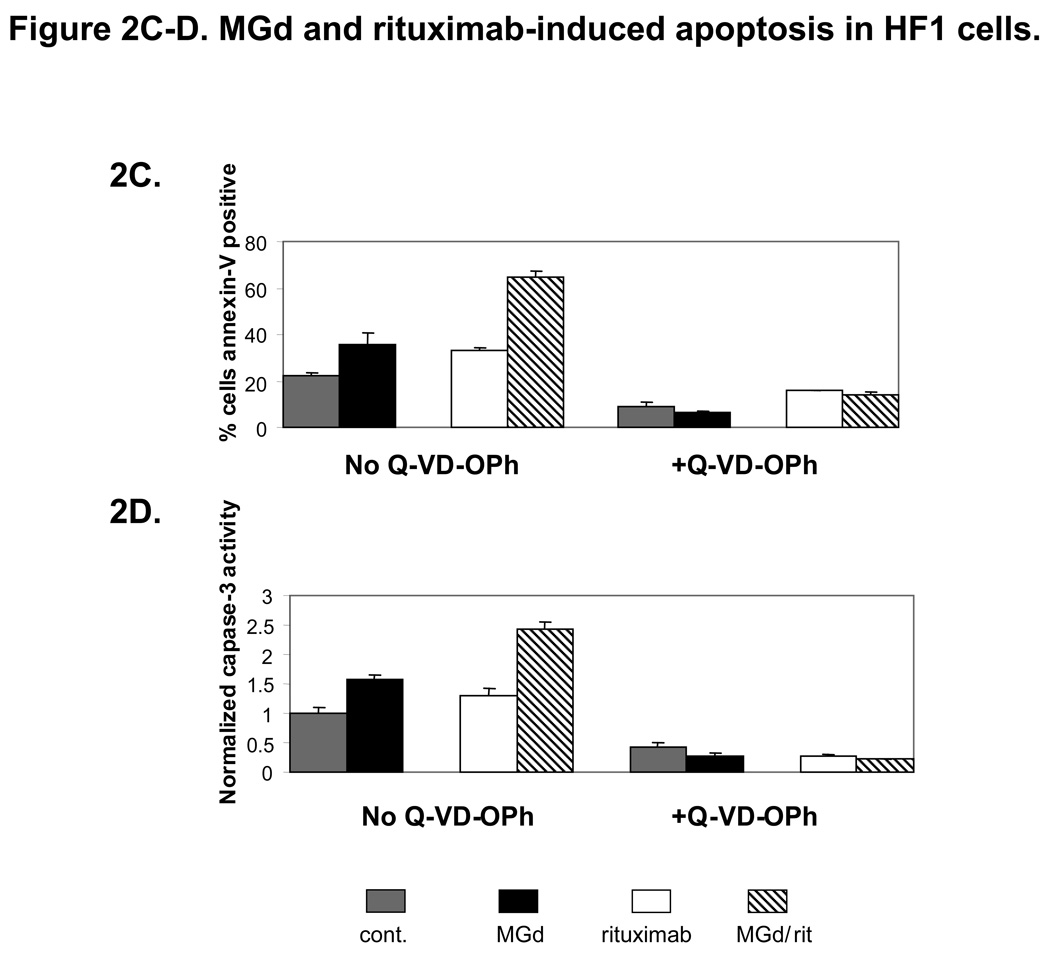
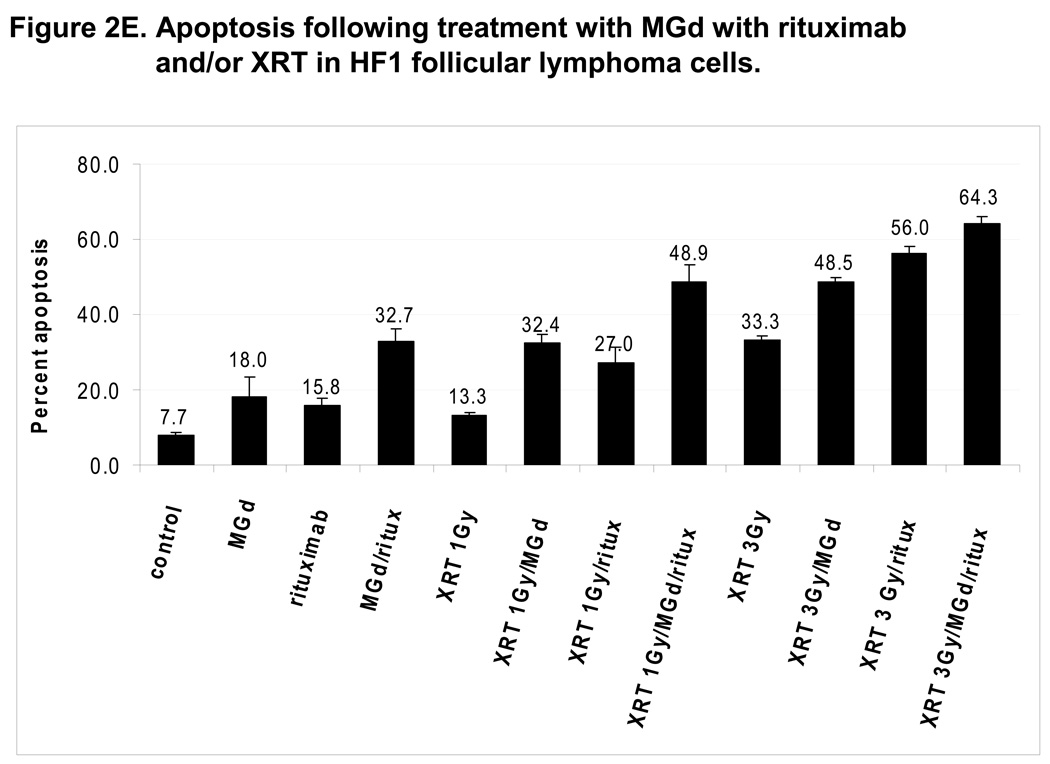
Using similar concentrations, we found further evidence of apoptosis with higher levels of cleaved PARP (Figure 2A) and >50% loss of MMP (Figure 2B) with combined MGd and rituximab (vs either agent alone). Apoptosis studies were also completed in SUDHL4 and Ramos lymphoma cell lines where additive cell death was seen (data not shown). In HF1 cells, caspase 3 activity was increased with MGd and to a lesser extent rituximab alone, while both agents combined resulted in additive increased caspase 3. Furthermore, the pan-caspase inhibitor Q-VD-OPh resulted in substantial blockage of MGd and/or rituximab-induced apoptosis, including abrogation of caspase 3 activation (Figure 2C and 2D). We also studied cell death combining MGd and/or radiation (with and without rituximab) in HF1 cells (Figure 2E). When MGd was combined with rituximab or radiation (1Gy–3Gy), an additive decrease in cell proliferation and induction of apoptosis were seen. The highest level of cell death was seen when MGd, radiation, and rituximab were combined together.
Figure 2. MGd combined with rituximab and/or radiation induced apoptosis.
Figure 2A shows that cleaved PARP was more than 4-fold increased in HF1 cells using MGd/rituximab combined vs either agent alone (MGd 40µm and rituximab 25ng/ml). Cleaved PARP ratios are shown relative to Hsc70. In Figure 2B, we examined loss of mitochondrial membrane potential, which was significantly decreased with combined MGd/rituximab vs either agent alone (p<0.001). In Figure 2C and 2D, the activation of caspase 3 and apoptosis (annexinV/PI) were measured in HF1 cells with MGd and/or rituximab in the presence of absence of the pan-caspase inhibitor Q-VD-OPh. Overall apoptosis and caspase 3 activity induced by MGd and/or rituximab was caspase-dependent. Figure 2E shows MGd combined with ionizing radiation (with/without rituximab) in HF1 cells using MGd 40µm, rituximab 25ng/ml, and radiation 1Gy and 3Gy. MGd combined with radiation showed additive apoptosis, while addition of rituximab led to further additive cell death. Abbreviations: NADP+, Nicotinamide adenine dinucleotide phosphate; NADPH is the reduced form of NADP+; O2, oxygen; FasL, Fas ligand; TRAIL, Tumor necrosis (TNF)-related apoptosis-inducing ligand; CI, combination index; SD, standard deviation; XRT, radiation; rit, rituximab.
Clinical trial patient characteristics
As shown in Table 1, 19/29 patients treated on trial had indolent lymphoma (18 follicular lymphoma). The remaining 10 patients had aggressive histologies. The median age for all patients was 64 years, while one-third were >75 years. The majority of patients had advanced stage disease at study entry, while 55% had bulky disease ≥5 cm. Median number of prior regimens was two (range, 1–4). Of note, 86% of all patients entered on this clinical trial had rituximab-refractory disease, defined as prior treatment with rituximab with no response or TTF of < 6 months. Seventy-eight percent (14/18) of follicular lymphoma patients had rituximab-refractory disease.
Table 1.
Patient demographics and clinical characteristics (n=29).
| Number | % | |
|---|---|---|
| Median age, years (range) | 65 (47–87) | 100 |
| Gender | ||
| Female | 15 | 52 |
| Male | 14 | 48 |
| Histology | ||
| Follicular | 18 | 62 |
| Marginal zone | 1 | 3 |
| DLBCL | 7* | 25 |
| Mantle cell | 3 | 10 |
| Performance status | ||
| Zero | 17 | 59 |
| One | 11 | 38 |
| Two | 1 | 3 |
| Stage (at study entry) | ||
| I/II | 7 | 24 |
| III/IV | 22 | 76 |
| Bulky disease | ||
| <5cm | 13 | 45 |
| 5cm–10cm | 12 | 41 |
| >10cm | 4 | 14 |
| Bone marrow involvement | ||
| <5% | 26 | 90 |
| 6%–24% | 3 | 10 |
| LDH | ||
| Elevated | 10 | 34 |
| Prior treatments** | ||
| One | 4 | 14 |
| Two | 11 | 38 |
| Three | 12 | 41 |
| Four | 2 | 7 |
|
Prior rituximab Yes |
28 | 97 |
|
Rituximab refractory Yes |
25 | 86 |
|
Prior radiotherapy Yes |
6 | 21 |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; LDH, lactate dehydrogenase.
Four patients with transformed DLBCL, three with de novo DLBCL.
Post-induction (or maintenance) rituximab was not considered a separate/different treatment regimen.
Among the group of rituximab-refractory follicular lymphoma, median age was 60 years (range 47–85), median prior regimens was 3 (range 1–4), 79% had stage III/IV disease, 55% had bulky disease ≥ 5cm, and 72% had intermediate or high FLIPI (≥ 3) at study entry. Twelve of the 14 of these patients had received prior alkylator-based chemotherapy, the most common regimen being R-CVP (9/12). Among the 10 evaluable patients with relapsed/refractory aggressive lymphoma, median age was 77 and 70% were stage III/IV at study entry. Ninety percent of these patients had received frontline R-CHOP-like (anthracycline-based) chemotherapy, while 90% of patients at time of study entry were rituximab-refractory.
Dosimetry
111Indium ibritumomab tiuxetan imaging and dosimetry were performed in 28 patients. The estimated radiation-absorbed doses were within acceptable levels, which allowed all patients to proceed with 90Y-ibritumomab tiuxetan treatment. The estimated radiation-absorbed doses are shown in Table 2, which do not appear different compared with prior “single agent” 90Y-ibritumomab tiuxetan studies.5,24
Table 2.
Dosimetry.*
| Radiation Dose (Gy) | ||||||
|---|---|---|---|---|---|---|
| Administered activity (MBq) |
Kidney | Liver | Lung | Spleen | Whole body | |
| Mean | 974.9 | 4.9 | 5.61 | 2.91 | 9.32 | 0.49 |
|
standard deviation |
177.7 | 1.99 | 1.56 | 1.23 | 4.56 | 0.17 |
| Minimum | 703 | 1.37 | 2.23 | 0.48 | 2.43 | 0.17 |
| Maximum | 1295 | 10.63 | 9.17 | 5.77 | 20.34 | 0.78 |
Following 111Indium ibritumomab tiuxetan imaging and dosimetry (n=29 patients).
Phase I results
Eighteen patients were enrolled and completed phase I MGd dose escalation (9 patients MGd 2.5 mg/kg, 6 patients MGd 3.5 mg/kg, and 3 patients MGd 5 mg/kg). Of all 29 patients who received 90Y-ibritumomab tiuxetan and MGd therapy, 25 had ≤5% marrow involvement. Thus, MGd dose escalation for the group of patients with >5% bone marrow involvement was not completed. No DLTs were seen for the 14 phase I patients with ≤5% bone marrow involvement or the 4 phase I patients with >5% marrow involvement. The remaining patients (n=11) received MGd at 5 mg/kg.
Efficacy
Twenty-eight patients were evaluable for response. The non-evaluable patient was a 75-year-old man with relapsed/refractory DLBCL who died 3 months following MGd/90Y-ibritumomab tiuxetan therapy from accidental causes (prior to 3-month restaging studies; stable disease at 1 month). The ORR for all evaluable patients was 57% (16/28); the majority of responses were complete (CR 43%). ORR by intent-to-treat for all patients (n=30) was 53% (16/30) with 40% CR (12/30). The efficacy data in the subgroup of patients with rituximab-refractory follicular lymphoma (n=14) showed an ORR of 86% with a CR rate of 64% (Table 3).
Table 3.
Hematologic and non-hematologic toxicity.*
| Hematologic | |||||||
|---|---|---|---|---|---|---|---|
| Median time to nadir (range) |
Median nadir value (range) |
Patients with grade 3 or 4 nadir |
Patients with grade 4 nadir |
||||
| Absolute neutrophil count (cells/mm3) |
7 weeks (5–9) | 1,000 (210–2,100) | 29% | 7% | |||
| Platelets (cells/mm3) | 5 weeks (3–8) | 51,000 (22,000– 129,000) |
46% | 0% | |||
| Hemoglobin (gm/dL) | 7 weeks (4–13) | 11.8 (7.4–13.5) | 18% | 0% | |||
| Non-hematologic adverse event | |||||||
| System | Grade 1 | Grade 2 | Grade 3 | Grade 4 | No. |
Total^ % |
|
| Skin | |||||||
| Green discoloration | 11 | 12 | 0 | 0 | 23 | 79 | |
| Pruritis | 5 | 0 | 0 | 0 | 5 | 17 | |
| Body as whole | |||||||
| Fatigue | 18 | 4 | 0 | 0 | 22 | 76 | |
| Hypoabluminema | 9 | 6 | 0 | 0 | 15 | 52 | |
| Infection | 2 | 7 | 1 | 0 | 9 | 31 | |
| Chills/rigors (during rituximab) | 5 | 2 | 0 | 0 | 7 | 24 | |
| Insomnia | 6 | 1 | 0 | 0 | 7 | 24 | |
| Headache | 3 | 3 | 0 | 0 | 6 | 21 | |
| Edema | 1 | 4 | 0 | 0 | 5 | 17 | |
| Pain (myalgia) | 2 | 2 | 0 | 0 | 4 | 14 | |
| Gastrointestinal | |||||||
| Constipation | 11 | 3 | 0 | 0 | 14 | 48 | |
| Nausea | 11 | 1 | 0 | 0 | 12 | 41 | |
| Diarrhea | 4 | 0 | 0 | 0 | 4 | 14 | |
| Vomiting | 4 | 0 | 0 | 0 | 4 | 14 | |
| Hepatic | |||||||
| Transaminases | 11 | 1 | 0 | 0 | 12 | 41 | |
| Alkaline phosphatase | 10 | 0 | 0 | 0 | 10 | 34 | |
| GGT elevation | 8 | 1 | 0 | 0 | 9 | 31 | |
| Bilirubin | 7 | 0 | 0 | 0 | 7 | 24 | |
| Cardiovascular | |||||||
| Hypertension | 8 | 0 | 0 | 0 | 8 | 28 | |
| Tachycardia | 6 | 0 | 0 | 0 | 6 | 21 | |
| Electrolytes | |||||||
| Hyponatremia | 7 | 0 | 0 | 0 | 7 | 24 | |
| Hypophosphatemia | 3 | 3 | 0 | 0 | 6 | 21 | |
| Hypokalemia | 4 | 0 | 1 | 0 | 6 | 21 | |
| Hypocalcemia | 5 | 0 | 0 | 0 | 5 | 17 | |
| Bicarbonate | 4 | 0 | 0 | 0 | 4 | 14 | |
| Hypomagnesemia | 4 | 0 | 0 | 0 | 4 | 14 | |
| Renal | |||||||
| Creatinine | 4 | 0 | 0 | 0 | 4 | 14 | |
The incidence included all grade 3 or 4 adverse events and those grade 1 and 2 that occurred in > 10%10% of patients relationship during the treatment period which were classified as probably or possibly related to study drug or relationship unknown.
Percent of total patients who received MGd/90Y ibritumomab tiuxetan (n=29).
Among the 10 evaluable patients with relapsed/refractory aggressive lymphomas, the ORR was 20%. The two responses, however, were both CRs in rituximab-refractory DLBCL patients (ORR in DLBCL: 29%). One of the related CRs lasted 11 months, while the other CR is ongoing (25+ months). Both of these cases were elderly men (72 and 87 years of age) with rituximab-refractory transformed DLBCL with no response to their prior chemotherapeutic regimen.
Of note, all responses in this trial were documented at 4 weeks following combined MGd/90Y-ibritumomab tiuxetan therapy. Six patients with PRs at 4 weeks improved to CR at subsequent (3-month) restaging. However, no “new” responders were identified after 4 weeks. Besides histology (i.e., indolent lymphoma vs aggressive, p<0.01), there were no predictive factors of response on univariate analysis (e.g., bulk disease, age, performance status, LDH, or rituximab-refractory vs rituximab-sensitive status).
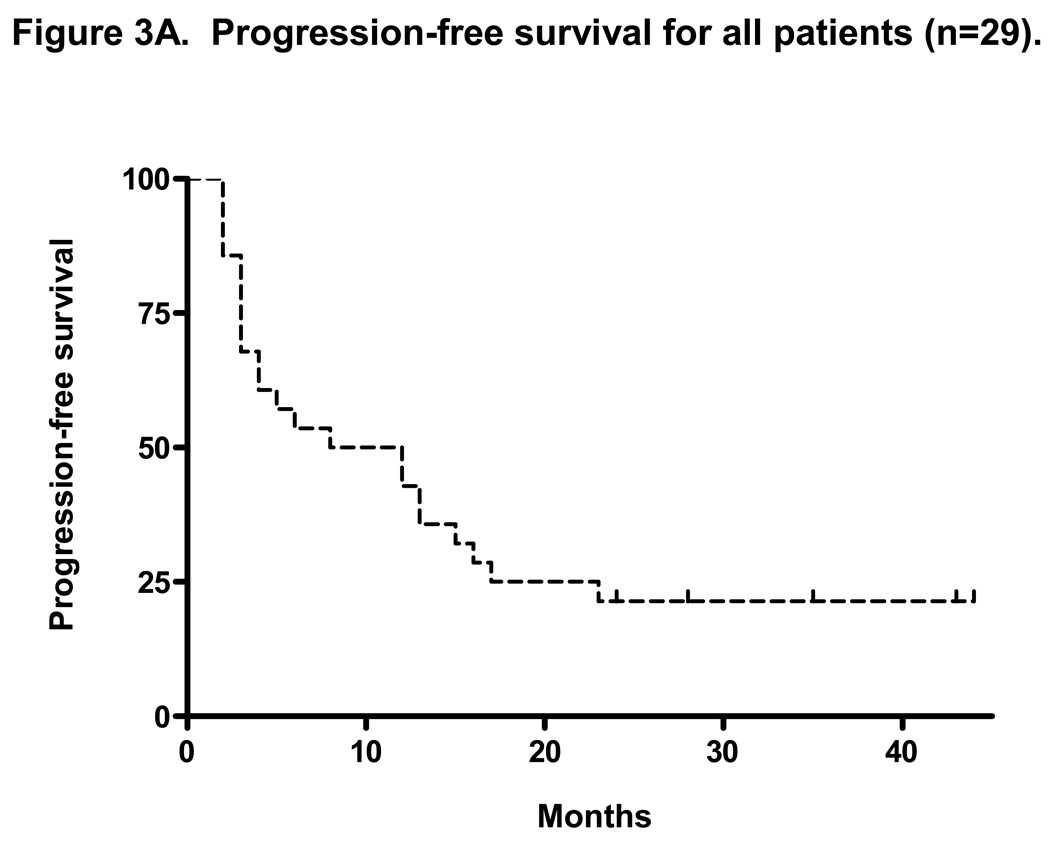
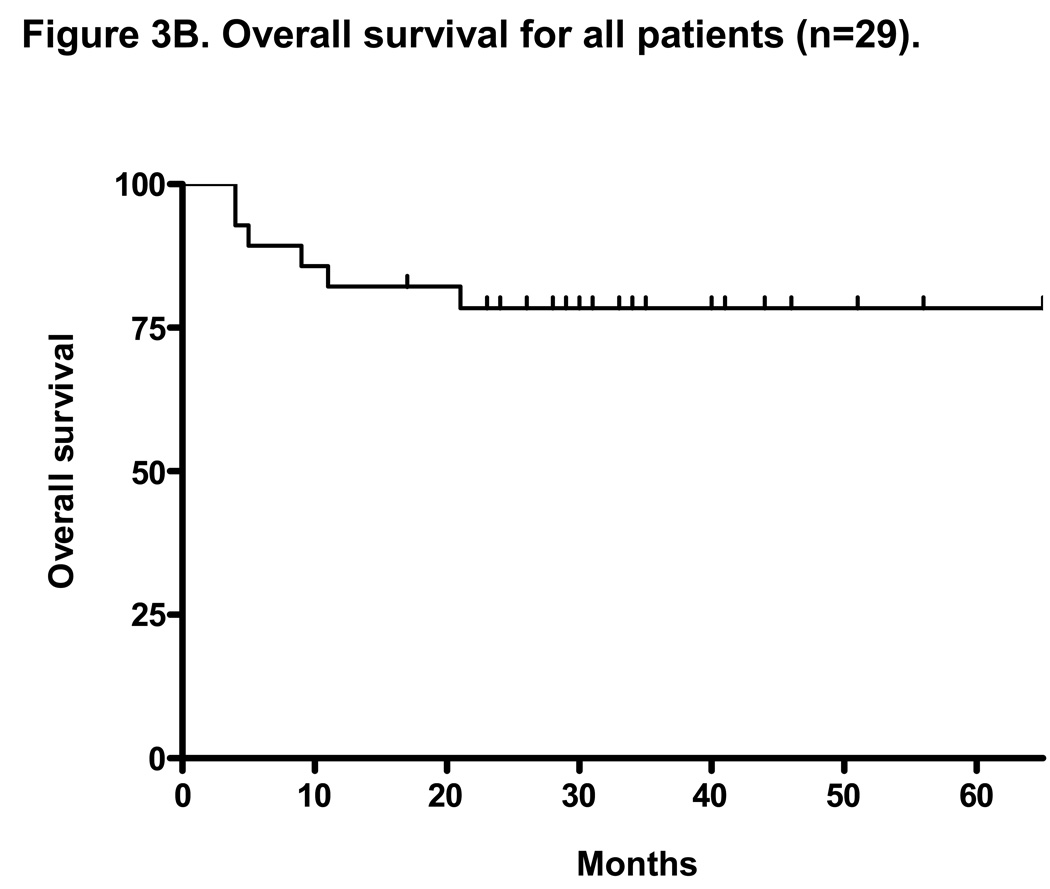
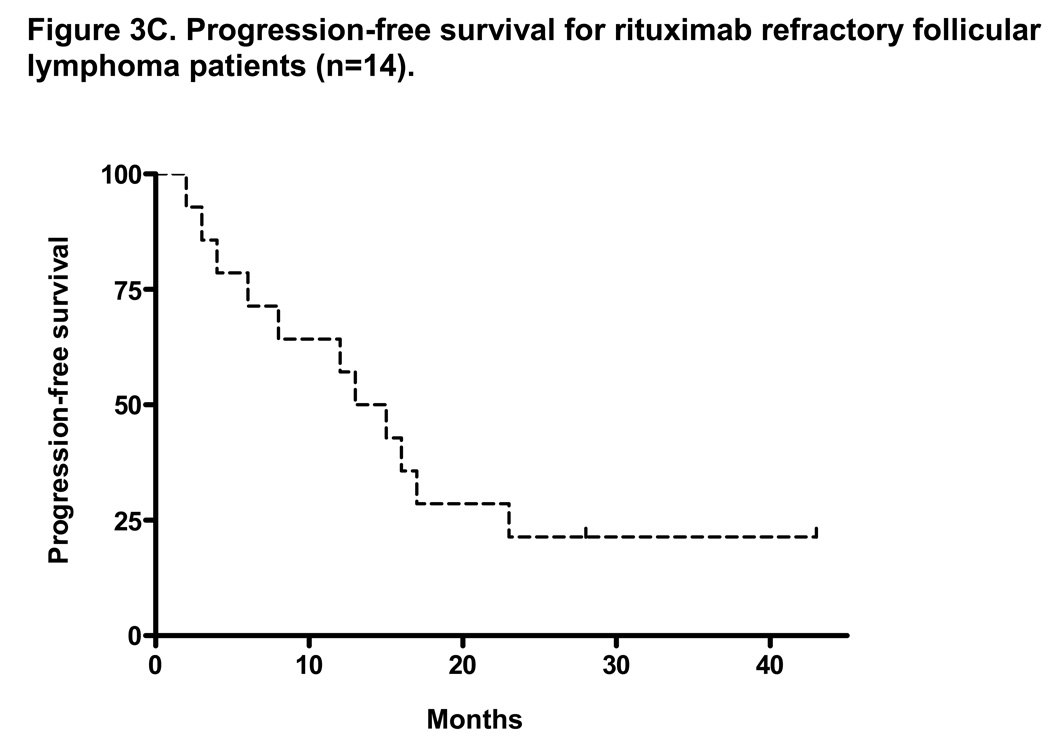
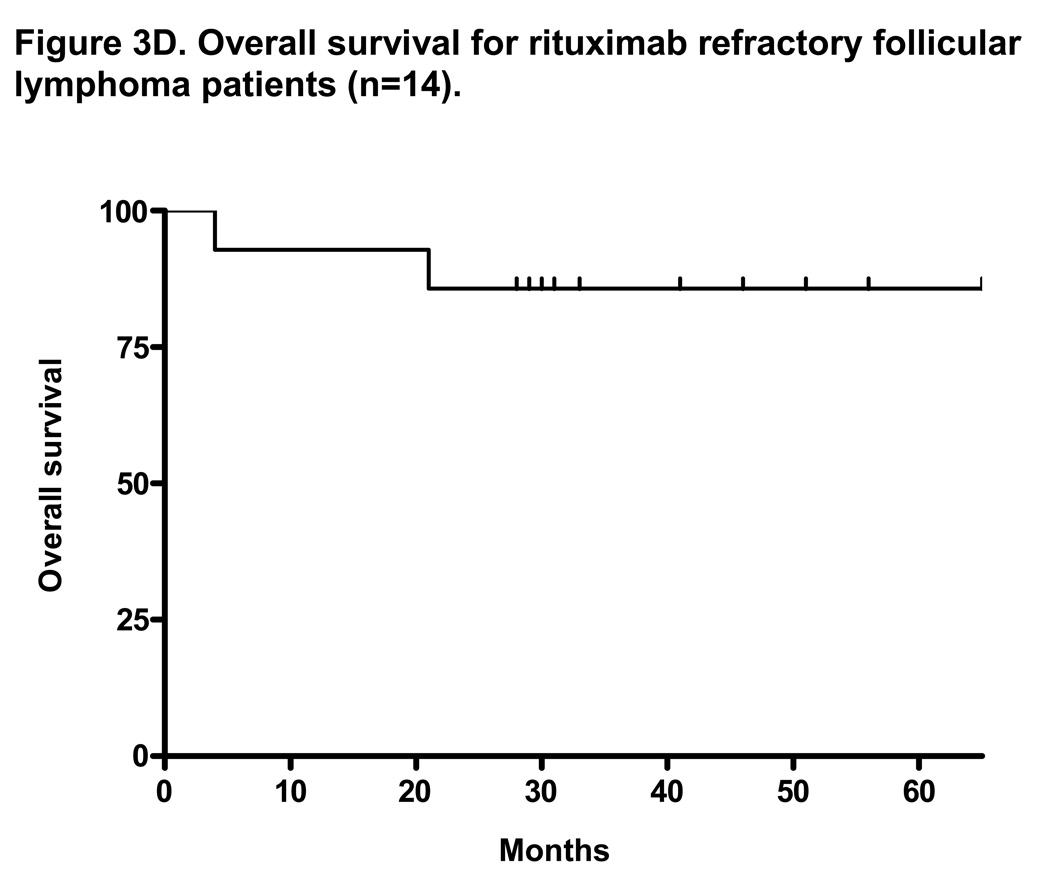
At a median follow-up of 35 months, the median TTP for all patients on intent-to-treat analysis (n=30) was 10 months (range 1–51+ months) and the median OS was not reached with 3-year OS of 77% (Figures 3A and 3B). The median duration of response for all patients was 17 months (range, 2–51+ months). With a median follow-up of 37 months, the median TTP for patients with rituximab-refractory follicular lymphoma was 14 months (range, 2–51 months) and the median OS was not reached with 3-year OS of 80% (Figures 3C and 3D). The median duration of response for this group was 18 months (range, 4–51+ months).
Figure 3. Intent-to-treat progression-free survival (PFS) and overall survival (OS) Kaplan-Meier curves for all patients (n=30) and for rituximab-refractory follicular lymphoma patients (n=14).
Figure 3A and 3B shows the PFS and OS for all patients enrolled on trial. At a median follow-up of 35 months, the median PFS was 10 months and the median OS was not reached (PFS and time to treatment failure (TTF) were identical). Figure 3C shows the median PFS for rituximab-refractory follicular lymphoma of 14 months. This compares to data by Witzig et al5 using “single agent” 90Y ibritumomab tiuxetan therapy in rituximab-refractory follicular lymphoma with median TTP of 6.8 months.5 Figure 3D shows median OS for rituximab refractory follicular lymphoma patients has not been reached.
Safety
Therapy was well tolerated. Most toxicity was hematologic, while the percentage of grade 3/4 hematologic adverse events and the median time to hematologic nadir levels appeared similar to single-agent 90Y-ibritumomab tiuxetan (Table 3). Grade 3/4 non-hematologic adverse events were uncommon (Table 3). Two grade 3 non-hematologic toxicities were seen (1 infection and 1 hypokalemia). At a median follow-up of 35 months, no secondary MDS or leukemia has been seen. The most common non-hematologic adverse event was a temporary olive-green discoloration of skin (80% of patients). The skin discoloration was due to the dark-green color of MGd. The discoloration developed gradually after repeated dosing of MGd and completely cleared within 4 to 5 days after the last dose. Some patients developed a transient pruritic rash on their fingertips (n=5) that also disappeared 3–4 days after completion of MGd. Other adverse events seen as shown in Table 3 included electrolyte abnormalities, liver enzyme abnormalities, and gastrointestinal symptoms, although the majority of symptoms were mild and self-limiting.
Discussion
One strategy to increase the effectiveness of radioimmunotherapy is through combination with non-cross resistant agents that may be additive or synergistic. Because of the activity of MGd combined with ionizing radiation in solid tumor studies,16,19,20 the absence of hematologic toxicity of MGd, our prior pre-clinical studies in hematologic malignancies,8,28,29 and the current pre-clinical studies in lymphoma, we reasoned that MGd might enhance the anti tumor activity of 90Y-ibritumomab tiuxetan. To our knowledge this report represents one of the first clinical trials combining a novel therapeutic agent combined concurrently with radioimmunotherapy for the treatment of lymphoma.
We and others have demonstrated that production of oxidative stress through novel non-chemotherapeutic agents results in cell death in hematologic cancer cell lines.8,28,30–35 MGd, also known as gadolinium texaphyrin, is a metalloporphyrin that had been developed as a radiation- and chemotherapy-enhancing agent.11,14,18 MGd is known to have selective tumor bio-localization, similar to naturally occurring porphyrins.12,14,36 Animal studies using magnetic resonance scanning and radiolabeled drug documented rapid clearance of MGd from blood and normal tissues with delayed clearance from tumors.21,37 Since this clinical study was initiated, MGd has been shown to induce apoptosis in lymphoma cell lines.28–30 Chen et al showed that MGd triggered the mitochondrial apoptotic pathway and activated the caspase system in NHL cells.29 In SUDHL4, Ramos, Raji, and HF1 cells, MGd was shown to induce oxidative stress as shown by oxidation of 2’,7’-dichlorofluorescin-diacetate to 2’,7’-dichlorofluorescein, while gene expression studies showed increased metal response element–binding transcription factor-1 (MTF-1)–regulated and hypoxia-inducible transcription factor-1 (HIF-1)-regulated genes.28 These genes were likely activated as an adaptive response to MGd-induced apoptosis. Ramos et al reported that MGd increased Akt phosphorylation, while specific inhibitors of Akt were synergistic combined with MGd.30 We found in the current pre-clinical studies that MGd combined with rituximab led to synergistic apoptosis and that cell death appeared to occur through a mitochondrial-related pathway that was caspase dependent. Furthermore, MGd combined with radiation resulted in an increase in apoptosis that was additive.
Dosing of MGd in this study was conservative. When this trial was opened in 2003, MGd had only been used clinically before as a radiation-sensitizing agent in combination with WBRT for the treatment of patients with metastatic brain disease. The MTD as determined by prior solid tumor phase Ib/II studies with MGd (given daily for 10 days) with concurrent WBRT was 6.3 mg/kg.17 Furthermore, plasma pharmacokinetics has shown no relationship between maximum concentration (Cmax) or area under the curve and MGd dose. In the phase III metastatic brain clinical trials, patients received 10 consecutive daily doses of MGd at 5mg/kg/day.16,19 MGd here was administered for 4 days following 111Indium ibritumomab tiuxetan and for 4 days immediately following 90Y ibritumomab tiuxetan therapy. The rationale for administering MGd following 111Indium ibritumomab tiuxetan was in part to determine if MGd altered the dosimetry of the radioimmunoconjugate. The rationale for continuing MGd 4 days following 90Y ibritumomab tiuxetan was in part related to the known biologic half-life of 90Y ibritumomab tiuxetan of 28 hours and the observation that the maximal binding of the 90Y ibritumomab tiuxetan antibody to the CD20 occurs at 96 to 144 hours.24 Continued treatment with MGd, beyond the 8 doses given here, was not done in part as the anti-lymphoma activity of MGd was not appreciated at the time this trial was planned.
The efficacy data from the phase I/II trial here using combined MGd/90Y-ibritumomab tiuxetan compares favorably to prior “single agent” radioimmunotherapy reports. In particular, there was a strong signal of clinical activity in the current clinical trial in the subset of rituximab-refractory follicular lymphoma. Witzig et al, using 90Y ibritumomab tiuxetan, reported an ORR of 74%, with associated 15% CR rate and median TTP of 6.8 months.5 In a similar patient population here, MGd/90Y-ibritumomab tiuxetan was associated with a marked increase in CR rate (64%) and approximate doubling of TTF (14 months). In DLBCL, the ORR here was 29% (CR 29%), both responses in transformed DLBCL. Morschhauser et al showed in relapsed/refractory DLBCL (non-transformed) that the ORR in patients who received prior rituximab-based chemotherapy was 19% (CR 8%).38
The time-to-response with radioimmunotherapy has never been examined. One notion has been that the time-to-response may be delayed, in part given the delayed myelosuppression associated with radioimmunotherapy. We found in the current clinical trial that all responses occurred very promptly- all by 4 weeks, following MGd/90Y-ibritumomab tiuxetan. It is important to note that it is not known to what degree MGd contributed to the clinical activity seen here. A randomized clinical trial of combined MGd/90Y-ibritumomab tiuxetan vs 90Y ibritumomab tiuxetan alone would be needed. Given the apparent single-agent cytotoxic activity of MGd in lymphoma seen in pre-clinical data, it would be rational in future trials to apply ‘extended dosing’ of MGd.
Delayed thrombocytopenia and neutropenia have been the most common hematologic side effects documented with 90Y ibritumomab tiuxetan therapy with median days from baseline to nadir of 43 and 50 days, respectively.5,39,40 The hematologic toxicity seen in this trial (Table 3) appeared similar to prior single-agent 90Y ibritumomab tiuxetan (median nadir counts with 0.4 mCi/kg): 50,000/mm3 for platelets, 1,100/mm3 for neutrophil count, and 9.9 g/dL for hemoglobin.24,40 The most common non-hematologic adverse event seen with MGd was a temporary greenish discoloration of skin as described before. This has been seen in all prior MGd clinical trials that used more than 3–4 consecutive daily doses,16,17,36,37 although the symptoms (including pruritis) are mostly mild and self-limited. Other non-hematologic adverse events seen here included several electrolyte changes, liver enzyme abnormalities, and gastrointestinal symptoms; although the vast majority of symptoms were mild (grade 1) and transient. It should be noted that patients were followed very closely in this clinical trial including daily history and physical examinations (during MGd dosing), while serum chemistry and hepatic function testing was performed every 2–3 days initially. Other adverse events in the current trial did not appear increased compared with prior radioimmunotherapy studies.
In conclusion, novel therapeutic agents exist, such as MGd, which may be safely combined with radioimmunotherapy. We found that MGd, when given together with 90Y-ibritumomab tiuxetan, did not appear to increase hematologic or other toxicity. Furthermore, all responses here occurred promptly (within 4 weeks), while a high rate of CRs were seen, especially in rituximab-refractory follicular lymphoma patients. Continued pre-clinical and clinical studies combining other novel therapeutic agents, such as bortezomib41 and CpG 7909,42 together with radioimmunotherapy are warranted.
Statement of translational relevance
We have had a long-standing interest the study of novel therapeutic agents that induce apoptosis through oxidative stress pathways. We published results before using the novel expanded porphyrin, motexafin gadolinium (MGd), in resistant myeloma cell lines; we showed that MGd induced intra-cellular oxidative stress with resultant apoptosis (Evens et al, Blood 2005). Since MGd had been shown to enhance ionizing radiation in solid tumor models, we hypothesized that MGd may enhance the efficacy of radioimmunotherapy in lymphoma. We examined MGd in vitro here combined with rituximab and/or radiation in lymphoma cell lines documenting additive or synergistic apoptosis, which occurred through mitochondrial-mediated pathways. We subsequently completed an investigator-initiated phase I/II clinical trial in relapsed/refractory NHL using escalating doses of MGd combined with standard radioimmunotherapy. Prior to this study, MGd had only been used clinically before combined with WBRT for solid-tumor brain metastases. The data presented here represents true bench-to-bedside clinical/translational research.
Supplementary Material
The treatment schedule is shown in Supplemental Figure 1A. Patients received eight total doses of MGd on days 1 through 4 and days 8 through 11. No additional MGd was administered. Gamma camera imaging with dosimetry was performed on days 1, 2, 4, and 7. Supplemental Figure 1B shows the planned abbreviated dose escalation of MGd with 3 dose levels (2.5mg/kg, 3.5mg/kg, and 5.0 mg/kg) and two treatment cohorts. Since the myelosuppression of radioimmunotherapy is related in part to the amount of lymphomatous marrow infiltration, the initial cohort accrued included only patients with ≤5% bone marrow involvement. Once this patient group completed MGd 2.5mg/kg, they continued to MGd 3.5mg/kg, while patients with 6%–24% bone marrow involvement began enrollment at dose level 1. MGd was based on actual body weight. Abbreviations: MGd, motexafin gadolinium; 90Yttrium-zevalin, 90Yttrium ibritumomab tiuxetan. 111Indium-zevalin, 111Indium ibritumomab tiuxetan.
Acknowledgements
To the AACR/ASCO Methods in Clinical Cancer Research Meeting (this clinical trial was developed/written at the 2002 Summer Workshop). To the NIH/NCI; this translational/clinical trial was the primary project of the K23 CA109613-A1 grant (AME).
To the staff of the Northwestern University General Clinical Research Center (GCRC) for the treatment and care of all patients.
Also to Biogen IDEC, Cambridge, MA, succeeded by Cell Therapeutics, Inc, Seattle, WA for supplying the 90Y ibritumomab tiuxetan (Zevalin®) for the clinical trial and to Pharmacyclics, Inc for supplying the MGd. The radioimmunoconjugate yttrium-90 ibritumomab tiuxetan is currently FDA approved for treatment of patients with relapsed or refractory, low grade or follicular B-cell NHL, including patients with follicular NHL who are refractory to rituximab. MGd is not an FDA approved treatment agent.
Grant support: National Cancer Institute, K23 CA109613-A1 for translational research/clinical trial (AME) and M01 RR-00048 National Center for Research Resources National Institutes of Health (clinical trial).
Footnotes
Presented: American Society of Hematology (ASH), 47 th Annual Meeting, Atlanta, Georgia, December 2005; ASH, 49th Annual Meeting, Atlanta, GA, December 2007; and Oral Presentation at the 10th International Conference on Malignant Lymphoma; Lugano, Switzerland,June 2008.
References
- 1.Vose JM, Wahl RL, Saleh M, et al. Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed lowgrade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2000;18:1316–1323. doi: 10.1200/JCO.2000.18.6.1316. [DOI] [PubMed] [Google Scholar]
- 2.Wiseman GA, Gordon LI, Multani PS, et al. Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin lymphoma and mild thrombocytopenia: a phase II multicenter trial. Blood. 2002;99:4336–4342. doi: 10.1182/blood.v99.12.4336. [DOI] [PubMed] [Google Scholar]
- 3.Gordon LI, Molina A, Witzig T, et al. Durable responses after ibritumomab tiuxetan radioimmunotherapy for CD20+ B-cell lymphoma: long-term follow-up of a phase 1/2 study. Blood. 2004;103:4429–4431. doi: 10.1182/blood-2003-11-3883. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RI, Kaminski MS, Wahl RL, et al. Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin's lymphomas. J Clin Oncol. 2005;23:7565–7573. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 5.Witzig TE, Flinn IW, Gordon LI, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:3262–3269. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 7.Horning SJ, Younes A, Jain V, et al. Efficacy and safety of tositumomab and iodine-131 tositumomab (Bexxar) in B-cell lymphoma, progressive after rituximab. J Clin Oncol. 2005;23:712–719. doi: 10.1200/JCO.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Evens AM, Lecane P, Magda D, et al. Motexafin gadolinium generates reactive oxygen species and induces apoptosis in sensitive and highly resistant multiple myeloma cells. Blood. 2005;105:1265–1273. doi: 10.1182/blood-2004-03-0964. [DOI] [PubMed] [Google Scholar]
- 9.Hashemy SI, Ungerstedt JS, Zahedi Avval F, Holmgren A. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J Biol Chem. 2006;281:10691–10697. doi: 10.1074/jbc.M511373200. [DOI] [PubMed] [Google Scholar]
- 10.Magda D, Miller RA. Motexafin gadolinium: a novel redox active drug for cancer therapy. Semin Cancer Biol. 2006;16:466–476. doi: 10.1016/j.semcancer.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Miller RA, Woodburn KW, Fan Q, et al. Motexafin gadolinium: a redox active drug that enhances the efficacy of bleomycin and doxorubicin. Clin Cancer Res. 2001;7:3215–3221. [PubMed] [Google Scholar]
- 12.Woodburn KW. Intracellular localization of the radiation enhancer motexafin gadolinium using interferometric Fourier fluorescence microscopy. J Pharmacol Exp Ther. 2001;297:888–894. [PubMed] [Google Scholar]
- 13.Magda D, Gerasimchuk N, Lecane P, Miller RA, Biaglow JE, Sessler JL. Motexafin gadolinium reacts with ascorbate to produce reactive oxygen species. Chem Commun (Camb) 2002:2730–2731. doi: 10.1039/b208760j. [DOI] [PubMed] [Google Scholar]
- 14.Magda D, Lepp C, Gerasimchuk N, et al. Redox cycling by motexafin gadolinium enhances cellular response to ionizing radiation by forming reactive oxygen species. Int J Radiat Oncol Biol Phys. 2001;51:1025–1036. doi: 10.1016/s0360-3016(01)01810-7. [DOI] [PubMed] [Google Scholar]
- 15.Mehta MP, Shapiro WR, Phan SC, et al. Motexafin Gadolinium Combined with Prompt Whole Brain Radiotherapy Prolongs Time to Neurologic Progression in Non-Small-Cell Lung Cancer Patients with Brain Metastases: Results of a Phase III Trial. Int J Radiat Oncol Biol Phys. 2008 doi: 10.1016/j.ijrobp.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 16.Mehta MP, Shapiro WR, Glantz MJ, et al. Lead-in phase to randomized trial of motexafin gadolinium and whole-brain radiation for patients with brain metastases: centralized assessment of magnetic resonance imaging, neurocognitive, and neurologic end points. J Clin Oncol. 2002;20:3445–3453. doi: 10.1200/JCO.2002.07.500. [DOI] [PubMed] [Google Scholar]
- 17.Carde P, Timmerman R, Mehta MP, et al. Multicenter phase Ib/II trial of the radiation enhancer motexafin gadolinium in patients with brain metastases. J Clin Oncol. 2001;19:2074–2083. doi: 10.1200/JCO.2001.19.7.2074. [DOI] [PubMed] [Google Scholar]
- 18.Xu S, Zakian K, Thaler H, et al. Effects of Motexafin gadolinium on tumor metabolism and radiation sensitivity. Int J Radiat Oncol Biol Phys. 2001;49:1381–1390. doi: 10.1016/s0360-3016(00)01566-2. [DOI] [PubMed] [Google Scholar]
- 19.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 20.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 21.Miller RA, Woodburn K, Fan Q, Renschler MF, Sessler JL, Koutcher JA. In vivo animal studies with gadolinium (III) texaphyrin as a radiation enhancer. Int J Radiat Oncol Biol Phys. 1999;45:981–989. doi: 10.1016/s0360-3016(99)00274-6. [DOI] [PubMed] [Google Scholar]
- 22.Gordon LI, Witzig TE, Wiseman GA, et al. Yttrium 90 ibritumomab tiuxetan radioimmunotherapy for relapsed or refractory low-grade non-Hodgkin's lymphoma. Semin Oncol. 2002;29:87–92. doi: 10.1053/sonc.2002.30148. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Horning SJ, Coiffier B, et al. NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 24.Witzig TE, White CA, Wiseman GA, et al. Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20(+) B-cell non-Hodgkin's lymphoma. J Clin Oncol. 1999;17:3793–3803. doi: 10.1200/JCO.1999.17.12.3793. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan ELMM. Nonparametric estimation from incomplete observation. JASA. 1958;53:457–481. [Google Scholar]
- 27.Cox. Regression models and life tables. J Royal Statist Soc (B) 1972;34:187–220. [Google Scholar]
- 28.Lecane PS, Karaman MW, Sirisawad M, et al. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-cell lymphoma lines. Cancer Res. 2005;65:11676–11688. doi: 10.1158/0008-5472.CAN-05-2754. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Ramos J, Sirisawad M, et al. Motexafin gadolinium induces mitochondrially-mediated caspase-dependent apoptosis. Apoptosis. 2005;10:1131–1142. doi: 10.1007/s10495-005-0887-2. [DOI] [PubMed] [Google Scholar]
- 30.Ramos J, Sirisawad M, Miller R, Naumovski L. Motexafin gadolinium modulates levels of phosphorylated Akt and synergizes with inhibitors of Akt phosphorylation. Mol Cancer Ther. 2006;5:1176–1182. doi: 10.1158/1535-7163.MCT-05-0280. [DOI] [PubMed] [Google Scholar]
- 31.Gartenhaus RB, Prachand SN, Paniaqua M, Li Y, Gordon LI. Arsenic trioxide cytotoxicity in steroid and chemotherapy-resistant myeloma cell lines: enhancement of apoptosis by manipulation of cellular redox state. Clin Cancer Res. 2002;8:566–572. [PubMed] [Google Scholar]
- 32.Bellosillo B, Villamor N, Lopez-Guillermo A, et al. Complement-mediated cell death induced by rituximab in B-cell lymphoproliferative disorders is mediated in vitro by a caspase-independent mechanism involving the generation of reactive oxygen species. Blood. 2001;98:2771–2777. doi: 10.1182/blood.v98.9.2771. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 34.Shanafelt TD, Lee YK, Bone ND, et al. Adaphostin-induced apoptosis in CLL B cells is associated with induction of oxidative stress and exhibits synergy with fludarabine. Blood. 2005;105:2099–2106. doi: 10.1182/blood-2004-06-2205. [DOI] [PubMed] [Google Scholar]
- 35.Miller CP, Ban K, Dujka ME, et al. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110:267–277. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viala J, Vanel D, Meingan P, Lartigau E, Carde P, Renschler M. Phases IB and II multidose trial of gadolinium texaphyrin, a radiation sensitizer detectable at MR imaging: preliminary results in brain metastases. Radiology. 1999;212:755–759. doi: 10.1148/radiology.212.3.r99se10755. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal DI, Nurenberg P, Becerra CR, et al. A phase I single-dose trial of gadolinium texaphyrin (Gd-Tex), a tumor selective radiation sensitizer detectable by magnetic resonance imaging. Clin Cancer Res. 1999;5:739–745. [PubMed] [Google Scholar]
- 38.Morschhauser F, Illidge T, Huglo D, et al. Efficacy and safety of yttrium-90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood. 2007;110:54–58. doi: 10.1182/blood-2007-01-068056. [DOI] [PubMed] [Google Scholar]
- 39.Gordon LI. Practical considerations and radiation safety in radioimmunotherapy with yttrium 90 ibritumomab tiuxetan (Zevalin) Semin Oncol. 2003;30:23–28. doi: 10.1053/j.seminoncol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Witzig TE, White CA, Gordon LI, et al. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-hodgkin's lymphoma. J Clin Oncol. 2003;21:1263–1270. doi: 10.1200/JCO.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 41.Pervan MCJ, Matso D, Said JW, McBride WH, de Vos S. Active Combination Therapy of Bortezomib (Velcade) and Ibritumomab Tiuxetan (Zevalin) in an In Vivo Diffuse Large B-Cell Lymphoma Model. Blood. 2005;106:2406a. [Google Scholar]
- 42.Witzig TEWG, Weiner G, Ansell SM, Micallef I, Habermann TM, Zent CS, Inwards DJ, Shanafelt T, Porrata L, Maurer MJ, Allmer C, Ballas C. A Phase I Trial of CpG-7909, Rituximab Immunotherapy, and Y90 Zevalin Radioimmunotherapy for Patients (Pts) with Previously Treated CD20+ Non-Hodgkin Lymphoma. Blood. 2007;110:124a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The treatment schedule is shown in Supplemental Figure 1A. Patients received eight total doses of MGd on days 1 through 4 and days 8 through 11. No additional MGd was administered. Gamma camera imaging with dosimetry was performed on days 1, 2, 4, and 7. Supplemental Figure 1B shows the planned abbreviated dose escalation of MGd with 3 dose levels (2.5mg/kg, 3.5mg/kg, and 5.0 mg/kg) and two treatment cohorts. Since the myelosuppression of radioimmunotherapy is related in part to the amount of lymphomatous marrow infiltration, the initial cohort accrued included only patients with ≤5% bone marrow involvement. Once this patient group completed MGd 2.5mg/kg, they continued to MGd 3.5mg/kg, while patients with 6%–24% bone marrow involvement began enrollment at dose level 1. MGd was based on actual body weight. Abbreviations: MGd, motexafin gadolinium; 90Yttrium-zevalin, 90Yttrium ibritumomab tiuxetan. 111Indium-zevalin, 111Indium ibritumomab tiuxetan.