Abstract
Background
Thrombin is an enzyme essential to the acceleration of the coagulation cascade and the conversion of fibrinogen to clottable fibrin.
Objectives
We evaluated the relation of basal peak thrombin generation to risk of future VTE, and determined whether associations were independent of other coagulation markers.
Methods
LITE ascertained VTE in two prospective population-based cohorts: the Atherosclerosis Risk in Communities (ARIC) study and the Cardiovascular Health Study (CHS). Peak thrombin generation was measured on stored plasma in a nested case-control sample (434 cases, 1,004 controls). Logistic regression was used to estimate the relation of peak thrombin generation to VTE, adjusted for age, sex, race, center and BMI. Mediation was evaluated by additionally adjusting for factor VIII and D-dimer.
Results
Relative to the first quartile of peak thrombin generation, the odds ratio (95% CI) of VTE for those above the median was 1.74 (1.28–2.37). The association was modestly attenuated by adjustment for factor VIII and D-dimer 1.47 (1.05–2.05). Associations appeared stronger for idiopathic than for secondary VTE. Elevated peak thrombin generation more than added to the VTE risk associated with Factor V Leiden or low aPTT.
Conclusions
In this prospective study of two independent cohorts, elevated basal peak thrombin generation was associated with subsequent risk of VTE, independent of established VTE risk factors.
Keywords: Longitudinal Investigation of Thromboembolism Etiology (LITE), thrombin generation, venous thromboembolism
Background
Deep vein thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are major sources of morbidity and mortality in the elderly[1]. Hypercoagulability is an established risk factor for VTE[2]. Thrombin is essential to the acceleration of the coagulation cascade, since it serves as an activator for platelets, factor V, and factor VIII, and is a critical component of a positive-feedback loop which results in the generation of large amounts of additional thrombin, the conversion of fibrinogen to fibrin, and ultimately clot formation[3].
While measuring thrombin generation has been possible since 1953[4, 5], only recently have assays been developed with which thrombin generation can be efficiently measured[6]. These functional assays activate the coagulation cascade using tissue factor and phospholipids, then monitor the concentration of thrombin generated over time. Using information from the thrombogram curve (thrombin generation graphed against time) produced by these assays, thrombin generation can be expressed in multiple ways[7]. Most pertinent to this manuscript is peak thrombin generation, which is the maximal concentration of thrombin formed at a given point in time. Endogenous thrombin potential (ETP), which is the area under the thrombin generation curve, is the other measure most commonly reported in the literature.
There is currently great interest in whether measurement of basal thrombin generation predicts VTE occurrence or recurrence. Of the five prospective studies (two using Austrian Study on Recurrent VTE data, with different thrombin generation assays) that have assessed the relation of thrombin generation to recurrent VTE, four have found greater peak thrombin generation[8, 9] and/or ETP[9–11] to be predictive of VTE recurrence. The Leiden Thrombophilia Study (LETS), however, observed no association between peak thrombin generation or ETP and risk of recurrent DVT[12].
Using their case-control data, the LETS also assessed whether measures of thrombin generation were predictive of first DVT[12]. Among cases, thrombin generation values were assessed at least three months after discontinuation of anticoagulant therapy for a first DVT. Individuals with an ETP above the 90th percentile measured in control subjects had an increased relative odds of DVT [OR (95% CI): 1.5 (0.9, 2.3)], which was stronger when the analyses were restricted to idiopathic DVT [OR: 1.7 (1.0–2.8)]. However, a dose-response relation was not demonstrated when ETP was modeled as quartiles, and no associations were observed between peak thrombin generation and risk of DVT.
Though not entirely consistent, the evidence suggesting that measures of thrombin generation may be related to VTE is provocative. To date, however, the relation of peak thrombin generation to incident VTE, using plasma collected prior to VTE, is unknown. Thus, using data from the Longitudinal Investigation of Thromboembolism Etiology (LITE) study we explored the association of basal peak thrombin generation to risk of future VTE. To build on previous studies, we further assessed whether this relationship was independent of other hemostatic factor levels. We hypothesized that associations of basal peak thrombin generation would be associated positively with VTE risk, but would be largely explained by levels of other hemostasis markers that contribute to higher thrombin generation.
Methods
LITE Study Design
LITE is a prospective study of VTE occurrence in two population-based cohorts: the Atherosclerosis Risk in Communities (ARIC) study[13] and the Cardiovascular Health Study (CHS) [14, 15]. Extensive CVD risk factor information was collected in both studies, generally in a similar fashion, using standard measures. Local institutional review boards approved the study protocols, and all participants gave informed consent.
The ARIC study is a multi-center population-based prospective cohort study designed to investigate the etiology and natural history of atherosclerosis in middle-aged adults[13]. Participants were recruited from four US communities: Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD. The study cohort included 15,972 white and black men and women aged 45–64 years at baseline, in 1987–1989.
CHS is a population-based longitudinal study of coronary heart disease and stroke in adults aged 65 years and older[14, 15]. A total of 5,201 men and women sampled from Medicare eligibility lists were recruited in 1989 to 1990 from four U.S. communities: Forysth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA. An additional 687 African-American participants were recruited from 1992 to 1993.
VTE Identification and Classification
ARIC participants were contacted annually by telephone or through clinic visits, and CHS participants were contacted twice a year, alternating clinic visits and telephone calls. Hospitalizations were identified by participants or proxy reports in both ARIC and CHS, by surveillance of local hospital discharge lists in ARIC, and by a search of Medicare records in CHS. For each hospitalization identified, all ICD-9-CM discharge codes were recorded.
All records with ICD-9-CM codes indicating possible VTE (415.1x, 451, 451.1x, 451.2, 451.8x, 451.9, 453.0, 453.1, 453.2, 453.8, 453.9, 996.7x, 997.2, and 999.2, and procedure code 38.7) were identified, copied, and independently reviewed by two physicians (ARF and MC) [16]. Deep vein thrombosis was defined on the basis of duplex ultrasound or venogram or, in rare cases, by impedance plethysmography, computed tomography, or autopsy. Definite PE required ventilation/perfusion scanning showing multiple segmental or subsegmental mismatched perfusion defects, or a positive pulmonary angiogram, computed tomography, or autopsy[16]. VTE events were further classified as idiopathic or secondary (occurring within 90 days of major trauma, surgery, hospitalization, or marked immobility or associated with active cancer or chemotherapy).
LITE Nested Case-Control Sample
A nested-case control design was employed to study associations between some blood parameters and VTE incidence. All VTE cases were included in the nested study. Controls were randomly selected at a ratio of two per case. They were frequency matched to the cases by age (5-year groupings), gender, race (African-American or Caucasian), follow-up time (cases’ event date within 2 years of controls assigned date), and study (ARIC or CHS).
Hemostatic Factor Assessment
The same phlebotomy and sample processing methods were applied across all the field centers for CHS and ARIC, with centralized training of technicians and quality control monitoring[17–19]. Blood samples were drawn into Vacutainer tubes with minimal stasis using atraumatic venipuncure, a tourniquet time <2 minutes and a 21-guage butterfly needle connected to a Vacutainer outlet via a Luer adaptor. In both cohorts, 32g/L citrate samples were spun at 3000xG for 10 minutes at 4C to remove platelets[17, 18]. Therefore, samples are appropriate for coagulation factor testing. We have previously shown good results for other assays that are very sensitive to sample handling/pre-analytical issues, such as prothrombin fragment 1.2, and especially fibrinopeptide A in the CHS[20]. Serum, citrate and EDTA plasma, and DNA were collected and stored at −70°C.
Some hemostatic factors were assessed in each cohort at baseline soon after blood drawing, using published methods: fibrinogen, factor VII, factor VIII, von Willebrand factor (vWF; ARIC only), activated partial thromboplastin times (aPTT; ARIC only)[17, 18]. Other factors were measured previously in the LITE nested case-control sample on stored blood: factors IX, X, XI, D-Dimer, factor V Leiden,[21] prothrombin 20210A[22] and ABO blood-type by genotyping[23]. Factor IX, X, XI, were measured using enzyme immunoassay kits from Enzyme Research Laboratories (South Bend, IN). D-Dimer was measured by the STAR automated coagulation analyzer (Diagnostica Stago), using an immuno-turbidometric assay (Liatest D-DI; Diagnostica Stago, Parsippany, NJ).
Peak thrombin generation, for both CHS and ARIC, was measured in platelet-poor citrate plasma, usually from the baseline exam but always before VTE, using the Technothrombin TGA kit (Technoclone, Vienna, Austria) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). The reaction was triggered with the TGA RC Low reagent, which contained a low concentration of phospholipid micelles containing 71.6 pM of rhTF in Tris-Herpes-NaCl buffer. The median within-sample coefficient of variation in ARIC was 10.9%, and in CHS 3.6%. Samples had been previously thawed and refrozen, but there was no difference in the number of freeze-thaw cycles between cases and controls. We evaluated freeze-thaw effect in 9 analyzed samples from local volunteers. After a second cycle of freeze thaw, mean thrombin generated decreased from 397 to 345 nM, but it did not change further after more cycles (3rd and 4th cycle means 369 and 334 nM, respectively). Other parameters of thrombin generation were not available.
Statistical Analysis
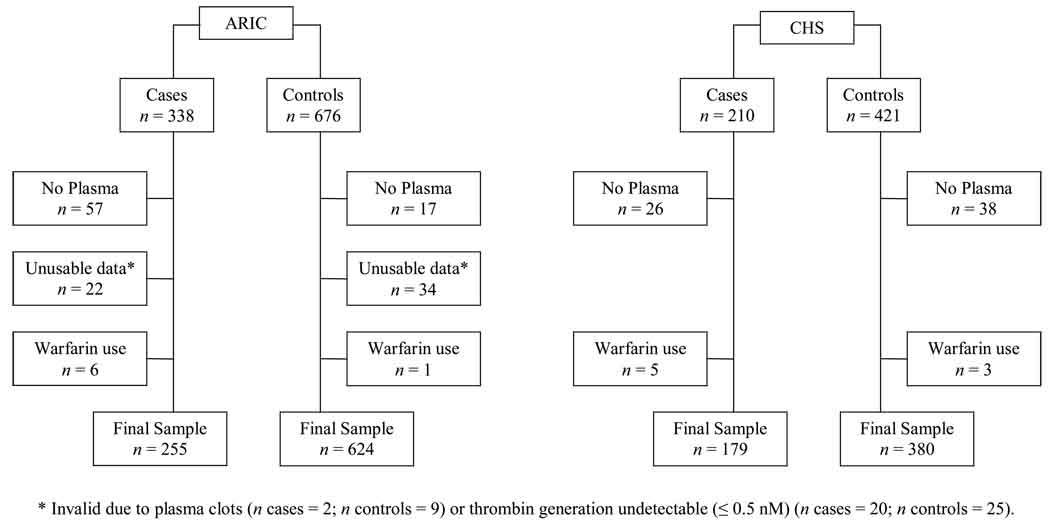
Participants were excluded from analysis if they had no plasma available, unusable peak thrombin generation data, or were using warfarin at baseline. A flow chart of study exclusions is presented in Figure 1. In ARIC, cases had a greater proportion of missing or unusable thrombin generation data than controls. For both ARIC and CHS, subject characteristics were compared in participants missing peak thrombin generation data or with undetectable peak thrombin generation levels, and those with valid peak thrombin generation data, stratified by case-control status. No consistent or remarkable differences existed between those included versus excluded from this study.
Figure 1.
Flow chart of LITE participant exclusions by cohort and case-control status.
Due to differences in the distribution of peak thrombin generation levels between ARIC and CHS, study-specific quartiles and standard deviations (SDs) of basal peak thrombin generation were derived according to the peak thrombin generation distribution among controls.
Frequencies and means of variables of interest were compared between VTE cases and controls, using chi-squared tests or Student’s t-tests. In controls, general linear regression was used to assess the relation of peak thrombin generation levels to demographics, physiologic characteristics, genetic risk factors, and hemostatic factor levels. Associations between hemostatic factor levels and peak thrombin generation were age-adjusted. Linear trends were assessed by numbering the peak thrombin generation quartiles (1 through 4) and entering this variable into the models as a continuous term.
For the primary analysis, odds ratios (ORs) and 95% confidence intervals (CIs) for the relation between peak thrombin generation and VTE were estimated using unconditional logistic regression. Model 1 adjusted for age (continuous), sex, race, center, and BMI (continuous). ORs were also estimated for idiopathic and secondary VTE. Peak thrombin generation was represented both as quartiles and per standard deviation increment. In further models we evaluated whether associations between thrombin generation and VTE were independent of other hemostatic factors by adjusting for these factors and determining if adjustment altered the beta coefficient for peak thrombin generation by 10% or more.
Interactions of peak thrombin generation with other VTE risk factors were also explored. Relative excess risk due to interactions (RERIs) were used to assess additive interaction[24, 25], while cross-product terms were used to evaluate multiplicative interaction.
All authors had access to the data; analyses were conducted by PLL.
Results
In this nested case-control sample, the mean baseline age of ARIC participants was 55 years, while in CHS the mean baseline age was 73 years. In ARIC, 43% of participants were men, and 31% black. These proportions in CHS were 44% and 21%, respectively. Included in this analysis were 255 VTE cases and 624 controls in ARIC during 16 years of follow-up (median = 13.2 years), and 179 cases and 380 controls in CHS during 14 years (median = 11.8 years). In both studies, 42% of cases were classified as idiopathic.
Unadjusted baseline characteristics of ARIC and CHS participants stratified by VTE case-control status are reported in Table 1. In ARIC, compared to controls, those who developed VTE had a higher mean BMI, a greater prevalence of the factor V Leiden mutation and non-O blood type, lower aPTT, and higher levels of factors VIII, IX, XI, D-dimer, vWF, and peak thrombin generation. In CHS, cases also had a higher prevalence of the prothrombin mutation than controls, but did not have higher levels of factor IX. aPTT and vWF were not assessed in CHS.
Table 1.
Baseline characteristics of LITE participants stratified by cohort (ARIC or CHS) and VTE case-control status.
| ARIC | CHS | ||||
|---|---|---|---|---|---|
| Controls | Cases | Controls | Cases | ||
| N | 624 | 255 | 380 | 179 | |
|
Demographics (matching factors) |
|||||
| Age (SD) | 55.9 (5.6) | 55.9 (5.5) | 73.1 (5.6) | 73.0 (5.5) | |
| Black Race (%) | 31.3 | 31.8 | 19.5 | 23.5 | |
| Male Gender (%) | 42.8 | 42.8 | 43.4 | 45.3 | |
| Physiologic Characteristics | |||||
| BMI (SD) | 28.0 (5.2) | 29.7 (5.8) | 26.5 (4.5) | 28.3 (5.3) | |
| Prevalent Diabetes (%) | 10.2 | 14.9 | 13.4 | 18.4 | |
| Prevalent CHD (%) | 5.2 | 7.2 | 13.7 | 10.6 | |
| VTE Genetic Risk Factors | |||||
| Factor V Leiden (%) | 2.9 | 10.9 | 3.4 | 7.9 | |
| Prothombin 20210A (%) | 2.0 | 2.1 | 1.7 | 6.1 | |
| Non-O Blood Type (%) | 53.8 | 67.0 | 52.3 | 64.6 | |
| Hemostatic Factors | |||||
| Factor VII (%, SD) | 118 (27) | 122 (30) | 123 (29) | 124 (30) | |
| Factor VIII (%, SD) | 131 (39) | 149 (48) | 122 (37) | 130 (43) | |
| Factor IX (%, SD) | 140 (30) | 146 (31) | 150 (31) | 154 (30) | |
| Factor X (%, SD) | 139 (29) | 141 (35) | 138 (30) | 139 (33) | |
| Factor XI (%, SD) | 135 (34) | 141 (37) | 126 (32) | 133 (33) | |
| Fibrinogen (mg/dL, SD) | 305 (61) | 308 (66) | 325 (66) | 313 (65) | |
| D-dimer* (ug/mL) | 0.35 | 0.42 | 0.52 | 0.66 | |
| vWF (%, SD) | 118 (47) | 140 (57) | -- | -- | |
| aPTT (seconds, SD) | 29.1 (2.8) | 28.0 (3.3) | -- | -- | |
| Peak Thrombin Generation | |||||
| Mean (nM, SD) | 107 (91) | 123 (97) | 245 (121) | 272 (149) | |
| Median (nM) | 80 | 98 | 232 | 253 | |
Geometric mean
Table 2 presents characteristics of ARIC and CHS controls according to study-specific peak thrombin generation quartiles. Overall, there was no relation of peak thrombin generation levels to demographics, physiologic characteristics, or VTE genetic risk factors. Following age adjustment, in ARIC there were associations between peak thrombin generation and factor VIII, vWF, and aPTT. The Spearman’s age-adjusted partial correlations between peak thrombin generation and these hemostatic factors were r = 0.17 (p < 0.0001), r = 0.13 (p = 0.0002) and r = −0.22 (p < 0.0001), respectively. In CHS, after age-adjustment, peak thrombin generation was positively related to factors VII, VIII, IX, and X. The age-adjusted Spearman’s correlations between peak thrombin generation and these factors were: factor VII r = 0.14 (p = 0.002); factor VIII r = 0.18 (p = 0.0001); factor IX r = 0.15 (p = 0.0009); factor X r = 0.12 (p = 0.01).
Table 2.
Characteristics [mean or %] of LITE control participants according to quartiles* of peak thrombin generation and cohort.
| ARIC | CHS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak Thrombin Generation | |||||||||||
| Quartiles | 1 | 2 | 3 | 4 | ptrend | 1 | 2 | 3 | 4 | ptrend | |
| N | 156 | 156 | 156 | 156 | 95 | 95 | 95 | 95 | |||
| Median (nM) | 26 | 59 | 107 | 205 | 110 | 200 | 267 | 378 | |||
| Range (nM) | (1–45) | (46–80) | (81–143) | (144–665) | (4–155) | (156–232) | (233–316) | (317–693) | |||
| Demographics | |||||||||||
| Age (years) | 55.6 | 55.5 | 56.0 | 56.4 | 0.18 | 73.4 | 73.6 | 72.6 | 73.1 | 0.47 | |
| Black Race† (%) | 27.6 | 34.0 | 24.4 | 39.1 | 0.02 | 16.7 | 19.0 | 22.1 | 20.0 | 0.83 | |
| Male Gender† (%) | 45.5 | 45.5 | 45.5 | 34.6 | 0.13 | 54.7 | 43.2 | 39.0 | 36.8 | 0.06 | |
| Physiologic | |||||||||||
| Characteristics‡ | |||||||||||
| BMI (kg/m2) | 27.6 | 28.1 | 27.8 | 28.3 | 0.33 | 26.8 | 25.9 | 26.5 | 26.9 | 0.68 | |
| Prevalent Diabetes (%) | 12.4 | 11.2 | 8.9 | 8.2 | 0.17 | 17.9 | 4.2 | 13.7 | 17.9 | 0.54 | |
| Prevalent CHD (%) | 3.3 | 4.1 | 6.5 | 6.9 | 0.10 | 14.6 | 14.5 | 8.7 | 16.9 | 0.95 | |
| VTE Genetic Risk Factors† | |||||||||||
| Factor V Leiden (%) | 2.0 | 3.4 | 3.4 | 2.8 | 0.88 | 4.4 | 4.6 | 2.3 | 2.2 | 0.72 | |
| Prothrombin 20210A (%) | 4.05 | 1.4 | 1.4 | 1.4 | 0.26 | 0.0 | 1.2 | 3.5 | 2.2 | 0.31 | |
| Non-O Blood Type (%) | 51.4 | 54.2 | 55.5 | 54.1 | 0.91 | 50.6 | 50.6 | 51.8 | 52.3 | 0.99 | |
| Hemostatic Factors‡ | |||||||||||
| Factor VII (%) | 119 | 116 | 117 | 121 | 0.49 | 118 | 120 | 120 | 132 | 0.003 | |
| Factor VIII (%) | 125 | 127 | 134 | 139 | 0.0004 | 114 | 121 | 121 | 132 | 0.004 | |
| Factor IX (%) | 138 | 143 | 137 | 143 | 0.37 | 150 | 142 | 151 | 158 | 0.02 | |
| Factor X (%) | 138 | 138 | 134 | 145 | 0.08 | 135 | 136 | 137 | 146 | 0.01 | |
| Factor XI (%) | 134 | 133 | 131 | 141 | 0.09 | 128 | 121 | 128 | 130 | 0.38 | |
| Fibrinogen (mg/dL) | 313 | 299 | 305 | 304 | 0.36 | 329 | 316 | 314 | 339 | 0.33 | |
| D-dimer§ (ug/mL) | 0.52 | 0.46 | 0.47 | 0.48 | 0.49 | 0.64 | 0.71 | 0.67 | 0.68 | 0.80 | |
| vWF (%) | 115 | 114 | 119 | 126 | 0.02 | -- | -- | -- | -- | -- | |
| aPTT (seconds) | 29.6 | 29.5 | 28.8 | 28.5 | <0.0001 | -- | -- | -- | -- | -- | |
Study-specific, based on the distribution among controls.
P-value for X2 test, other p-values by ANOVA.
Adjusted for age.
Geometric mean.
In ARIC, CHS and LITE as a whole, peak thrombin generation levels were associated positively with VTE risk (Table 3). In LITE, after adjustment for demographics and BMI, there was a positive linear trend (p = 0.0003). Those with thrombin generation values above the median were at a 74% greater risk of VTE, compared to those in the lowest quartile. The odds of VTE increased 20% (7%, 34%) per 1 SD increment of peak thrombin generation level. Factor VIII and D-dimer were identified statistically as potential “mediators” of the LITE thrombin generation-VTE association; after adjustment for these factors, the association of peak thrombin generation with VTE was attenuated modestly [OR per 1 SD: 1.16 (1.03, 1.32)].
Table 3.
Odds ratios (95% CI) of VTE according to peak thrombin generation quartile and per 1 standard deviation increment in peak thrombin generation*† The LITE study.
| Peak Thrombin Quartiles | Per 1 SD | ||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Ptrend | |||
| LITE | |||||||
| Total VTE | Cases (n) | 79 | 95 | 126 | 134 | ||
| Controls (n) | 251 | 251 | 251 | 251 | |||
| OR (95% CI) | 1.00 | 1.24 (0.87–1.76) | 1.70 (1.21–2.40) | 1.78 (1.26–2.51) | 0.0003 | 1.20 (1.07–1.34) | |
| Idiopathic VTE | Cases (n) | 29 | 38 | 58 | 58 | ||
| OR (95% CI) | 1.00 | 1.33 (0.79–2.24) | 2.10 (1.28–3.42) | 2.05 (1.25–3.37) | 0.001 | 1.24 (1.07–1.44) | |
| Secondary VTE | Cases (n) | 50 | 57 | 68 | 76 | ||
| OR (95% CI) | 1.00 | 1.19 (0.78–1.82) | 1.47 (0.97–2.23) | 1.64 (1.08–2.49) | 0.01 | 1.17 (1.02–1.33) | |
| ARIC | |||||||
| Total VTE | Cases (n) | 44 | 49 | 82 | 80 | ||
| Controls (n) | 156 | 156 | 156 | 156 | SD = 91 | ||
| OR (95% CI) | 1.00 | 1.09 (0.68–1.75) | 1.82 (1.17–2.81) | 1.77 (1.14–2.75) | 0.002 | 1.17 (1.01–1.35) | |
| Idiopathic VTE | Cases (n) | 17 | 16 | 33 | 42 | ||
| OR (95% CI) | 1.00 | 0.92 (0.45–1.90) | 1.88 (1.00–3.53) | 2.33 (1.26–4.31) | 0.0007 | 1.28 (1.07–1.53) | |
| Secondary VTE | Cases (n) | 27 | 33 | 49 | 38 | ||
| OR (95% CI) | 1.00 | 1.18 (0.67–2.08) | 1.79 (1.05–3.03) | 1.40 (0.80–2.44) | 0.11 | 1.06 (0.88–1.28) | |
| CHS | |||||||
| Total VTE | Cases (n) | 35 | 46 | 44 | 54 | ||
| Controls (n) | 95 | 95 | 95 | 95 | SD = 121 | ||
| OR (95% CI) | 1.00 | 1.48 (0.86–2.55) | 1.52 (0.87–2.65) | 1.81 (1.04–3.15) | 0.05 | 1.26 (1.05–1.50) | |
| Idiopathic VTE | Cases (n) | 12 | 22 | 25 | 16 | ||
| OR (95% CI) | 1.00 | 1.99 (0.91–4.34) | 2.42 (1.11–5.28) | 1.52 (0.65–3.54) | 0.32 | 1.18 (0.91–1.52) | |
| Secondary VTE | Cases (n) | 23 | 24 | 19 | 38 | ||
| OR (95% CI) | 1.00 | 1.23 (0.63–2.40) | 1.00 (0.49–2.03) | 2.06 (1.08–3.93) | 0.04 | 1.33 (1.08–1.63) | |
Adjusted for age, sex, race, center, and BMI.
Quartile and SD based on the study-specific control distribution.
In ARIC, after demographic and BMI adjustments, participants with peak thrombin generation values above the median were at nearly 80% greater risk of VTE, as compared to those in the lowest quartile. Per 1 SD increment of peak thrombin generation, the odds of VTE increased 17% (1%, 35%). Factor VIII and aPTT were statistical “mediators” of the ARIC thrombin generation-VTE association; after adjustment for these factors, the association of peak thrombin generation with VTE was modestly attenuated [OR per 1 SD: 1.12 (0.96, 1.31)].
Likewise, in CHS the demographic- and BMI-adjusted OR for extreme quartiles of peak thrombin generation was 1.81 (1.04, 3.15), while the odds of VTE increased 26% (5%, 50%) per 1 SD increment in peak thrombin generation. Factor X and D-dimer were statistical “mediators” of the association; adjustment for these factors attenuated the peak thrombin generation-VTE relation in CHS to a larger extent than in ARIC [OR per 1 SD: 1.14 (0.93, 1.39)].
The median peak thrombin generation level of the CHS Pittsburgh center (181 nM) was lower than that of the other three CHS centers (258 nM). In sensitivity analyses in which we excluded Pittsburgh data, the results were somewhat stronger. ORs of VTE per 1 SD increment of peak thrombin generation were 1.32 (1.09, 1.61) following demographic- and BMI-adjustment, and 1.21 (0.96, 1.54) after additional adjustment for factor X and D-dimer. Center differences were also present in ARIC, but were less pronounced. Stratifying ARIC analyses by centers with high and low means yielded similar results (data not shown).
In LITE as a whole, and both individual cohorts, associations of peak thrombin generation with risk of idiopathic VTE and secondary VTE were similar, though at times these subgroup associations did not achieve statistical significance. Associations tended to be larger for idiopathic than secondary VTE in ARIC, but the opposite was observed in CHS (albeit smaller numbers).
Table 4 presents ORs for combinations of peak thrombin generation greater than the median and other VTE risk factors, adjusted for demographics and BMI. For factor V Leiden, compared to those without both risk factors, the OR of VTE in the presence of both factors was 6.93 (95% CI: 3.38, 14.22) (p = 0.04). The RERI suggested that 61% of the excess VTE risk for factor V Leiden and high peak thrombin generation was due to the combination of these two factors. The aPTT also interacted synergistically with peak thrombin generation, with an RERI% of 67% (p = 0.03). Compared to those with low aPTT and low peak thrombin generation, the OR of VTE with both factors was 3.17 (95% CI: 2.06, 4.89). No other additive or multiplicative interactions between peak thrombin generation and VTE genetic risk factors or hemostatic factors were observed (data not shown).
Table 4.
Odds ratios and relative excess risk due to interactions (RERI) for VTE in relation jointly to peak thrombin generation and other factors*† The LITE study.
| Variable | Variable Level |
Peak Thrombin Generation (median cut-point†) |
OR (95% CI) | RERI | RERI%‡ | p-value§ |
|---|---|---|---|---|---|---|
| VTE Genetic Risk Factors | ||||||
| Factor V Leiden | ||||||
| Yes | High | 6.93 (3.38, 14.22) | 3.64 | 61 | 0.04 | |
| No | High | 1.56 (1.20, 2.02) | ||||
| Yes | Low | 2.73 (1.25, 5.94) | ||||
| No | Low | 1.00 (Reference) | ||||
| Prothrombin Mutation | ||||||
| Yes | High | 2.93 (1.13, 7.63) | 0.41 | 21 | 0.81 | |
| No | High | 1.60 (1.24, 2.06) | ||||
| Yes | Low | 1.93 (0.66, 5.60) | ||||
| No | Low | 1.00 (Reference) | ||||
| ABO Blood Type | ||||||
| Yes | High | 2.66 (1.85, 3.81) | 0.52 | 31 | 0.20 | |
| No | High | 1.51 (1.01, 2.24) | ||||
| Yes | Low | 1.63 (1.11, 2.38) | ||||
| No | Low | 1.00 (Reference) | ||||
| Biomarkers | ||||||
| Factor VIII | (Top 25%) | |||||
| High | High | 2.38 (1.65, 3.42) | 0.76 | 55 | 0.14 | |
| Low | High | 1.39 (1.04, 1.85) | ||||
| High | Low | 1.23 (0.77, 1.95) | ||||
| Low | Low | 1.00 (Reference) | ||||
| D-dimer | (> Median) | |||||
| High | High | 2.53 (1.78, 3.61) | 0.09 | 6 | 0.83 | |
| Low | High | 1.71 (1.22, 2.39) | ||||
| High | Low | 1.73 (1.21, 2.48) | ||||
| Low | Low | 1.00 (Reference) | ||||
| aPTT | (Bottom 25%) | |||||
| Low | High | 3.17 (2.06, 4.89) | 1.45 | 67 | 0.03 | |
| High | High | 1.36 (0.94, 1.97) | ||||
| Low | Low | 1.36 (0.75, 2.44) | ||||
| High | Low | 1.00 (Reference) | ||||
Adjusted for age, sex, race, center, and BMI.
Median based on the study-specific control distribution.
RERI% estimates the proportion of VTE caused by two factors that can be attributed to their additive interaction.
P values for additive interaction.
Sensitivity analyses were conducted in which we excluded individuals with a self-reported history of VTE at baseline. In ARIC 23 cases and 17 controls reported a history of VTE, while in CHS 22 cases and 12 controls reported prior VTE. Results of these analyses were similar to those including the entire LITE case-control sample (data not shown). Additional sensitivity analyses were conducted in which we 1) included individuals with levels below the manufacturer’s 0.5 nM lower limit of detection (n = 45), and 2) excluded individuals with extremely low (but detectable) peak thrombin generation values of ≤ 4 nM (n = 19). In both instances, results were virtually identical to those inclusive of the entire cohort.
Discussion
In this prospective study which combined data from two independent cohorts, elevated basal peak thrombin generation was associated with an increased risk of subsequent VTE. In the combined LITE data, participants with peak thrombin generation values above the median were at 74% greater risk of VTE, as compared to those in the lowest quartile. In ARIC, the associations were more apparent for idiopathic VTE than for secondary VTE, suggesting a possible genetic basis for the association. Peak thrombin generation was positively associated with some other hemostatic factors related to VTE risk, and the association of peak thrombin generation with VTE was partly attenuated after accounting for levels of some hemostatic factors.
The relation of thrombin generation to first DVT has been explored in the LETS case-control study[12]. The LETS investigators reported that participants with high levels of ETP (90th percentile measured in controls) were at increased risk of incident DVT, with associations being stronger for idiopathic DVT. However, a dose-response relation was not demonstrated when ETP was modeled as quartiles, and no associations were observed between peak thrombin generation and incident DVT. It is unclear why our results were more robust than LETS, though methodological differences may provide some explanation. While similar, the assays used to measure peak thrombin generation in these studies were not identical; LETS used the Thrombinoscope™ assay[7] (Synapse, Maastricht, Netherlands), while LITE used the Technothrombin® TGA assay (Technoclone, Vienna, Austria). Also, LITE enrolled participants and collected plasma samples before the onset of VTE, while in LETS case participants were sampled after their first VTE and at least three months after the discontinuation of anticoagulant therapy[12]. Blood storage duration also varied between studies. Most LITE findings for other hemostatic factors have been similar to LETS findings, but these design differences might still be a partial explanation for the different findings.
Elevated peak thrombin generation interacted synergistically with both factor V Leiden and low aPTT. Participants with both elevated peak thrombin generation and factor V Leiden were at greatly increased risk of VTE (OR: 6.93). Those with low aPTT and elevated peak thrombin generation had a two-fold greater VTE risk. Screening for these risk factor combinations may be clinically useful in identifying individuals at greatest risk of VTE.
In ARIC and CHS, demographic variables, BMI, and genetic VTE risk factors were unrelated to peak thrombin generation levels. Published data evaluating the relation of thrombin generation to these variables has been inconclusive. Most[8, 26, 27], but not all[9, 10], prior studies have reported a positive association with age. Sex has generally been unrelated[8, 10, 12], though one study found higher peak thrombin generation in females[26]. The Factor V Leiden mutation had no relation to thrombin generation studies using assays similar to the one used here[8, 10, 12], but a positive relation in another, utilizing an assay measuring the effect of thrombomodulin on thrombin generation [28]. Likewise, relations of thrombin generation with the prothrombin mutation have been mixed, with reports showing both a positive relation[10, 12], and no relation[8, 28]. The absence of an association of peak thrombin generation with BMI, race, or ABO blood group has not previously been reported. It is difficult to compare studies since assay methodology differences, such as presence or absence of thrombomodulin or activated protein C, and phospholipid or tissue factor concentration, likely explain differences in findings. Any clinical application of peak thrombin generation testing requires assay standardization across methods and further clinical research data comparing different assays for clinical utility.
Thrombin generation plays a central role in hemostasis and thrombosis, as the entire coagulation system is engaged in its generation and subsequent inactivation[29]. Consistent with published findings[26, 30], in ARIC and CHS peak thrombin generation levels were positively correlated with levels of other hemostatic factors. Specifically, in ARIC there was a positive association between peak thrombin generation levels and factor VIII, and vWF, and an inverse association with the aPTT, while in CHS peak thrombin generation was positively related to concentrations of factors VII, VIII, IX, and X.
Given the shared pathway of hemostatic factors and thrombin generation, we explored whether peak thrombin generation was predictive of VTE risk, independent of hemostatic factors related to thrombosis. In LITE, factor VIII and D-dimer were identified statistically as potential “mediators” of the association between peak thrombin generation and VTE. Factor VIII is activated by thrombin[3], while D-dimer is a marker of fibrin formation; both are VTE risk factors[31–38]. Levels of these “mediators” only partly explained associations of peak thrombin generation with VTE, thereby suggesting that peak thrombin generation measurement may add independent information to current thrombotic risk assessment. Findings from two recent VTE recurrence cohorts also reported that thrombin generation and D-Dimer were independently associated with risk of recurrence[10, 11]. Further, the odds of VTE were qualitatively elevated among LITE participants with high peak thrombin generation in conjunction with high factor VIII or D-dimer, as compared to those with a single elevated hemostatic factor. This, too, indicates that thrombin generation assessment may provide complementary clinical information.
The peak thrombin generation distributions in ARIC and CHS were different enough that we were reluctant to combine the cohorts using absolute thrombin generation values. The amount of missing data also differed between cohorts. Nevertheless, as hypothesized, peak thrombin generation was positively associated with VTE incidence in both cohorts, and in the data combined using relative values. Thus, while the absolute levels of peak thrombin generation in LITE may be questioned, peak thrombin generation was moderately effective in rank-ordering participants according to future VTE risk.
While the ARIC and CHS study methods were very similar, minor differences in blood collection, processing, or storage may have influenced thrombin generation levels. Peak thrombin generation levels may also have been influenced by prior thawing and refreezing of the samples, or the long duration of storage at −70°C (up to 20 years). Any impact of pre-analytical or analytical factors for this assay on our results, however, would presumably be distributed comparably in cases and controls, and would most likely weaken associations between peak thrombin generation and VTE, thereby biasing our results toward the null hypothesis. This suggests that the risk estimates we observed are underestimates of the true risk of VTE associated with peak thrombin generation levels.
Given the novelty of this assay, little has been published regarding how factors such as storage and processing may affect values. Our differing mean values between cohorts is not without precedent. A review of published clinical studies that measured thrombin generation indicated large variability in results, likely due to assay differences, making comparisons among studies difficult[39]. It has been suggested that between-study variability may be improved through normalization via use of reference plasma, by standardizing protocols and using standard reagents[39, 40]. If clinical applications are considered, these issues require resolution.
There are limitations of this study, aside from those already discussed pertaining assay factors. Inclusion as a case in this study required a clinical diagnosis of VTE. Cases of VTE that were asymptomatic or not clinically recognized would not have been detected. Likewise, some cases of fatal pulmonary embolism may have been missed. Symptomatic DVT may also have been missed if it was treated in an outpatient setting, although this would have been rare during the time-period of this study[41]. Strengths of this study include the prospective investigation of VTE with assessment of peak thrombin generation before VTE onset, systematic case-validation, relatively large sample size and number of hemostatic factors assessed, and the population-based sample from a wide geographic distribution in the United States. Further, this study is unique in its exploration of whether the VTE-peak thrombin generation association was independent of other hemostatic factors, and its assessment of additive interaction.
To conclude, in these prospective LITE data elevated basal peak thrombin generation was associated with a 74% increased risk of VTE. While additional research is needed, the observation that the association of peak thrombin generation was only partly explained by other measured hemostatic factors is intriguing, and suggests that thrombin generation assessment may have clinical utility in stratifying individuals according to their risk of incident VTE. Factor V Leiden and low aPTT were found to interact synergistically with elevated peak thrombin generation. Patients with relevant combinations of these adverse risk factors may be worthwhile targets for intervention. Overall, our findings on risk of first VTE are consistent with previous studies of recurrent VTE[8, 9], which reported that thrombin generation potential was predictive of future thrombosis.
Acknowledgments
The LITE study was funded by National Heart, Lung, and Blood Institute (NHLBI) grant R01 HL59367. The ARIC study is carried out as a collaborative study supported by NHLBI contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. CHS was funded by NHLBI contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, NHLBI grant U01 HL080295, with additional contribution from the National Institute of Neurological Disorders and Stroke. PLL was supported as a predoctoral fellow on NHLBI training grant T32 HL07779. The authors thank the staff and participants of the ARIC and CHS studies for their important contributions. We have no conflicts to declare.
References
- 1.White RH. The Epidemiology of Venous Thromboembolism. Circulation. 2003;107(I):4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 2.Crowther MGJS. Venous Thromboembolism: Chapter 128. In: Hoffman RBE, Shattil S, Furie B, Cohen H, editors. Hematology: Basic Principles and Practices. 4th edn. London: Churchill Livingstone; 2004. [Google Scholar]
- 3.Mann KG. Thrombin Formation. Chest. 2003;124:4S–10S. doi: 10.1378/chest.124.3_suppl.4s. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane RG, Biggs R. A thrombin generation test; the application in haemophilia and thrombocytopenia. J Clin Pathol. 1953;6:3–8. doi: 10.1136/jcp.6.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitney WR, Dacie JV. A simple method of studying the generation of thrombin in recalcified plasma; application in the investigation of haemophilia. J Clin Pathol. 1953;6:9–14. doi: 10.1136/jcp.6.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Beguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 7.Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost. 2006;96:553–561. [PubMed] [Google Scholar]
- 8.Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of Patients at Low Risk for Recurrent Venous Thromboembolism by Measuring Thrombin Generation. JAMA. 2006;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 9.Tripodi A, Legnani C, Chantarangkul V, Cosmi B, Palareti G, Mannucci PM. High thrombin generation measured in the presence of thrombomodulin is associated with an increased risk of recurrent venous thromboembolism. J Thromb Haemost. 2008;6:1327–1333. doi: 10.1111/j.1538-7836.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 10.Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clin Chem. 2008;54:2042–2048. doi: 10.1373/clinchem.2008.112243. [DOI] [PubMed] [Google Scholar]
- 11.Besser M, Baglin C, Luddington R, van Hylckama Vlieg A, Baglin T. High rate of unprovoked recurrent venous thrombosis is associated with high thrombin-generating potential in a prospective cohort study. J Thromb Haemost. 2008;6:1720–1725. doi: 10.1111/j.1538-7836.2008.03117.x. [DOI] [PubMed] [Google Scholar]
- 12.van Hylckama Vlieg A, Christiansen SC, Luddington R, Cannegieter SC, Rosendaal FR, Baglin TP. Elevated endogenous thrombin potential is associated with an increased risk of a first deep venous thrombosis but not with the risk of recurrence. Br J Haematol. 2007;138:769–774. doi: 10.1111/j.1365-2141.2007.06738.x. [DOI] [PubMed] [Google Scholar]
- 13.The Aric I. The Atherosclerosis Risk in Communities (ARIC) Study: Design and Objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. The American Journal of Medicine. 2004;117:19. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 18.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study--I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61:15–19. [PubMed] [Google Scholar]
- 19.Chambless LE, McMahon R, Finch A, Sorlie P, Heiss G, Lyles R, Wu KK. ARIC hemostasis study--III. Quality control. Atherosclerosis Risk in Communities. Thromb Haemost. 1993;70:588–594. [PubMed] [Google Scholar]
- 20.Cushman M, Psaty BM, Macy E, Bovill EG, Cornell ES, Kuller LH, Tracy RP. Correlates of thrombin markers in an elderly cohort free of clinical cardiovascular disease. Arterioscler Thromb Vasc Biol. 1996;16:1163–1169. doi: 10.1161/01.atv.16.9.1163. [DOI] [PubMed] [Google Scholar]
- 21.Folsom AR, Cushman M, Tsai MY, Aleksic N, Heckbert SR, Boland LL, Tsai AW, Yanez ND, Rosamond WD. A prospective study of venous thromboembolism in relation to factor V Leiden and related factors. Blood. 2002;99:2720–2725. doi: 10.1182/blood.v99.8.2720. [DOI] [PubMed] [Google Scholar]
- 22.Folsom AR, Cushman M, Tsai MY, Heckbert SR, Aleksic N. Prospective study of the G20210A polymorphism in the prothrombin gene, plasma prothrombin concentration, and incidence of venous thromboembolism. American Journal Of Hematology. 2002;71:285–290. doi: 10.1002/ajh.10229. [DOI] [PubMed] [Google Scholar]
- 23.Ohira T, Cushman M, Tsai MY, Zhang Y, Heckbert SR, Zakai NA, Rosamond WD, Folsom AR. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) J Thromb Haemost. 2007;5:1455–1461. doi: 10.1111/j.1538-7836.2007.02579.x. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz SW, Carlucci C, Chambless LE, Rosamond WD. Synergism between smoking and vital exhaustion in the risk of ischemic stroke: evidence from the ARIC study. Ann Epidemiol. 2004;14:416–424. doi: 10.1016/j.annepidem.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Assmann SF, Hosmer DW, Lemeshow S, Mundt KA. Confidence intervals for measures of interaction. Epidemiology. 1996;7:286–290. doi: 10.1097/00001648-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Dielis AW, Castoldi E, Spronk HM, van Oerle R, Hamulyak K, Ten Cate H, Rosing J. Coagulation factors and the protein C system as determinants of thrombin generation in a normal population. J Thromb Haemost. 2008;6:125–131. doi: 10.1111/j.1538-7836.2007.02824.x. [DOI] [PubMed] [Google Scholar]
- 27.Haidl H, Cimenti C, Leschnik B, Zach D, Muntean W. Age-dependency of thrombin generation measured by means of calibrated automated thrombography (CAT) Thromb Haemost. 2006;95:772–775. [PubMed] [Google Scholar]
- 28.Couturaud F, Duchemin J, Leroyer C, Delahousse B, Abgrall JF, Mottier D A pilot study. Thrombin generation in first-degree relatives of patients with venous thromboembolism who have factor V Leiden. A pilot study. Thromb Haemost. 2008;99:223–228. doi: 10.1160/TH07-08-0515. [DOI] [PubMed] [Google Scholar]
- 29.Hemker HC, Beguin S. Phenotyping the clotting system. Thromb Haemost. 2000;84:747–751. [PubMed] [Google Scholar]
- 30.Duchemin J, Pan-Petesch B, Arnaud B, Blouch MT, Abgrall JF. Influence of coagulation factors and tissue factor concentration on the thrombin generation test in plasma. Thromb Haemost. 2008;99:767–773. doi: 10.1160/TH07-09-0581. [DOI] [PubMed] [Google Scholar]
- 31.Nossent AY, Eikenboom JC, Bertina RM. Plasma coagulation factor levels in venous thrombosis. Semin Hematol. 2007;44:77–84. doi: 10.1053/j.seminhematol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Tripodi A. Levels of coagulation factors and venous thromboembolism. Haematologica. 2003;88:705–711. [PubMed] [Google Scholar]
- 33.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, Folsom AR. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) The American Journal of Medicine. 2002;113:636. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 34.Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, Schneider B, Weltermann A, Speiser W, Lechner K, Eichinger S. High Plasma Levels of Factor VIII and the Risk of Recurrent Venous Thromboembolism. N Engl J Med. 2000;343:457–462. doi: 10.1056/NEJM200008173430702. [DOI] [PubMed] [Google Scholar]
- 35.Andreescu AC, Cushman M, Rosendaal FR. D-dimer as a risk factor for deep vein thrombosis: the Leiden Thrombophilia Study. Thromb Haemost. 2002;87:47–51. [PubMed] [Google Scholar]
- 36.Lowe G, Woodward M, Vessey M, Rumley A, Gough P, Daly E. Thrombotic variables and risk of idiopathic venous thromboembolism in women aged 45–64 years. Relationships to hormone replacement therapy. Thromb Haemost. 2000;83:530–535. [PubMed] [Google Scholar]
- 37.Palareti G, Legnani C, Cosmi B, Guazzaloca G, Pancani C, Coccheri S. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost. 2002;87:7–12. [PubMed] [Google Scholar]
- 38.Cushman M, Folsom AR, Wang L, Aleksic N, Rosamond WD, Tracy RP, Heckbert SR. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101:1243–1248. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- 39.Baglin T. The measurement and application of thrombin generation. Br J Haematol. 2005;130:653–661. doi: 10.1111/j.1365-2141.2005.05612.x. [DOI] [PubMed] [Google Scholar]
- 40.Dargaud Y, Luddington R, Gray E, Negrier C, Lecompte T, Petros S, Hogwood J, Bordet JC, Regnault V, Siegemund A, Baglin T. Effect of standardization and normalization on imprecision of calibrated automated thrombography: an international multicentre study. Br J Haematol. 2007;139:303–309. doi: 10.1111/j.1365-2141.2007.06785.x. [DOI] [PubMed] [Google Scholar]
- 41.de Lissovoy G. Economic issues in the treatment and prevention of deep vein thrombosis from a managed care perspective. Am J Manag Care. 2001;7:S535–S538. discussion S8–44. [PubMed] [Google Scholar]