Abstract
Nonmyeloablative conditioning is less toxic and results in initial establishment of mixed hematopoietic T cell chimerism for up to half a year with prolonged presence of host T cell immunity. In this study, we examined whether this translates into differences in the risks and/or severity of cytomegalovirus (CMV) infection and disease. We analyzed data from 537 nonmyeloablative (NM-HCT) and contemporaneous 2489 myeloablative hematopoietic cell transplant (M-HCT) recipients. In CMV seropositive recipients, no difference in the overall hazards of CMV infection at any level [adjusted hazard ratio (adj. HR) 0.9, 95% confidence interval (95%CI): 0.7-1.0, P=0.14] was noted; however, NM-HCT was associated with a lower risk of high-grade CMV infection (adj. HR 0.7, 95%CI: 0.5-0.9, P=0.02). CMV disease rates were similar between the groups during the first 100 days after HCT but NM-HCT recipients had an increased risk of late CMV disease (adj. HR 2.0, 95% CI 1.2-3.4). The increased risk of late CMV disease after NM-HCT was pronounced during the earlier years of the study period but not detectable in more recent years. Contrary to earlier reports, survival following CMV disease was not reduced after NM-HCT when compared to M-HCT recipients. These results suggest that residual host cells after NM-HCT reduce progression to higher CMV viral load in NM-HCT recipients; however, this effect does not appear to protect against serious complications of CMV. Therefore, CMV prevention strategies in NM-HCT recipients should be similar to those used in M-HCT recipients.
Keywords: nonmyeloablative allogeneic hematopoietic stem cell transplantation, CMV infection, CMV diseases
Introduction
Nonmyeloablative HCT (NM-HCT) is now widely used in patients with hematological and non-hematological malignancies who are ineligible for myeloablative HCT (M-HCT) due to advanced age or comorbidities (1-3). NM-HCT includes reduced or minimally intensive conditioning therapy before transplantation combined with more intensive immunosuppression after transplantation. The nonmyeloablative regimen used in Seattle consists of low-dose total body irradiation (TBI) 2 Gy with or without fludarabine followed by cyclosporine (CSP) or tacrolimus and mycophenolate mofetil (MMF) as post-grafting immunosuppression (3-9). This regimen causes minimal toxicity and results in initial establishment of mixed host/donor T cell chimerism for up to approximately 6 months. Thus, risk or severity of viral infections, such as cytomegalovirus (CMV) infection, may be reduced.
We initially reported that the time of CMV disease onset was delayed after HLA matched related NM-HCT, supporting the hypothesis that extended presence of host memory immune responses after NM-HCT might play a role in protection against early CMV infection (10-12). We also reported that the risk of CMV disease was similar after NM-HCT from HLA-matched-unrelated and related donors (13). Our study also suggested that CMV disease might be associated with a lower mortality in NM-HCT recipients (10). These studies were done early after the technique was introduced, which limited the power of statistical analyses due to small sample sizes.
This report examines the incidence, risk, and outcome of CMV infection in a large cohort of recipients undergoing NM-HCT and compared the outcomes with those of contemporaneous M-HCT recipients.
Patients and Methods
This retrospective analysis was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA). Informed consent was provided according to the Declaration of Helsinki.
Patients
We compared outcomes in 537 consecutive patients who received NM-HCT between December 1997 and December 2005 at the FHCRC and 2489 patients who received M-HCT between January 1995 and December 2005 at the FHCRC (Table 1). Recipient and donor age, proportions of a prior transplant history, peripheral stem cell source, CMV high-risk patients and MMF use as a post-grafting immune suppressant were significantly higher in NM-HCT compared to M-HCT. NM-HCT became more common in the later years and no T-depletion regimen was used in NM-HCT.
Table1.
Characteristics of the Study Cohort
| M-HCT (n=2489 ) | NM-HCT (n=537 ) | ||
|---|---|---|---|
| Variable | Number (%) | Number (%) | p-value |
| Median (range) age,yrs | |||
| Patient | 39.8 (0.5-67.0) | 54.2 (0.5-74.5) | <0.0001 |
| Donor | 38.2 (0.7-81.7) | 42.5 (1.3-83.3) | <0.0001 |
| Gender | |||
| Male | 1422 (57.1) | 334 (62.2) | |
| Female | 1067 (42.9) | 203 (38.6) | 0.03 |
| Donor Gender | |||
| Male | 1399 (56.2) | 284 (52.9) | |
| Female | 1087 (43.7) | 253 (47.1) | 0.26 |
| Recipient Race | |||
| Caucasian | 2015 (81.0) | 470 (87.5) | |
| Other/unknown | 474 (19.0) | 67 (12.5) | <0.001 |
| Donor Race | |||
| Caucasian | 1457 (58.5) | 269 (50.1) | |
| Other/unknown | 1032 (41.5) | 268 (49.9) | <0.001 |
| Year of Transplantation | |||
| 1995-1997 | 834 (33.5) | 1 (0.2) | |
| 1998-2000 | 716 (28.8) | 116 (21.6) | |
| 2001-2003 | 578 (23.2) | 225 (41.9) | |
| 2004-2005 | 361 (14.5) | 195 (36.3) | <0.0001 |
| Prior Transplant (auto and/or allo) | |||
| Yes | 74 (3.0) | 202 (37.6) | |
| No | 2415 (97.0) | 335 (62.4) | <0.0001 |
| Source of stem cell | |||
| BM | 1431 (57.5) | 49 (9.1) | |
| PBSC | 1015 (40.8) | 487 (90.7) | |
| Cord | 43 (1.7) | 1 (0.2) | <0.0001 |
| HLA Matching | |||
| Matched related | 1017 (42.0) | 221 (44.0) | |
| Mismatched related/Unrelated | 1404 (56.4) | 281 (52.3) | 0.28 |
| CMV seropositive | |||
| Yes | 1260 (50.6) | 311 (57.9) | |
| No | 1228 (49.3) | 225 (41.9) | <0.01 |
| Donor CMV seropositive | |||
| Yes | 984 (39.5) | 230 (42.8) | |
| No | 1503 (60.4) | 307 (57.2) | 0.30 |
| CMV Risk | |||
| Low | 894 (35.9) | 152 (28.3) | |
| Intermediate | 334 (13.4) | 73 (13.6) | |
| High | 1260 (50.6) | 311 (57.9) | <0.01 |
| Disease Diagnosis | |||
| AA | 67 (2.7) | 2 (0.4) | |
| ALL | 346 (13.9) | 21 (3.9) | |
| AML | 702 (28.2) | 143 (26.6) | |
| CLL | 18 (0.7) | 40 (7.5) | |
| CML | 669 (26.9) | 18 (3.4) | |
| HL | 14 (0.6) | 46 (8.6) | |
| MDS | 465 (18.7) | 41 (7.6) | |
| MM | 38 (1.5) | 83 (15.5) | |
| NHL | 106 (4.3) | 98 (18.3) | |
| Congenital disorders | 19 (0.8) | 16 (3.0) | |
| Other | 44 (1.8) | 29 (5.4) | <0.0001 |
| T-cell Depletion Regimen | |||
| Yes | 270 (10.9) | 0 (0.0) | |
| No | 2219 (89.2) | 537 (100.0) | <0.0001 |
| Acute GVHD Prophylaxis | |||
| Calcineurin inhibitor only | 27 (1.1) | 17 (3.2) | |
| Calcineurin inhibitor +MMF | 66 (2.7) | 495 (92.2) | |
| Calcineurin inhibitor +MTX | 2294 (92.2) | 5 (0.9) | |
| Other | 101 (4.1) | 20 (3.7) | <0.0001 |
AA indicates aplastic anemia, ALL: acute lymphocytic leukemia; AML: acute myeloid leukemia; CLL: chronic lymphocytic leukemia; CML: chronic myeloid leukemia; HL: Hodgkin lymphoma; MDS: myelodysplastic syndrome; MM: multiple myeloma; NHL: non-Hodgkin lymphoma; CMV: cytomegalovirus; BM: bone marrow; PBSC: peripheral blood stem cell; T-cell depletion regimens are those containing antithymocyte globulin or anti-CD3 antibody, BC3; acute GVH: acute graft-versus-host disease and MMF: mycophenolate mofetil and MTX: methotrexate. P-values from chi-square test, Fisher’s exact test or Wilcoxon rank-sum test as appropriate.
The CMV risk was stratified into three groups: low (recipient negative and donor negative); intermediate (recipient negative and donor positive); and high (recipient positive and either donor negative or positive) based on recipient and donor CMV serostatus before HCT.
Preparative conditioning regimens and sources for HCT
Three hundred forty-eight (64.8%) NM-HCT recipients received fludarabine (30 mg /m2/day for 3 consecutive days) and low-dose TBI (2 Gy, day 0), while 90 patients (16.8%) received low-dose TBI (2 Gy, day 0) alone. Patients in the M-HCT group received different types of conditioning. The most common regimens consisted of cyclophosphamide (60 mg/kg/day for 2 consecutive days) followed by TBI (12 - 13.2 Gy) or busulfan (4 mg/kg/day for 4 consecutive days) followed by cyclophosphamide (60 mg/kg/d for 2 consecutive days) in 805 (32.3%) and 779 (31.3%) patients, respectively (data not shown). The distribution of stem cell sources used in NM-HCT and M-HCT is shown in Table 1.
Prophylaxis and diagnosis of graft-versus-host disease (GVHD)
GVHD prophylaxis regimens are shown in Table 1. NM-HCT patients most commonly received CSP and MMF orally as immune suppressants post HCT (n= 457, 85.1%) (data not shown). MMF was administered 15 mg/kg orally twice a day from day 0 to day 27 and discontinued for the HLA-match related NM-HCTs and MMF 15 mg/kg orally two or three times a day from day 0 to day 40 with a taper to day 96 for the unrelated NM-HCTs were given. For the single HLA-antigen and combined HLA-antigen and allele mismatched NM-HCTs, 15 mg/kg MMF was given 3 times a day and was tapered at day 100 over 2 months (5-9).
M-HCT patients most commonly received the combination of CSP and MTX (n=2122, 85.3%) (data not shown). CSP was given at a dose of 1.5 mg/kg intravenously twice a day or 6.25 mg/kg orally twice a day. MTX was administered intravenously at a dose of 15 mg/m2 on day 1, and 10 mg/m2 on day 3, 6, and 11. Diagnoses of acute GVHD or chronic GVHD were performed according to established criteria (14, 15).
Infection surveillance and pre-emptive therapy against CMV
CMV surveillance including polymerase chain reaction (PCR), pp65 antigenemia (AG) and blood culture was performed on a weekly basis until day 100. After day 100, surveillance and preemptive therapy were recommended for CMV intermediate and high-risk patients on a weekly or biweekly basis. Patients were monitored for the development of CMV infection and diseases until day 365. CMV pp65 AG was quantified as the average number of positive cells per 200,000 peripheral blood leukocytes and quantitative detection of CMV DNA in plasma by PCR was performed as described (16).
Ganciclovir (GCV) treatment was started when CMV AG/PCR became positive during the first 100 days after HCT. All patients with CMV AG at any level received GCV (5 mg/kg IV twice daily) for 7 to 14 days as induction therapy, followed by maintenance therapy with a half dose of GCV (5 mg/kg IV daily) or valganciclovir 900 mg once a day orally until negative surveillance testing was detected or day 100. After day 100, pre-emptive therapy consisting of IV GCV or valganciclovir induction, followed by maintenance therapy, was recommended when CMV AG became positive or when PCR was greater than 1000 copies/mL. GCV was substituted with foscarnet in patients with neutropenia.
Between January 1995 and November 1998, no patients received acyclovir for varicella zoster virus (VZV) prevention; but herpes simplex virus (HSV) positive recipients were given acyclovir, 250 mg/m2 twice daily from day -7 until engraftment and resolution of mucositis. From November 1998 until May 2002, VZV seropositive HCT recipients received prophylaxis against VZV (acyclovir 250 mg/m2 intravenously followed by 800 mg orally or valacyclovir 500 mg orally, all drugs given twice per day for 1 year after transplantation (valacyclovir was preferred for patients who received more than 0.5 mg/kg per day of steroids). HCT patients undergoing transplantation after May 2002 received the same regimen until 1 year after transplantation. In patients who were still receiving inmmunosuppression at one year, acyclovir/valacyclovir prophylaxis was continued until 6 months after discontinuation of all immunosuppression (17).
Definitions of CMV infection and disease
CMV AG was diagnosed by blood pp65 antigen testing, CMV viremia by positive blood culture or shell vial centrifugation culture (10), and detection of CMV DNA by PCR (16). CMV disease was defined by established criteria (18).
Study endpoints
In the present study, we evaluated the following 6 endpoints: 1) CMV infection (any CMV AG/DNA detection) by day 100; 2) high-grade CMV infection (CMV AG > 10/200,000 peripheral blood leukocytes or PCR > 1000 copies/ml by day 100; 3) CMV viremia (culture) by day 100; 4) CMV disease by day 100 and 1 year; 5) late CMV disease which occurred after day 100 after HCT; 6) survival after CMV disease.
Statistical analysis
Characteristics of NM-HCT and M-HCT patients were summarized using frequency counts and percentages for categorical variables and medians and ranges for continuous variables.
The cumulative incidences of CMV infection, CMV high-grade infection, CMV disease and late CMV disease were compared between NM-HCT and M-HCT groups, with subsequent transplantation or death considered competing risks. Cumulative incidence curves for these endpoints also were stratified by CMV risk groups defined by donor and recipient seropositivity and by transplant year groupings between 1995 and 2005. The probability of survival was estimated by the Kaplan-Meier method. Hazards were compared using log-rank tests. The median times to onset of CMV disease were compared by the Wilcoxon rank sum test.
Univariate and multivariate Cox regression models were used to estimate hazard ratios and 95% confidence intervals (95% CI). Cox regression analyses for CMV infection, high-grade CMV infection, CMV viremia and CMV disease were performed in the CMV high-risk group.CMV viremia was analyzed in just a slightly smaller subset because CMV viremia was tested only through 12/2003. Covariates included recipient/donor age and sex, recipient/donor race, donor CMV serostatus, sex mismatch, HLA disparity, donor relationship, intensity of conditioning, stem cell source, HSV type I, II serostatus, T cell depleted conditioning, transplantation year, disease risk, GVHD prophylaxis, acute GVHD and chronic GVHD. Additionally, CMV risk group and maximum values of CMV AG and PCR testing were evaluated as risk factors for CMV disease.
Acute and chronic GVHD, CMV AG and PCR testing were analyzed as time-dependent variables. Variables less than P=0.05 in the univariate models were candidates for the multivariate models. Nonmyeloablative vs. myeloablative conditioning was forced into multivariate models of all endpoints.
Results
CMV Infection and Viremia
Low and intermediate-risk group (D−/R−, D+/R−)
The cumulative incidences of CMV infection by day 100 were similar between NM-HCT and M-HCT in CMV low and intermediate-risk groups. High-grade CMV infection was very rare, particularly in CMV low-risk groups and was not noted in any low-risk NM-HCT patient (Table 2)
Table 2.
The Incidences of CMV infection and disease
| Endpoints | Incidence in M-HCT |
Incidence in NM-HCT |
P value |
|---|---|---|---|
| CMV infection by day 100 | |||
| Low-risk | 0.03 | 0.02 | .64 |
| Intermediate-risk | 0.15 | 0.21 | .27 |
| High-risk | 0.66 | 0.62 | .08 |
| High-grade CMV infection by day 100 | |||
| Low-risk | 0.01 | 0.00 | .33 |
| Intermediate-risk | 0.05 | 0.09 | .20 |
| High-risk | 0.25 | 0.14 | <.0001 |
| CMV disease by day 100 | |||
| Low-risk | 0.01 | 0.01 | .52 |
| Intermediate-risk | 0.01 | 0.02 | .68 |
| High-risk | 0.10 | 0.07 | .11 |
| CMV disease by 1 year | |||
| Low-risk | 0.01 | 0.01 | .96 |
| Intermediate-risk | 0.03 | 0.03 | .95 |
| High-risk | 0.15 | 0.15 | .50 |
| Late CMV disease | |||
| Low-risk | 0.01 | 0.00 | .35 |
| Intermediate-risk | 0.02 | 0.04 | .68 |
| High-risk | 0.09 | 0.11 | .52 |
High-risk group (D−/R+, D+/R+)
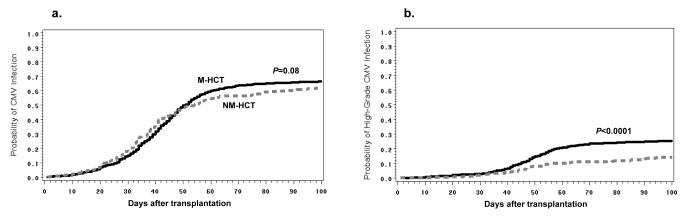
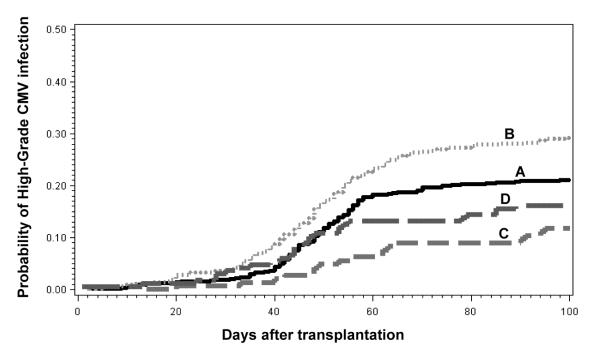
In CMV high-risk group (n=1571), there were trends towards lower CMV infection and high-grade CMV infection rates in NM-HCT (Table 2, Figure 1). When high viral load was analyzed stratified by HLA-matched related vs. unrelated/HLA-mismatched related donor status in the CMV high-risk group, the lower incidence of high-grade CMV infection in NM-HCT was seen both in the HLA-matched related and unrelated/HLA-mismatched related settings (Figure 2). Unrelated and HLA-mismatched donor status was associated with a somewhat higher cumulative incidence of high-grade CMV infection at day 100 than HLA-matched related donor status (Figure 2). This effect was seen in both M-HCT and NM-HCT recipients. In the CMV high-risk group, other factors associated with increased risks of any CMV infection were recipient age and acute GVHD (III or IV). Other factors associated with an increased risk of high-grade CMV infection were recipient race (other than Caucasian) and acute GVHD (Table 3).
Figure 1. Cumulative incidences of any and high-grade CMV infections in CMV high-risk patients.
The probabilities of a) any CMV infection and b) high-grade CMV infection (CMV AG > 10 cells per 200,000 PBL or CMV DNA > 1000 copies per mL of plasma) in CMV high-risk patients are displayed. The dashed line indicates nonmyeloablative hematopoietic cell transplant (NM-HCT) and the solid line indicates myeloablative hematopoietic cell transplant (M-HCT). P values were calculated by the log-rank test.
Figure 2. Cumulative incidences of CMV high-grade infection in CMV high-risk patients stratified by matched-related vs. unrelated/HLA-mismatched donor.
A) myeloablative HLA-matched related donor, B) myeloablative unrelated/ HLA-mismatched related donor, C) nonmyeloablative HLA-matched related donor, D) nonmyeloablative unrelated/ HLA-mismatched related donor.
Table 3.
Univariate and Multivarite Analyses of Risk Factors for CMV infection in CMV high-risk group
| CMV infection* |
CMV high-grade infection* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Factors | Univariate HR (95%CI) |
P | adj. HR (95%CI) |
P | Univariate HR (95%CI) |
P | adj. HR (95%CI) |
P |
| NM-HCT | 0.9 (0.7-1.0) | .08 | 0.9 (0.7-1.0) | .14 | 0.5 (0.4-0.7) | <.0001 | 0.7 (0.5-0.9) | .02 |
| Recipient age ≤41 yrs | 1.3 (1.1-1.4) | <.001 | 1.5 (1.3-1.7) | <.0001 | 0.9 (0.8-1.1) | .46 | - | - |
| Recipient Race (other than Caucasian) |
1.2 (1.0-1.4) | .01 | 1.1 (0.9-1.4) | .23 | 1.2 (1.0-1.6) | .08 | 1.4 (1.1-1.9) | <.01 |
| Donor CMV positive | 1.0 (0.9-1.1) | .56 | - | - | 0.7 (0.6-0.9) | .002 | 0.7 (0.6-0.9) | <.01 |
| Related Donor | 0.8 (0.7-0.9) | <.0001 | 0.9 (0.7-1.2) | .46 | 0.7 (0.5-0.8) | .0001 | 0.6 (0.4-1.0) | .04 |
| HSV1 positive (recipient) | 0.7 (0.5-0.8) | <.001 | 0.6 (0.5-0.8) | <.001 | 1.0 (0.7-1.6) | .92 | - | - |
| Acute GVHD (III or IV) | 1.5 (1.3-1.8) | <.0001 | 1.4 (1.2-1.6) | <.0001 | 2.2 (1.8-2.8) | <.0001 | 1.8 (1.4-2.3) | <.0001 |
| Year of transplant | ||||||||
| 1995-1997 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 1998-2000 | 0.8 (0.7-1.0) | .01 | 0.8 (0.7-1.0) | .01 | 0.7 (0.5-0.9) | .006 | 0.7 (0.5-0.9) | <.01 |
| 2001-2003 | 0.8 (0.7-1.0) | .02 | 0.9 (0.7-1.1) | .20 | 0.5 (0.4-0.7) | <.0001 | 0.6 (0.4-0.8) | <.001 |
| 2004-2005 | 0.8 (0.7-1.0) | .04 | 0.8 (0.6-1.0) | .05 | 0.4 (0.3-0.5) | <.0001 | 0.4 (0.3-0.6) | <.0001 |
adj. HR: adjusted hazard ratio; CI: confidence interval; NM-HCT: nonmyeloablative allogeneic hematopoietic stem cell transplantation; CMV: cytomegalovirus. HSV1: herpes simplex virus type1; GVHD: graft-versus-host disease. The factors which were statistically significant in either multivariate model of CMV infection or high-grade CMV infection, and NM-HCT are displayed.
Total sample size of CMV high-risk patients is 1571.
Among seropositive patients transplanted between 1995 and 2003 (n=1263), the incidence of CMV viremia (culture proven) by day 100 was significantly lower in NM-HCT compared with M-HCT (10% vs. 19%, P <0.001). However, in the multivariate model, the significance was not sustained. Other risk factors for CMV viremia were recipient race (other than Caucasian) (adj. HR 1.9, 95%CI: 1.2-3.0, P<0.01) and acute GVHD II to IV (adj. HR 3.4, 95%CI: 2.2-5.2, P<0.0001). CMV viremia was less common in the later years of the study period (2001-2003) compared to 1995-1997 (adj. HR 0.4, 95%CI: 0.6-0.6, P<0.0001) (data not shown).
Time to CMV Negativity after Start of Preemptive Therapy
As a surrogate marker for duration of anti-CMV treatment, we compared the duration from first positive to the first negative AG/PCR result between NM-HCT and M-HCT and found no difference in all patients [median (range):7 (0-13) vs.7 (0-50) days, respectively, P=0.98] or in patients at high risk for CMV (seropositive recipients) [median (range):7 (0-13) vs.7 (0-50) days, respectively, P=0.97]
CMV Disease and Survival after CMV Disease
Low and intermediate-risk group (D−/R−, D+/R−)
Among CMV low and intermediate-risk groups, there was no significant statistical difference in the risk for CMV disease by day 100 and by 1 year between NM-HCT and M-HCT (Table 2).
High-risk (D−/R+, D+/R+)
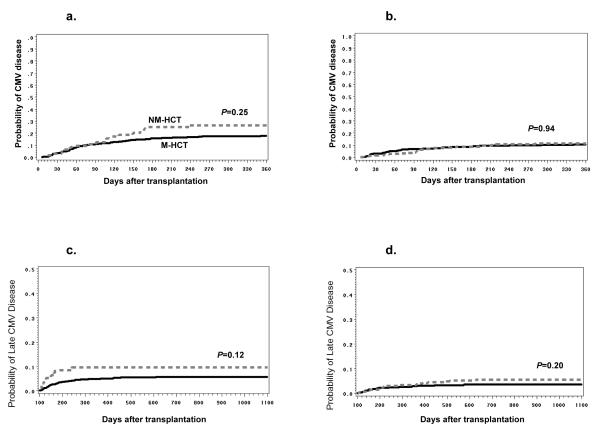
In the CMV high-risk group, the cumulative incidence of CMV disease by day 100 (but not by 1 year) tended to be less frequent in NM-HCT compared with M-HCT (Table 2, 4). A significant decline of CMV disease incidence was noted after 2001 compared to 1995-2000 (Figure 3a, b). The cumulative incidence for CMV disease in CMV high-risk NM-and M-HCT patients from 1995 to 2000 were 27% vs. 18% (P=0.25) compared to 12% vs. 11% (P=0.94) between 2001 and 2005 (Figure 3a, b). A significant delay in the onset of CMV disease was observed in NM-HCT in the high-risk CMV group [median day of onset; 106.5 (6.0-1273.0) vs. 69.5 days (6.0-1775.0) P=0.02]. Among NM-HCT, the cumulative incidences of CMV disease at 1 year between HLA-matched related and unrelated/HLA-mismatched related donors were very similar (15% vs. 14%, P=0.05).
Table 4.
Univariate and Multivariate Analyses of Risk Factors for CMV Disease in CMV high-risk group
| CMV infection* |
CMV high-grade infection* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Factors | Univariate HR (95%CI) |
P | adj. HR (95%CI) |
P | Univariate HR (95%CI) |
P | adj. HR (95%CI) |
P |
| NM-HCT | 0.9 (0.7-1.3) | .74 | 1.3 (0.9-1.9) | .12 | 1.2 (0.7-1.8) | .52 | 2.0 (1.2-3.4) | .01 |
| Donor sex (female) | 1.3 (1.0-1.6) | .08 | 1.4 (1.1-1.7) | .02 | 1.2 (0.8-1.8) | .31 | - | - |
| Unrelated/HLA-mismatched | 1.4 (1.1-1.8) | <.01 | 1.2 (0.7-2.1) | .44 | 2.5 (1.7-3.9) | <.0001 | 2.1 (1.4-3.3) | <.01 |
| HSV1 positive | 2.0 (1.0-3.9) | .04 | 2.3 (1.2-4.5) | .01 | 2.6 (0.8-8.5) | .12 | - | - |
| Acute GVHD (III or IV) | 2.7 (2.1-3.6) | <.0001 | 2.1 (1.6-2.7) | <.0001 | - | - | - | - |
| Chronic GVHD | 2.6 (1.8-3.8) | <.0001 | 2.1 (1.4-3.0) | <.0001 | - | - | - | - |
| Acute (III or IV) or Chronic GVHD |
- | - | - | - | 6.0 (2.6-13.6) | <.0001 | 4.1 (1.8-9.5) | <.01 |
| Year of transplant | ||||||||
| 1995-1997 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 1998-2000 | 1.2 (0.9-1.7) | .24 | 1.3 (0.9-1.8) | .14 | 0.7 (0.4-1.8) | .14 | 0.7 (0.4-1.1) | .12 |
| 2001-2003 | 0.7 (0.5-0.9) | .02 | 0.8 (0.5-1.4) | .44 | 0.5 (0.3-0.8) | <.01 | 0.4 (0.2-0.8) | <.01 |
| 2004-2005 | 0.6 (0.4-0.9) | .02 | 0.8 (0.5-1.3) | .31 | 0.6 (0.3-1.0) | .05 | 0.5 (0.3-0.9) | .03 |
| Max. CMV AG | ||||||||
| 0 | 1.0 | 1.0 | - | - | ||||
| >0-2/200,000 | 1.7 (1.1-2.5) | .01 | 1.7 (1.1-2.5) | .01 | - | - | - | - |
| >2-10/200,000 | 1.6 (1.0-2.6) | .05 | 1.4 (0.9-2.3) | .16 | - | - | - | - |
| >10/200,000 | 4.7 (3.3-6.8) | <.0001 | 3.7 (2.5-5.4) | <.0001 | - | - | - | - |
| Max. CMV PCR (copies/ml) | ||||||||
| 0 | 1.0 | 1.0 | - | - | ||||
| 0-1000 | 2.3 (1.4-3.8) | <.01 | 2.4 (1.4-4.1) | <.01 | - | - | - | - |
| >1000 | 2.4 (1.2-4.6) | .01 | 3.2 (1.6-6.5) | <.01 | - | - | - | - |
| Max. CMV AG>10 /PCR>1000 before day 100 (copies/ml) |
- | - | - | - | 2.8 (1.9-4.1) | <.0001 | 2.0 (1.4-3.0) | <.01 |
adj. HR: adjusted hazard ratio; CI: confidence interval; NM-HCT: nonmyeloablative allogeneic hematopoietic stem cell transplantation; CMV: cytomegalovirus. HSV1: herpes simplex virus type1; GVHD: graft-versus-host disease; AG: antigenemia; PCR: polymerase chain reaction. The factors which were statistically significant in either multivariate model of CMV disease or late CMV disease, and NM-HCT are displayed
Thel sample size of CMV high-risk patients at risk for CMV disease is 1571; the sample size of CMV high-risk patients who survived CMV-disease-free to day 100 and were at risk for late CMV disease is 1109.
Figure 3. Cumulative incidences of CMV disease in CMV high-risk patients and late CMV disease.
The probabilities of a) CMV disease from1995 to 2000, b) CMV disease from 2001 to 2005 in CMV high-risk patients, c) late CMV disease from1995 to 2000, and d) late CMV disease from 2000 to 2005 are displayed. The probability curves for late CMV were generated from all CMV high risk patients who survived beyond day 100 without underlying disease relapse. The dashed line indicates nonmyeloablative hematopoietic cell transplant (NM-HCT) and the solid line indicates myeloablative hematopoietic cell transplant (M-HCT). P values were calculated by the log-rank test.
Other risk factors associated with CMV disease in multivariate analysis were donor female sex, acute GVHD, chronic GVHD, HSV type I seropositivity, and positivity of CMV AG and/or PCR (Table 4).
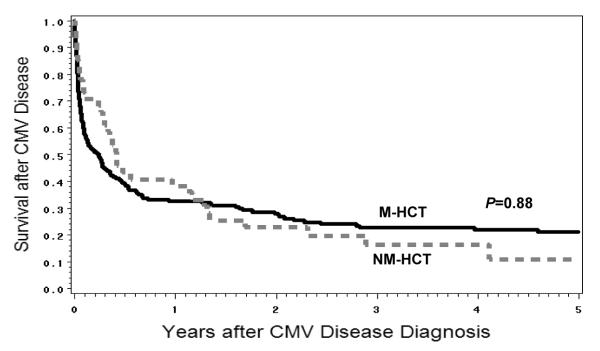
The probability of survival in CMV high-risk patients who developed CMV disease (n=226) was not significantly different between NM-HCT and M-HCT recipients (P=0.88) (Figure 4).
Figure 4. Survival after CMV disease in CMV high-risk patients.
The probability of survival after CMV disease in 226 high-risk patients who had CMV disease. The dashed line indicates nonmyeloablative hematopoietic cell transplant (NM-HCT) and the solid line indicates myeloablative hematopoietic cell transplant (M-HCT). P values were calculated by the log-rank test.
Late CMV Disease
Low and intermediate-risk group (D−/R−, D+/R−)
No significant statistical differences in incidences were observed between MN-HCT and M-HCT. Late CMV disease was not observed in any CMV low-risk NM-HCT patient (Table 2).
High-risk group (D−/R+, D+/R+)
No statistically significant differences in late CMV disease incidences were detected between MN-HCT and M-HCT in CMV high-risk group (Table 2).
Among all the risk groups, NM-HCT was significantly associated with late CMV disease after adjustment of multiple covariates (adj. HR 1.8, P=0.01) (Table 4). However, this was mainly driven by a high incidence of late CMV disease during the earlier years of the study period (Figure 3c, d). Additional factors for late CMV disease were: HLA-mismatch or unrelated donor, acute GVHD (III or IV) or chronic GVHD before day 100, and maximum CMV AG >10/ PCR > 1000 copies/ml before day 100. Furthermore, late CMV disease was less common in more recent years (2001-2003) compared to 1995-1997 (Table 4).
Secondary invasive bacterial and fungal infection after CMV infection
We compared the incidences of secondary bacterial infection before day 100 and fungal infections before 1 year after HCT between NM-HCT and M-HCT. There was no significant difference in risk of probable and definite invasive fungal infection between NM-HCT and NM-HCT in all CMV risk groups (p=0.77), nor in high-CMV-risk group (p=0.83).
Secondary invasive bacterial infections were less common in NM-HCT (23% vs. 28%, Chi square p-value <0.0001). There was a significant difference in hazard of bacterial infection between NM-HCT and M-HCT adjusted for CMV risk group (HR=0.6, 95% CI=0.5-0.8, P<0.0001); when the analysis was restricted to the CMV high risk group (seropositive recipients), a similar effect was seen (HR=0.7, 95% CI=0.5-0.9, P<0.01).
Discussion
We comprehensively examined risks and outcomes of CMV infection and disease in a large cohort of uniformly treated patients that provided the necessary power to analyze CMV endpoints in NM-HCT recipients. NM-HCT recipients had similar rates of CMV infection and disease compared to M-HCT, although a delayed timing of disease and lower maximum CMV viral loads were noted. Contrary to an earlier small study that showed a trend towards improved outcome of CMV disease in NM-HCT (10), the present study did not show evidence of such an effect.
In a previous study, we demonstrated that NM-HCT showed trends towards lower incidence of CMV infection pp65 antigenemia, CMV viremia, and CMV disease during the first 100 days after HCT. However, we did not show statistically significant differences in the incidence of these CMV events between NM-HCT and M-HCT, possibly due to the small sample size (10). In the present study, we were able to provide statistical evidence that the incidence of high CMV viral load in NM-HCT is lower compared with M-HCT. This effect was seen in both HLA-mismatched-related or unrelated HCT and HLA-matched-related HCT recipients (Figure 2). Similar to an earlier study, there was a trend towards a more profound reduction of high CMV load in HLA-matched related NM-HCT recipients than in HLA-mismatched related or unrelated NM-HCT recipients, but even with this large sample size, this did not reach statistical significance (Figure 2). We speculate that the strong immunosuppressants and/or the high incidence and severity of GVHD in HLA-mismatched related or unrelated HCT might somewhat diminish the protection from persisting host T cell immunity in NM-HCT. We confirmed that the onset of CMV disease was delayed, which resulted in a trend towards less CMV disease before day 100 in NM-HCT recipients (Table 2). Collectively, these data suggest that the residual CMV specific host memory cells had a limited or no effect on reactivation of CMV but contributed to preventing progression to higher levels of viral load, at least early after less intensive conditioning. This is consistent with laboratory studies that showed a persistence of host memory T cells in HLA-matched related NM-HCT recipients (11, 12). However, after complete donor chimerism has been achieved in NM-HCT recipients, the benefits of protection against CMV infection seem to disappear. Our previous studies of CMV immunity showed that, after day 100, there was no difference in CMV-specific T cell immunity between NM-HCT and M-HCT recipients (11, 12).
Somewhat surprisingly, more patients developed late CMV disease following NM-HCT compared to M-HCT. Further analysis suggested that the effect was driven by the earlier years of the study period (10) (Figure 3c). This was likely due to less virologic surveillance for late CMV infection and less use of late preemptive therapy, possibly due to the perception that infectious complications were less frequent and/or severe (10). Additionally, the prolonged MMF prophylaxis or treatment, particularly in the unrelated NM-HCT, possibly contributed to more frequent incidence of late CMV disease in NM-HCT than in M-HCT. In more recent years, there was no increased risk of late CMV disease in NM-HCT recipients (Figure 3d). Also, the overall incidence of late CMV disease declined in both NM and M-HCT recipients, likely due to extended monitoring of CMV by PCR and increased use of preemptive anti-CMV treatment beyond day 100.
Risk factors for late CMV disease seen in this study (Table 4) were consistent with earlier reports by our group and others (20, 21, 22).
Although we were unable to separate the effect of GVHD on the risk of CMV endpoints in our previous studies (10, 20), in the current study, both acute GVHD and chronic GVHD were statistically significant risk factors for early and late CMV disease in the current study. This was consistent with previous reports (21).
The strengths of this study were the large sample size permitting multivariate modeling, well defined and homogenous transplant protocols, highly standardized supportive care, CMV surveillance, and a comprehensive and standardized workup of BAL and biopsy specimens (including autopsy specimens). Limitations were the retrospective nature of the analysis and that co-medications could only be analyzed by protocol (as supposed to on a per-patient basis). Also, the data might not extend to different reduced-intensity protocols. Furthermore, in this study, the majority of the NM-HCT patients received peripheral blood stem cells (Table 1). Although we could not detect a significant effect of the stem cell on CMV outcomes in the multivariate models, NM-HCT and peripheral stem cell use are tightly linked so we cannot conclusively rule out an interaction between the two modalities (19).
In conclusion, this large study provided robust data on the risk of CMV infection and disease in recipients of NM-HCT. The study confirmed and added statistical strength to some of the earlier findings, including the delayed onset of CMV disease and a lower risk of progression to higher viral loads during the first 100 days. Of note, the earlier reported trend towards an improved outcome of CMV disease after NM-HCT could not be confirmed in this study. In addition, the study showed that the survival rate after CMV disease was still very unfavorable and emphasizes the need for improved prevention and treatment strategies for CMV. Maribavir, a novel antiviral agent (23) and immune enhancement strategies with CMV-specific T cells (24) or CMV vaccination (25) or preemptive strategies that combine virologic and immunologic monitoring may be options in this regard.
Acknowledgements
We thank Daniel Stachel, MD, for chart review, and Gary Schoch and Craig Silva for database services.
Grant support: This study was supported by the National Institutes of Health (CA 18029, CA15704, HL36444, CA78902). Hirohisa Nakamae was funded by the Graduate School of Medicine, Osaka City University, Osaka, Japan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablativetherapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 2).Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 3).McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 4).Feinstein L, Sandmaier B, Maloney D, et al. Nonmyeloablative hematopoietic cell transplantation. Replacing high-dose cytotoxic therapy by the graft-versus-tumor effect. Ann N Y Acad Sci. 2001;938:328–337. [PubMed] [Google Scholar]
- 5).Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 6).Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HSCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1609. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 7).Maloney DG, Molina AJ, Sahebi F, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 8).Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 9).Scott BL, Sandmaier BM, Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous\ leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 10).Junghanss C, Boeckh M, Carter RA, et al. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood. 2002;99:1978–1985. doi: 10.1182/blood.v99.6.1978. [DOI] [PubMed] [Google Scholar]
- 11).Maris M, Boeckh M, Storer B, et al. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol. 2003;31:941–952. doi: 10.1016/s0301-472x(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 12).Baron F, Storer B, Maris MB, et al. Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12:1176–1187. doi: 10.1016/j.bbmt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13).Junghanss C, Storb R, Maris MB, et al. Impact of unrelated donor status on the incidence and outcome of cytomegalovirus infections after non-myeloablative allogeneic stem cell transplantation. Br J Haematol. 2003;123:662–670. doi: 10.1046/j.1365-2141.2003.04671.x. [DOI] [PubMed] [Google Scholar]
- 14).Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 15).Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 16).Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004;42:1142–1148. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110:3071–3077. doi: 10.1182/blood-2007-03-077644. [DOI] [PubMed] [Google Scholar]
- 18).Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 19).Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97:3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 20).Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 21).Einsele H, Hebart H, Kauffmann-Schneider C, et al. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant. 2000;25:757–763. doi: 10.1038/sj.bmt.1702226. [DOI] [PubMed] [Google Scholar]
- 22).Ozdemir E, Saliba RM, Champlin RE, et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;40:125–136. doi: 10.1038/sj.bmt.1705699. [DOI] [PubMed] [Google Scholar]
- 23).Winston DJ, Young JA, Pullarkat V, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem-cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008;111(11):5403–5410. doi: 10.1182/blood-2007-11-121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 25).Wloch MK, Smith LR, Boutsaboualoy S, et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis. 2008;197:1634–1642. doi: 10.1086/588385. [DOI] [PMC free article] [PubMed] [Google Scholar]