Abstract
BCR-ABL plays an essential role in the pathogenesis of chronic myeloid leukemia (CML) and some cases of acute lymphocytic leukemia (ALL). Even though ABL kinase inhibitors have shown great promise in the treatment of CML, the persistence of residual disease and the occurrence of resistance have prompted investigations into the molecular effectors of BCR-ABL. Here we show that BCR-ABL stimulates the proteasome-dependent degradation of members of the Forkhead family of tumor suppressors in vitro, in an in vivo animal model, and in samples from patients with BCR-ABL-positive CML or ALL. As several downstream mediators of BCR-ABL are regulated by the proteasome degradation pathway, we also demonstrate that inhibition of this pathway, using bortezomib, causes regression of CML-like disease. Bortezomib treatment led to inhibition of BCR-ABL-induced suppression of FoxO proteins and their pro-apoptotic targets, TNF-Related Apoptosis Inducing Ligand (TRAIL) and BIM, thereby providing novel insights into the molecular effects of proteasome inhibitor therapy. We additionally show sensitivity of imatinib resistant BCR-ABL T315I cells to bortezomib. Our data delineates the involvement of FoxO proteins in BCR-ABL-induced evasion of apoptosis and provides evidence that bortezomib is a candidate therapeutic in the treatment of BCR-ABL-induced leukemia.
Keywords: BCR-ABL, Bim, Bortezomib, FoxO, Proteasome, TRAIL
INTRODUCTION
The BCR-ABL oncoprotein plays a central role in the pathogenesis of virtually all chronic myeloid leukemia (CML) and 15-30% of acute lymphoblastic leukemia (ALL) cases (1-3). A s a constitutively active tyrosine kinase, BCR-ABL induces the hyperactivation of various signaling pathways that promote cell growth and suppress apoptosis, ultimately resulting in leukemogenesis (2, 4). In recent years, there has been remarkable progress in the treatment of myeloproliferative diseases, especially with the development of the ABL kinase inhibitor, imatinib mesylate (Gleevec, STI-571) (5, 6).. Whereas clinical data from imatinib treatment appears promising, the development of resistance due to primary or acquired point mutations in BCR-ABL (7, 8) is a growing problem. Although highly potent kinase inhibitors, such as AMN107 (9) and BMS-354825 (10), have been recently developed to target imatinib resistance, these compounds do not inhibit all possible imatinib-resistant mutants of BCR-ABL (i.e., a commonly occurring threonine-to-isoleucine mutation at residue 315 (T315I), located within the kinase domain of BCR-ABL). Alternative strategies, such as Aurora Kinase inhibitor, VX680, which also targets ABL, as well as combination therapies using chemotherapeutic agents and imatinib have shown some success in the treatment of the T315I mutant (11, 12). However, since these strategies also target the ABL kinase, a genetic pressure may promote the emergence of additional resistant mutants. Therefore, the identification of novel strategies for the treatment of leukemia are of high priority (13).
As an alternative to targeting the ABL kinase, a promising approach involves the inhibition of downstream cellular pathways critical for BCR-ABL-mediated leukemogenesis. The activation of the PI3-K/Akt pathway plays a significant role in BCR-ABL-mediated leukemogenesis (14). One class of PI3-K/Akt effectors that are key regulators of cellular fate is the FoxO sub-family of forkhead transcription factors, consisting of FoxO3a, FoxO1, FoxO4, and FoxO6 (15-18). Recent evidence suggests that FoxO proteins function as tumor suppressors (19) and promote their growth-suppressive effects by up-regulating the expression of cell-cycle inhibitory genes and pro-apoptotic genes, such as FasL (18) TRAIL (20, 21), and Bim (22-24). Therefore, the transcriptional activity of FoxO3a is inhibited by Akt-dependent phosphorylation, which causes retention of FoxO3a in the cytoplasm (25). We and others have previously shown that BCR-ABL expression promotes FoxO3a phosphorylation at Akt-consensus sites leading to its persistent localization in the cytoplasm and evasion of apoptosis (20, 23). The expression of a FoxO3a triple mutant, in which all three Akt phosphorylation sites have been mutated, results in constitutive activity of FoxO3a and promotes the death of BCR-ABL-transformed cells (20, 23). Further, it has been demonstrated that silencing of FoxO3a in BCR-ABL-transformed cells prevents apoptosis induced by imatinib, thereby providing additional evidence towards the significance of FoxO3a inhibition in BCR-ABL transformation (23).
Here, we test the hypothesis that BCR-ABL stimulates the proteasome-dependent inhibition of members of the Forkhead family of tumor suppressors. Consequently, as FoxO proteins and several other downstream mediators of BCR-ABL are regulated by the proteasome degradation pathway, we investigate whether the inhibition of the proteasome pathway, using bortezomib (velcade, PS-341), causes regression of leukemia. Overall, our results provide novel evidence towards the involvement of the proteasome pathway in the inhibition of FOXO tumor suppressors in the context of leukemogenesis, and demonstrate for the first time using an in vivo model, that the proteasome pathway plays a role in BCR-ABL mediated leukemogenesis. Our results also further indicate the potential for proteasome inhibition therapy in the context of imatinib-resistant BCR-ABL mutations.
MATERIALS AND METHODS
Plasmids and Cell lines
pMSCV-IRES-GFP and pMSCV-BCR-ABL-IRES-GFP, have been described in our previous work (26). BaF3 cells containing either the control vector pMSCV-neomycin resistance, or pMSCV-BCR-ABL (P210)-neomycin resistance were provided by Dr. David Baltimore (California Institute of Technology). BaF3/BCR-ABL T315I cells were provided by Drs. Azam Mohammad and George Daley (Children's Hospital, Harvard Medical School).
Reagents
Imatinib mesylate (Gleevec, STI-571, Novartis) and bortezomib (Velcade, PS-341, Millennium) were purchased from the Beth Israel Deaconess Medical Center Pharmacy approved for research purposes only. Antibodies include FKHRL-1 (FoxO3a), 4G10-phospho-tyrosine, HSP-90 (Upstate Biotech); -β-actin (Sigma), phospho-FKHR (FoxO1)-(Thr24)/FKHRL1 (FoxO3a)-(Thr32), phospho-AKT (Ser 473) (Cell Signaling Technologies); c-AKT, c-ABL, (Santa Cruz Biotech); -BIM (Affinity Biolabs). Additional antibodies used for immunohistochemistry are TRAIL (ICL labs Inc.), BIM, (Santa Cruz Biotech), and myeloperoxidase (DAKO).
Bone marrow transduction, transplantation (BMT) and bortezomib treatment
BMT was carried out according to standard protocols (27, 28). 10 days post BMT, treatments via tail-vein injection with either vehicle control (0.9% saline) or bortezomib was done twice-weekly. Blood was obtained from the tail vein and blood smear slides were prepared with Wright Giemsa stain solution HEMA-QUIK II (Fisher Scientific) according to manufacturer's instructions.
Subcutaneous xenograft tumor model and treatment
BaF3-BCR-ABL or BaF3-BCR-ABL (T315I) cells were mixed with matrigel (BD Biosciences) at 1:1 v/v, and 100 μl of the mixture containing 5×106 BaF3/p210 or 5×106 BaF3/p210 (T315I) cells was injected subcutaneously into the right flank of NU/NU mice (8 weeks old, female) (Charles River Laboratories, Inc.). When tumor volumes reached 150 - 200 mm3, mice were randomized to obtain 12 mice into each treatment group. Imatinib was dissolved in distilled water and delivered at 100 mg/kg in 100 μl by gavage twice a day. Bortezomib was dissolved in 0.9% saline and delivered at 0.8 mg/kg in 100 μl by tail vein (i.v.) injection twice weekly. Tumor volume was measured using calipers in two dimensions and calculated using the formula (width2 × length) / 2.
Immunohistochemistry
Immunohistochemistry was performed as described previously(29).
Patient sample isolation and analysis
8 cc. of discarded whole blood samples from CML, ALL patients or from healthy individuals were collected in Heparin vacutainer tubes. Mononuclear cells (MNCs) were separated using 4 ml of Ficoll-Paque gradient using standard procedures.
RESULTS
FoxO tumor suppressors are down-regulated in BCR-ABL-transformed cells
Studies in mice with different levels of FoxO deficiency demonstrated that germ line and somatic FoxO null mutations of up to five alleles result in mild neoplastic phenotypes (19, 30). Thus, an oncogenic challenge may be necessary to reveal the transformation-promoting effects of an incomplete loss of FoxO. The inhibition of FoxO activity constitutes an important mechanism by which BCR-ABL suppresses apoptosis, and induces transformation (20, 23). Therefore, it is essential to determine the molecular mechanisms by which BCR-ABL negatively regulates FoxO proteins. In these studies, we will primarily show our data with FoxO3a, however, in some key studies we also provide data for other members of this family.
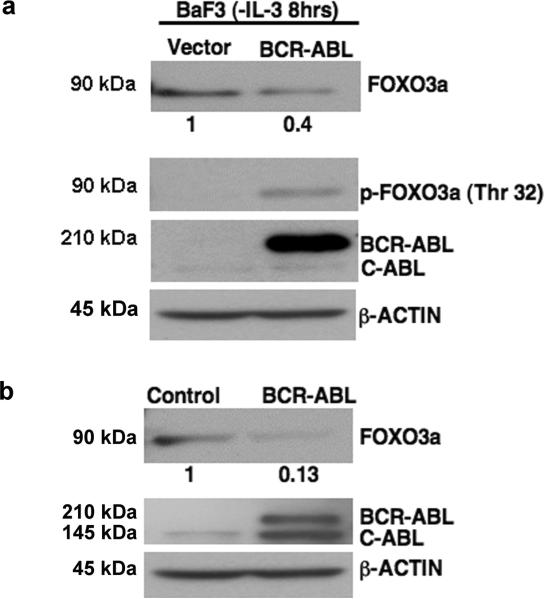
Phosphorylation of FoxO proteins at their Akt-consensus sites inhibits their activity. BCR-ABL-transformed BaF3 cells maintain FoxO3a phosphorylation at an Akt-mediated phosphorylation site (Threonine 32) despite the absence of IL-3 (Fig. 1a). In contrast, non-transformed BaF3 cells (dependent on IL-3 for survival) lose this phosphorylation (Fig. 1a). Additionally, an approximate 60% decrease in total FoxO3a protein expression levels is detected in BCR-ABL-transformed, as compared to non-transformed BaF3 cells (Fig. 1a). Therefore, the inhibition of FoxO3a in BCR-ABL-transformed cells extends beyond phosphorylation and also results in the suppression of FoxO3a protein expression. Consistent with this observation, treatment of BaF3/BCR-ABL cells with imatinib resulted in increased expression of FoxO3a (Sup. Fig. 1a,b), indicating that suppression of FoxO3a expression is, at least in part, dependent on BCR-ABL kinase activity.
Figure 1. BCR-ABL inhibits the protein expression of FoxO3a.
a) Western blot indicates decreased FoxO3a expression in BCR-ABL expressing BaF3 cells. Values below the blot are determined by densitometry with β-ACTIN normalization. p-FoxO3a, BCR-ABL, and β-ACTIN are included as controls. b) Western blot of whole cell lysates from vector or BCR-ABL-transduced primary murine bone marrow cells showing a similar pattern of FoxO3a expression as in a.
In order to determine whether BCR-ABL-induced inhibition of FoxO3a expression is a primary event in response to BCR-ABL expression, we transduced primary murine bone marrow (BM) cells with retroviruses containing either control IRES-GFP or BCR-ABL-IRES-GFP. Cells were subsequently sorted for GFP expression via flow cytometry, and their GFP positivity was ascertained with fluorescence microscopy (Sup. Fig.2). Notably, FoxO3a expression was suppressed by approximately 87% in BCR-ABL-BM cells, as compared to vector control BM cells (Fig. 1b). Importantly, both types of cells were grown in the presence of growth factors, yet FoxO3a expression remained higher in control BM cells than in BCR-ABL-BM cells. These data indicate that normal growth factor receptor signaling does not reduce the levels of FoxO3a expression to that observed in BCR-ABL-expressing cells, and thereby support a role for BCR-ABL induced suppression of FoxO3a expression. In addition to FoxO3a, we also observed attenuation of FoxO1 and FoXO4 expression in BCR-ABL-transformed cells (Sup. Fig. 3).
The proteasome inhibitor, bortezomib, restores FoxO3a expression in BCR-ABL transformed cells
Phosphorylation of substrates by activated Akt lead to their degradation via the proteasome (31). The constitutive activation of the PI3-K/Akt pathway has been well established in BCR-ABL transformation (14, 32, 33). We observed that FoxO3a suppression in BCR-ABL-transformed cells is dependent on the activation of the PI3-K pathway, as chemical inhibition of this pathway with LY 294002 not only reduced FoxO3a phosphorylation but also restored FoxO3a protein expression (Sup. Fig. 1c). Such observations suggested that FoxO3a down-regulation in BCR-ABL-transformed cells could be proteasome-dependent. In order to test this hypothesis, we investigated whether the proteasome inhibitor, bortezomib, can reverse BCR-ABL-induced suppression of FoxO3a.
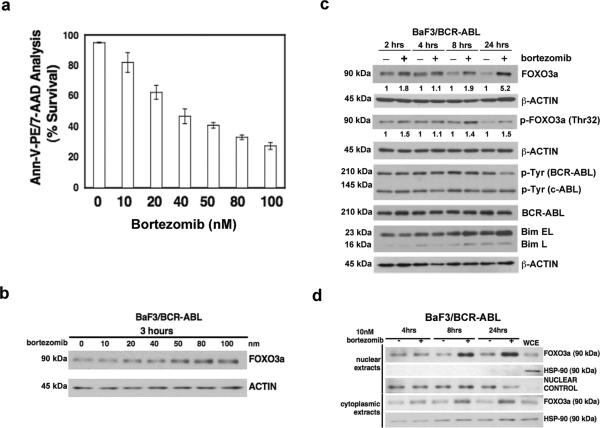
Bortezomib is the first highly potent inhibitor of the proteasome to enter the clinic, and is FDA approved for the treatment of relapsed and refractory multiple myeloma (34-36) as well as relapsed and refractory mantle cell lymphoma (37). Treatment of BaF3/BCR-ABL cells with bortezomib showed a dose-dependent increase in apoptosis, as measured by Annexin-V-PE/7-AAD staining (Fig. 2a). Bortezomib treatment showed a dose-dependent increase in FoxO3a protein expression as early as 3 hours (Fig. 2b). In addition, even at the lowest concentration (10nM) used in these studies, bortezomib resulted in an approximately 5-fold increase in FoxO3a expression over a 24-hour time course (Fig. 2c). We confirmed proteasome inhibition-induced increases in FoxO3a protein expression by treatment with another proteasome inhibitor, epoxomicin (Sup. Fig. 4). Taken together, these results show that inhibition of the proteasome induces apoptosis and up-regulates FoxO3a levels in BCR-ABL-transformed cells.
Figure 2. The proteasome inhibitor bortezomib restores expression of FoxO3a and induces apoptosis in BCR-ABL transformed cells.
a) Quantitative cell survival data from annexin-V staining 24 hours after bortezomib treatment of BaF3/BCR-ABL cells. Data shown are averages +/- std. dev from three independent experiments. b) Western blot showing elevated FoxO3a protein expression with increasing concentrations of bortezomib. c) Western blot indicates an increase in FoxO3a expression in BaF3/BCRABL cells treated with bortezomib over a 24-hour time course. Values below the blot are determined by densitometry with β-ACTIN normalization. d) Western blot demonstrating an increase in FoxO3a protein in nuclear extracts from BaF3/BCR-ABL cells treated with bortezomib. Whole cell extract (WCE) from untreated cells is included as a control. Successful compartmentalization is indicated by a lack of HSP-90 detection in the nuclear extracts, with its positive detection in WCE and cytoplasmic extracts.
Because Akt-mediated phosphorylation of FoxO3a targets it for proteasomal degradation (31), we expected that proteasome inhibition in BaF3/BCR-ABL cells would result in accumulation of phosphorylated FoxO3a. Phosphorylated FoxO3a levels initially increased after bortezomib treatment, but by 24h the increase in total FoxO3a expression exceeded the increase in phosphorylated FoxO3a (Fig. 2c). These results suggest that bortezomib treatment promotes the accumulation of non-phosphorylated FoxO3a, which is localized in the nucleus, where it can serve its transcriptional inducing activity. We therefore analyzed FoxO3a expression in nuclear extracts from cells treated with bortezomib. In contrast to untreated controls, FoxO3a significantly accumulated in the nucleus of bortezomib-treated BaF3/BCR-ABL cells (Fig. 2d).
In order to assess the effect of bortezomib on pro-apoptotic factors downstream of FoxO3a, we analyzed the expression of BIM, which is regulated by FoxO3a (22) and is suppressed in a FoxO3a-dependent manner in BCR-ABL-transformed cells (23). We observed that BIM expression increased in response to bortezomib (Fig. 2c). Since BIM is also regulated by proteasomal-degradation, the observed increase in BIM protein expression could be due to both FoxO3a-dependent and -independent effects. TRAIL is another target of FoxO3a that is suppressed in BCR-ABL-transformed cells (20). We found that bortezomib treatment of BaF3/BCR-ABL cells led to a modest but statistically significant increase in TRAIL mRNA levels(Sup. Fig. 5). Therefore, the apoptosis-inducing effects of bortezomib treatment in BCR-ABL-transformed cells is, at least in part, caused by an increase in the expression of the pro-apoptotic factors, BIM and TRAIL, a likely consequence of the restoration of nuclear FoxO3a.
BCR-ABL-expressing primary bone marrow cells are highly sensitive to bortezomib
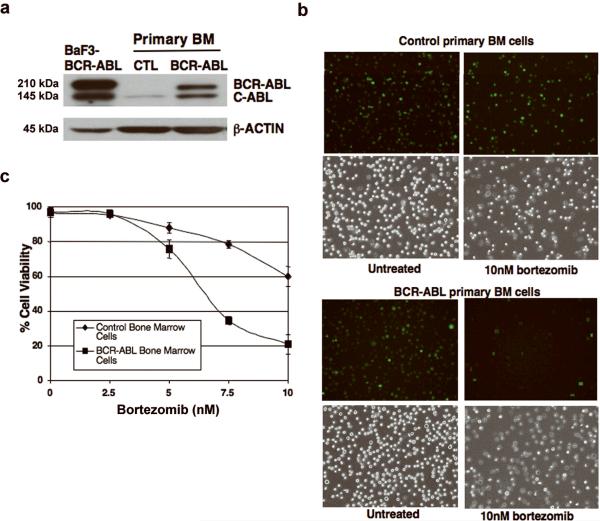
As several downstream mediators of BCR-ABL, including FoxO proteins, are regulated by proteasomal degradation, we then hypothesized that inhibition of the proteasomal degradation pathway could suppress BCR-ABL-induced leukemia. Tumor cells have been shown to display greater sensitivity to the effects of bortezomib than normal cells (38, 39). One explanation for this differential sensitivity is that cancer cells rely upon the proteasome to a greater extent than normal cells in order to perturb the expression of proteins involved in regulating the cell cycle and apoptosis (40). We investigated whether bortezomib selectively affects BCR-ABL-expressing cells over normal primary BM cells. Importantly, BCR-ABL-expressing BM cells (Fig. 3a) treated with 10 nM bortezomib showed a significant reduction in survival, as compared to vector control cells. This was observed by the significant reduction in GFP-positive cells, which correlates with the appearance of dead BCR-ABL-expressing BM cells (Fig. 3b). In order to quantify these differences, we measured cell viability in response to bortezomib treatment over a 0-10 nM range in both normal and BCR-ABL- expressing primary BM cells, and observed that BCR-ABL-expressing cells are approximately three times more sensitive to bortezomib than normal cells (Fig. 3c).
Figure 3. BCR-ABL-expressing primary bone marrow cells are more sensitive to bortezomib than normal cells.
a) Western blot confirming expression of BCR-ABL in BCR-ABL-transduced primary BM cells used in (b) and (c) below. b) Fluorescent microscope images (original magnification, 200x) with corresponding phase-contrast images showing increased sensitivity of BCR-ABL-transduced BM cells (BCR-ABLGFP) to 10nM bortezomib (24hrs) in contrast to vector control (GFP) BM cells. A representative area of each sample is shown. c) Cell viability (trypan blue) is significantly lower after 24hr bortezomib treatment in the presence of IL-3, IL-6, and SCF in BCR-ABL-transduced BM cells compared to vector control BM cells.
Bortezomib inhibits CML-like disease and prolongs survival in a BCR-ABL murine bone marrow transplant model
Human CML can be faithfully modeled in mice by retroviral transduction of the BCR-ABL oncogene into mouse BM cells, followed by transplantation into irradiated syngeneic recipient mice (27, 28). Mice that receive BCR-ABL-transduced BM develop a fatal CML-like myeloproliferative disease in three to four weeks. This system has been useful both in determining molecular mechanisms by which the BCR-ABL oncogene acts in the pathogenesis of CML and for the testing of potential therapies (2, 9, 28, 41).
We used this model to test whether inhibition of the proteasome-degradation pathway by bortezomib can reverse BCR-ABL-induced CML progression. Previous studies have reported the effectiveness of bortezomib at doses ranging from 0.5 mg/kg to 1.0 mg/kg (42-45). In order to determine the optimal dose for our studies, we generated vector control and BCR-ABL-transduced mice and treated the mice twice weekly with 0 mg/kg (vehicle), 0.1 mg/kg, 0.5 mg/kg, or 1.0 mg/kg of bortezomib. After three treatments, we observed maximal suppression of splenomegaly, or the enlarged spleen that is typically observed in this CML model, from mice treated with bortezomib at 1.0 mg/kg (n=3) (Sup. Fig. 6). As previous studies have found 0.8 mg/kg to be optimal (43, 45), we also tested this dose and found a similar response as with 1.0 mg/kg (Fig. 4b). Therefore, we chose 0.8 mg/kg for the following studies.
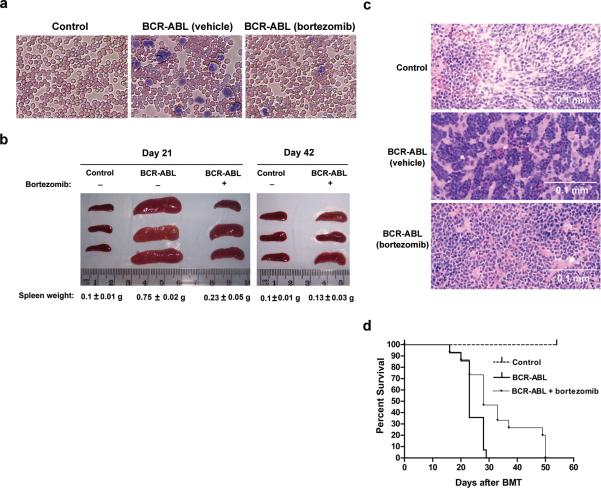
Figure 4. Treatment of BCR-ABL-transduced mice with bortezomib decreases signs of CML-like illness and prolongs survival.
a) Wright-Giemsa staining of blood smears from vehicle-treated vector control, vehicle-treated BCR-ABL and bortezomib-treated BCR-ABL-transduced mice (on day 21 after BMT, and after 4 treatments of 0.8mg/kg bortezomib) show an excess of granulocytes (dark purple) in BCR-ABL mice but decreased staining of granulocytes in bortezomib-treated BCR-ABL-transduced mice, comparable to vector control mice. Original magnification, 200X. b) Bortezomib treatment reduces splenomegaly in BCR-ABL-transduced mice. Spleens were isolated on day 21 after BMT and 4 bortezomib treatments (left panel) and on day 42 after BMT and 11 treatments (right panel). Groups treated with vehicle only are marked as (-), and groups treated with bortezomib are marked as (+). Spleen weight was indicated at the bottom of each group. The statistical significance of differences among various groups of spleen weight was determined by analysis of variance (ANOVA). N = 3 for all groups. Significant values are as follows: p < 0.001 for Day 21 BCR-ABL group treated with vehicle only versus all other groups (including Day 42 groups); p < 0.05 for Day 21 BCR-ABL group treated with bortezomib versus control groups; p < 0.05 for Day 42 BCR-ABL group treated with bortezomib versus Day 21 BCR-ABL group treated with bortezomib. c) H & E staining of spleen samples obtained from the same groups of mice described in (b) shows reduced myeloid infiltration in the spleens of BCR-ABL-transduced mice treated with bortezomib. d) Bortezomib treatment prolongs survival of BCR-ABL-transduced mice. BCR-ABL-transduced mice were treated with either vehicle (0.9% saline) or bortezomib (0.8mg/kg). Survival of mice was plotted using the Kaplan-Meier method (nonparametric cumulated survival fraction plot), and statistical comparison between the curves obtained using the log rank test. p < 0.01 for BCR-ABL group treated with bortezomib (n = 15) versus BCR-ABL group treated with vehicle only (n = 14).
Either vehicle control (0.9% saline) or bortezomib (0.8 mg/kg) was administered by intravenous injection (i.v.) into BCR-ABL-transduced mice 10 days post bone marrow transplantation (BMT) and continued on a twice-weekly treatment schedule. Blood samples were collected on day 21 after BMT and also after each of 4 bortezomib treatments to determine the level of leukocytes. In contrast to vector control mice, BCR-ABL-transduced mice had an excess of leukocytes detected by Wright-Giemsa staining of peripheral blood smears (Fig. 4a). However, bortezomib-treated BCR-ABL-transduced mice had fewer leukocytes, resembling vector control mice (Fig. 4a). Similarly, the complete blood count (CBC) showed a statistically significant (p=0.019; two tailed Student t-test) increase in white blood cell count among the BCR-ABL transduced (untreated) mice (range:11.48-34.56 ×103cells /ul; n=3) compared to vehicle only control or BCR-ABL mice treated with bortezomib (n=13; 2.14-9.18 X103 cells/μl; One outlier at 26.18 ×103 cells/μl was also included in the analysis). The normal reference range at the laboratory tested is 5.4-16×103 cells/μl.
Yet another significant symptom of CML-like disease in this model is splenomegaly, and by 21 days post-BMT, we clearly observed splenomegaly in vehicle-treated BCR-ABL-transduced mice, but not in vector control mice. Bortezomib treatment significantly reduced splenomegaly in BCR-ABL-transduced mice (p < 0.001) by day 21 and resulted in further reduction by day 42 (p < 0.05) (Fig. 4b). Upon examination of the spleen tissue by hematoxylin and eosin (H&E) staining, we found that spleen samples from BCR-ABL mice treated with vehicle control displayed heavy infiltration of myeloid cells (Fig. 4c). In contrast, bortezomib-treated BCR-ABL spleen samples showed little myeloid infiltration (Fig. 4c). We also observed similar pathological changes in the liver (data not shown).
We next determined whether the attenuation of CML-like pathophysiology in the BCR-ABL-induced CML mouse model correlated with increased survival. BCR-ABL-transduced mice treated with vehicle control began to die as early as 16 days after BMT, but the majority of mice in this group died during the third and the fourth weeks after BMT (Fig. 4d, broad solid line, n = 14). In the bortezomib-treated BCR-ABL group, 47% of the mice had survived at the end of one month after BMT, and the last mouse in this group died by day 49 after BMT (Fig. 4d, fine solid line, n = 15). These data show that the survival of bortezomib-treated, BCR-ABL-transduced mice was significantly prolonged (p < 0.01). Finally, we determined that bortezomib treatment did result in a molecular regression of leukemia, as qRT-PCR analysis of BM cells on Day 21 revealed that the normalized copy number of BCR-ABL in bortezomib treated mice was significantly reduced compared to that of an untreated mouse (Sup. Fig. 7).
Bortezomib treatment of BCR-ABL-transduced leukemic mice restores normal expression of FoxO3a and its targets TRAIL and BIM
We analyzed the effect of bortezomib treatment on FoxO, TRAIL and Bim expression. We could barely detect FoxO3a protein in the BCR-ABL-transduced mice, whereas moderate expression was found in control mice, providing further support for inhibition of FoxO3a tumor suppressor by the BCR-ABL oncogene (Fig. 5a). The positive staining of myeloperoxidase (MPO) in vehicle-treated BCR-ABL mice confirmed that overall protein expression was not affected in these mice (Fig. 5a). Bortezomib treatment completely restored FoxO3a protein expression to a level comparable in vehicle-treated control mice (Fig. 5a and 5b). We observed similar results in the marrow where FoxO3a protein expression was virtually absent in vehicle-treated BCR-ABL-transduced mice but was restored in response to treatment with bortezomib (Fig. 5b). Taken together, these results provide novel in vivo evidence that greatly diminished protein expression of FoxO3a tumor suppressor is associated with BCR-ABL-mediated leukemogenesis.
Figure 5. FoxO3a, TRAIL, and BIM are suppressed in a BCR-ABL-induced CML mouse model, with bortezomib treatment abrogating these effects.
a) 2 mice were analyzed from each treatment group through all the immunohistochemistry experiments and the same results were received for both within each group. Immunohistochemical (IHC) staining for FoxO3a using a FoxO3a-specific antibody on spleen sections from vehicle or bortezomib-treated vector control and BCR-ABL-transduced mice. Original magnification 400x. Note presence of myeloperoxidase on H&E stained spleen section from vehicle-treated BCR-ABL-transduced mice. Original magnification 400x. b) IHC staining for FoxO3a on marrow sections from vehicle or bortezomib-treated vector control and BCR-ABL-transduced mice showing a similar FoxO3a expression pattern as in a. Original magnification 1000x. c) IHC staining for phospho-FoxO3a (Thr32)/FoxO1 (Thr24) on spleen sections from vehicle or bortezomib-treated vector control and BCRABL-transduced mice. Original magnification 400x. d) Western blot of mononuclear cells from CML patients (P1-P3) and a Ph+ positive ALL patient (P4) and healthy individuals (N) demonstrates a loss of FoxO3a and FoxO1 expression in CML and ALL patients.
Given that the phosphorylation of FoxO3a is an important mechanism for its negative regulation (25, 31), we compared FoxO3a phosphorylation in control and BCRABL-transduced mice, using an antibody that detects the phosphorylated forms of FoxO3a (Threonine 32) as well as that of the FoxO family member FoxO1 (Threonine 24). This analysis indicated that BCR-ABL mice express phosphorylated FoxO3a and FoxO1, albeit at low levels, whereas similar or slightly higher levels were observed in control mice (Fig. 5c). Consistent with our in vitro findings (Fig. 1a), these results suggest that the lower level of FoxO3a that is present in BCR-ABL mice is predominantly phosphorylated and is therefore inactive.
We also observed that the expression of the FoxO3a targets, observed TRAIL and BIM were greatly diminished in leukemic BCR-ABL mice, in contrast to vector control mice (Sup. Fig. 8b,c). Importantly, bortezomib-treated BCR-ABL-transduced mice indicated a clear restoration of expression of these proteins to the levels found in control mice. (Sup. Fig 8b, c).
Bortezomib restores expression of FoxO3a in imatinib-resistant T315I cells
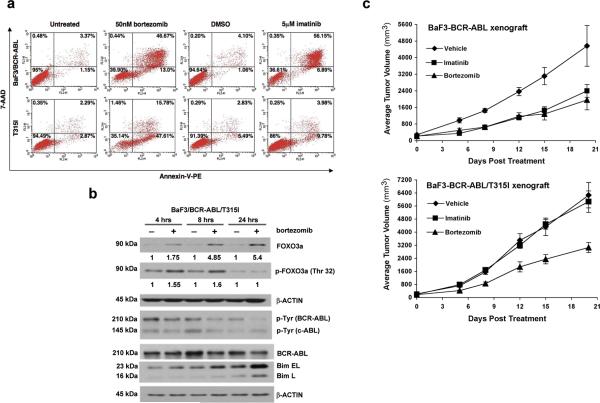
The T315I BCR-ABL mutation frequently occurs in CML patients that are resistant to imatinib (11), and even remains refractory to the more potent second generation kinase inhibitors (9-11). Bortezomib treatment of imatinib-resistant BCRABL T315I cells signifcantly reduced cell survival and induced apoptosis, as measured by the MTT assay (Sup. Fig 9a) and Annexin-V-PE/7-AAD staining, respectively (Fig. 6a). By 48h of bortezomib treatment, both BaF3/BCR-ABL and BaF3/BCR-ABL T315I cells showed similar percentages of predominantly late apoptotic cells (Sup. Fig 9b).
Figure 6. Imatinib-resistant T315I BCR-ABL transformed cells are sensitive to bortezomib.
a) Representative flow cytometry dot plots (Annexin-V-PE (x-axis) and 7-AAD (y-axis) staining) comparing BaF3/BCR-ABL and BaF3/BCR-ABL T315I cells, treated for 24 hours with 50nM bortezomib, and showing significant induction of apoptosis (cells represented in the LR, UR, and UL quadrants). Imatinib resistance is confirmed in T315I cells. b) Western blot indicating an increase in FoxO3a expression upon bortezomib treatment of BaF3/BCR-ABL T315I cells. c) Bortezomib inhibits both BaF3-BCR-ABL and BaF3-BCR-ABL (T315I) xenograft tumor growth while BaF3/p210 (T315I) tumors do not respond to imatinib inhibition.
Similar to imatinib-sensitive BCR-ABL cells (Fig. 2c), FoxO3a protein expression was also restored in T315I cells upon treatment with bortezomib (Fig. 6b). As an indicator of downstream apoptotic events in response to bortezomib treatment, the protein expression of BIM (particularly the Extra Long (EL) splice variant) was increased upon bortezomib treatment (Fig. 6b). These results indicate that FoxO3a is repressed in imatinib-resistant T315I cells, and that proteasome inhibition restores FoxO3a and induces apoptosis. FoxO3a depletion via siRNAs resulted in a partial rescue against bortezomib sensitivity in BCR-ABL T315I cells, suggesting that FoxO3a activity in part contributes to the effects of bortezomib (Sup. Fig 9c,d). We then examined the effect of bortezomib on BCR-ABL (T315I) expressing tumors in vivo. BaF3-BCR-ABL or BaF3-BCR-ABL (T315I) cells were utilized to generate xenograft tumor in nude mice by subcutaneous injection. Either imatinib or bortzomib treatment resulted in inhibition of wild type BCR-ABL-expressing tumors as early as 1 week (Fig. 6c). 20 days of imatinib or bortezomib treatment caused significant inhibition of tumor growth compared with vehicle group (p < 0.001 for imatinib vs. vehicle, p < 0.001 for bortezomib vs. vehicle, by One-way Analysis of Variance). In the BaF3-BCR-ABL (T315I) tumors, bortezomib treatment started to show tumor inhibition as early as 1 week after treatment (Figure 6c), while imatinib did not show any effect. By 20 days after treatment, bortezomib caused significant inhibition on tumor growth compared with vehicle group and imatinib group (p < 0.001 for bortezomib vs. vehicle, p < 0.01 for bortezomib vs. imatinib, by One-way Analysis of Variance). These results show that while BaF3-BCR-ABL (T315I) xenograft tumors are resistant to imatinib, their growth can be significantly inhibited by bortezomib.
FoxO3a expression is suppressed in Philadelphia chromosome positive CML and ALL patients
In order to investigate whether FoxO3a protein expression is also lost in Ph+ leukemia patients, we analyzed the peripheral blood samples of several CML and ALL patients. Peripheral blood (PB) was obtained from newly diagnosed CML patients (P1, P2, P3) who had not yet initiated any therapy. We also obtained peripheral blood from an ALL patient (P4) who was failing the standard therapy for ALL. Fluorescent in situ hybridization confirmed that these patients carried the BCR-ABL translocation (Sup. Fig. 10a,b). Western blot analysis of PB mononuclear cell preparations showed a significantly reduced expression of FoxO3a in leukemic samples, in contrast to healthy individuals (N) (Figure 5d). H & E staining of the diagnostic BM biopsy from the ALL patient shows a massive blast, with few normal hematopoietic cells (Sup. Fig. 10c). Immunohistochemical analysis of the BM biopsy demonstrates that FoxO3a is mainly expressed in normal hematopoietic cells and not in the blast cells. These observations provide evidence that FoxO3a is suppressed in these hematological malignancies, whereby loss of its expression is correlated with the leukemic disease.
DISCUSSION
An understanding of the molecular basis for the survival of tumor cells through resistance to apoptosis is critical for the development of rational and suitably targeted anti-neoplastic therapies. An increasing number of studies have demonstrated that activation of the PI3-K/Akt survival pathway plays an important role in BCR-ABL-mediated leukemogenesis. More recently, the significance of Akt activation in CML was investigated in the context of imatinib resistance. Interestingly, while the BCR-ABL T315I imatinib-resistant mutation is refractory to the combination of imatinib and numerous compounds, inhibition of Akt signaling with a phosphoinositide-dependent kinase-1 inhibitor, OSU-03012, synergizes with imatinib to induce apoptosis in BCRABL T315I cells (46). These studies suggest that Akt activation is an important event even in imatinib-resistant CML.
The FoxO3a transcription factor represents a critical substrate that is inhibited by Akt during growth factor-induced survival (25). We and other groups have previously shown that BCR-ABL imposes negative regulation of FoxO3a by promoting its constitutive phosphorylation and retention in the cytoplasm. Indeed, expression of a FoxO3a mutant that cannot be phosphorylated results in apoptosis of BCR-ABL-transformed cells (20, 23). Therefore, FoxO3a acts as a tumor suppressor, and its negative regulation is an important aspect of BCR-ABL-induced leukemia. In this study, we report that FoxO3a protein levels are greatly suppressed in BCR-ABL-transformed cells and BCR-ABL-expressing primary BM cells as well as in a BCR-ABL murine BM transplant model for CML. Importantly, we also observed this dramatic reduction in FoxO3a in newly diagnosed (prior to any treatment) CML patients and a BCR-ABL positive ALL patient. We show that treatment with bortezomib restores normal FoxO3a expression, and this restoration of FoxO3a was associated with a significant reduction of CML-like illness and prolonged survival of BCR-ABL-transduced mice.
Constitutive FoxO3a inhibition via phosphorylation and cytoplasmic retention in BCR-ABL-transformed cells leads to the suppressed expression of pro-apoptotic genes, such as BIM and TRAIL, and is therefore an important mechanism for BCR-ABL transformation (20, 23). Our in vitro findings revealed that bortezomib treatment led to the nuclear accumulation of FoxO3a, which correlated with an increase in the expression level of the FoxO3a gene targets TRAIL and BIM, and the induction of apoptosis in BCRABL-transformed cells. As TRAIL is a key mediator of tumor surveillance, the BCRABL-induced suppression of FoxO3a and TRAIL could interfere with tumor surveillance and thereby promote tumor growth and metastasis. Additionally, our findings that BIM expression is normalized in response to bortezomib treatment of BCR-ABL-transduced mice is consistent with a recent report which showed BIM as an important mediator of apoptosis induced by the combination of paclitaxel and bortezomib in epithelial tumor cells (47). In general, our demonstration that FoxO3a, TRAIL and BIM suppression in BCR-ABL-induced leukemogenesis is abrogated by proteasome inhibition in cell culture studies and in an in vivo mice model reveals novel mechanistic insights into the molecular effects and mechanisms of action of the anti-neoplastic proteasome inhibitor bortezomib, not only in leukemia but potentially in other malignancies.
Our studies also reveal that FoxO3a is also suppressed in BCR-ABL T315I cells and demonstrate that proteasome inhibition with bortezomib reverses FoxO3a suppression as well as potently induces apoptosis in these cells. Given that FoxO3a is emerging as a potential tumor suppressor, with its inactivation recently observed in several transformed cell lines and cancers (19, 48-50), it remains possible that FoxO3a activation is one of the important mechanisms for the anti-neoplastic activity of bortezomib in other cancers as well. In the future, it will be important to determine the role of additional signaling pathways that contribute towards the effects of bortezomib against BCR-ABL-transformed cells.
Therefore, these results, in principle, demonstrate the ability of a clinically approved proteasome inhibitor to reduce the burden of BCR-ABL-induced leukemic disease and carry important therapeutic implications regarding the management of resistance to imatinib therapy.
Supplementary Material
Acknowledgements
The IRB approval for the human studies is based on Protocol # 2007-P-000080/6). We would like to thank Susan Glueck for editing this manuscript, Susan Merrill (BD Biosciences) for assistance on multi-color flow cytometry experiments, Drs. Kenneth Ndebele and Nordine Benhaga for technical advice, Ms. Melissa Gorman, cytogenetics division for providing FISH images and Drs. David Baltimore, George Daley, Azam Mohammad, Michael Greenberg and Anne Brunnet for valuable reagents. This work was supported by NIH grants CA105306, HL080192 and investigator-initiated grant from Millennium Pharmaceutical to RKF. RKF is an American Cancer Society Scholar. Immunohistochemical methodology was supported by the Specialized Histopathology Core Lab of the Dana Farber/Harvard Cancer Center (NIH-P30CA6516).
REFERENCES
- 1.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96(10):3343–56. [PubMed] [Google Scholar]
- 2.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nature reviews. 2005;5(3):172–83. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 3.Van Etten RA. Mechanisms of transformation by the BCR-ABL oncogene: new perspectives in the post-imatinib era. Leukemia research. 2004;28(Suppl 1):S21–8. doi: 10.1016/j.leukres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Neshat MS, Raitano AB, Wang HG, Reed JC, Sawyers CL. The survival function of the Bcr-Abl oncogene is mediated by Bad-dependent and -independent pathways: roles for phosphatidylinositol 3-kinase and Raf. Molecular and cellular biology. 2000;20(4):1179–86. doi: 10.1128/mcb.20.4.1179-1186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 6.Carroll M, Ohno-Jones S, Tamura S, et al. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TELPDGFR fusion proteins. Blood. 1997;90(12):4947–52. [PubMed] [Google Scholar]
- 7.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293(5531):876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 8.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2(2):117–25. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 9.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(2):129–41. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 11.Shah NP, Skaggs BJ, Branford S, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. The Journal of clinical investigation. 2007;117(9):2562–9. doi: 10.1172/JCI30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nature reviews. 2007;7(5):345–56. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- 13.O'Hare T, Eide CA, Deininger MW. New Bcr-Abl inhibitors in chronic myeloid leukemia: keeping resistance in check. Expert opinion on investigational drugs. 2008;17(6):865–78. doi: 10.1517/13543784.17.6.865. [DOI] [PubMed] [Google Scholar]
- 14.Skorski T, Bellacosa A, Nieborowska-Skorska M, et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. Embo J. 1997;16(20):6151–61. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8(6):440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 16.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96(13):7421–6. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003;278(38):35959–67. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- 18.Greer EL, Brunet A. FOXO transcription factors in ageing and cancer. Acta physiologica (Oxford, England) 2008;192(1):19–28. doi: 10.1111/j.1748-1716.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 19.Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128(2):309–23. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaffari S, Jagani Z, Kitidis C, Lodish HF, Khosravi-Far R. Cytokines and BCRABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor. Proc Natl Acad Sci U S A. 2003;100(11):6523–8. doi: 10.1073/pnas.0731871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277(49):47928–37. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 22.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10(19):1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 23.Essafi A, Fernandez de Mattos S, Hassen YA, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24(14):2317–29. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 24.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162(4):613–22. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 26.Ghaffari S, Kitidis C, Fleming MD, Neubauer H, Pfeffer K, Lodish HF. Erythropoiesis in the absence of janus-kinase 2: BCR-ABL induces red cell formation in JAK2(-/-) hematopoietic progenitors. Blood. 2001;98(10):2948–57. doi: 10.1182/blood.v98.10.2948. [DOI] [PubMed] [Google Scholar]
- 27.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247(4944):824–30. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92(10):3829–40. [PubMed] [Google Scholar]
- 29.Song K, Benhaga N, Anderson RL, Khosravi-Far R. Transduction of tumor necrosis factor-related apoptosis-inducing ligand into hematopoietic cells leads to inhibition of syngeneic tumor growth in vivo. Cancer Res. 2006;66(12):6304–11. doi: 10.1158/0008-5472.CAN-05-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278(14):12361–6. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 32.Varticovski L, Daley GQ, Jackson P, Baltimore D, Cantley LC. Activation of phosphatidylinositol 3-kinase in cells expressing abl oncogene variants. Mol Cell Biol. 1991;11(2):1107–13. doi: 10.1128/mcb.11.2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skorski T, Kanakaraj P, Nieborowska-Skorska M, et al. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood. 1995;86(2):726–36. [PubMed] [Google Scholar]
- 34.Adams J. Proteasome inhibition in cancer: development of PS-341. Semin Oncol. 2001;28(6):613–9. doi: 10.1016/s0093-7754(01)90034-x. [DOI] [PubMed] [Google Scholar]
- 35.Chauhan D, Hideshima T, Mitsiades C, Richardson P, Anderson KC. Proteasome inhibitor therapy in multiple myeloma. Mol Cancer Ther. 2005;4(4):686–92. doi: 10.1158/1535-7163.MCT-04-0338. [DOI] [PubMed] [Google Scholar]
- 36.Ghobrial J, Ghobrial IM, Mitsiades C, et al. Novel therapeutic avenues in myeloma: changing the treatment paradigm. Oncology (Williston Park, NY. 2007;21(7):785–92. discussion 98-800. [PubMed] [Google Scholar]
- 37.Goy A. Mantle cell lymphoma: evolving novel options. Current oncology reports. 2007;9(5):391–8. doi: 10.1007/s11912-007-0053-9. [DOI] [PubMed] [Google Scholar]
- 38.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23(3):630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 39.Adams J. Proteasome inhibition: a novel approach to cancer therapy. Trends Mol Med. 2002;8(4 Suppl):S49–54. doi: 10.1016/s1471-4914(02)02315-8. [DOI] [PubMed] [Google Scholar]
- 40.Magill L, Lynas J, Morris TC, Walker B, Irvine AE. Proteasome proteolytic activity in hematopoietic cells from patients with chronic myeloid leukemia and multiple myeloma. Haematologica. 2004;89(12):1428–33. [PubMed] [Google Scholar]
- 41.Van Etten RA. Models of chronic myeloid leukemia. Curr Oncol Rep. 2001;3(3):228–37. doi: 10.1007/s11912-001-0055-y. [DOI] [PubMed] [Google Scholar]
- 42.Goel A, Dispenzieri A, Geyer SM, Greiner S, Peng KW, Russell SJ. Synergistic activity of the proteasome inhibitor PS-341 with non-myeloablative 153-Sm-EDTMP skeletally targeted radiotherapy in an orthotopic model of multiple myeloma. Blood. 2006;107(10):4063–70. doi: 10.1182/blood-2005-09-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan T, Stauffer JK, Williams R, et al. Proteasome inhibition to maximize the apoptotic potential of cytokine therapy for murine neuroblastoma tumors. J Immunol. 2006;176(10):6302–12. doi: 10.4049/jimmunol.176.10.6302. [DOI] [PubMed] [Google Scholar]
- 44.Schumacher LY, Vo DD, Garban HJ, et al. Immunosensitization of tumor cells to dendritic cell-activated immune responses with the proteasome inhibitor bortezomib (PS-341, Velcade) J Immunol. 2006;176(8):4757–65. doi: 10.4049/jimmunol.176.8.4757. [DOI] [PubMed] [Google Scholar]
- 45.Wagner-Ballon O, Pisani DF, Gastinne T, et al. Proteasome inhibitor bortezomib impairs both myelofibrosis and osteosclerosis induced by high thrombopoietin levels in mice. Blood. 2007;110(1):345–53. doi: 10.1182/blood-2006-10-054502. [DOI] [PubMed] [Google Scholar]
- 46.Tseng PH, Lin HP, Zhu J, et al. Synergistic interactions between imatinib mesylate and the novel phosphoinositide-dependent kinase-1 inhibitor OSU-03012 in overcoming imatinib mesylate resistance. Blood. 2005;105(10):4021–7. doi: 10.1182/blood-2004-07-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan TT, Degenhardt K, Nelson DA, et al. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7(3):227–38. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Hu MC, Lee DF, Xia W, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117(2):225–37. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 49.Scheijen B, Ngo HT, Kang H, Griffin JD. FLT3 receptors with internal tandem duplications promote cell viability and proliferation by signaling through Foxo proteins. Oncogene. 2004;23(19):3338–49. doi: 10.1038/sj.onc.1207456. [DOI] [PubMed] [Google Scholar]
- 50.Gu TL, Tothova Z, Scheijen B, Griffin JD, Gilliland DG, Sternberg DW. NPMALK fusion kinase of anaplastic large-cell lymphoma regulates survival and proliferative signaling through modulation of FOXO3a. Blood. 2004;103(12):4622–9. doi: 10.1182/blood-2003-03-0820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.