Abstract
HIV diagnosis and presentation to appropriate medical care during early stages of disease has substantial clinical and public health benefits. However, a large proportion of HIV-infected Americans experience diagnosis and treatment related delays. Prior research evaluating barriers to early HIV diagnosis and care presentation have been published primarily from large East and West coast urban centers. Therefore, predictors of delayed presentation to HIV care identified by these studies may not be generalizable to the South where infected persons are increasingly non-white, female, poor, non-urban, and possibly exposed to HIV heterosexually. We review here the benefits conferred by HIV care, descriptive epidemiology of delayed HIV diagnosis and care, and potential barriers to early medical care with special reference to conditions prevalent in the South.
Introduction
The United States (US) Southern region (Table I) has suffered a disproportionate burden of the HIV/AIDS epidemic, particularly in recent years. In 2004, the South accounted for 46.6% of incident AIDS cases and 45.5% of AIDS-related deaths, the most of any geographic region (CDC, 2005). Eight of the ten states with the highest HIV-related death rates per 100,000 population are Southern states (Table I).
Table I.
Burden of HIV/AIDS Among States Comprising the Southern Region of the United States†, 2004.
| AIDS Case Rate/100,000 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-Related Death Rate/100,000‡ |
All |
Males |
Females |
Black |
Incident AIDS Cases -% Black |
|||||||
| Southern States | National Ranking | Rate | National Ranking | Rate | National Ranking | Rate | National Ranking | Rate | National Ranking | Rate | National Ranking | % |
| Alabama | 15 | 4.3 | 22 | 10.3 | 24 | 17.6 | 16 | 7.7 | 41 | 34.5 | 7 | 70.2 |
| Arkansas | 23 | 3.2 | 31 | 6.7 | 31 | 13.1 | 25 | 3.5 | 47 | 23.2 | 20 | 42.2 |
| Delaware | 5 (tie) | 8.5 | 7 | 18.9 | 13 | 27.8 | 5 | 17.8 | 10 | 77.1 | 9 | 65.6 |
| D. C¶ | 1 | 40.8 | 1 | 179.2 | 1 | 320.0 | 1 | 113.3 | 1 | 335.1 | 1 | 87.8 |
| Florida | 3 | 10.4 | 3 | 33.5 | 3 | 57.5 | 3 | 23.1 | 3 | 148.7 | 16 | 52.0 |
| Georgia | 8 | 8.2 | 8 | 18.6 | 7 | 34.1 | 10 | 12.1 | 15 | 61.8 | 3 | 76.6 |
| Kentucky | 27 (tie) | 2.3 | 34 | 6.1 | 34 | 12.2 | 33 | 2.6 | 37 | 37.1 | 26 | 36.7 |
| Louisiana | 7 | 8.4 | 5 | 22.4 | 5 | 39.3 | 7 | 16.3 | 13 | 65.8 | 5 | 75.1 |
| Maryland | 2 | 10.7 | 4 | 26.1 | 4 | 41.5 | 4 | 22.5 | 7 | 92.6 | 2 | 82.2 |
| Mississippi | 10 | 6.8 | 11 | 16.5 | 9 | 29.4 | 11 | 11.8 | 30 | 42.8 | 6 | 73.9 |
| North Carolina | 12 | 5.8 | 13 | 13.3 | 18 | 23.3 | 12 | 9.4 | 23 | 54.1 | 8 | 69.8 |
| Oklahoma | 24 | 2.7 | 36 | 5.5 | 36 | 11.2 | 37 | 2.4 | 46 | 25.3 | 31 | 28.2 |
| South Carolina | 9 | 7.4 | 10 | 18.1 | 8 | 31.1 | 9 | 12.8 | 20 | 57.8 | 4 | 75.5 |
| Tennessee | 11 | 5.9 | 15 (tie) | 13.1 | 16 | 24.7 | 18 | 7.4 | 17 | 59.6 | 12 | 59.9 |
| Texas | 14 | 5.1 | 12 | 14.7 | 12 | 28.2 | 14 | 8.7 | 12 | 68.4 | 22 | 41.8 |
| Virginia | 19 (tie) | 3.5 | 21 | 10.7 | 23 | 18.3 | 16 (tie) | 7.7 | 32 (tie) | 42.2 | 10 | 62.8 |
| West Virginia | 39 | 1.1 | 37 | 5.1 | 37 | 9.2 | 28 | 3.0 | 32 (tie) | 42.2 | 36 | 22.6 |
| United States | 4.9 | 15.0 | 27.4 | 9.5 | 27.1 | 48.0 | ||||||
Source: Henry J. Kaiser Family Foundation, www.statehealthfacts.org.
Centers for Disease Control and Prevention Defined Southern Region.
Age-adjusted death rate per 100,000 for year 2002. Source: Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics Report. 53(5); October 12, 2004.
District of Columbia.
Note: Shading is for ease of reading only.
From 1995 to 1998, the period following initial use of antiretroviral therapy (ART) in so-called highly active ART (HAART) regimens, AIDS incidence rates and AIDS-related deaths in the US declined 38% and 63%, respectively. Since 1999, AIDS-related deaths have alternately increased and decreased slightly between successive years, with a recent increase of 2.6% between 2002 and 2003 (CDC, 2004a; CDC, 2005). The South is the only region in the US to experience an increase in AIDS incidence every year since 2000, with a cumulative increase of 25% (CDC, 2005).
Engaging HIV medical care during early stages of disease and maintaining ongoing care provides numerous clinical benefits including receipt of HAART (Hogg et al., 2001; McNaghten et al., 1999; Palella et al., 1998, 2003; Phillips et al., 2001); OI prophylaxis (Kaplan et al., 2000; McNaghten et al., 1999); and treatment of co-infections, including other STDs. Treating HIV-infected persons co-infected with other STDs provides public health benefits by reducing HIV transmissibility (Cohen et al., 1997; Ghys et al., 1997). Other potential public health benefits provided by HIV medical care include reduced HIV transmissibility with consistent ART use (Gupta et al., 1997; Quinn et al., 2000; Vernazza et al., 1997a; Vernazza et al., 1997b), and promoting the adoption of safer sexual practices including condom use and decreased number of sex partners (CDC, 2003b; Schreibman et al., 2003).
Recent mortality trends and reversal of prior incidence trends in the US can be attributed to many factors, including delays in making the HIV diagnosis and seeking medical care. Prior research assessing predictors of delayed HIV diagnosis and presentation to medical care in the US has been published from large urban centers, primarily on the East and West coasts. Therefore, predictors of delayed presentation to HIV medical care identified by these studies may not be generalizable to the South where infected persons are increasingly non-white, female, poor, non-urban, and exposed to HIV heterosexually. Additionally, the tendency for multiple potential barriers to care exist among individual HIV-infected Southerners and the relative impact of these barriers is not fully understood. We briefly review the descriptive epidemiology of delayed HIV diagnosis and delayed HIV medical care; then provide a review of potential barriers to early receipt of HIV-medical care, with special reference to conditions prevalent in the South.
Delayed HIV diagnosis and medical care
Based on extrapolations from US population samples, one-quarter of HIV-infected persons are unaware of their infection (Fleming et al., 2002; Holtgrave et al., 2004). Furthermore, the CDC has estimated that 31% of HIV-ELISA and Western blot test-positive persons do not return for their results (CDC, 2003a). Recent FDA approval of rapid HIV testing strategies with same-hour results (CDC, 2003a) will likely increase the proportion of HIV-positive persons diagnosed early and who receive their results (Sy et al., 1998). However, the present circumstance with 30–40% of persons newly diagnosed receiving a concurrent AIDS diagnosis (Castilla et al., 2002; Dybul et al., 2002; Klein et al., 2003) severely limits the potential benefits of HIV medical care (Hogg et al., 2001; Palella et al., 2003; Phillips et al., 2001). At the University of Alabama at Birmingham (UAB) Outpatient Clinic for HIV/AIDS, referred to as the UAB 1917 Clinic, 53.6% of patients who presented with CDC-defined AIDS (n=498) between January, 1996 and December, 2004 were diagnosed with HIV in the year preceding their entry to care (Krawczyk et al., 2006).
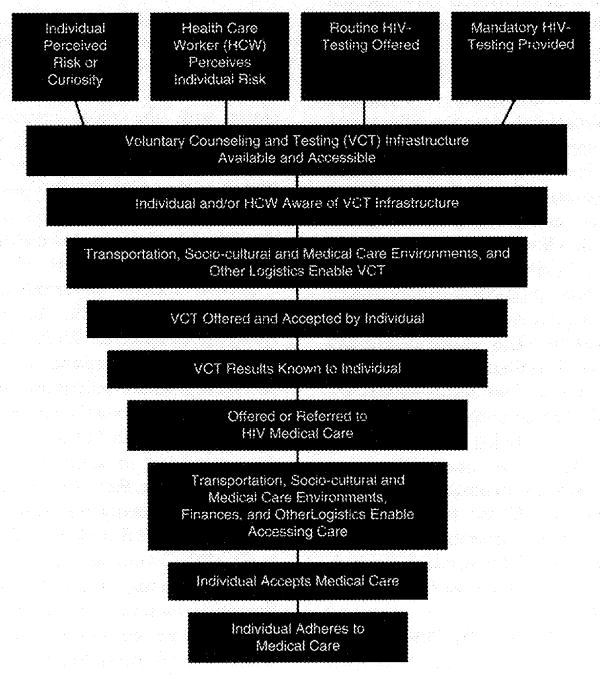
Delayed HIV diagnosis can be viewed as one of several key missing links in a care-prevention continuum (Figure 1). Following an HIV diagnosis, 25% of infected persons substantially delay accessing care, even for five years or more (Kilmarx et al., 1998; Samet et al., 1998; Siegel et al., 1997). At the UAB 1917 Clinic, 33.9% of HIV-positive persons delayed their first clinic visit more than one year following diagnosis, 43.4% of whom presented with CDC-defined AIDS (Krawczyk et al., 2006). Several studies have reported that nearly 75% of patients present for initial care with CD4+ cell counts already <500/μL, and 23–30% with counts ≤200/μL (Samet et al., 1998; Samet et al., 1994). A population-based estimate for any large sample of HIV-infected Southerners was not identified in the current literature.
Figure 1.
The HIV/AIDS Care/Prevention Continuum†
†:Horizontal scale represents attrition between stages among HIV-positive persons.
Potential barriers to HIV medical care in the Southern US
Research focusing on predictors of delayed HIV diagnosis and delayed presentation to medical care in the Southern US is limited. Therefore, here we will describe characteristics prevalent among HIV-infected Southerners that may be relevant to delayed HIV medical care, and provide supportive evidence extrapolated from documented experiences in other US regions. The potential barriers reviewed include: income and insurance, rural residence, HIV care for women, African-American race, and HIV risk exposure group. Of particular note in the following review, are the presence of multiple potential barriers among HIV-infected Southerners and the absence of prior research assessing their combined impact.
Income and Insurance
Persons of lower income and those at poverty level are more likely to report prior testing (Holtzman et al., 1998; Phillips, 1993) yet less likely to receive early diagnosis compared to persons of higher income (CDC, 2003c; Sorvillo et al., 1998). With the exception of HIV Cost and Services Utilization Study (HCSUS) results (Turner et al., 2000), most studies have suggested that uninsured HIV-infected persons and those with Medicaid receive comparatively suboptimal care (Schwarcz et al., 1997a; Shapiro et al., 1999; Siegel et al., 1997). Discrepancies across studies with regard to Medicaid recipients receiving suboptimal care may reflect state variation in services supported by Medicaid.
Poverty and the reliance of many HIV-infected Southerners on public assistance may have a tremendous impact on delayed HIV medical care but is not fully understood. Based upon three-year averages (2001–2003), the Southern region includes nine of the ten states with the highest proportion of individuals living in poverty, ranging from 7.7% to 18.5% of the population (U.S. Census Bureau 2004). Approximately 17 million southerners are uninsured (Southern States Manifesto 2003), including many HIV-infected persons who must rely on Medicaid, Medicare, disability insurance, the Ryan White CARE Act, and the AIDS Drug Assistance Program (ADAP) to receive necessary treatment. Among UAB 1917 Clinic patients, 51.9% are receiving publicly funded insurance; 45.1% of whom presented with CDC-defined AIDS, including 49.7% of those receiving Medicare or Medicaid (Krawczyk et al., 2006).
Medicaid is the largest source of public funding for HIV medical care in the US covering more than half of HIV-infected persons (HIV/AIDS Policy Fact Sheet. Medicaid and HIV/AIDS, 2004). However, some low income HIV-infected persons needing Medicaid benefits are ineligible until their medical expenses meet eligibility requirements (CMS, 2005), or their HIV disease progresses to meet the Social Security Administration’s definition of disability (AIDS Action, 2004). Medicaid eligibility and the services covered also vary substantially by state. For example, Medically Needy (MN) is an optional Medicaid eligibility group allowed by 35 states to spend down on medical expenses to meet income eligibility. Optional HIV/AIDS services states may support under Medicaid include prescription drug coverage, dental care, clinic services, case management, and hospice care (HIV/AIDS Policy Fact Sheet. Medicaid and HIV/AIDS, 2004).
ADAP serves as additional security for HIV-infected persons with each state administering its own ADAP, setting financial and clinical eligibility requirements, and authorizing HIV/AIDS drug availability. In the Southeast, financial eligibility criteria in 2004 ranged from 125% to 500% of the federal poverty level, with ADAP formularies ranging from 25 to 230 approved prescription drugs. Of the 18 states with ADAP restrictions, such as capped enrollments and waiting lists, nine are in the South (Table II) (NASTAD 2006). Data from the Healthcare Cost and Utilization Project (HCUP) suggest ADAP restrictions correlate with adverse health outcomes. From 1996 to 2000, HIV-related hospitalizations decreased by 33% in the eight states that were involved in the HCUP study. However, the two states with the most restrictive ADAP policies, Florida and South Carolina, achieved the smallest declines, 11% and 13% respectively (Hellinger, 2004). Given the large proportion of African-American ADAP clients in the South (Table II), these restrictions may have a disproportionate effect among African-Americans.
Table II.
Summary of Medicaid and AIDS Drug Assistance Program (ADAP) Restrictions, and ADAP Usage Among Southern States†.
| Proportions of ADAP Clients Who Are Black, 2004§ |
|||||
|---|---|---|---|---|---|
| Southern States | ADAP Restrictions(January, 2006)‡ | Receiving ADAP Supplemental Grant, 2004¶ | Receiving ADAP Emerging Communities Reward, 2004¶ | National Ranking | % |
| United States | 18 States Total (22 States Anticipated by March 2007) | 15 States Total | 20 States Total | 34 | |
| Alabama | Yes | Yes | Yes | 8 | 58 |
| Arkansas | Yes | 3 (tie) | 69 | ||
| Delaware | Yes | 11 | 54 | ||
| D.C.†† | 1 | 77 | |||
| Florida | Yes | 15 (tie) | 40 | ||
| Georgia | (Anticipated by 2007) | Yes | 6 | 64 | |
| Kentucky | Yes | Yes | Yes | 24 | 26 |
| Louisiana | Yes | Yes | Yes | 9 | 57 |
| Maryland | 5 | 67 | |||
| Mississippi | Yes | 2 | 70 | ||
| North Carolina | Yes | Yes | Yes | 10 | 55 |
| Oklahoma | Yes | Yes | Yes | 31 (tie) | 17 |
| South Carolina | (Anticipated by 2007) | Yes | Yes | 3 (tie) | 69 |
| Tennessee | Yes | Yes | 7 | 61 | |
| Texas | Yes | Yes | 23 | 27 | |
| Virginia | Yes | Yes | 12 (tie) | 52 | |
| West Virginia | Yes | Yes | 40 | 9 | |
Centers for Disease Control and Prevention Defined Southern Region.
ADAP restrictions include ADAP waiting lists, capped enrollments, income eligibility restrictions, medical eligibility restrictions, reduced formulary restrictions, and annual per capita expenditure limits. Source: National Alliance of State and Territorial AIDS Directors. (NASTAD). The ADAP Watch. February 1, 2006. www.nastad.org/documents/public/publicpolicy/2006-02-NASTAD_ADAP_Watch.pdf.
United States Government Accountability Office (GAO). Changes Needed to Improve the Distribution of Ryan White Care Act and Housing Funds; February, 2006.
Henry J. Kaiser Family Foundation, www.statehealthfacts.org.
District of Columbia.
Note: Shading is for ease of reading only.
Several federal government efforts have been implemented in response to unmet financial needs. As of 2000, all Ryan White Care Act grantees have been required to respond to “unmet need” defined as “HIV positive individuals that are aware of their status and not receiving regular medical care” (HRSA, 2004). In 2004, an additional $20 million were provided to states with ADAP waiting lists (White House Fact Sheet, 2004; White House, 2004). Additionally for fiscal year 2004, 15 states received ADAP Supplemental Grants earmarked to assist states with severe need, 10 of which were Southern states; and 20 states received ADAP Emerging Communities Reward funds awarded to states with a metropolitan area not receiving Title I Ryan White CARE Act funds despite having substantial AIDS burden, 11 of which were Southern states (GAO, 2006) (Table II).
Rural Residence
The CDC received 18,308 reports of AIDS diagnoses from non-metropolitan statistical areas (non-MSAs) during the first 13 years of the HIV-epidemic (1981–1993). This number more than doubled to 45,643 by the year 2000 (McKinney, 2002). The South accounted for more than two-thirds of non-MSA AIDS cases in 2001, with non-MSA Southerners accounting for 21% of the regions incident HIV-diagnoses (CDC, unpublished data). At the UAB 1917 Clinic, 25.8% of patients were from Alabama non-MSA, and 43.3% of these patients already had CDC-defined AIDS at first presentation (Krawczyk et al., 2006).
A higher proportion of HIV-infected rural residents are uninsured (19.6%) or publicly insured (13.4%) when compared to urban residents (14.3% and 11.1%, respectively) (McKinney 2002). The adverse impact of uninsured status among rural HIV-infected Southerners is clear; in a survey of non-urban HIV-infected Alabamians, 39% had no form of insurance and 16% had lost their insurance since becoming HIV-infected (Beltrami et al., 1999). In a separate survey of rural HIV-infected Alabama and Mississippi women, 36% reported unmet needs for HIV treatment as a result of being uninsured or having limited assistance from Medicaid (Moon et al., 2001). This additional reliance of many HIV-infected Southerners on public assistance further emphasizes the need to eliminate income and insurance-related barriers.
Most Federal funds for HIV medical care are allocated to locales based upon where a person was diagnosed. Many HIV-infected persons are diagnosed in large urban areas and then return home to rural areas for family support (Southern States Manifesto, 2003). Therefore, the already limited pool of Federal funds allocated to HIV medical care is disproportionately directed away from rural regions where many infected persons reside. Providers and facilities offering HIV medical care are also limited in rural regions. In 2001, the median number of physicians per 100,000 population was 85.6 (range: 57.3–125.1) in Northeastern non-MSAs compared to 55 (range: 42.2–70.5) in Southern non-MSAs (McKinney, 2002). One HIV clinic in Albany, Georgia covers 14 different counties with some patients traveling three hours to receive care (AIDS Alert, 2003). Rural areas also typically lack medical practitioners specializing in the treatment of HIV and AIDS (Southern States Manifesto, 2003). Due to the limited availability of physicians, facilities, and care funds, many HIV-infected rural residents elect to receive care in large urban areas. In a study of six urban infectious disease clinics in North Carolina, South Carolina, and Alabama, 75% of HIV-infected patients lived in rural areas, and the median commute to receive care was 43 miles (Whetten-Goldstein et al., 2001). At the UAB 1917 Clinic, 33.7% of patients traveled 50 miles or more to receive HIV care (Krawczyk et al., 2006). Concerns about confidentiality are an additional incentive for rural residents to seek care elsewhere (Reif et al., 2005; Whetten-Goldstein et al., 2001). The reliance of rural residents on urban clinics introduces additional potential barriers, such as transportation (Reif et al., 2005), time off work, lost wages, and childcare. These may also, in turn, affect HIV treatment adherence (Mohammed et al., 2004).
HIV Care for Women
In the 1980s and 1990s, the index of suspicion for HIV diagnosis was higher for men than women (Schoenbaum et al., 1993). More recently, HIV-infected women have sought earlier testing due to known or suspected sexual contact with a high risk or infected partner. In contrast, men are more likely to delay testing until they note symptoms (Biber et al., 1999; Klein et al., 2003). Combined with increased HIV-testing among pregnant women (Stringer et al., 2001), earlier trends have reversed such that women are now likely to be diagnosed earlier than men (Klein et al., 2003). In 2002, 41% of males and 32% of females received an AIDS diagnosis within 12 months of their HIV diagnosis (CDC, 2004a). While some studies suggest women also seek HIV medical care sooner than men (Guenter et al., 1999; Samet et al., 1998; Samet et al., 1994), others suggest that women may defer their own medical needs (Siegel et al., 1997; Siegel et al., 1998; Solomon et al., 1996; Sorvillo et al., 1998; Ungaro et al., 1998), particularly when they are caring for children (Shapiro et al., 1999; Siegel et al., 1997; Solomon et al., 1998). HCSUS data found 71% of HIV-infected women age 30–39 had one or more children under age 18, compared to only 19% of HIV-infected men; 70% of these women were unemployed (Schuster et al., 2000).
Women accounted for only 7% of nationwide AIDS cases in 1986 but represented 27% in 2004 (CDC, 2005). Among HIV-infected persons, AIDS-related mortality among women has increased almost 10% since 1999 compared to a 6.1% decrease among men (CDC, 2004a). An increasing proportion of female AIDS cases occur among those with heterosexual risk (63.4% in 1999, 70.7% in 2003), of African-American race/ethnicity (67% in 2003), and those residing in the South (41%) (CDC, 2002; Hader et al., 2001). Data from 2003 indicated that eight of the 10 states with the highest AIDS case rates among women were located in the South (Table I), likely due in part to poorer access to early diagnosis and medical care. African-Americans are disproportionately represented in the Southern epidemic among women, accounting for 77% of incident female AIDS cases in 2001 (CDC, unpublished data). Women are also more likely than other groups, according to HCSUS data, to experience physical harm related to their positive HIV status (10.3% of women, 4.5% of MSM, and 3.2% of other men) (Zierler et al., 2000). Given the increasing proportion of HIV-infected females in the South, and the tendency for many Southern infected females to be African-American, poor, lacking insurance, and/or residing in rural areas, it is likely that their unmet needs even exceed the considerable needs of women elsewhere in the US (Holmes et al., 1997).
African-Americans
Evidence in the US is inconsistent regarding race/ethnicity and the timing of HIV diagnosis (Holtzman et al., 1998; Phillips, 1993), though non-Hispanic whites generally receive earlier testing than minorities. African-Americans may be more likely to refuse testing (Simon et al., 1996), fail to receive test results (Valdiserri et al., 1993; Wiley et al., 1998), report no prior testing history (Grinstead et al., 1997; Schwarcz et al., 1997b), and delay seeking HIV medical care (Turner et al., 2000).
HIV and AIDS are increasingly prevalent among minorities, particularly among African-Americans. Among persons living with AIDS (PLWA) in the US at the end of 2004, 42.9% were African-American resulting in an AIDS rate of 56.4 per 100,000 population among African-Americans compared to the national rate of 14.1 per 100,000 (CDC, 2005). In 2002, African-Americans represented 12% of the US population, but accounted for 50% of new AIDS diagnoses (CDC, 2002). Similar to national data, African-Americans in the South accounted for 53% of PLWA despite comprising just 19% of the region’s population (U.S. Census Bureau, 2002). However, in 2004 the South accounted for all ten states with the highest proportion of incident AIDS cases occuring among African-Americans (Table I). HIV/AIDS incidence data from Alabama in the year 2003 further illustrates the disparity between races where the rates per 100,000 were: White males–11.9, African-American males–73.3; White females–2.8, African-American females–34.6 (ADPH, 2003).
Black Americans have an uninsured rate that is 50% greater than the rate for non-Hispanic Whites (Southern States Manifesto, 2003). Although many HIV-infected African-Americans do receive publicly financed support for their HIV medical care, about 30% of those living below 200% federal poverty level are not covered by Medicaid (Southern States Manifesto, 2003). The tendency for HIV-infected African-Americans to be uninsured and ineligible for needed Medicaid benefits may increase the likelihood of delayed presentation to care. At the UAB 1917 Clinic, African-Americans represented 54.8% of publicly insured persons presenting to care with CDC-defined AIDS; non-Hispanic Whites accounted for 40.6% (Krawczyk et al., 2006). African-Americans may also be reluctant to access medical care because of injustices in the past by physicians and researchers, most notably the Tuskegee Syphilis Study, and the widespread belief that HIV is a form of government genocide (AIDS Alert, 2003; Whetten et al., 2004). The persistent legacy of the Tuskegee Syphilis Study is especially pronounced in the South where the study took place. Stigma associated with HIV risk behaviors may further reduce access to HIV medical care among African-Americans (Southern States Manifesto, 2003). This is particularly true among IDUs and young African-American MSM, the latter of which represented 29% of all new AIDS diagnoses and 32% of all new HIV diagnoses among Southerners in 2001 (CDC, unpublished data). At the UAB 1917 Clinic, Black MSM were 22% more likely than White MSM to present for initial care with CDC-defined AIDS (p = 0.06) (Krawczyk et al., 2006).
Given the combined stigma of being HIV-infected and a minority, common suspicions about health care providers, and the prevalence of female gender, poverty, and being uninsured or publicly insured, infected African-Americans in the South may be especially susceptible to delayed medical care. However, the combined effects of these barriers on delayed HIV care are not fully understood, particularly among African-Americans in the South.
HIV Risk Exposure Group
Heterosexually exposed persons have been shown to seek testing late in disease (CDC, 2003c). Persons exposed to HIV through homosexual behavior or IDU may also delay seeking care (Guenter et al., 1999; Samet et al., 1998). The proportion of incident AIDS diagnoses among different HIV risk exposure groups is similar when comparing the South (CDC, unpublished data) to the entire US (CDC, 2002) with approximately 40%, 30%, and 20% occurring among MSM, persons with heterosexual risk, and IDUs, respectively. African-Americans accounted for 46% of Southern MSM AIDS diagnoses in 2001 (CDC, unpublished data), compared to 36% among nationwide MSM AIDS diagnoses (CDC 2002). Statistics to support regional variation in HIV incidence are less readily available, and as a result, regional variations in incidence trends by risk exposure group are under-documented. Related evidence does anecdotally conclude that incident cases of heterosexually acquired HIV are increasing in the Southern US. Evidence includes the incidence of other STD’s (CDC, 2004b); trends in HIV incidence by race/ethnicity, gender, and area of residence in the South (ADPH, 2003; CDC, 2004a); the proportion of population who are minority women (CDC, 2002; CDC, 2004a; Hader et al., 2001); and expert opinion (Southern States Manifesto, 2003).
Fear of being diagnosed is a common deterrent to HIV-testing, especially among high-risk non-whites (Sy et al., 1998). Among 5649 young MSM surveyed in six US cities between 1994 and 2000, 91% of HIV-infected African-Americans were unaware of their HIV status, and 59% of this subgroup perceived themselves to be at low or very low risk of acquiring HIV (MacKellar et al., 2005). MSMs are more likely to seek testing than other high risk groups (Norton et al., 1997; Phillips et al., 1995), but some MSMs delay testing due to a fear of a positive result (Billington et al., 1998; Stall et al., 1996). MSMs who are well integrated in the gay community and visit gay venues regularly are more likely to be tested for HIV (Rebchook et al., 1998). However, these gay social structures are relatively lacking in many areas of the South, and are virtually non-existent in the Black community. Many MSM, particularly African-Americans in the South, frequently encounter community, family, and faith-based stigma and frank hostility. Among UAB 1917 Clinic patients, 46.3% of MSM presented for initial care with CDC-defined AIDS, compared to 39.8% of heterosexuals (p = 0.07). African-Americans accounted for a larger proportion of MSM presenting with AIDS than Whites, as previously mentioned (Krawczyk et al., 2006). The combined effects of HIV risk exposure group and other potential barriers to care, such as race and gender, have not been fully evaluated in the South. Additional understanding might be expected to improve seeking of HIV diagnosis and care among certain risk populations, such as minority MSM and heterosexually infected women, through interventions specifically targeting the needs of these groups.
Summary and recommendations
We have provided here a review of the clinical and public health benefits related to early HIV diagnosis and entry into HIV medical care, the descriptive epidemiology of observed delays, and the prevalence of known barriers to delayed care in the Southern US. The extent to which these barriers exist in the South undoubtedly inhibits the HIV care-prevention continuum (Figure 1). Risk perception, access to generalized medical care, prior or current incarceration, social support, HIV/AIDS-related stigma, negative physical and emotional consequences following diagnosis, and transient residence may influence delayed HIV medical care, but are also not well understood in the South. To comprehend and alleviate the barriers described herein and other barriers to HIV testing and care, additional research focusing on the Southern region is much needed. Such research includes: (1) first identifying and better understanding the specific barriers to timely receipt of HIV diagnosis and medical care in the South; (2) assessing the independent, synergistic, and/or modifying effects of these barriers; and (3) developing and evaluating prevention interventions and medical care models to reduce barriers to early HIV diagnosis and receipt of medical care.
Acknowledgments
This work was supported in part by National Institutes of Health grant number 5P30AI027767-17. The authors would also like to thank the following for their contributions to this work: from The University of Alabama at Birmingham School of Public Health, Dr. Richard Kaslow, Dr. Christopher Coffey, and Amita Bey; from the 1917 Outpatient Clinic in Birmingham, Alabama: Dr. Michael Saag, Dr. Jim Raper, Davendra Sohal, Ashlee Chatham, the health care providers and patients; and from Cooper Green Hospital St. Georges Clinic: Ann Atkinson.
References
- ADPH. State of Alabama Integrated Epidemiologic Profile HIV/AIDS Prevention and Care, 2003 2003 [Google Scholar]
- AIDS directors seek help to fight epidemic in the South. AIDS Alert. 2003;18:54–57. [PubMed] [Google Scholar]
- Beltrami JF, Vermund SH, Fawal HJ, et al. HIV/AIDS in nonurban Alabama: Risk activities and access to services among HIV-infected persons. Southern Medical Journal. 1999;92:677–683. doi: 10.1097/00007611-199907000-00006. [DOI] [PubMed] [Google Scholar]
- Biber CL, Jaker MA, Kloser P, et al. A study of sex differences in presentation for care of HIV. AIDS Patient Care and STDs. 1999;13:103–110. doi: 10.1089/apc.1999.13.103. [DOI] [PubMed] [Google Scholar]
- Billington A, Imrie JC, McOwan AG, et al. Young gay men attending a dedicated service are less likely to test for HIV than peers using routine clinic services: why?. 12th World AIDS Conference; Geneva. 1998. [Google Scholar]
- Castilla J, Sobrino P, de la Fuente L, et al. Late diagnosis of HIV infection in the era of highly active antiretroviral therapy: Consequences for AIDS incidence. AIDS. 2002;16:1945–1951. doi: 10.1097/00002030-200209270-00012. [DOI] [PubMed] [Google Scholar]
- CDC. HIV/AIDS Surveillance Report 2002. Centers for Disease Control and Prevention. 2002:14. [Google Scholar]
- CDC. Advancing HIV Prevention: New strategies for a changing epidemic-United States, 2003. MMWR. 2003a;52:329–332. [PubMed] [Google Scholar]
- CDC. Incorporating HIV prevention into the medical care of persons living with HIV. MMWR. 2003b;52(RR12):1–24. [Google Scholar]
- CDC. Late versus early testing of HIV-16 sites, United States, 2000–2003. MMWR. 2003c;52:581–586. [PubMed] [Google Scholar]
- CDC. HIV/AIDS Surveillance Report 2003. Centers for Disease Control and Prevention. 2004a:15. [Google Scholar]
- CDC. Sexually Transmitted Disease Surveillance, 2003 2004b [Google Scholar]
- CDC. HIV/AIDS Surveillance Report 2004. Centers for Disease Control and Prevention. 2005:16. [Google Scholar]
- CMS. Centers for Medicare & Medicaid Services; 2005. www.cms.hhs.gov/hiv/hivfs.asp. [PubMed] [Google Scholar]
- Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- Dybul M, Bolan R, Condoluci D, et al. Evaluation of initial CD4+ T cell counts in individuals with newly diagnosed human immunodeficiency virus infection, by sex and race, in urban settings. The Journal of Infectious Diseases. 2002;185:1818–1821. doi: 10.1086/340650. [DOI] [PubMed] [Google Scholar]
- Early Treatment for HIV Act: Expanding care through Medicaid. Until It’s Over AIDS Action Policy Brief. 2004 [PubMed] [Google Scholar]
- Fact sheet: Extending and improving the lives of those living with HIV/AIDS. 2004 [Google Scholar]
- Fleming P, Byers RH, Sweeney PA, et al. HIV prevalence in the United States, 2000. 9th Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. 2002. [Google Scholar]
- GAO. Changes needed to improve the distribution of Ryan White CARE Act and housing funds. United States Government Accountability Office. Report to Congressional Requesters. 2006;Appendix III:82–83. [Google Scholar]
- Ghys PD, Fransen K, Diallo MO, et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosupression in female sex workers in Abidjan, Cote d’Ivoire. AIDS. 1997;11:F85–93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- Grinstead OA, Peterson JL, Faigeles B, et al. Antibody testing and condom use among heterosexual African Americans at risk for HIV infection: the National AIDS Behavioral Surveys. American Journal of Public Health. 1997;87:857–859. doi: 10.2105/ajph.87.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenter CD, Gill MJ. A population with short delay from diagnosis of human immunodeficiency virus to medical care. Archives of Internal Medicine. 1999;159:758–759. doi: 10.1001/archinte.159.7.758. [DOI] [PubMed] [Google Scholar]
- Gupta P, Mellors JW, Kingsley L, et al. High viral load in semen of human immunodeficiency virus type-1 infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. Journal of Virology. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hader SL, Smith DK, Moore JS, et al. HIV infection among women in the United States: Status at the millenium. JAMA. 2001;285:1186–1192. doi: 10.1001/jama.285.9.1186. [DOI] [PubMed] [Google Scholar]
- Hellinger FJ. HIV patients in the HCUP database: A study of hospital utilization and cost. Inquiry. 2004;41:95–105. doi: 10.5034/inquiryjrnl_41.1.95. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- Holmes R, Fawal H, Moon TD, et al. Acquired immunodeficiency syndrome in Alabama: Special concerns for African-American women. Southern Medical Journal. 1997;90:697–701. doi: 10.1097/00007611-199707000-00009. [DOI] [PubMed] [Google Scholar]
- Holtgrave DR, Anderson T. Utilizing HIV transmission rates to assist in prioritizing HIV prevention services. International Journal of STD & AIDS. 2004;15:789–792. doi: 10.1258/0956462042563639. [DOI] [PubMed] [Google Scholar]
- Holtzman D, Rubinson R, Bland SD, et al. HIV testing behavior and associated characteristics among US adults, 1993–1994. AIDS Behavior. 1998;2:269–281. [Google Scholar]
- HRSA. Health Resources and Services Administration; 2004. Available at www.hrsa.gov. [Google Scholar]
- Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2000;30:S5–14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- Kilmarx PH, Hamers FF, Peterman TA. Living with HIV: experience and perspectives of HIV-infected sexually transmitted disease clinic patients after posttest counseling. Sexually Transmitted Diseases. 1998;25:28–37. doi: 10.1097/00007435-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Klein D, Hurley LB, Merrill D, et al. Review of medical encounters in the 5 years before a diagnosis of HIV-1 infection: Implications for early detection. Journal of Acquired Immunodeficiency Syndromes. 2003;32:143–152. doi: 10.1097/00126334-200302010-00005. [DOI] [PubMed] [Google Scholar]
- Krawczyk CS, Funkhouser E, Kilby MJ, et al. Factors associated with delayed initiation of HIV medical care among infected persons attending a southern HIV/AIDS Clinic. Southern Medical Journal. 2006;99 doi: 10.1097/01.smj.0000215639.59563.83. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKellar DA, Valleroy LA, Secura GM, et al. Unrecognized HIV infection, risk behaviors, and perceptions of risk among young men who have sex with men. Journal of Acquired Immunodeficiency Syndromes. 2005;38:603–614. doi: 10.1097/01.qai.0000141481.48348.7e. [DOI] [PubMed] [Google Scholar]
- McKinney M. Variations in rural AIDS epidemiology and service delivery models in the United States. The Journal of Rural Health. 2002;18:455–465. doi: 10.1111/j.1748-0361.2002.tb00910.x. [DOI] [PubMed] [Google Scholar]
- McNaghten AD, Hanson DL, Jones JL, et al. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis. AIDS. 1999;13:1687–1695. doi: 10.1097/00002030-199909100-00012. [DOI] [PubMed] [Google Scholar]
- Medicaid and HIV/AIDS. HIV/AIDS Policy Fact Sheet. The Henry J. Kaiser Family Foundation; Sep, 2004. Available at: www.kff.org. [Google Scholar]
- Mohammed H, Kieltyka L, Richardson-Alston G, et al. Adherence to HAART among HIV-infected persons in rural Louisiana. AIDS Patient Care and STDs. 2004;18:289–296. doi: 10.1089/108729104323076025. [DOI] [PubMed] [Google Scholar]
- Moon TD, Vermund SH, Tong TC, et al. Opportunities to improve prevention and services for HIV-infected women in nonurban Alabama and Mississippi. Journal of Acquired Immunodeficiency Syndromes. 2001;28:279–281. doi: 10.1097/00042560-200111010-00013. [DOI] [PubMed] [Google Scholar]
- NASTAD. The ADAP Watch. National Alliance of State and Territorial AIDS Directors. 2006 February 1, 2006, Available at: www.nastad.org/documents/public/publicpolicy/2006-02-NAS-TAD_ADAP_Watch.pdf.
- Norton J, Elford J, Sherr L, et al. Repeat HIV testers at a London same-day testing clinic. AIDS. 1997;11:731–781. [PubMed] [Google Scholar]
- Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Annals of Internal Medicine. 2003;138:620–626. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- Phillips AN, Staszewski S, Weber R, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA. 2001;286:2560–2567. doi: 10.1001/jama.286.20.2560. [DOI] [PubMed] [Google Scholar]
- Phillips KA. Factors associated with voluntary HIV testing for African-Americans and Hispanics. AIDS Education and Prevention. 1993;5:95–103. [PubMed] [Google Scholar]
- Phillips KA, Paul J, Kegeles S, et al. Predictors of repeat HIV testing among gay and bisexual men. AIDS. 1995;9:769–775. doi: 10.1097/00002030-199507000-00015. [DOI] [PubMed] [Google Scholar]
- President’s remarks: President Bush discusses HIV/AIDS initiatives. Philadelphia: 2004. [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New England Journal of Medicine. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Rebchook G, Hays RB, Kegeles SB. Gay community integration predicts HIV testing among US young gay men. 12th World AIDS Conference; Geneva. 1998. [Google Scholar]
- Reif S, Golin CE, Smith SR. Barriers to accessing HIV/AIDS care in North Carolina: rural and urban differences. AIDS Care. 2005;17:558–565. doi: 10.1080/09540120412331319750. [DOI] [PubMed] [Google Scholar]
- Samet JH, Freedberg KA, Stein MD, et al. Trillion virion delay: Time from testing positive for HIV to presentation for primary care. Archives of Internal Medicine. 1998;158:734–740. doi: 10.1001/archinte.158.7.734. [DOI] [PubMed] [Google Scholar]
- Samet JH, Retondo MJ, Freedberg KA, et al. Factors associated with initiation of primary medical care for HIV-infected persons. The American Journal of Medicine. 1994;97:347–353. doi: 10.1016/0002-9343(94)90301-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum EE, Webber MP. The underrecognition of HIV infection in women in an inner-city emergency room. American Journal of Public Health. 1993;83:363–368. doi: 10.2105/ajph.83.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibman T, Friedland G. Human immunodeficiency virus infection prevention: Strategies for clinicians. Clinical Infectious Diseases. 2003;36:1171–1176. doi: 10.1086/374359. [DOI] [PubMed] [Google Scholar]
- Schuster MA, et al. HIV-infected parents and their children in the United States. American Journal of Public Health. 2000;90:1074–1081. doi: 10.2105/ajph.90.7.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz SK, Katz MH, Hirozawa A, et al. Prevention of Pneumocystis carinii pneumonia: who are we missing. AIDS. 1997a;11:1263–1268. doi: 10.1097/00002030-199710000-00010. [DOI] [PubMed] [Google Scholar]
- Schwarcz SK, Spitters C, Ginsberg MM, et al. Predictors of human immunodeficiency virus counselling and testing among sexually transmitted disease clinic patients. Sexually Transmitted Diseases. 1997b;24:347–352. doi: 10.1097/00007435-199707000-00007. [DOI] [PubMed] [Google Scholar]
- Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: Results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- Siegel K, Karus D, Raveis VH. Testing and treatment behavior of HIV-infected women: white, African-American, Puerto Rican comparisons. AIDS Care. 1997;9:297–309. doi: 10.1080/713613154. [DOI] [PubMed] [Google Scholar]
- Siegel K, Raveis VH, Gorey E. Barriers and pathways to testing among HIV-infected women. AIDS Education and Prevention. 1998;10:114–127. [PubMed] [Google Scholar]
- Simon PA, Weber M, Ford WL, et al. Reasons for HIV antibody test refusal in a heterosexual sexually transmitted disease clinic population. AIDS. 1996;10:1549–1553. doi: 10.1097/00002030-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Solomon L, Frank R, Vlahov D, et al. Utilization of health services in a cohort of intravenous drug users with known HIV-1 serostatus. American Journal of Public Health. 1991;81:1285–1290. doi: 10.2105/ajph.81.10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L, Moore J, Gleghorn A, et al. HIV testing behaviors in a population of inner-city women at high risk for HIV infection. Journal of Acquired Immunodeficiency Syndromes. 1996;13:267–272. doi: 10.1097/00042560-199611010-00009. [DOI] [PubMed] [Google Scholar]
- Solomon L, Stein M, Flynn C, et al. Health services use by urban women with or at risk for HIV-1 infection: the HIV Epidemiology Research Study (HERS) Journal of Acquired Immunodeficiency Syndromes. 1998;17:253–261. doi: 10.1097/00042560-199803010-00011. [DOI] [PubMed] [Google Scholar]
- Sorvillo F, Kerndt P, Bunch G, et al. Early HIV detection: successes and failures. 12th World AIDS Conference; Geneva. 1998. [Google Scholar]
- Southern States Manifesto. HIV/AIDS and STDs in the South: A Call to Action. 2003 [Google Scholar]
- Stall R, Hoff C, Coates TJ, et al. Decisions to get HIV tested and to accept antiretroviral therapies among gay/bisexual men: implications for secondary prevention efforts. Journal of Acquired Immunodeficiency Syndromes. 1996;11:151–160. doi: 10.1097/00042560-199602010-00006. [DOI] [PubMed] [Google Scholar]
- Stringer EM, Stringer JS, Cliver SP, et al. Evaluation of a new testing policy for human immunodeficiency virus to improve screening rates. Obstetrics and Gynecology. 2001;98:1104–1108. doi: 10.1016/s0029-7844(01)01631-3. [DOI] [PubMed] [Google Scholar]
- Sy F, Smith D, Thompson S, et al. The association of perceived risk for HIV infection and self-reports of HIV antibody testing in a predominantly rural US southern state. 12th World AIDS Conference; Geneva. 1998. [Google Scholar]
- Sy FS, Rhodes SD, Choi ST, et al. The acceptability of oral fluid testing for HIV antibodies: a pilot study in gay bars in a predominantly rural state. Sexually Transmitted Diseases. 1998;25:211–215. doi: 10.1097/00007435-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Time Series of State Population Estimates. United States Bureau of the Census 2002 2002 [Google Scholar]
- Turner BJ, Cunningham WE, Duan N, et al. Delayed medical care after diagnosis in a US national probability sample of persons infected with human immunodeficiency virus. Archives of Internal Medicine. 2000;160:2614–2622. doi: 10.1001/archinte.160.17.2614. [DOI] [PubMed] [Google Scholar]
- Ungaro AB, Luppi CG, Buccheri V, et al. CD4+ T lymphocyte counts of HIV infected women seeking an anonymous counseling/testing service in Sao Paulo, Brazil. 12th World AIDS Conference; Geneva. 1998. [Google Scholar]
- U.S. Census Bureau. 2004 Available at: www.census.gov.
- Valdiserri RO, Moore M, Gerber AR, et al. A study of clients returning for counselling after HIV testing: implications for improving rates of return. Public Health Reports. 1993;108:12–18. [PMC free article] [PubMed] [Google Scholar]
- Vernazza PL, Gilliam BL, Dyer J, et al. Quantification of HIV in semen: correlation with antiretroviral treatment and immune status. AIDS. 1997a;11:987–993. [PubMed] [Google Scholar]
- Vernazza PL, Gilliam BL, Flepp M, et al. Effect of antiviral treatment on the shedding of HIV-1 in semen. AIDS. 1997b;11:1249–1254. doi: 10.1097/00002030-199710000-00008. [DOI] [PubMed] [Google Scholar]
- Whetten K, Reif S, Lowe K, et al. Gender differences in knowledge and perceptions of HIV resources among individuals living with HIV in the Southeast. Southern Medical Journal. 2004;97:342–349. doi: 10.1097/01.SMJ.0000118902.64603.A5. [DOI] [PubMed] [Google Scholar]
- Whetten-Goldstein K, Quyen Nguyen T, Heald AE. Characteristics of individuals infected with the human immunodeficiency virus and provider interaction in the predominantly rural Southeast. Southern Medical Journal. 2001;94:212–222. [PubMed] [Google Scholar]
- Wiley DJ, Frerichs RR, Ford WL, et al. Failure to learn human immunodeficiency virus test results in Los Angeles public sexually transmitted disease clinics. Sexually Transmitted Diseases. 1998;25:342–345. doi: 10.1097/00007435-199808000-00003. [DOI] [PubMed] [Google Scholar]
- Zierler S, Cunningham WE, Andersen R, et al. Violence victimaztion after HIV infection in a US probablilty sample of adult patients in primary care. American Journal of Public Health. 2000;90:208–215. doi: 10.2105/ajph.90.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]