Abstract
The human visual system undergoes continuous anatomical, physiological and functional changes throughout the life span. There is also continuous change in the spectral distribution and intensity of light reaching the retina from infancy through senescence, primarily due to changes in the absorption of short-wave light by the lens. Despite these changes in the retinal stimulus and the signals leaving the retina for perceptual analysis, color appearance is relatively stable during aging as measured by broadband reflective or self-luminous samples, the wavelengths of unique blue and yellow, and the achromatic locus. Measures of ocular media density for younger and older observers show, indeed, that color appearance is independent of ocular media density. This may be explained by a renormalization process that was demonstrated by measuring the chromaticity of the achromatic point before and after cataract surgery. There was a shift following cataract surgery (removal of a brunescent lens) that was initially toward yellow in color space, but over the course of months, drifted back in the direction of the achromatic point before surgery. The spatial characteristics of color mechanisms were quantified for younger and older observers in terms of chromatic perceptive fields and the chromatic contrast sensitivity functions. Younger and older observers differed with small spots or with chromatic spatial gratings near threshold, but there were no significant differences with larger spots or suprathreshold spatial gratings.
Keywords: color appearance, aging, photoreceptors, chromatic contrast sensitivity, ocular media, white point
1. Introduction
The fundoscopic image of the normal retina appears stable until about the age of 50 years, or even longer in many cases. We now recognize, however, that this stability is an illusion. Beneath this apparent stability is a struggle between senescent degradation on the one hand, and molecular renewal of ocular and neural structures on the other. Senescence is so apparent to those who study it that it is easy to lose track of the wonder that the visual system functions so well for as long as it does. This is possible, in part, because visual cells are engaged in virtually constant molecular reconstruction from the first days of life.1 These molecular processes allow for the repair and replacement of all the cellular constituents except for the DNA following the inevitable insult that occurs from several sources in the retina, including the radiation it was designed to detect.2 The organization of neural networks also supports stability of vision during senescence by virtue of processing algorithms that must operate over a wide range of light levels and noisy inputs. Related to these algorithms is the ability of the visual system to adapt over variable time scales to changes in afferent signals due to changes in the eye’s optics and loss of cells. Taken together, the balance between senescent degradation and molecular renewal is tipped in the direction of senescence such that blindness itself is thought by some authorities to be inevitable by the age of 120 years.3
Among the most salient anatomical and physiological changes affecting the signals generated by the central retina are reductions in the retinal4 and choroidal blood supply,5 a decrease in cell density of the retinal pigment epithelium,6 a reduction in photoreceptor numbers7 and changes in photoreceptor morphology,8,9 and a loss of retinal ganglion cells.10,11 While large losses in ganglion cell density are needed to produce measurable changes in visual fields,12 the consequences of retinal aging are readily demonstrated by direct recordings of the global rod and cone high-intensity a-wave electroretinogram (ERG) responses13 and in the more localized multifocal ERG response.14 The latter functionally oriented studies support the view that aging is continuous from adolescence or early adulthood.
Psychophysical studies provide a clearer picture of senescent changes in human vision, perhaps because they depend upon an intact system doing what it was designed to do. Numerous psychophysical studies have examined age-related changes in sensitivity of the three classes of cone photoreceptors that define normal trichromatic vision. Absolute sensitivity of each cone class increases after infancy15 and continues through adolescence.16 Beyond the teenage years, numerous studies have demonstrated S-cone sensitivity decreases with age,17,18 and some,16,19,20 but not all,21 studies have shown similar losses in the sensitivity of M- and L-cone mechanisms.
The most common method for measuring cone sensitivity (or its inverse, threshold) is to vary the intensity of a monochromatic light superimposed on a chromatic adapting field until the test stimulus reaches detection threshold. The purpose of the adapting field is to suppress the sensitivity of two of the three classes of cones and the rods, leaving the third cone type isolated to mediate sensitivity.22,23 For example, an intense yellow adapting background will suppress M- and L-cone pathways, rendering the S-cone pathway relatively more sensitive. Under these conditions of chromatic adaptation, an S-cone pathway can be isolated for wavelengths below about 510 nm, depending on the intensity of the background. At longer wavelengths, sensitivity is mediated by M- and/or L-cone pathways. Although univariant cone mechanisms are not isolated completely with these methods,24 it is nevertheless possible to measure cone sensitivity to a good first approximation over parts of the spectrum. The quality of cone pathway isolation can be improved by judicious choice of temporal parameters of the test stimuli owing to the slower response of S-cone mechanisms under most25 but not all26 conditions. For mechanism isolation at longer wavelengths, brief test flashes are more likely to be determined by the quantum catch of a single class of cone,27 whereas longer test flash durations are more likely to be detected through a chromatically-opponent pathway.28 From these considerations, it is apparent that variations in stimulus parameters among studies may have led to assessing sensitivity under conditions that complicate the comparison of different mechanisms and hence have produced somewhat varying results in the literature.
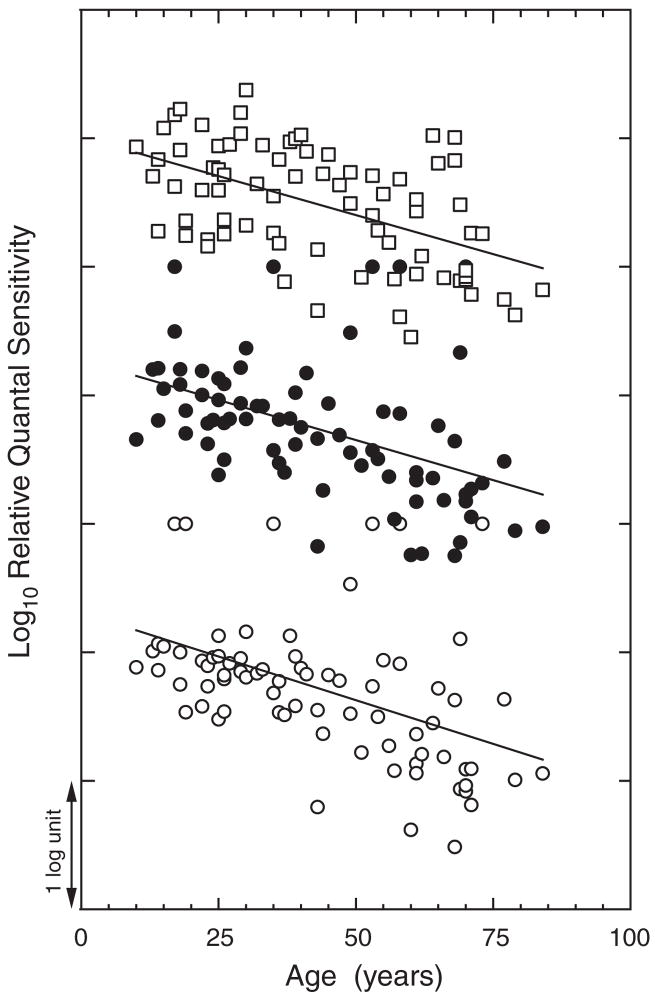
An example of age-related changes in sensitivity of S-, M-and L-cone mechanisms is presented in Fig. 1. Subjects were sampled evenly over the age range of about 12 to 88 years. Each datum represents average sensitivity across wavelengths for which one cone class dominated sensitivity: Scones (440, 460, 500 nm); M- and L-cones (500, 530, 560, 590 and 620 nm). The decline in sensitivity with advancing age is significant for each of the three cone classes, but there is no significant difference among the slopes for the different cone types. Results consistent with these have been reported by Knoblauch Vital-Durand and Barbur16 based on a different task, detection of chromatic stimuli falling on dichromatic confusion lines that were embedded in a spatiotemporal achromatic noise field. There are two difficulties in interpreting these and related studies. First, age-related changes in sensitivity depend on the level of cone pathway adaptation.29,30 Cone adaptation can be controlled by more elaborate methods such as by measuring cone thresholds on the plateau of the threshold vs. intensity function determined separately for each individual and each cone mechanism.20 A second factor that must be taken into account to understand age-related changes in cone sensitivity is the contribution of lens and macular pigmentation, as variation in these preretinal pigments will alter the intensity of short-wave lights reaching the retina. Macular pigment density depends upon dietary factors more than upon age,20 while the ocular media (mostly lens) increases substantially with age.31,32 Only a few studies have obtained individual measures to correct sensitivities of cone mechanisms for ocular media and macular pigment density, although many have included estimates of preretinal factors. It is clear from all of these approaches that the loss in sensitivity of the individual cone pathways is due to both optical and neural factors. To these considerations it may be added that when stimuli are presented in Newtonian view, as opposed to Maxwellian view, a correction for age-related changes in pupillary area33 is needed if one wishes to specify sensitivity in terms of the retinal stimulus. It is also evident from considering these factors and numerous studies that have included individuals covering the entire life span that what we call normal cone sensitivity changes continuously with age, and what we call aging begins at least by adolescence.
Fig. 1.
Log relative sensitivity (quanta delivered to the cornea) is plotted as a function of age for mechanisms dominated by either S-(unfilled squares), M-(filled circles), or L-(unfilled circles) cone mechanisms. The data are normalized arbitrarily along the sensitivity axis for each cone mechanism. Least-squares linear regression lines are shown for each cone type. [Data from Werner and Steele19].
2. Hue Mechanisms and Aging
The large losses in sensitivity of cone mechanisms described in the previous section and elsewhere (see reviews by Weale,34 Knau and Werner35) have been important for understanding senescent physiological changes in the retina and visual pathways. From both a theoretical and practical perspective, it is also important to ask what age-related changes take place in the suprathreshold world in which we usually function. Until recently, relatively little research has addressed this issue.
Color appearance depends upon the post-receptoral combinations of cone signals. Psychophysically, these post-receptoral combinations give rise to blue-yellow, red-green and black-white dimensions of color appearance, with each pair organized in an opponent manner.36 In opponent-color theory, a hue that is uniquely blue or yellow defines a state in which a red-green process is in equilibrium. Similarly, unique red and green define the hue equilibria for a theoretical blue-yellow process.37 Because of their importance in color theory, we measured the wavelength of the spectral unique hues as a function of age. One set of results is shown in Fig. 2. Unique blue and unique yellow differed among individuals of the same age as expected from previous literature, but there was no significant shift across the age range tested. The same results were found in this study at other light levels tested38 and with a replication using other methods.39 One interpretation of this finding is based on evidence that the red-green process is derived from a linear combination of signals from the three cone classes under the conditions of our measurements. The invariance across age might be expected if the receptor signals to a red-green process are reduced equally in amplitude for all three cone types, hence maintaining constant cone signal ratios across the life span. Figure 2 also shows that unique green does change as a function of age. This result is difficult to interpret because the blue-yellow chromatic process combines the cone signals nonlinearly.40,41 Thus, with stimuli equated at the cornea for younger and older observers, the latter group was tested at a lower retinal illuminance which might have produced a shift in unique green with age.
Fig. 2.
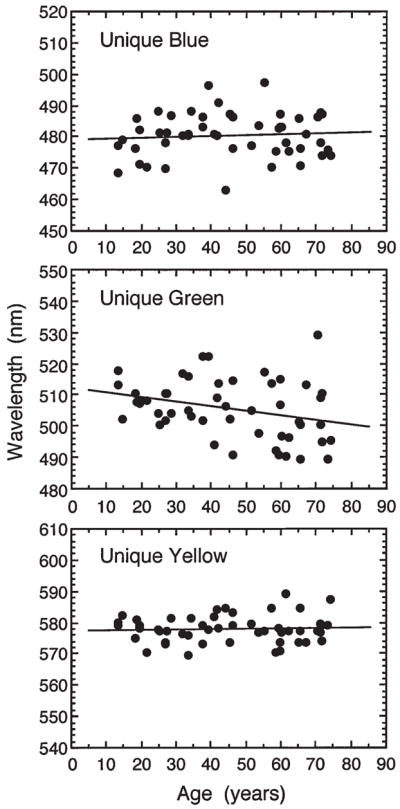
Wavelengths of the spectral unique hues plotted as a function of age for 0.95° diameter, foveally-viewed stimuli (7.1 cd·m−2). Solid lines are based on a least-squares linear regression equation. [Data from Schefrin and Werner38].
Stability of unique hues would be expected to promote constancy of color appearance across the life span as other stimuli may be considered combinations of these fundamental hues. Tests of unique hues are special cases, however, because they are measured with monochromatic light and consequently are not sensitive to the effects of age-related changes in the ocular media that might influence the perception of more natural stimuli. This is because the only influence of the ocular media on a monochromatic light is to reduce its intensity. With broadband stimuli, however, the ocular media shift the intensity and, importantly for color appearance, the relative spectral distribution of the retinal stimulus.
Several studies of color appearance in younger and older observers have been carried out using broadband stimuli, surfaces or computer-simulations of surfaces. For example, Schefrin and Werner42 presented 2° test surfaces taken from the Optical Society of America’s Uniform Color Scales. Each stimulus was presented separately in a Ganzfeld-like hemisphere within a uniform middle-gray surround illumination. Each of five stimuli was presented at three different lightness levels. In addition, the stimuli were selected such that there was one pair that plotted along a tritan axis in MacLeod-Boynton43 receptor-excitation space and another pair that plotted approximately on an axis of constant S-cone stimulation. Following Gordon and Abramov,44 the subjects were asked to first scale the percentage of each fundamental hue (red, green, yellow, and blue) and then to scale the proportion of overall chromatic and achromatic content. Hue-naming percentages used by groups of younger and older observers did not differ by more than 5% for any hue category. There was a small but statistically reliable difference between the groups in the achromatic proportions assigned to the stimuli. Specifically, older subjects perceived the colors of all stimuli to have more achromatic content (less chromatic content) than demonstrated by younger observers. Differences between the two age groups in perceived chromatic content of the test stimuli progressively increased as the lightness and luminance levels decreased. In other words, the two groups differed primarily for darker colors. This may have been due to a lower effective retinal illuminance for the older group when tested under these more natural conditions. Okajima et al.45 reported similar results using a larger set of 75 chips. Essentially no difference between young and old was found when the subjects chose one of 11 color names for each stimulus. When the task involved assigning percentages to each hue term, there were small differences that depended on saturation.
The results of these experiments are surprising when one considers that the retinal stimulus in the older population would be expected to contain substantially less short-wave light. In other words, older and younger observers attach the same labels to different retinal stimuli (albeit the same corneal stimuli). Lindsey and Brown46 have recently considered the effects of lens brunescence in a different context. They noted that the age-related increase in ocular media density with age is accelerated by exposure to ultraviolet-B (UV-B) radiation. Thus, as one moves toward latitudes closer to the equator and/or higher elevations, ambient UV-B radiation is higher and there is more rapid aging of the ocular media.47 This implies that the relative reduction in S-cone stimulation associated with normal aging is accelerated in people residing at lower latitudes. Lindsey and Brown further noted that the color lexicon is also different in many cultures closer to the equator in that some lack separate terms for “green” and “blue”;48 these categories are sometimes collapsed into a single term called “grue.” Therefore, Lindsey and Brown hypothesized that premature lens brunescence was causally related to a loss in the ability to see “blue” and the use of the term “grue.” They tested their hypothesis by presenting simulated Munsell chips on a computer monitor, with one set filtered based on the lens density of older observers. Consistent with their hypothesis, there was a reduction in the use of the term blue for the latter set of stimuli. Note, however, that this experiment is a simulation of the effects of the aging lens, but this is not necessarily the same as testing elderly people. Schefrin and Werner42 noted that filters that simulate the aging lens do not alter the verbal reports of younger observers in a way that matches older observers. In the latter simulation, younger subjects adapted for 15 minutes which might be long enough for many psychophysical experiments, but which is a trivial duration compared to the decades of adaptation in an older person.
In all the previous studies, the differences in ocular media density for younger and older observers was estimated from the literature, but not measured individually. A recent study by Hardy, Frederick, Kay and Werner49 used a psychophysical procedure to determine ocular media density in ten younger (18–35 years) and ten older (65–85 years) normal trichromats. From this information, it was possible to present the same set of stimuli to the two groups (i.e., equated at the cornea) as well as a set having essentially the same retinal spectral distribution that would be appropriate for the other age group. The condition in which the younger group was tested with stimuli that were filtered by the aging lens is comparable to the simulated lens brunescence condition of the Lindsay and Brown study. Each set of stimuli included 40 simulated Munsell chips presented as 4.4° disks on a gray (CIE illuminant C) background. As expected from Lindsey and Brown’s46 results, younger subjects used the term “blue” significantly less often in the simulated aging condition. However, when the stimuli were identical at the cornea for the two groups, older subjects used “blue” just as often as younger subjects did. In fact, color naming in the younger and older groups was virtually identical for the first set of stimuli. As shown in Fig. 3, there was no correlation between the proportion of “blue” or “green” responses and the density of the ocular media. Thus, while lens brunescence is likely to be accelerated in individuals with higher UV-B exposure, this does not explain the existence of “grue” in high UV-B areas. It appears than neither the term “grue” nor the stability of color perception of older observers with high ocular media density can be explained by the retinal stimulus alone. Indeed, some other factors, presumably neural, must compensate for senescent changes in the ocular media to maintain constancy of color appearance over a wide range of conditions.
Fig. 3.
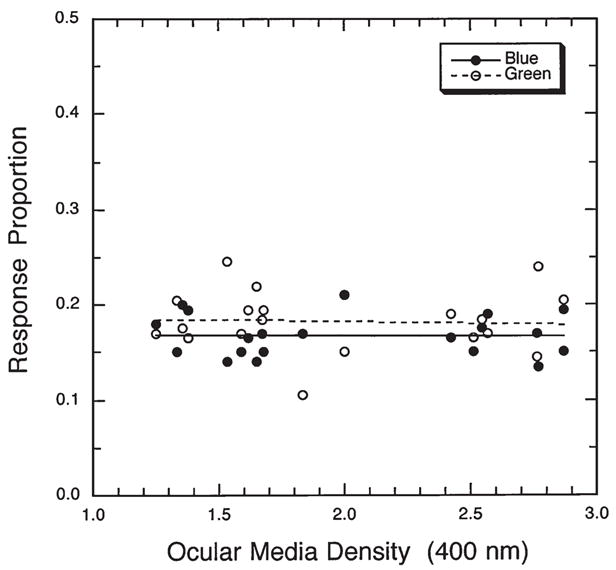
Proportion of blue (filled symbols) and green (unfilled symbols) responses to 40 simulated Munsell chips presented on a computer screen are plotted as a function of ocular media density. The slopes of the least-squares linear regression lines did not differ significantly from zero. The range shown here spans the lowest values typically found in an adult population to values typically diagnosed as early-stage cataract. [Data from Hardy, Frederick, Kay and Werner49].
3. Renormalization of Chromatic Mechanisms
While the unique hues specify the equilibrium state for a single opponent-chromatic process, the achromatic point represents a null state for all chromatic mechanisms. The achromatic point is defined as a stimulus that is devoid of all hue. Depending upon the intensity of this stimulus and its surround, it may vary from white to black. Here we consider the achromatic point measured under conditions in which it appears white. If a chromatic mechanism has been altered by changed inputs or by adaptation, a shift in the white point would be expected. The achromatic point can thus provide a sensitive indicator of color appearance changes with age in any direction in color space; it is a probe for all color directions simultaneously.
While individual spectral unique hues are unaffected by the density of the ocular media, mixtures of these wavelengths are strongly affected. Consider a theoretical observer who is asked to mix the two wavelengths corresponding to that observer’s unique blue and unique yellow. A ratio can be found that is neither yellow nor blue, but achromatic. Now suppose that person is again tested with a lens that has aged for 50 years and is therefore of higher optical density. To produce the same chromaticity on the retina, the observer with the more brunescent lens must choose a different ratio of the two monochromatic lights because of the selective loss of short-wave light reaching the retina. In a chromaticity diagram, the achromatic point would, therefore, shift in the direction of the short-wave spectrum locus. That is, higher radiance of the unique blue stimulus would be required with increasing age to compensate for absorption by the lens. The results of this experiment would also be affected by any age-related changes in relative cone sensitivities and/or in opponent-chromatic processes because the achromatic point represents a balanced state of these systems.
Werner and Schefrin39 measured the achromatic point for 50 normal observers ranging in age from 11-to-78 years. The observer’s task was to vary the intensity ratio of their individually determined unique blue and unique yellow while maintaining constant overall retinal illuminance. The method of constant stimuli was used, with data collected at three illuminance levels (10, 100, 1,000 tds). The shift in the achromatic locus expected on the basis of lenticular senescence was not observed. Indeed, there was no significant change in the achromatic point as a function of age. At first glance, it might be thought that the stability of unique blue, unique yellow (see Fig. 2), and the achromatic locus can be interpreted as evidence that aging is simply not associated with changes in color mechanisms. This is not the case. In order to keep the achromatic locus constant with age, there must be one or more processes that compensate for the changes in the retinal stimulus caused by lenticular senescence.
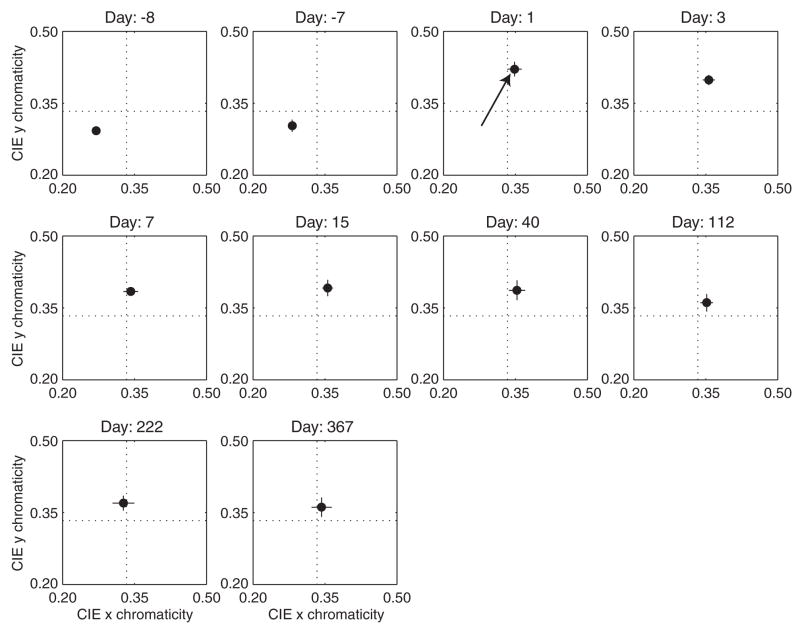
To test the hypothesis that there is active renormalization to compensate for lenticular senescence, Delahunt, Webster, Ma and Werner50 measured the locus of the achromatic point before and after cataract surgery. A person with a brunescent cataract may be considered a case of extreme lens aging. Stimuli were presented on a calibrated computer screen and adjusted in chromaticity space with luminance fixed at 30 cd·m−2. The 9.5° test stimulus flashed on and off at 3 s intervals to reduce adaptation to the stimulus. The starting chromaticity was selected randomly. Settings were made before surgery and at various intervals after surgery up to one year. The stimuli were viewed monocularly.
The locus of the achromatic point for one observer (age 71) is shown in Fig. 4 for different times before and after surgery. Note that following surgery to remove the cataractous lens, there is a large shift in the achromatic point in the yellow direction, as shown by the arrow. Much of this effect is expected from removal of the cataractous lens and the change in the retinal stimulus. However, the achromatic point slowly drifts back in the direction of the pre-surgery chromaticity, reaching a fairly stable value at about three months after surgery. Tests on three other subjects show a similar pattern of results.
Fig. 4.
Locus of the achromatic point for one observer as a function of time before and after cataract extraction plotted in CIE x,y coordinates. The dashed lines cross at equal-energy white. [Data from Delahunt, Webster, Ma and Werner50].
Neitz et al.51 reported shifts in unique yellow settings by about ±4 nm after subjects adapted to ‘red’ or ‘green’ light for a number of hours per day over several days. A return to the original unique yellow locus required as much as ten days. For even longer adaptation to a cataractous lens, renormalization of the achromatic point, and hence rebalancing of all chromatic mechanisms, required as long as three months and the shifts in color space were quite large. Neitz et al. reported inter-ocular transfer of color appearance shifts due to adaptation under their test conditions, consistent with mediation at a cortical level. In contrast, Delahunt et al. found that the shifts in the achromatic point proceeded in the cataractous eye without influencing the achromatic point in the fellow eye. The subject presented in Fig. 4 had a cataractous lens removed 8 months prior to the ‘test’ eye, but there was little change in the settings made with this eye over the same one-year test period as the test eye. This suggests that most of the long-term adaptation effects studied here, and by inference associated with normal aging, occur at a site prior to binocular combination. The post-surgery settings for the subject presented in Fig. 4 were converted to cone excitation values using Smith-Pokorny52 fundamentals. Most of the differences in achromatic settings can be accounted for by changes in S-cone response which might be expected in view of normal lens aging having its greatest effect on S-cone stimulation.53
4. Spatio-Chromatic Mechanisms
4.1 Chromatic perceptive field size
Color is processed in the visual pathways in a manner that depends on the spatial and temporal characteristics of a stimulus.54 Just as hue and saturation vary with intensity, they also vary with stimulus area. Moderately intense monochromatic lights presented as small fields (less than 15 minutes of arc) and short durations (50–200 ms), are called white 50% of the time.55 Abramov et al.56 showed that hue-scaling may be used to determine the critical size necessary to sustain a percept of a particular hue. They found that hue increased with field size up to an asymptotic level “as if some perceptive field was being filled, by analogy to similar results from receptive fields of neurons” (ref. 56). The size of these perceptive fields increased with retinal eccentricity as would be expected from spatial summation studies,57,58 but at each retinal locus, the perceptive fields were larger than those obtained from threshold experiments. At threshold, Volbrecht et al.58 found a relation between ganglion cell density and psychophysically measured spatial summation of short-wave sensitive and long-wave sensitive cone pathways. These findings invite the hypothesis that age-related retinal losses in ganglion cell density might influence hue perception if a stimulus falls below a critical size. As stimulus size is increased, differences between younger and older observers would be expected to diminish.
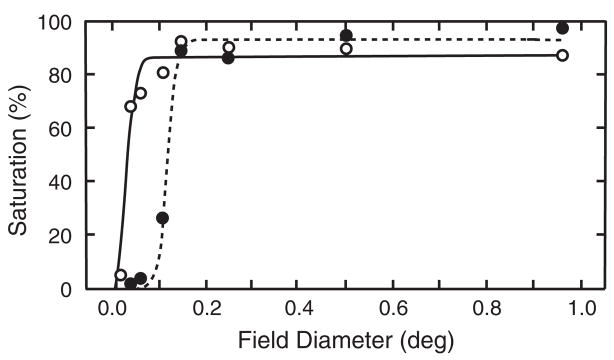
This hypothesis was tested by Knau and Werner.59 Fifty-eight observers (18–83 years) scaled the chromatic content (saturation percentage) of monochromatic stimuli (480, 505, 577, and 650 nm) that were equated for each observer to a 2.6 log troland, 560-nm standard by heterochromatic flicker photometry. Nine field sizes ranging from 0.0096 to 0.96 deg in diameter were tested at each of three retinal locations: 2.5° inferior, where there is known to be age-related loss in cone numbers,8 6° nasal, corresponding to a location with significant age-related loss in ganglion cell density,10 and 6° temporal as a control, a location with no significant age-related cone or ganglion cell loss.
Figure 5 presents data for a younger and older subject tested at 2.5° in the inferior retina. At all locations and test wavelengths, there was a rise in saturation with increasing field size. Smooth curves show fits with a Naka-Rushton60 equation, which is useful for summarizing the data with a small number of parameters. Note the displacement of the rising portions of the two curves, demonstrating a difference between these two observers in the critical field size necessary for hue perception. Contrary to expectations, there was no dependency on retinal location associated with senescent losses of photoreceptors or ganglion cells. As retinal imaging methods are improved, this issue should be reconsidered by measuring anatomical and psychophysical parameters for the same younger and older individuals. The results did demonstrate clearly, however, that the minimum field size for perception of hue is larger in older than young individuals. Senescent changes in color perception are evident when probed with small field sizes, are reduced for increasing field sizes, and not detectable at larger field sizes.
Fig. 5.
Percent saturation is plotted as a function of field size for two observers, ages 19 (open symbols) and 79 (closed symbols) years. These results were obtained with a 480 nm light presented at 2.5° in the inferior retina. [Data from Knau & Werner59].
4.2 Chromatic contrast sensitivity
The previous section provided evidence for senescent changes in chromatic vision when the test stimulus was small, corresponding to higher spatial frequencies. A more general characterization of the spatial properties of chromatic mechanisms is provided by the chromatic contrast sensitivity function. Several previous studies61–64 have examined age-related changes in contrast sensitivity along nominal cardinal axes. These studies are useful in specifying changes in contrast sensitivity for a set of standard conditions, but the stimuli were not strictly chromatic as the effects of chromatic aberration and individual differences in luminosity functions were not controlled. As such, it is not possible from previous literature to separate age-related changes due to optical vs. neural factors. To address this issue, and to compare well-controlled threshold measures with naturalistic (less controlled) suprathreshold measures, we have recently obtained new tests of senescence of the chromatic contrast sensitivity function.
Chromatic contrast sensitivity functions were obtained by Hardy, Delahunt, Okajima and Werner65 using temporally ramped Gabor patches of constant spatial bandwidth that were modulated along separate S-cone and L-M-cone axes at a space-average luminance level of 15 cd·m−2. Thresholds were measured for ten younger (mean age of 24 years) and ten older (mean age of 73 years) observers using four, vertically oriented, spatial frequencies (0.5–4 cycles per degree). These tests were carried out under conditions in which the stimuli were equated at the cornea for all observers based upon a cone-contrast space of a standard observer. A second set of measurements was obtained for the same observers with cone contrast spaces adjusted separately based upon individual measurements of ocular media density and spectral efficiency using heterochromatic flicker photometry. In all cases, the observers viewed a calibrated CRT through an air-spaced achromatizing lens to control for axial chromatic aberration and auxiliary optics to create a small exit pupil, thereby controlling for age-related miosis. A dental-impression mount was required to maintain stable head and eye position during these experiments.
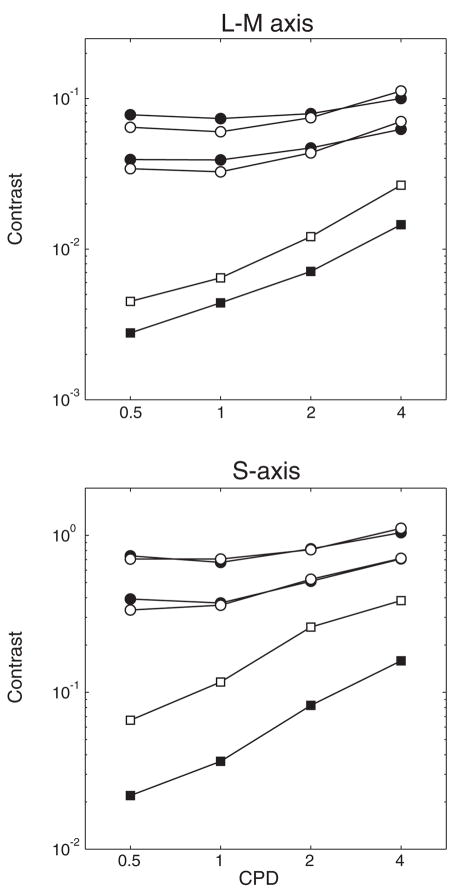
Separate panels in Fig. 6 show S-cone and L-M-cone contrast threshold functions (squares) plotted for younger (filled symbols) and older subjects (open symbols). Modulations were calculated by combining contrasts for each cone class66 using an equation suggested by Brainard.67
Fig. 6.
Contrast threshold sensitivity functions (squares) and contrast matching functions (circles) are plotted for younger subjects (filled symbols) and older subjects (open symbols). These data are from two studies that used different methodologies as described in the text. However, S-axis thresholds were obtained in both studies for a 2 cpd stimulus, and the CMFs were scaled based on a comparison of these thresholds. [Data from Hardy, Delahunt, Okajima and Werner65 and Delahunt, Hardy, Okajima and Werner72].
| (1) |
where ci is the Michelson contrast for each cone class and c̄ is the normalized cone contrast vector length.
As expected, the chromatic contrast functions are low-pass.68 The data presented in Fig. 6 are based upon measurements with stimuli equated at the cornea. There is a significant difference in chromatic contrast sensitivity between younger and older observers at all spatial frequencies and for both chromatic axes. The difference in sensitivity is greater for S-axis stimuli than for L-M varying stimuli, prior to adjustments based upon ocular media density. Contrast sensitivities measured with controls for variation in ocular media density show that differences between young and old are reduced, at least for S-cone modulation. Nevertheless, when stimuli are equated at the retina, significant differences between younger and older observers remain. The difference is similar across spatial frequency and for the two chromatic axes. From these results, it may be concluded that both optical and neural factors contribute to age-related losses in chromatic contrast sensitivity.
Research summarized in previous sections shows that the color appearance of spatially uniform stimuli remains relatively stable despite substantial age-related losses in cone sensitivity. In the spatio-chromatic domain one may ask whether a similar relation holds for suprathreshold conditions by measuring the contrast-matching function. With this task, the chromatic contrast of patterns varying in spatial frequency is adjusted to match a standard grating of fixed spatial frequency. The resultant functions are similar to those obtained in a detection task when the standard itself is close to threshold, but become flatter as the contrast of the standard is increased.69,70 In both the chromatic and the luminance domains, this has been called contrast constancy.71
To evaluate contrast sensitivity under suprathreshold and naturalistic conditions of viewing, Delahunt, Hardy, Okajima and Werner72 measured contrast-matching functions for groups of younger (mean age, 24 years) and older (mean age, 72 years) observers. Chromatic gratings were presented on a computer monitor viewed freely with a chin rest, and without an artificial pupil or achromatizing lens. Heterochromatic flicker photometry was measured for each observer so that the chromatic stimuli could be modulated at isoluminance. Two S-axis stimuli (2 cpd) were selected as standards and subjects matched their contrast to create L-M axis standards at the same spatial frequency. Using these standards, subjects adjusted the contrast of patterns having other spatial frequencies modulated along the same chromatic axis as one of the standards. The stimuli were square with a fixed visual angle and no temporal ramp. Contrast-matching functions were measured at two luminance levels (5 and 30 cd·m−2) and in each case stimuli were flashed on (0.75 s) and off (0.75 s) continuously until the subject found a satisfactory match.
The contrast-matching functions are shown in separate panels of Fig. 6 for L-M-cone and S-cone modulation. Data for younger and older observers are shown by filled and unfilled circles, respectively. The contrast-matching functions were similar for the two light levels so only the data from the 30 cd·m−2 luminance level are presented. Because the stimuli were not adjusted for absolute differences caused by senescent changes in the ocular media and pupil, they were closer to threshold for older subjects. Nevertheless, differences between the two groups that characterized threshold contrast sensitivity are absent at suprathreshold levels for all spatial frequencies tested.
Although different methods were used to measure threshold and suprathreshold contrast sensitivity, the two sets of data can be compared based upon the same cone-contrast metric and a control experiment in which S-cone thresholds were obtained with the subjects used for contrast matching. The contrast-matching functions were then scaled based on a comparison of these thresholds. It can be seen that above threshold, spatio-chromatic mechanisms operate similarly in younger and older observers.
5. Conclusions
Senescent changes in color appearance and spatial-color processing have been probed by a variety of methods and in different regions of color space. At threshold, there are significant age-related changes, but at suprathreshold levels there is a remarkable degree of stability in spatial-color vision across the life span. Discovering how the visual system accomplishes this stability with senescent changes in the ocular media and visual pathways is a major challenge. The answers will lend insight not only into aging processes but to fundamental design principles of visual coding that may be more difficult to capture if the visual system is not examined in its various forms across the life span.
Research so far has demonstrated that the visual system recalibrates itself for changing retinal stimuli and altered signals from the visual pathways. This has been extensively studied in other contexts such as in color constancy, but the renormalization of color mechanisms associated with aging takes place over a more protracted time scale (months following cataract removal) than has been studied before. This result would not have been discovered from studies of threshold. Although much more is to be learned through investigations of visual thresholds, they are often not good predictors of visual experience and thus quantitative measures of visual experience are essential for characterizing visual aging.
The ability of the visual system to maintain stable color appearance and spatio-color perception despite the large physiological variations with aging has still other important implications. Researchers interested in aging are often inspired by what changes during development or senescence, but what is stable may be equally important and more difficult to understand. The stability of certain visual functions in aging may serve to better distinguish between normal aging and age-related pathology. The compensations discussed in this paper are not restricted to aging or to color vision and it may be useful to consider them more broadly. For example, color sensitivity varies with retinal eccentricity and is influenced by spatial variations in macular pigment density. Color appearance also varies with retinal eccentricity when probed with small spots,56,59 yet during fixation and eye movements, the color of objects viewed naturally does not seem to change under most conditions. This may imply some compensation processes to normalize perception across retinal eccentricity. Compensation processes during senescence appear to be instantiated at multiple levels of visual processing as discussed in the introduction in the context of molecular renewal. Another example, combining optics (waveguide properties), photoreceptors and the visual stimulus is in the Stiles-Crawford effect of the first kind. Photoreceptors tend to change their alignment with respect to the center of the pupil where the entering light is most effective as a stimulus for vision.73 Photoreceptor alignment can change, adaptively, to optimize detection of light and contrast. Despite local forces within the eye, microadhesions and strains on the underlying choroid, changes in photoreceptor morphology and deposits of materials inside the cones associated with aging, the Stiles-Crawford effect is stable across the life span for most individuals.74 Other functions that seem stable with visual aging include certain hyperacuities75 despite senescent losses in photoreceptors and ganglion cells.
Finally, if the visual system is recalibrating some of its functions across the life span, it will be instructive to discover what it uses as an external reference. Calibration for physical properties of the world ought not only to serve perceptual stability across the life span, but across observers.
Acknowledgments
Supported by the National Institute on Aging (grant AG04058) and a Jules and Doris Stein Research to Prevent Blindness Professorship.
References
- 1.Young RW. Trans Ophthal Soc UK. 1982;102:42. [PubMed] [Google Scholar]
- 2.Werner JS. In: The Changing Visual System: Maturation and Aging in the Central Nervous System. Bagnoli P, Hodos W, editors. Plenum Publishing Corp.; New York: 1991. p. 295. [Google Scholar]
- 3.Weale RA. In: The Visual Neurosciences. Chalupa LM, Werner JS, editors. MIT Press; Cambridge: 2004. p. 205. [Google Scholar]
- 4.Groh MJ, Michelson G, Langhans MJ, Harazny J. Ophthalmol. 1996;103:529. doi: 10.1016/s0161-6420(96)30662-3. [DOI] [PubMed] [Google Scholar]
- 5.Lam AK, Chan ST, Chan H, Chan B. Optom Vision Sci. 2003;80:305. doi: 10.1097/00006324-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Am J Ophthalmol. 1996;121:181. doi: 10.1016/s0002-9394(14)70583-5. [DOI] [PubMed] [Google Scholar]
- 7.Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Ophthalmol. 1995;102:1853. doi: 10.1016/s0161-6420(95)30784-1. [DOI] [PubMed] [Google Scholar]
- 8.Curcio CA, Millican CL, Allen KA, Kalina RE. Invest Ophthalmol Visual Sci. 1993;34:3278. [PubMed] [Google Scholar]
- 9.Marshall J. In: XXIII Concilium Ophthalmologicum. Shimizu K, Oosterhuis JA, editors. Elsevier North-Holland; Amsterdam: 1978. p. 375. [Google Scholar]
- 10.Curcio CA, Drucker DN. Ann Neurol. 1993;33:248. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- 11.Harman A, Abrahams B, Moore S, Hoskins R. Anat Rec. 2000;260:124. doi: 10.1002/1097-0185(20001001)260:2<124::AID-AR20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Quigley HA, Addicks EM, Green WR. Arch Ophthalmol. 1982;100:135. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 13.Birch DG, Hood DC, Locke KG, Hoffman DR, Tzekov RT. Arch Ophthalmol. 2002;120:1045. doi: 10.1001/archopht.120.8.1045. [DOI] [PubMed] [Google Scholar]
- 14.Gerth C, Garcia SM, Ma L, Keltner JL, Werner JS. Graefe’s Arch Clin Exp Ophthalmol. 2002;240:202. doi: 10.1007/s00417-002-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abramov I, Hainline L, Turkel J, Lemerise E, Smith H, Gordon J, Petry S. Invest Ophthalmol Vis Sci. 1984;25:1307. [PubMed] [Google Scholar]
- 16.Knoblauch K, Vital-Durand F, Barbur JF. Vision Res. 2001;41:23. doi: 10.1016/s0042-6989(00)00205-4. [DOI] [PubMed] [Google Scholar]
- 17.Eisner A, Fleming SA, Klein ML, Mauldin WM. Invest Ophthalmol Vis Sci. 1987;28:1824. [PubMed] [Google Scholar]
- 18.Johnson CA, Adams AJ, Twelker JD, Quigg JM. J Opt Soc Am A. 1988;5:2131. doi: 10.1364/josaa.5.002131. [DOI] [PubMed] [Google Scholar]
- 19.Werner JS, Steele VG. J Opt Soc Am A. 1988;5:2122. doi: 10.1364/josaa.5.002122. [DOI] [PubMed] [Google Scholar]
- 20.Werner JS, Bieber ML, Schefrin BE. J Opt Soc Am A. 2000;17:1918. doi: 10.1364/josaa.17.001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haegerstrom-Portnoy G, Hewlett SE, Barr SAN. In: Colour Vision Deficiencies IX. Drum B, Verriest G, editors. Kluwer Academic Publishers; Dordrecht: 1989. p. 345. [Google Scholar]
- 22.Stiles WS. Coloquio sobre Problemas Opticos de la Vision (Madrid) Union Internationale Physique pure et appliquée. 1953;1:65. [Google Scholar]
- 23.Wald G. Science. 1964;145:1007. doi: 10.1126/science.145.3636.1007. [DOI] [PubMed] [Google Scholar]
- 24.van Norren D, Bouman M. Mod Prob Ophthalmol. 1976;17:27. [PubMed] [Google Scholar]
- 25.Brindley GS, Du Croz JJ, Rushton WAH. J Physiol (London) 1966;183:497. doi: 10.1113/jphysiol.1966.sp007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stockman A, MacLeod DIA, DePriest DD. Vision Res. 1991;31:189. doi: 10.1016/0042-6989(91)90111-h. [DOI] [PubMed] [Google Scholar]
- 27.Wandell BA, Pugh EN., Jr Vision Res. 1980;20:613. doi: 10.1016/0042-6989(80)90118-2. [DOI] [PubMed] [Google Scholar]
- 28.Wandell BA, Pugh EN., Jr Vision Res. 1980;20:625. doi: 10.1016/0042-6989(80)90119-4. [DOI] [PubMed] [Google Scholar]
- 29.Schefrin BE, Werner JS, Plach M, Utlaut N, Switkes E. J Opt Soc Am A. 1992;9:355. doi: 10.1364/josaa.9.000355. [DOI] [PubMed] [Google Scholar]
- 30.Schefrin BE, Shinomori K, Werner JS. J Opt Soc Am A. 1995;12:1233. doi: 10.1364/josaa.12.001233. [DOI] [PubMed] [Google Scholar]
- 31.Werner JS. J Opt Soc Am. 1982;72:247. doi: 10.1364/josa.72.000247. [DOI] [PubMed] [Google Scholar]
- 32.Weale RA. J Physiol (London) 1988;395:577. doi: 10.1113/jphysiol.1988.sp016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loewenfeld IE. In: Topics in Neuro-Ophthalmology. Thompson HS, Daroff R, Frisén L, Glaser JS, Sanders MD, editors. Williams and Wilkens; Baltimore, MD: 1979. p. 124. [Google Scholar]
- 34.Weale RA. The Senescence of Human Vision. Oxford University Press; Oxford, England: 1992. [Google Scholar]
- 35.Knau H, Werner JS. In: Trends in Optics and Photonics, Vision Science and Its Applications. Lakshminarayanan V, editor. Vol. 35. Optical Society of America; Washington, DC: 2000. p. 382. [Google Scholar]
- 36.Hering E. In: Outlines of a Theory of the Light Sense. Hurvich LM, Jameson D, translators. Harvard University Press; Cambridge: 19201964. [Google Scholar]
- 37.Hurvich LM, Jameson D. Psychol Rev. 1957;64:384. doi: 10.1037/h0041403. [DOI] [PubMed] [Google Scholar]
- 38.Schefrin BE, Werner JS. J Opt Soc Am A. 1990;7:305. doi: 10.1364/josaa.7.000305. [DOI] [PubMed] [Google Scholar]
- 39.Werner JS, Schefrin BE. J Opt Soc Am A. 1993;10:1509. doi: 10.1364/josaa.10.001509. [DOI] [PubMed] [Google Scholar]
- 40.Larimer J, Krantz DH, Cicerone CM. Vision Res. 1974;14:1127. [Google Scholar]
- 41.Werner JS, Wooten BR. J Opt Soc Am. 1979;69:422. doi: 10.1364/josa.69.000422. [DOI] [PubMed] [Google Scholar]
- 42.Schefrin BE, Werner JS. Color Res Appl. 1993;18:380. doi: 10.1002/1520-6378(2001)26:1+<::AID-COL8>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacLeod DIA, Boynton RM. J Opt Soc Am. 1979;69:1183. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- 44.Gordon J, Abramov I. Color Res Appl. 1988;13:146. [Google Scholar]
- 45.Okajima K, Tsuchiya N, Yamashita K. Proc SPIE. 2002;4421:259. [Google Scholar]
- 46.Lindsey DT, Brown AM. Psychol Sci. 2002;13:506. doi: 10.1111/1467-9280.00489. [DOI] [PubMed] [Google Scholar]
- 47.Young RW. Age-Related Cataract. Oxford University Press; Oxford, England: 1991. [Google Scholar]
- 48.Kay P, Berlin B, Maffi L, Merrifield W. In: Color Categories in Thought and Language. Hardin CL, Maffi L, editors. Cambridge University Press; Cambridge, England: 1997. p. 21. [Google Scholar]
- 49.Hardy JL, Frederick CM, Kay P, Werner JS. doi: 10.1111/j.0956-7976.2005.01534.x. to be published in Psychol. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delahunt PB, Webster MA, Ma L, Werner JS. doi: 10.1017/S0952523804213025. to be published in Visual Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neitz J, Carroll J, Yamouchi Y, Neitz M, Williams DR. Neuron. 2002;35:783. doi: 10.1016/s0896-6273(02)00818-8. [DOI] [PubMed] [Google Scholar]
- 52.Smith V, Pokorny J. Vision Res. 1975;15:161. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- 53.Werner JS. In: Progress in Retinal and Eye Research. Osborne NN, Chader J, editors. 15/2. Pergamon Press; Oxford, UK: 1996. p. 621. [Google Scholar]
- 54.DeValois RL, DeValois KK. Spatial Vision. Oxford University Press; New York: 1988. [Google Scholar]
- 55.Bouman MA, Walraven PL. J Opt Soc Am. 1957;47:834. doi: 10.1364/josa.47.000834. [DOI] [PubMed] [Google Scholar]
- 56.Abramov I, Gordon J, Chan H. J Opt Soc Am. 1991;8:404. doi: 10.1364/josaa.8.000404. [DOI] [PubMed] [Google Scholar]
- 57.Nagy AL, Doyal JA. J Opt Soc Am A. 1993;10:1147. doi: 10.1364/josaa.10.001147. [DOI] [PubMed] [Google Scholar]
- 58.Volbrecht VJ, Shrago EE, Schefrin BE, Werner JS. J Opt Soc Am A. 2000;17:641. doi: 10.1364/josaa.17.000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knau H, Werner JS. J Opt Soc Am A. 2002;19:208. doi: 10.1364/josaa.19.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naka KI, Rushton WA. J Physiol (London) 1966;185:587. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steen R, Whitaker D, Elliott DB, Wild JM. Optom Vis Sci. 1994;71:792. doi: 10.1097/00006324-199412000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Werner A, Schwarz G, Paulus W. In: Colour Vision Deficiencies XII. Drum B, editor. Kluwer Academic Publishers; Dordrecht: 1995. p. 235. [Google Scholar]
- 63.Fiorentini A, Porciatti V, Morrone MC, Burr DC. Vision Res. 1996;36:3557. doi: 10.1016/0042-6989(96)00032-6. [DOI] [PubMed] [Google Scholar]
- 64.Isono H, Kurata K. Proc Internat Workshop on Gerontechnology. National Institute of Biosicence and Human Technology; Tsukuba, Japan: 2001. p. 83. [Google Scholar]
- 65.Hardy JL, Delahunt PB, Okajima K, Werner JS. to be published in J. Opt. Soc. Am. A. [Google Scholar]
- 66.Chaparro A, Stromeyer CFI, Huang EP, Kronauer RE, Eskew RTJ. Nature. 1993;361:348. doi: 10.1038/361348a0. [DOI] [PubMed] [Google Scholar]
- 67.Brainard DH. In: Human Color Vision. Kaiser PK, Boynton RM, editors. Optical Society of America; Washington: 1996. p. 563. [Google Scholar]
- 68.Mullen KT. J Physiol (London) 1985;359:381. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tiippana K, Rovamo J, Nasanen R, Whitaker D, Makela P. Vision Res. 2000;40:2159. doi: 10.1016/s0042-6989(00)00076-6. [DOI] [PubMed] [Google Scholar]
- 70.Vimal RL. Vision Res. 2000;40:3231. doi: 10.1016/s0042-6989(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 71.Georgeson MA, Sullivan GD. J Physiol (London) 1975;253:627. doi: 10.1113/jphysiol.1975.sp011162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delahunt PB, Hardy JL, Okajima K, Werner JS. to be published in J. Opt. Soc. Am. A. [Google Scholar]
- 73.Stiles WS, Crawford BH. Proc Royal Society (London) 1933;112-B:428. [Google Scholar]
- 74.DeLint PJ, Vos JJ, Berendschot TTJM, van Norren DA. Invest Opthalmol Vis Sci. 1997;38:1271. [PubMed] [Google Scholar]
- 75.Enoch JM, Werner JS, Haegerstrom-Portnoy G, Lakshminarayanan V, Rynders M. J Gerontol Biol Sci. 1999;54A:336. doi: 10.1093/gerona/54.8.b336. [DOI] [PubMed] [Google Scholar]