Abstract
Brain-gut axis represents a complex reflex circuit that integrates the communication between cortex and the digestive system. Disturbances of the neuromodulatory processes in the brain-gut axis generate functional digestive disorders mainly centered on the pain symptoms and motility disorders. This article reviews structural and patho-physiological aspects of the brain-gut axis and explains how the neuromodulatory interventions currently used in order to treat GI conditions related to the brain-gut axis disturbances. The neuromodulation can be realized by pharmacological targeting mainly receptors in the periphery or using electrical stimulation applied at different levels of the nervous system or directly in the muscular layers of the bowels resulting in modulation of the digestive system activity. The efficacy of the methods using electrostimulation is dependent on the parameters of the physical system used: amplitude, frequency, burst time of the electrical current and also the positioning of the electrodes. While pharmacological interventions are largely used at the moment, neuromodulatory interventions involving electrical stimulation showed clinical efficacy in research trials and have promise.
Keywords: neuromodulation, brain-gut axis, electrical stimulation
Introduction
Bowel activity is directly connected and neuromodulated by the cortical activity throughout efferent and afferent neural pathways. This integrated bidirectional entero-cortical activity can be defined as the brain-gut axis. Physiologically, normal bowel activity requires a balanced normal brain-gut axis. Disturbances of the brain-gut axis are among the key factors in generating multiple gastrointestinal conditions that can be of primary etiology or exacerbations of other primary conditions. These disorders are classified as functional disorders (Irritable Bowel Syndrome (IBS), functional dyspepsia, functional biliary pain, and chronic abdominal pain) and motility disorders such as gastroparesis, constipation, gastroesophageal reflux disease (GERD), Cyclic Vomiting Syndrome (CVS).
The Functional Gastrointestinal Disorders (FGID) represent conditions characterized mainly by the presence of pain or discomfort, generated by an interaction with various conditions (environmental, bacterial flora, genetic predisposition, hypersensitivity) but lacking gross structural abnormalities. The advances in this specific research area suggest that the functional disorders could be generated by abnormalities in neuromodulatory processes involving the brain-gut axis. Gastrointestinal motility disorders are abnormalities of the neuroenteric system causing delayed or rapid transit that can be either of myogenic or neurogenic origin.
Understanding the neuromodulatory processes of the brain-gut axis at different levels can shed insight to the pathophysiology of some specific conditions as well as define opportunities for novel methods of treatment. This review will approach different aspects of the brain-gut axis such as structural, genetic, neurotransmission and brain imaging that can explain the role and mode of action of some neuromodulatory interventions such as gastric electric stimulation (GES), spinal cord stimulation (SCS) and sacral nerve stimulation (SNS)
Structural aspects of the brain-gut axis
The brain is connected to the bowels through neural pathways that collect the information from the receptors in the periphery (structures in the bowel) and relay it to the cortical areas. After the information is integrated and analyzed at cortical levels, a response is generated downstream, further neuromodulating the actions of the enteric system. As a visceral structure, the bowel function is controlled and modulated mainly by the autonomic nervous system with its two main subdivisions: sympathetic and parasympathetic. Behavioral and cognitive processes can influence bowel activity throughout indirect and complex pathways: anxiety and depression can exacerbate irritable bowel syndrome, non-ulcer dyspepsia [1] or chronic abdominal pain while relaxation techniques and behavioral therapies can improve them [2, 3]
As a general physiologic model, brain-gut axis can be compared with a complex reflex circuit composed of receptors, afferent fibers projecting to the integrative central areas and efferent fibers projecting to the effector structures (smooth muscles and glands) (see figure 1). The bowel activity can be also neuromodulated by intrinsic neural systems that bypass the cortical networks: myenteric and submucosal plexuses and other reflex circuits (i.e.-gastro-colic, ileo-colic).
Figure 1.
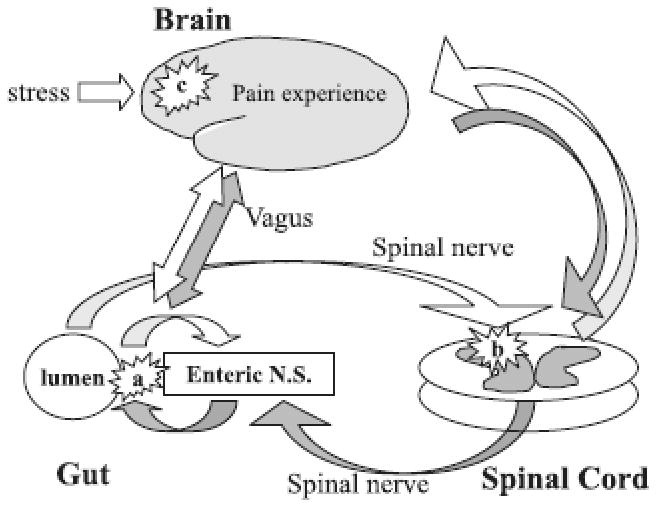
Schematic representation of the brain-gut axis and involvement of stress and pain in the neuromodulatory processes. White arrows represent afferent sensorial pathways and dark arrows efferent pathways, ultimately distributing to the muscles and glands in the bowels.
Multiple types of information from the GI tract is first collected and transformed into electrical potential at the level of the nervous receptor structures. The receptors of the GI tract are mostly specialized afferent neural terminations capable of directly collecting the specific information: chemical, mechanical, thermal and pain.
The chemosensitive afferent fibers detect pH, osmolality, or presence of different chemical compounds resulting from digestion. (amino-acids, glucose, lipids). Chemosensitivity is particularly very important in the small bowel, where detecting the chemical composition of the digested food further regulates digestive processes. There are specific afferent endings that produce responses dependent to hydrogen ion concentration [4]. Glucose sensitive vagal endings are potentially located in the submucosa of the jejuno-ileal segment because they are activated only by absorbable carbohydrates [5]. The role of the glucose sensitive endings could be to stimulate the secretion of the insulin by activating entero-pancreatic reflex circuits and also to create the sensation of satiety [6]. Short and long chain fatty acid vagal receptor endings respond in a dependent manner to the fatty acid luminal concentration[7]. Activation of these endings inhibits the gastric emptying and this effect can be abolished by bilateral vagotomy [8]. More recent studies in humans, suggested that abnormalities in neuromodulation at the level of chemoreceptor endings could be the key factor in generating functional dyspepsia. Schwartz and colleagues [9] found that antral infusion of acid is associated with significantly diminished motility and higher symptoms such as nausea in patients with functional dyspepsia when compared to the controls. In this context, the pharmacologic action of proton pump inhibitors resulting in decreasing the intragastric acidity can also be seen as a neuromodulatory intervention because these drugs can influence the sensorial input at the nerve endings level and further influencing the bowel activity.
The presence of specialized enteroendocrine cells acting as receptor taste structures and neuromodulating the digestive activities was documented in a number of very recent studies [10, 11]. These receptors identified with imunohistochemical methods are located in the mucosa of the jejunum and they are able to detect the presence of beneficial or harmful substances. From these levels, the information is transmitted via vagal fibers to the central areas as well as directly to the intrinsic neural plexuses. By increasing motility, these reflexes can generate vomiting and aversive behavior [10]. Clearly defining the neural circuits that are involved with reactions to harmful substances can open the possibility for neuromodulatory interventions that can influence these type of responses.
The mechanoreceptor endings are particularly important in the regions of the GI tract that have a “reservoir” role (stomach, colon and gallbladder). Different pressure levels and stretch of the bowel wall activate these types of receptors and generate changes in the motility and secretory activity [12, 13] as well as influence sensation of hunger and satiation by integrating the afferents at hypothalamic levels. Previous studies demonstrated that lesions in the lateral and medial hypothalamic nuclei are associated with dramatic changes in eating behavior[14-16]. Chen and colleagues [17] suggested that the gastric electric stimulation (GES) could be used in the treatment of obesity by stimulating gastric distention sensitive neurons in the ventromedial hypothalamic nucleus via vagal mechanoreceptor endings. This can further neuromodulate central activity and digestive motility to induce satiation.
The hypersensitivity to intraluminal distensions demonstrated by a considerable number of studies[18, 19] could be due specifically to abnormalities at the level of mechanosensitive intestinal afferents [20]. Gebhart and coll. [21] found that stimulation of vanilloid 1 (TRPV1) and acid sensing ion channel 3 receptors, considered visceral mechanoreceptors have an important role in inducing hypersensitivity to colon distensions in mice. Electrophysiologic studies suggested that pharmacologic neuromodulation could be efficient in reducing the colonic hypersensitivity by inhibition of transduction processes in the mechanosensitive endings from the rectum or by blocking the propagation of the conduction potentials [22].
Activation of different subsets of mechanoreceptors could elicit specific sensation of the GI tract. Low threshold mechanoreceptors may be involved in transmission of non-painful sensations (i.e. –fullness) while high-threshold mechanoreceptors may be involved in transmission of sharp, localized conscious painful sensation [23]. Some animal studies demonstrated the involvement of the spinal cord in inhibiting the nociceptive reflexes that are triggered at the level of these receptors [24, 25]. These observations could have a direct clinical applicability in humans: for example, in one case report study Mousad et all.[26] found that the stimulation of thoracic spinal cord improved the pain and diarrhea symptoms in a patient with IBS refractory to medication.
The information collected by the receptors is transmitted via autonomic nerve fibers: sympathetic and parasympathetic system. The parasympathetic preganglionic afferent fibers receiving information from the proximal esophagus to the proximal colon are dendrites of the neurons from the brainstem located mainly in the nodose ganglia and forming a large division of the vagus nerve. The parasympathetic afferent innervation of the distal colon and rectum is provided by fibers that travel along the sacral nerves S2-S4 and terminate in the dorsal horns. From the sacral segments the information is sent through spinothalamic and spinoreticular tracts to the subcortical and cortical structures. The motor (efferent) preganglionic parasympathetic fibers originate in the dorsal vagal nucleus in the brainstem and in the intermediolateral columns at S2-S4 spinal levels. These fibers travel along the vagus and pelvic nerves and synapse with the second order neurons in the proximity of the intestinal walls, modulating motility and secretory activity. Vagus and pelvic nerves are very important sites for neuromodulatory interventions: for example, severe peptic ulcers refractory to medical therapy can be cured by surgical interruption performed at the level of the distal vagal endings (vagotomy). A number of studies used electrical stimulation of peripheral nerves or dorsal column to improve conditions like fecal incontinence [27], severe refractory constipation [28], irritable bowel syndrome [26] or chronic abdominal pain [29, 30].
The sympathetic afferent fibers are dendrites of the unipolar neurons located in the dorsal root of the ganglia of the thoracic spinal cord (T1-T10). The sympathetic efferent fibers that coordinate the activity in the gastro-intestinal system start in the lateral horn of the thoraco-lumbar spinal cord (T1-L3) where the first order neuron is located. The axons of the first order sympathetic neurons (preganglionic) gather and form the splanchnic nerves which synapse with the second order neuron in three major ganglia: celiac, mesenteric superior and mesenteric inferior. From here, the axons of the second order sympathetic neuron (postganglionic) project to the end innervation targets, muscles and glands. From the spinal levels the information is transferred to subcortical structures via spinothalamic and spinoreticular tracts but also via fibers of the dorsal columns[31]
Pain plays an important role in the brain-gut axis neuromodulatory processes. Besides the spinothalamic tracts, classically considered the major nociceptive pathway, the involvement of the dorsal columns (DC) has been documented as well. Myelotomy performed at thoracic levels in human subjects [32] and lesions performed in the DC after inducing colon inflammation in rats [33] decreased consequently the pain. Palecek [34] described the DC as part of a nociceptive amplification circuit where descending supraspinal afferents originating in the rostroventral medulla facilitate the nociceptive ascending inputs from the DC. This neural model may explain the efficacy of intraspinal neuromodulatory interventions used in pain conditions.
From the hypothalamus and thalamus, the information is sent, integrated and neuromodulated at different central cortical area such as anterior and posterior cingulatecortexes, insular and amygdalian areas, as shown using brain imaging methods. Besides the circuits involving cortical and sub-cortical projections, bowel activity is also regulated by intrinsic neural plexuses (myenteric and submucosal). The intrinsic plexuses are embedded in the intestinal wall and are composed mainly of a ganglionated neural networks that neuromodulate the sensory and motor activity. Even if these plexuses have an independent action on bowel activity, their proper activity requires a normal functioning connection with the central cortical and sub-cortical structures [35].
Neuromodulatory interventions at any level on the neurotransmission pathway can potentially influence the clinical outcome. These interventions would target receptors in the periphery by pharmacological means or centrally where specific nerve fibers influence downstream the activity in the neural circuitry as a whole.
Neurotransmitters
The connection between nerve fibers and bowel is realized by different excitatory and inhibitory molecules that neuromodulate gut activity. Besides molecules secreted at nerve endings, some of the molecules are secreted directly by cells from the intestinal wall with local effects.
Acetylcholine is the main mediator of the parasympathetetic system modulating its actions as described above. Despite the discovery in the recent years of many other neurotransmitters, acetylcholine is still one of the most important excitatory neuromodulators because the cholinergic motor neurons are the main drivers of motility in the enteric system. Disturbances in the acetylcholine activity and metabolism (i.e.- anesthetics, organophosphoric compounds) considerably affect the motility and secretion in the gut. Trout [36] demonstrated that colonic smooth muscle stimulation with electric field produced a release of acetylcholine proportional with the intensity of the electric stimulus. Similar outcomes were reported when muscle stripes from the stomach were stimulated using electric field stimulation [37]. These animal studies may explain the biochemical mechanism of action of electric stimulation in humans, used to treat conditions like refractory gastroparesis or chronic constipation.
Biologic amines (serotonine, norepinephrine, dopamine) are also important molecules that participate to the neuromodulation of the brain-gut axis. These amines can act in periphery mediating the effects of the sympathetic system. By inhibiting the activity of the cholinergic neurons, they decrease secretion, motility and relax the sphincters. Besides their involvement in regulating sympathetic system, these substances seemed to neuromodulate the pain threshold in the brain-gut axis. Numerous studies support the concept that biologic amines are involved in the pathophysiology of the IBS or other pain syndromes (i.e.-fibromylagia) [38-40]. The neuromodulatory interventions on the aminergic system are particularly pharmacologic interventions, targeting specific receptor that enhance or block the actions of these substances in periphery (bowels) or centrally (brain areas). Specifically in the case of Irritable Bowel Syndrome associated with constipation, modulation of serotoninergic system with 5HT4 receptor agonists such as tegaserod improves the symptoms related to pain respectively the frequency and consistency of the bowel movements [39]. Stimulation of these receptors further activates the cholinergic neurons, modulating the intestinal motility [41], [42]. Molecules like tricyclic antidepressants (amitriptyline, doxepine, imipramine) also modulate the activity of these substances targeting possibly specific areas in the brain. By increasing the sensory threshold, these substances can reduce pain and improve the symptoms in Irritable Bowel Syndrome or other visceral pain syndromes [38, 43].
Calcitonin-gene related peptide (CGRP), bradykinins and tachykinins (Substance P) are molecules involved in visceral hyperalgesia and pain syndromes. Lee and collaborators [44] showed that repetitive painful rectal stimulation in rats without intestinal inflammation induced an acute increase in the spinal levels of CGRP and SP and an up-regulation of their gene expression. Spinal release of these substances can induce visceral hypersensitivity, especially when the noxious stimulus is repeated. Neuromodulatory interventions on the kinin system are mainly pharmacologic, consisting of designed molecules to block kinin receptors. Inatomi and collaborators [45] demonstrated that intrathecal injection of a kinin antagonist TAK-637 reduced consistently the pain symptoms in rabbits that experienced colorectal distensions and mucosal inflammation. The mechanism of action is more likely mediated by spinal cord and not by periphery. Tachykinin antagonists have been shown to modulate GI sensations such as nausea with the NK1 antagonist such as aprepitant [46].
The opioid system plays an important role in pain threshold in brain-gut axis. Opioids influence the bowel activity mainly by inhibiting the peristalsis and the secretion [47, 48]. The opioids mediate these effects by two proposed mechanisms: 1. inhibition of release of acetylcholine from the neurons in myenteric plexus [49] 2. neuromodulation of the acetylcholine activity on the bowel muscle [48]. Kromer demonstrated that naloxone and normorphine influence the peristalsis in guinea pigs only if the muscles stripes are already primed by acetylcholine. The same study also showed that a total blockage of acetylcholine using tetrodotoxine does not allow the opiod agonist or antagonist to act.
The endogenous opioid system seems to have a central role in pain syndromes related to brain-gut axis disturbances. Recently, novel neuromodulatory pharmacotherapies using molecules that target the kappa-opioid receptors (Fedozotine) have been shown to be effective in irritable bowel syndrome and functional dyspepsia [50].
Placebo effect has also been attributed to the action of endogenous opioid system [51], although it is debatable if nalaxone can reverse the placebo effect or not. In clinical trials, the placebo rate in patients with IBS is relatively high (46%) and independent of the intervention used, pharmacological or Complementary and Alternative Medicine (CAM) [52]. The placebo effect needs to be considered when approaching a specific treatment and assessing its success rate.
Brain imaging
In the last 10 years, brain-gut axis has been investigated also using brain-imaging methods such as fMRI and PET. These studies demonstrated that the abnormalities are not entirely confined to the gut periphery but also can be triggered by different patterns of cortical activation. The main concept of these experiments was to use models that are exposed to noxious stimuli in order to induce painful states similar to those experienced by patients with IBS, functional dyspepsia or other visceral pain syndromes. Analyzing brain activity during such conditions and comparing between groups (healthy versus patients with conditions) can shed light on the mechanisms that may possibly trigger these disorders. Even though the results of the studies present some heterogeneity, it is clear that CNS activity has changed in patients with chronic pain. A majority of the study results show that areas such as thalamus, anterior and posterior insula and hippocampus/amygdala are more activated in patients with IBS than controls during painful rectal stimulation using balloon distensions[53]. Interestingly, even if Anterior Cingulate Cortex represents a key area for autonomic processes, the analysis done by Gaman at al. failed to show a consistent pattern of activation across the studies reviewed [54-65]. Different cognitive processes such as placebo activate frontal cortex and participate in modulation of sensorial afferent inputs from the periphery inducing analgesia [66].
A recent brain-imaging study on patients with functional dyspepsia [67] using stimulation with balloon distensions demonstrated a significant lower pain threshold in this patient population but failed to show any significant cortical activity difference between the two groups. Another group [68] demonstrated that during acidic exposure of esophageal mucosa, healthy subjects and patients with GERD activate similar regions of the brain, although the patient group shows a more rapid spike and a more intense brain activity. These findings could be explained by the existence of a damaged esophageal mucosa, allowing for denudation of neural afferents and consequently a more rapid activation of the brain. The higher intensity of the brain activity could be due to a prior sensitization of specific brain areas in patients with GERD. Of note, the areas activated during acidic exposure are relatively similar with area activated during painful stimulation of rectal segment suggesting the existence of a common brain matrix that integrates the visceral pain sensation.
The studies using brain imaging are particularly of a considerable importance for multiple reasons. Documenting pathological brain mechanisms could help define targets for therapies at a pharmacological level or for other means of neuromodulation such as Transcranial Magnetic Stimulation [69-71] which is an external intervention using external magnetic field and acting at the cortical level. Hamdy and colleagues [69] demonstrated that stimulating the swallowing motor cortex using TMS could enhance cortical excitability and influence downstream the pharyngeal motor behavior. TMS has improved pain symptoms related to different conditions (stroke, neuralgia). Particularly, for the brain-gut axis TMS demonstrated improvement in chronic visceral pain due to chronic pancreatitis when secondary somatosensory area was stimulated [70]. This application may have a role in the future to impact visceral pain but more experience is needed. Finding consistent abnormal patterns in disease with brain imaging techniques may allow neurological phenotyping, which may personalize the treatment regimen for each specific condition.
Neuromodulatory interventions
Brain-gut axis abnormalities can manifest as gastrointestinal motility and functional disorders. Because the neural activity plays a major role in these disorders, interventions aiming to modulate these processes can possibly improve the symptoms. Currently, different types of neuromodulatory interventions are being used. Pharmacologic therapies have been largely available and used because they present some benefits such as familiarity of the patient to the use of drugs and titrability. Drug development for the functional GI disorders is surging because of an enormous unmet medical need of effective treatment need for improving the visceral pain symptoms. These drugs still have disadvantages such as intolerance due to side effects and equally common is clinical ineffectiveness in some patient populations. Many drugs used to treat functional digestive disorders are not bowel specific and this may result in numerous potential side effects. As an example, tricyclic antidepressants used for pain syndromes (IBS, visceral pain syndrome, chronic abdominal pain) can decrease the pain but also they can produce hypotension, dryness of the mouth and eyes, difficulty to urination or somnolence and dizziness via anticholinergic side effects. Implantable devices (electrodes, pacemakers) that produce electrical stimulation represent another potential therapeutic alternative. These devices are still not mainstream in clinical practice. The overall concept is the generation of controlled electrical stimulation in the nerve fibers which can modulate downstream the bowel activity- secretion and motility. We previously described the theoretical foundation that may explain the utility and functionality of the neuromodulatory interventions that are being reviewed in this study.
Gastro-esophageal reflux disease (GERD) is a very common clinical condition. The main pathophysiologic mechanism of GERD is a decreased tonus of the lower esophageal sphincter (LES) and decreased esophageal motility, allowing to the gastric acid to remain prolonged in the esophagus, inducing mucosal damage. The acidic milieu attacks the ill prepared esophageal mucosa and consequently produces heartburn symptoms and increases potentially the risk for esophageal adenocarcinoma.
The therapies widely used may decrease the pH of the gastric acid (proton pump inhibitors and histamine receptor blockers) or increase the strength of the esophageal sphincter (cisapride, metoclopramide, surgical fundoplication). Several studies have examined the effect of the electrical stimulation on the tonus of the LES. Xing and collaborators [72] used electrodes implanted directly in the muscular wall of the stomachs of eight healthy dogs, 14 cm above the pylorus. Two different types of electric current have been used: low frequency/wide pulse (0.1 Hz/375 ms) and high frequency/narrow pulse (14 Hz/330 μs). Both types of stimulation significantly increased the pressure in the LES. Low frequency/wide pulse current increased from a mean of 21.5 mmHg to 30.9 mmHg (p<0.01) while high frequency/narrow pulse current increased from 19.6 mmHg to 33.4 mmHg (p<0.01). The plasma level of specific regulatory peptides (pancreatic polypeptide, neurotensin, motilin and gastrin) was not significantly affected so a pressure increase due to a vagovagal cholinergic reflex intervention was excluded. Possible mechanisms that increased the strength of the LES could be a direct alpha-adrenergic stimulation and local release of excitatory kinins such as neurokinin-A producing a slow and long lasting effect and substance P producing an immediate and short lasting effect.[72]
In a recent study [73], using the same concept, a 3.3mm×28mm micro-stimulator (Bion ®) endoscopic implanted directly in the muscular layers of the lower esophagus of three dogs and guided by remote control, increased significantly the LES pressures in all three dogs (p<0.02) with a current of 10mA, 20 Hz and 200 μs. Electric stimulation of the LES is at the moment used in animal models but these studies demonstrate that there is a potential for treatment of GERD in humans for the future.
Gastroparesis (delayed gastric emptying) can be idiopathic or it may appear as a consequence of vagal damage in patients with advanced diabetes mellitus or as a post-surgical complication. The muscular layers of the stomach lose their capacity of generating efficient and organized contractions resulting in food emptying delay. In comparison with GERD, impact of gastric stimulation upon gastroparesis has been more studied in humans. Treatments for this condition are more in need because of the paucity of effective pharmacological options. Advanced cases of gastroparesis may result in gastrostomy, jejunostomy or even in total parenteral nutrition due to gut motility failure.
A number of studies using gastroparetic and healthy canine models demonstrated the potential impact of gastric electrical stimulation upon gastric motility. Ross and collaborators [74] compared gastric activity between one-channel versus four-channel gastric stimulation. One-channel stimulation (4mA/550ms/110% slow wave intrinsic frequency) did not accelerate the gastric emptying of a liquid while the four-channel stimulation (1, 0.8, 0.6, 0.4 mA/40mS/110% slow wave intrinsic frequency Hz) significantly increased it at 30 and respectively 60 minutes after the meal ingestion. The multi-channel stimulation most likely created a propagation pattern more similar with the natural peristalsis. A canine model with vagotomy and glucagons administration to induce gastroparesis demonstrated a significant increase of the gastric emptying using afour-channel system with three silver electrodes placed in the stomach (midcorpus, proximal and distal antrum) and one in the duodenum. An asynchronous current of 2mA amplitude and 300mS pulse width was used [75].
In a double-blind study, a cohort of gastroparetic patients (diabetics and idiopathics) received continuous gastric electric stimulation through electrodes implanted directly in the stomach wall. GES improved significantly vomiting symptoms and the quality of life for these patients. Gastric emptying was moderately but significant accelerated at 2h but failed to show significance at 4h [76].
Interestingly, GES could be used to induce opposite symptoms than those described above, which may serve a role in patients with intractable obesity. The proposed mechanisms for treatment of obesity by decreasing food intake could be an impaired gastric motility especially in the fed state and a lower capacity of the stomach to distend resulting in a fast inductance of satiation [77]. Cigaina [78, 79] was one of the first to use the gastric myo-electric stimulation in animals and humans with electrodes surgically implanted in the lesser curvature of the stomach using a current with the frequency of 12 cycles per minute and burst time of 2s. A variable follow up of up to 5 years after placing the electric stimulation demonstrated that all 22 patients recruited from 1995 to 2000 lost weight. The article reported the excess body mass index loss (EBL) at 5 years for 3 patients was on average 24.5%. Two other patient cohorts followed at 12 and 30 months, demonstrated a weight decrease of 19.5% and 24.5% respectively. The author hypothesized that an increased inter-digestive intragastric pressure resulted because of the changes in motility determined a re-set of the satiety threshold. Neurohormonal mechanisms activated through parasympathetic plexuses were also proposed but this remains an area for further research because some other articles failed to demonstrate an involvement of parasympathetic neurohormonal axis but rather a sympathetic one [80]. In a French study [81], Transcend® Intragastric Stimulator using two electrodes placed laparoscopically in the lesser curvature near the gastroesophageal junction in one group of patients and low in the lesser curvature near the pylorus in another group of patients delivered a current of 10 mA/40 Hz/208 μS pulse width. This study failed though to show weight loss in female patients at 4 months (weight gain of 6.2% EBL). Weight loss was seen only in male subset of the population (mean weight loss of 25.1 % EBL). The result is preliminary because the male sample size was smaller than the female one and also the follow up was shorter for the males. A comparison presenting the weight loss according with the position of the electrodes was not presented. The authors of this study concluded that intragastric electrical stimulation can be efficient in selected subjects with a BMI between 35 and 40 and after some other surgical procedures such as gastric banding failed to show improvement. In another multicenter placebo controlled study [82], 103 patients with a BMI (Body Mass Index) between 40 and 55, had surgically implanted a Transcend ® device in the anterior wall of the stomach lateral to the pes anserinus. The current delivered by this device has 6.0 mA amplitudes, a pulse width of 208 μs, a burst rate of 40 Hz and on/off epochs of 2/3s. This study failed to show a significant weight loss difference at 6 months follow up between study and control groups although a subset of 34 patients presented with more than 20% weight loss at 29 months follow up. This study demonstrated that introduction of a preoperative screening questionnaire can select subjects that would probably respond to the gastric stimulation by decreasing with more than 12% their weight during the therapy. Factors such as age, BMI and patient perception about physical and emotional state are the most important predictors for the implantable gastric stimulation (IGS) therapy to succeed in treating obesity.
An interesting brain imaging study [83] using Positron Emission Tomography in patients that had a Implantable Gastric Stimulator (IGS) found that the periods of time when the stimulator was “on” correlated with a decreased of uncontrolled eating (r=.81,p<0.01) and also with an increased activation (18% more) of right hippocampus, an area involved in the reward system processing. This finding emphasize that gastric stimulation can induce satiation through direct stimulation in the periphery but also through central brain circuits.
Hormonal interactions are another proposed mechanism of action for the GES [84]. Two-hour gastric stimulation on the lesser curvature of the serosal surface of the stomach at 1 cm proximal to the pyloric ring induced an increased expression of neurons reactive to oxytocin which is an anorexigenic peptide [85] and decreased the expression of neurons reactive to orexin which is a peptide that stimulates the food intake [86, 87]. The current used to induce this changes had an intensity of 2mA, the width pulse 0.3 mS and the on/off sequence was 2s/3s, continuously for 2h. Delivering GES continuously for 2.5h in dogs on the greater curvature of the stomach, 2 cm above the pylorus using a current with 5mA/40 Hz/2mS, delayed the gastric emptying of solids and decreased significantly the insulin levels but not the leptin, glucagons and glucose. This result contrast with the ones previously presented (decreasing the gastric emptying) and one explanation could be the position of the electrodes. A decreased level of insulin can be explained by the activation of sympathetic system via alpha and beta-adrenergic receptors. The hormonal variations induced by the GES seem to be an important aspect but its understanding needs further research. All the mechanisms mentioned above will lead to a decreased food intake and possible nausea symptoms leading to a potential benefit for patients with intractable obesity.
Regarding the pain syndromes (Irritable Bowel Syndrome, Visceral Pain Sydrome, Chronic Abdominal pain, Chest Pain), the efficacy of various neuromodulatory interventions was reported. Greenwood-Van Meerveld and colleagues [24] found that spinal cord stimulation (SCS) in normal rats at T12/L1 levels with a monophasic rectangular current of 50 Hz and width pulse of 0.2mS produced decrease in pain sensations to 60 mmHg colonic distensions when the intensity of the current was 90% of the motor threshold. Even after the implanted SCS system was turned off the delayed effect lasted between 70 to 90 minutes. Using the same model they sensitized the colonic mucosa of the rats using acetic acid [24] or trinitrobenzensulfonic acid [88] and observed also that in these situations, applying electrical stimulation at the spinal level will decrease pain symptoms, mirrored by the decreased number of abdominal contractions. SCS could interfere at the central levels to suppress neuronal hyperexcitability induced by experimental conditions (acid stimulation, painful distention) or/and possibly to depress sympathetic activity. This will result in a decreased cortical pain perception.
Reports that assessed the efficacy of the SCS in human subjects with Irritable Bowel Syndromes are scarce at the moment. Mousad and Krames [26] reported a significant improvement of diarrhea symptoms and overall functionality in a patient with Irritable Bowel Syndrome, even though after 6 months of spinal stimulation with a permanent Pisces® qudropolar electrode she experienced relapse of the pain symptoms and readjustment of the opioid dosage was needed. This outcome may suggest possible adaptive mechanism involved with pain.
Ceballos and colleagues [89] reported long lasting analgesia in a patient with chronic mesenteric ischemia after SCS using a tetrapolar system with the tip of the electrode placed at the level of T16. SCS was also successfully used in abdominal pain associated with Familial Mediterranean Fever (FMF) [90]. In 2 cases of FMF, implantation of a single octopolar stimulator at T8-T9 levels improved significantly the pain symptoms at 3 and 6 months, even though in the first patient a follow up at 12 months found the efficacy of SCS to have decreased. Khan and colleagues [91] applied SCS using different stimulation systems (PISCES or Genesis Implantable Pulse Generators) percutaneously introduced so that the tip of the lead reached T5-T7 spinal level producing good truncal paresthesias in the T7-T12 dermatomes area in patients with visceral pain syndromes of various causes. Follow-ups for 6 to 8 months showed a decrease in the pain symptoms of at least 44% in the Visual Analogue Scale in all the patients and at least 50% in the consumption of narcotic intake. Using SCS, Jackson and colleagues [92] reported also a 50-70% improvement of symptoms in a patient with severe (pain score 9/10) chest pain syndrome. Considering these results, the use of a direct spinal stimulation seems to be an appealing method. The efficiency of SCS in visceral pain syndromes should be assessed in a more homogenous clinical population.
Neuromodulatory interventions have been used with some success to improve conditions affecting the colon such as refractory constipation, defined as less than three bowel movements per week with straining or sensation of incomplete evacuation (Rome III criteria). Physiologically, constipation may occur by a generalized hypomotility of the colon and/or pelvic floor dyssynergia, a situation in which the normal sequence of neuromuscular function during defecation goes awry.
A number of studies looked for impact of sacral nerve stimulation (SNS) in humans [28, 93-95] with the use of electrodes implanted on the nerve fibers under general anesthesia. In one of the studies [95] electric stimulation of S2 produced a significant (p<0.01) increase of anterograde peristalsis all over the colon, while stimulation of S3 produced significant increase (p=0.03) of retrograde peristalsis. Individualized parameters such as stool frequency or laxative use demonstrated also improvement with the use of SNS in patients with refractory constipation. Even if the stimulation was applied on S2 and S3 roots, the improvement of propulsion was noted in the proximal and distal colon and not only in the distal part, which is what would have been expected according to the proximal extent of the sacral parasympathetic innervation [96, 97]. One of the explanations offered by the authors is the existence of recto-colonic reflexes capable of inducing proximal colonic activity when the rectum is stimulated. Another study [93] though, has less promising results, showing clinical improvement during stimulation in only 2 patients out of 8. The majority of the patients that participated in these studies experienced slow-transit constipation but improvement of symptoms was also noticed in pelvic floor dysinergic constipation [28]. SNS also demonstrated an increase of the peristaltic activity in sigmoid colon, rectum and anal sphincter in the patients with bowel atonia after injury of the spinal cord [98]. The placebo effect in the studies mentioned above could not be totally ruled out because they were not performed in a blinded, randomized fashion. A well defined neural network by which the SNS generates the beneficial changes mentioned above could not be exactly identified and this will need further investigation. Overall, the use of SNS in the patients with intractable constipation was beneficial for the patients and total colostomy could be delayed or potentially avoided, decreasing the morbidity associated with such a radical surgical procedure.
SNS is an efficient method in treating fecal incontinence produced by trauma at the spinal level with subsequent lost of sphincter control or generated by different conditions (systemic sclerosis, scleroderma). In one study [99] after screening for any response with temporary stimulation, a group of patients were exposed to permanent SNS. 4 out of 5 were good responders and SNS was used. In this group of selected patients, the incontinent episodes decreased to 0. Another cohort of women with fecal incontinence of various causes experienced marked improvement after 16 months after implantation of the permanent electrodes. 4 out of 5 reached a total cessation of the incontinent episodes [94]. These reports involve small sample sizes and they still need further validation in larger cohorts.
In conclusion, the brain-gut axis represents a bidirectional interaction and disturbances at central or peripheral levels will generate gut dysmotility and functional digestive disorders. The neuromodulatory interventions can impact at any level of the brain-gut axis, influencing the neural activity, attempting to regain the balance of this system. Neuromodulation can occur pharmacologically influencing the receptor activity or by electrical stimulation on the neural circuits targeting mechanisms related to membrane excitability. The technical configuration of the system, the parameters of the current used and also the specific position of the impulse generator within the brain-gut axis influence the clinical outcomes, mandating a careful clinical approach and an accurate diagnosis. Neuromodulation using electric stimulation with implantable devices is not yet widely used but clinical research shows promise for these methods.
Acknowledgments
Founded by National Institute of Health, grant K23 DK069614-01
Footnotes
The authors reported no conflict of interest.
References
- 1.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65(4):528–33. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 2.Lackner JM, et al. Cognitive therapy for irritable bowel syndrome is associated with reduced limbic activity, GI symptoms, and anxiety. Behav Res Ther. 2006;44(5):621–38. doi: 10.1016/j.brat.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lackner JM, et al. How does cognitive behavior therapy for irritable bowel syndrome work? A mediational analysis of a randomized clinical trial. Gastroenterology. 2007;133(2):433–44. doi: 10.1053/j.gastro.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews CJ, Andrews WH. Chemoreceptors, sensitive to a low pH, in the duodenum of the rabbit. J Physiol. 1970;210(1):39P–40P. [PubMed] [Google Scholar]
- 5.Mei N, Garnier L. Osmosensitive vagal receptors in the small intestine of the cat. J Auton Nerv Syst. 1986;16(3):159–70. doi: 10.1016/0165-1838(86)90022-6. [DOI] [PubMed] [Google Scholar]
- 6.Novin D, Sanderson JD, Vanderweele DA. The effect of isotonic glucose on eating as a function of feeding condition and infusion site. Physiol Behav. 1974;13(1):3–7. doi: 10.1016/0031-9384(74)90298-4. [DOI] [PubMed] [Google Scholar]
- 7.Melone J. Vagal receptors sensitive to lipids in the small intestine of the cat. J Auton Nerv Syst. 1986;17(3):231–41. doi: 10.1016/0165-1838(86)90060-3. [DOI] [PubMed] [Google Scholar]
- 8.Roze C, et al. Inhibition of gastric electrical and mechanical activity by intraduodenal agents in pigs and the effects of vagotomy. Digestion. 1977;15(6):526–39. doi: 10.1159/000198043. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz MP, Samsom M, Smout AJ. Chemospecific alterations in duodenal perception and motor response in functional dyspepsia. Am J Gastroenterol. 2001;96(9):2596–602. doi: 10.1111/j.1572-0241.2001.04103.x. [DOI] [PubMed] [Google Scholar]
- 10.Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292(2):G457–61. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland K, et al. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292(5):G1420–8. doi: 10.1152/ajpgi.00504.2006. [DOI] [PubMed] [Google Scholar]
- 12.Abrahamsson H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol Scand Suppl. 1973;390:1–38. [PubMed] [Google Scholar]
- 13.Grossman MI. Secretion of acid and pepsin in response to distention of vagally innervated fundic gland area in dogs. Gastroenterology. 1962;42:718–21. [PubMed] [Google Scholar]
- 14.Teitelbaum P, Stellar E. Recovery from the failure to eat produced by hypothalamic lesions. Science. 1954;120(3126):894–5. doi: 10.1126/science.120.3126.894. [DOI] [PubMed] [Google Scholar]
- 15.Williams DR, Teitelbaum P. Some observations on the starvation resulting from lateral hypothalamic lesions. J Comp Physiol Psychol. 1959;52:459–65. [PubMed] [Google Scholar]
- 16.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24(2):123–40. [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, et al. Excitatory effects of gastric electrical stimulation on gastric distension responsive neurons in ventromedial hypothalamus (VMH) in rats. Neurosci Res. 2006;55(4):451–7. doi: 10.1016/j.neures.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Bouin M, et al. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122(7):1771–7. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 19.Mertz H, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109(1):40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 20.Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995;108(3):636–43. doi: 10.1016/0016-5085(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 21.Jones RC, 3rd, et al. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133(1):184–94. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Su X, Gebhart GF. Effects of tricyclic antidepressants on mechanosensitive pelvic nerve afferent fibers innervating the rat colon. Pain. 1998;76(1-2):105–14. doi: 10.1016/s0304-3959(98)00031-1. [DOI] [PubMed] [Google Scholar]
- 23.Cervero F, Janig W. Visceral nociceptors: a new world order? Trends Neurosci. 1992;15(10):374–8. doi: 10.1016/0166-2236(92)90182-8. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood-Van Meerveld B, et al. Attenuation by spinal cord stimulation of a nociceptive reflex generated by colorectal distention in a rat model. Auton Neurosci. 2003;104(1):17–24. doi: 10.1016/s1566-0702(02)00262-x. [DOI] [PubMed] [Google Scholar]
- 25.Qin C, et al. Spinal cord stimulation modulates intraspinal colorectal visceroreceptive transmission in rats. Neurosci Res. 2007;58(1):58–66. doi: 10.1016/j.neures.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mousad D. Spinal cord stimulation reverses pain and diarrheal episodes of irritable bowel syndrome: a case report. Neuromodulation. 2004;7(2):82–88. doi: 10.1111/j.1094-7159.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 27.Ganio E, et al. Sacral nerve stimulation for treatment of fecal incontinence: a novel approach for intractable fecal incontinence. Dis Colon Rectum. 2001;44(5):619–29. doi: 10.1007/BF02234555. discussion 629-31. [DOI] [PubMed] [Google Scholar]
- 28.Kenefick NJ, et al. Permanent sacral nerve stimulation for treatment of idiopathic constipation. Br J Surg. 2002;89(7):882–8. doi: 10.1046/j.1365-2168.2002.02132.x. [DOI] [PubMed] [Google Scholar]
- 29.Tiede JM, et al. The use of spinal cord stimulation in refractory abdominal visceral pain: case reports and literature review. Pain Pract. 2006;6(3):197–202. doi: 10.1111/j.1533-2500.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 30.Kapural L, et al. Spinal cord stimulation is an effective treatment for the chronic intractable visceral pelvic pain. Pain Med. 2006;7(5):440–3. doi: 10.1111/j.1526-4637.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 31.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 32.Hirshberg RM, et al. Is there a pathway in the posterior funiculus that signals visceral pain? Pain. 1996;67(2-3):291–305. doi: 10.1016/0304-3959(96)03127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palecek J, Willis WD. The dorsal column pathway facilitates visceromotor responses to colorectal distention after colon inflammation in rats. Pain. 2003;104(3):501–7. doi: 10.1016/S0304-3959(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 34.Palecek J. The role of dorsal columns pathway in visceral pain. Physiol Res. 2004;53 1:S125–30. [PubMed] [Google Scholar]
- 35.Aaronson MJ, Freed MM, Burakoff R. Colonic myoelectric activity in persons with spinal cord injury. Dig Dis Sci. 1985;30(4):295–300. doi: 10.1007/BF01403836. [DOI] [PubMed] [Google Scholar]
- 36.Trout SJ. Assessment of acetylcholine release from myenteric plexus of guinea-pig colon. J Pharm Pharmacol. 1986;38(4):310–2. doi: 10.1111/j.2042-7158.1986.tb04575.x. [DOI] [PubMed] [Google Scholar]
- 37.Smits GJ, Lefebvre RA. Development of cholinergic and inhibitory non-adrenergic non-cholinergic responses in the rat gastric funds. Br J Pharmacol. 1996;118(8):1987–94. doi: 10.1111/j.1476-5381.1996.tb15634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowell MD, et al. Antidepressants in the treatment of irritable bowel syndrome and visceral pain syndromes. Curr Opin Investig Drugs. 2004;5(7):736–42. [PubMed] [Google Scholar]
- 39.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Morgan V, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54(5):601–7. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuurkes JA, et al. Motor-stimulating properties of cisapride on isolated gastrointestinal preparations of the guinea pig. J Pharmacol Exp Ther. 1985;234(3):775–83. [PubMed] [Google Scholar]
- 42.Tonini M, Galligan JJ, North RA. Effects of cisapride on cholinergic neurotransmission and propulsive motility in the guinea pig ileum. Gastroenterology. 1989;96(5 Pt 1):1257–64. doi: 10.1016/s0016-5085(89)80012-5. [DOI] [PubMed] [Google Scholar]
- 43.Spiller R, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56(12):1770–98. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu CL, et al. Changes of the neuropeptides content and gene expression in spinal cord and dorsal root ganglion after noxious colorectal distension. Regul Pept. 2005;131(1-3):66–73. doi: 10.1016/j.regpep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Okano S, Ikeura Y, Inatomi N. Effects of tachykinin NK1 receptor antagonists on the viscerosensory response caused by colorectal distention in rabbits. J Pharmacol Exp Ther. 2002;300(3):925–31. doi: 10.1124/jpet.300.3.925. [DOI] [PubMed] [Google Scholar]
- 46.Massaro AM, Lenz KL. Aprepitant: a novel antiemetic for chemotherapy-induced nausea and vomiting. Ann Pharmacother. 2005;39(1):77–85. doi: 10.1345/aph.1E242. [DOI] [PubMed] [Google Scholar]
- 47.Waterman SA, Costa M, Tonini M. Modulation of peristalsis in the guinea-pig isolated small intestine by exogenous and endogenous opioids. Br J Pharmacol. 1992;106(4):1004–10. doi: 10.1111/j.1476-5381.1992.tb14448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kromer W, Schmidt H. Opioids modulate intestinal peristalsis at a site of action additional to that modulating acetylcholine release. J Pharmacol Exp Ther. 1982;223(1):271–4. [PubMed] [Google Scholar]
- 49.Paton WD. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957;12(1):119–27. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lembo A. Peripheral opioids for functional GI disease: a reappraisal. Dig Dis. 2006;24(1-2):91–8. doi: 10.1159/000090312. [DOI] [PubMed] [Google Scholar]
- 51.Zubieta JK, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–62. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorn SD, et al. A meta-analysis of the placebo response in complementary and alternative medicine trials of irritable bowel syndrome. Neurogastroenterol Motil. 2007;19(8):630–7. doi: 10.1111/j.1365-2982.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- 53.Gaman A, Kuo B. A review of CNS activation patterns from fMRI studies in IBS patients versus healthy controls. Gastroenterology. 2007;32(4, supplement 2):A-602. [Google Scholar]
- 54.Yuan YZ, et al. Functional brain imaging in irritable bowel syndrome with rectal balloon-distention by using fMRI. World J Gastroenterol. 2003;9(6):1356–60. doi: 10.3748/wjg.v9.i6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andresen V, et al. Brain activation responses to subliminal or supraliminal rectal stimuli and to auditory stimuli in irritable bowel syndrome. Neurogastroenterol Motil. 2005;17(6):827–37. doi: 10.1111/j.1365-2982.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 56.Ringel Y, et al. Regional brain activation in response to rectal distension in patients with irritable bowel syndrome and the effect of a history of abuse. Dig Dis Sci. 2003;48(9):1774–81. doi: 10.1023/a:1025455330704. [DOI] [PubMed] [Google Scholar]
- 57.Silverman DH, et al. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112(1):64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 58.Naliboff BD, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63(3):365–75. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Kwan CL, et al. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology. 2005;65(8):1268–77. doi: 10.1212/01.wnl.0000180971.95473.cc. [DOI] [PubMed] [Google Scholar]
- 60.Mertz H, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118(5):842–8. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 61.Verne GN, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103(1-2):99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 62.Wilder-Smith CH, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53(11):1595–601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernstein CN, et al. Cortical mapping of visceral pain in patients with GI disorders using functional magnetic resonance imaging. Am J Gastroenterol. 2002;97(2):319–27. doi: 10.1111/j.1572-0241.2002.05464.x. [DOI] [PubMed] [Google Scholar]
- 64.Mayer EA, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115(3):398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 65.Song GH, et al. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126(1-3):79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 66.Kong J, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26(2):381–8. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vandenberghe J, et al. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology. 2007;132(5):1684–93. doi: 10.1053/j.gastro.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 68.Kern M, et al. Characterization of the cerebral cortical representation of heartburn in GERD patients. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G174–81. doi: 10.1152/ajpgi.00184.2003. [DOI] [PubMed] [Google Scholar]
- 69.Gow D, et al. Characterising the central mechanisms of sensory modulation in human swallowing motor cortex. Clin Neurophysiol. 2004;115(10):2382–90. doi: 10.1016/j.clinph.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 70.Fregni F, et al. Treatment of chronic visceral pain with brain stimulation. Ann Neurol. 2005;58(6):971–2. doi: 10.1002/ana.20651. [DOI] [PubMed] [Google Scholar]
- 71.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 72.Xing J, et al. Gastric electrical stimulation significantly increases canine lower esophageal sphincter pressure. Dig Dis Sci. 2005;50(8):1481–7. doi: 10.1007/s10620-005-2866-4. [DOI] [PubMed] [Google Scholar]
- 73.Clarke JO, et al. An endoscopically implantable device stimulates the lower esophageal sphincter on demand by remote control: a study using a canine model. Endoscopy. 2007;39(1):72–6. doi: 10.1055/s-2006-945102. [DOI] [PubMed] [Google Scholar]
- 74.Chen JD, et al. Efficiency and efficacy of multi-channel gastric electrical stimulation. Neurogastroenterol Motil. 2005;17(6):878–82. doi: 10.1111/j.1365-2982.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 75.Bellahsene BE, et al. Acceleration of gastric emptying with electrical stimulation in a canine model of gastroparesis. Am J Physiol. 1992;262(5 Pt 1):G826–34. doi: 10.1152/ajpgi.1992.262.5.G826. [DOI] [PubMed] [Google Scholar]
- 76.Abell T, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125(2):421–8. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 77.Chen J. Mechanisms of action of the implantable gastric stimulator for obesity. Obes Surg. 2004;14 1:S28–32. doi: 10.1007/BF03342135. [DOI] [PubMed] [Google Scholar]
- 78.Cigaina VV, et al. Long-term Effects of Gastric Pacing to Reduce Feed Intake in Swine. Obes Surg. 1996;6(3):250–253. doi: 10.1381/096089296765556854. [DOI] [PubMed] [Google Scholar]
- 79.Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12 1:12S–16S. doi: 10.1007/BF03342141. [DOI] [PubMed] [Google Scholar]
- 80.Ouyang H, Xing J, Chen JD. Tachygastria induced by gastric electrical stimulation is mediated via alpha- and beta-adrenergic pathway and inhibits antral motility in dogs. Neurogastroenterol Motil. 2005;17(6):846–53. doi: 10.1111/j.1365-2982.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 81.D'Argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002;12 1:21S–25S. doi: 10.1381/096089202762552638. [DOI] [PubMed] [Google Scholar]
- 82.Shikora SA. “What are the yanks doing?” the U.S. experience with implantable gastric stimulation (IGS) for the treatment of obesity - update on the ongoing clinical trials. Obes Surg. 2004;14 1:S40–8. doi: 10.1007/BF03342137. [DOI] [PubMed] [Google Scholar]
- 83.Wang GJ, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci U S A. 2006;103(42):15641–5. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang M, et al. Implantable gastric stimulation alters expression of oxytocin- and orexin-containing neurons in the hypothalamus of rats. Obes Surg. 2006;16(6):762–9. doi: 10.1381/096089206777346745. [DOI] [PubMed] [Google Scholar]
- 85.Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R99–106. doi: 10.1152/ajpregu.00008.2002. [DOI] [PubMed] [Google Scholar]
- 86.Schwartz GJ. Biology of eating behavior in obesity. Obes Res. 2004;12 2:102S–6S. doi: 10.1038/oby.2004.274. [DOI] [PubMed] [Google Scholar]
- 87.Kiwaki K, et al. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286(4):E551–9. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- 88.Greenwood-Van Meerveld B, et al. Spinal cord stimulation attenuates visceromotor reflexes in a rat model of post-inflammatory colonic hypersensitivity. Auton Neurosci. 2005;122(1-2):69–76. doi: 10.1016/j.autneu.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Ceballos A, et al. Spinal cord stimulation: a possible therapeutic alternative for chronic mesenteric ischaemia. Pain. 2000;87(1):99–101. doi: 10.1016/S0304-3959(00)00266-9. [DOI] [PubMed] [Google Scholar]
- 90.Kapur S, Mutagi H, Raphael J. Spinal cord stimulation for relief of abdominal pain in two patients with familial Mediterranean fever. Br J Anaesth. 2006;97(6):866–8. doi: 10.1093/bja/ael279. [DOI] [PubMed] [Google Scholar]
- 91.Khan EA. Application of Spinal Cord Stimulation for the treatment of abdominal visceral pain syndromes: case reports. Neuromodulation. 2005;8(1):14–27. doi: 10.1111/j.1094-7159.2005.05216.x. [DOI] [PubMed] [Google Scholar]
- 92.Jackson M, Simpson KH. Spinal cord stimulation in a patient with persistent oesophageal pain. Pain. 2004;112(3):406–8. doi: 10.1016/j.pain.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 93.Malouf AJ, et al. Short-term effects of sacral nerve stimulation for idiopathic slow transit constipation. World J Surg. 2002;26(2):166–70. doi: 10.1007/s00268-001-0202-5. [DOI] [PubMed] [Google Scholar]
- 94.Malouf AJ, et al. Permanent sacral nerve stimulation for fecal incontinence. Ann Surg. 2000;232(1):143–8. doi: 10.1097/00000658-200007000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dinning PG, et al. Sacral nerve stimulation induces pan-colonic propagating pressure waves and increases defecation frequency in patients with slow-transit constipation. Colorectal Dis. 2007;9(2):123–32. doi: 10.1111/j.1463-1318.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 96.Lannon J. The parasympathetic supply of the distal colon. British Journal of Surgery. 1947;34(136):373–378. doi: 10.1002/bjs.18003413606. [DOI] [PubMed] [Google Scholar]
- 97.Felten D, Jozefowicz R. Netter's Atlas of Human Neuroscience. 2003:131. [Google Scholar]
- 98.Varma JS, et al. Differential effects of sacral anterior root stimulation on anal sphincter and colorectal motility in spinally injured man. Br J Surg. 1986;73(6):478–82. doi: 10.1002/bjs.1800730619. [DOI] [PubMed] [Google Scholar]
- 99.Kenefick NJ, et al. Sacral nerve stimulation for faecal incontinence due to systemic sclerosis. Gut. 2002;51(6):881–3. doi: 10.1136/gut.51.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mertz H. Review article: visceral hypersensitivity. Aliment Pharmacol Ther. 2003;17(5):623–33. doi: 10.1046/j.1365-2036.2003.01447.x. [DOI] [PubMed] [Google Scholar]


