Abstract
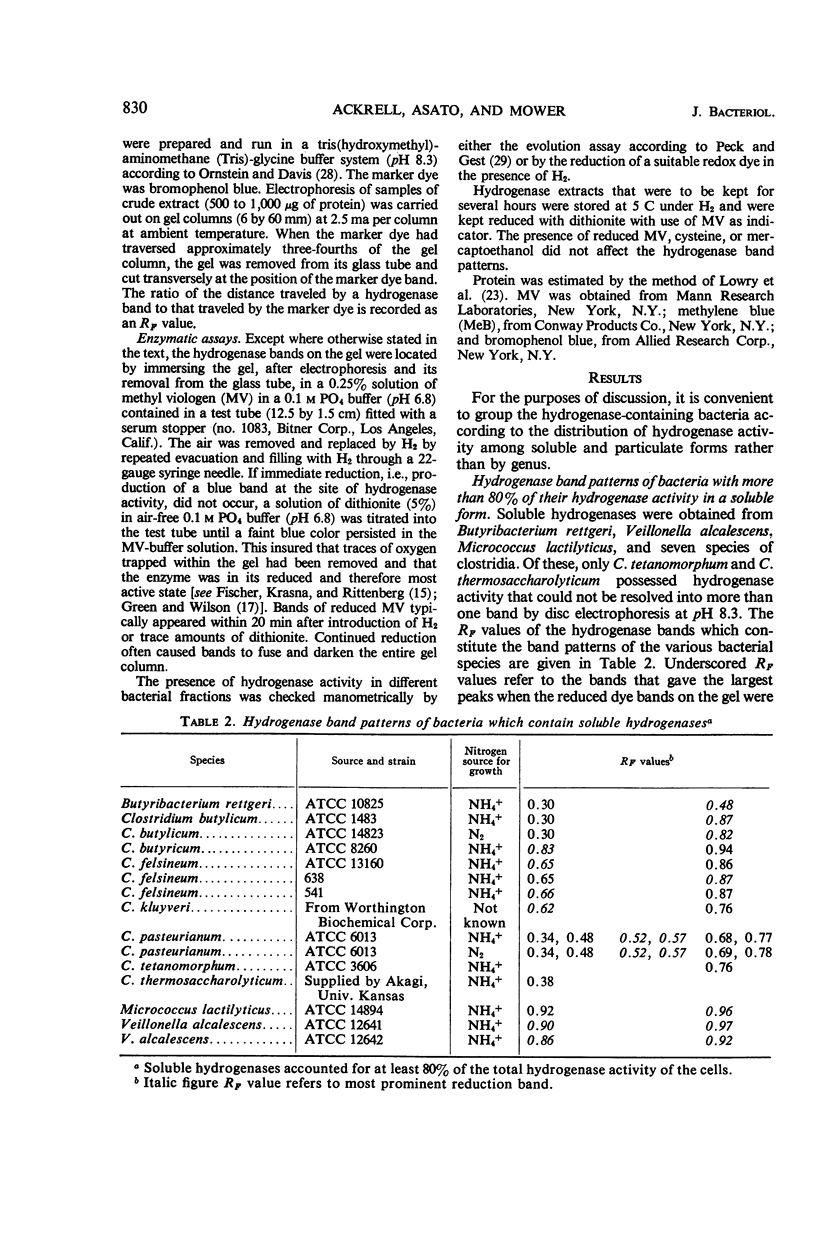
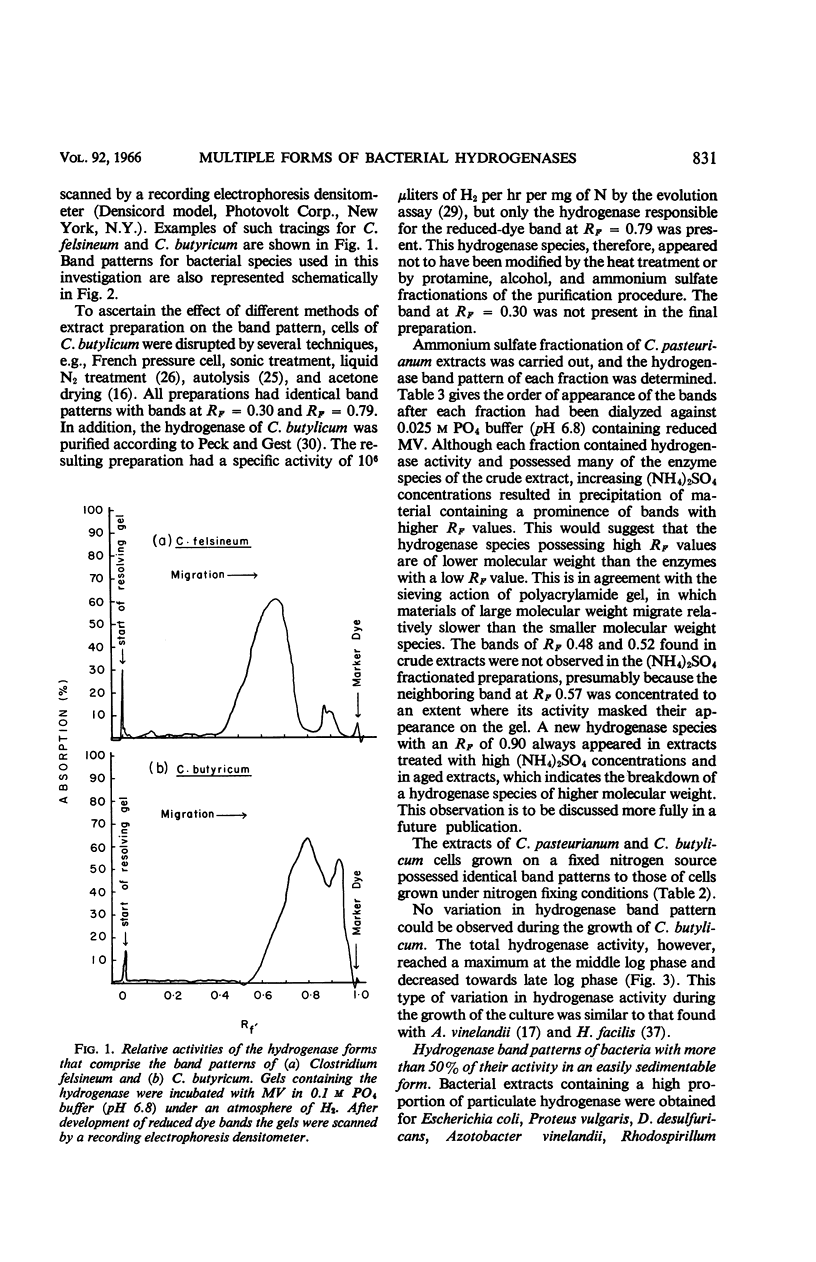
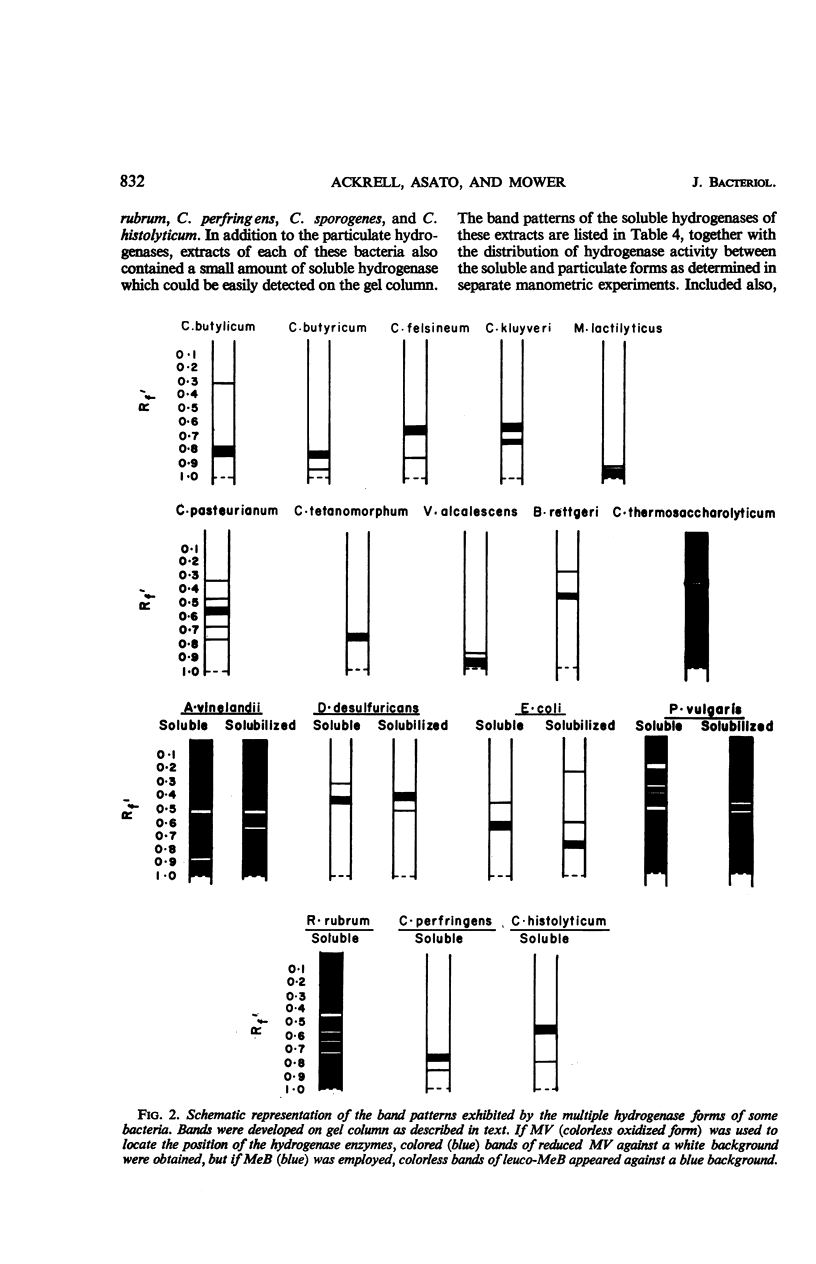
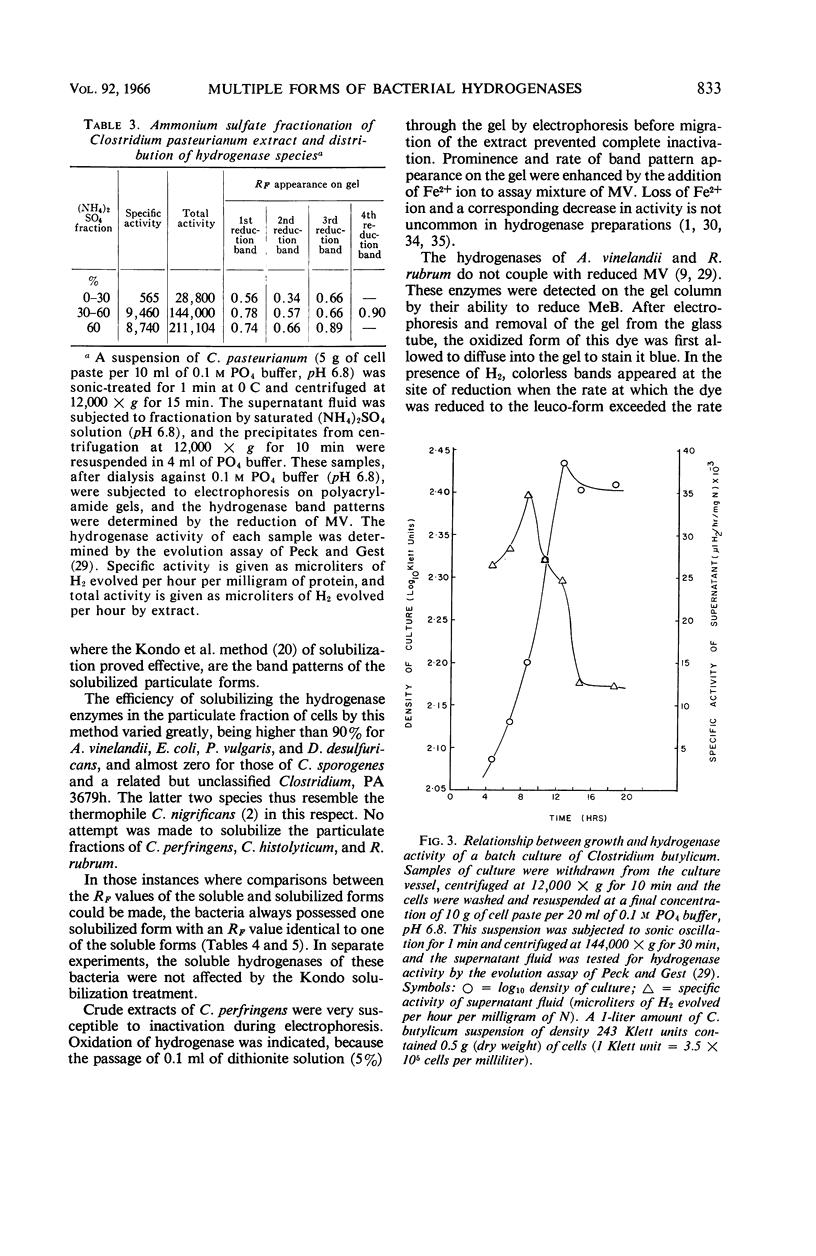
Ackrell, B. A. C. (University of Hawaii, Honolulu), R. N. Asato, and H. F. Mower. Multiple forms of bacterial hydrogenases. J. Bacteriol. 92:828–838. 1966.—Extracts of certain bacterial species have been shown by disc electrophoresis on polyacrylamide gel to contain multiple hydrogenase systems. The hydrogenase enzymes comprising these systems have different electrophoretic mobilities and produce a band pattern that is unique for each bacterial species. Of 20 bacterial species known to possess hydrogenase activity and which were examined by this technique, only the activities of Clostridium tetanomorphum and C. thermosaccharolyticum could be attributed, at pH 8.3, to a single hydrogenase enzyme. This multiplicity of hydrogenase forms was found both in bacteria which contain mostly soluble hydrogenases and in those where the hydrogenase is predominantly associated with particulate material. When solubilization of this particulate material could be effected, at least two solubilized hydrogenases were released, and, of these, one would have the same electrophoretic properties (i.e., RF) as one of the soluble hydrogenases already present in small amounts within the cell. Different growth conditions for various types of bacteria, such as the nitrogen source, the degree of aeration, and photosynthetic versus aerobic growth in the dark, as well as the conditions under which the cells were stored, markedly affected the hydrogenase activity of the cells, but not their hydrogenase band pattern. The disc electrophoresis technique proved to be 10 times more sensitive than the manometric technique in detecting hydrogenase activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKAGI J. M., CAMPBELL L. L. Studies on thermophilic sulfate-reducing bacteria. II. Hydrogenase activity of Clostridium nigrificans. J Bacteriol. 1961 Dec;82:927–932. doi: 10.1128/jb.82.6.927-932.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER F. D., PAPISKA H. R., CAMPBELL L. L. Choline fermentation by Desulfovibrio desulfuricans. J Bacteriol. 1962 Nov;84:973–978. doi: 10.1128/jb.84.5.973-978.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER H. A., SMYTH R. D., WILSON R. M., WEISSBACH H. The purification and properties of beta-methylaspartase. J Biol Chem. 1959 Feb;234(2):320–328. [PubMed] [Google Scholar]
- BONE D. H., BERNSTEIN S., VISHNIAC W. Purification and some properties of different forms of hydrogen dehydrogenase. Biochim Biophys Acta. 1963 Apr 9;67:581–588. doi: 10.1016/0006-3002(63)91868-7. [DOI] [PubMed] [Google Scholar]
- Bulen W. A. EFFECT OF TUNGSTATE ON THE UPTAKE AND FUNCTION OF MOLYBDATE IN AZOTOBACTER AGILIS. J Bacteriol. 1961 Jul;82(1):130–134. doi: 10.1128/jb.82.1.130-134.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. C., Bulen W. A. ATP-dependent hydrogen evolution by cell-free preparations of Azotobacter vinelandii. Biochim Biophys Acta. 1965 Sep 20;105(3):437–445. doi: 10.1016/s0926-6593(65)80229-6. [DOI] [PubMed] [Google Scholar]
- CARNAHAN J. E., CASTLE J. E. Some requirements of biological nitrogen fixation. J Bacteriol. 1958 Feb;75(2):121–124. doi: 10.1128/jb.75.2.121-124.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Duchow E., Douglas H. C. RHODOMICROBIUM VANNIELII, A NEW PHOTOHETEROTROPHIC BACTERIUM. J Bacteriol. 1949 Oct;58(4):409–416. doi: 10.1128/jb.58.4.409-416.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., WILSON P. W. Hydrogenase and nitrogenase in Azotobacter. J Bacteriol. 1953 May;65(5):511–517. doi: 10.1128/jb.65.5.511-517.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Needham D. M., Dewan J. G. Dismutations and oxidoreductions. Biochem J. 1937 Dec;31(12):2327–2352. doi: 10.1042/bj0312327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINE L., BARKER H. A. A new growth factor required by Butyribacterium rettgeri. J Bacteriol. 1950 Sep;60(3):349–363. doi: 10.1128/jb.60.3.349-363.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHLMILLER E. F., Jr, GEST H. A comparative study of the light and dark fermentations of organic acids by Rhodo-spirillum rubrum. J Bacteriol. 1951 Mar;61(3):269–282. doi: 10.1128/jb.61.3.269-282.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER E. C., MILLER J. A. In vivo combinations between carcinogens and tissue constituents and their possible role in carcinogenesis. Cancer Res. 1952 Aug;12(8):547–556. [PubMed] [Google Scholar]
- MORTENSON L. E. FERREDOXIN REQUIREMENT FOR NITROGEN FIXATION BY EXTRACTS OF CLOSTRIDIUM PASTEURIANUM. Biochim Biophys Acta. 1964 Mar 9;81:473–478. doi: 10.1016/0926-6569(64)90132-4. [DOI] [PubMed] [Google Scholar]
- MOSES V. Tricarboxylic acid cycle reactions in the fungus Zygorrhynchus moelleri. J Gen Microbiol. 1955 Oct;13(2):235–251. doi: 10.1099/00221287-13-2-235. [DOI] [PubMed] [Google Scholar]
- OKUDA K., EDWARDS G. C., WINNICK T. Biosynthesis of gramicidin and tryocidine in the Dubos strain of Bacillus brevis. I. Experiments with growing cultures. J Bacteriol. 1963 Feb;85:329–338. doi: 10.1128/jb.85.2.329-338.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. A new procedure for assay of bacterial hydrogenases. J Bacteriol. 1956 Jan;71(1):70–80. doi: 10.1128/jb.71.1.70-80.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Hydrogenase of Clostridium butylicum. J Bacteriol. 1957 Apr;73(4):569–580. doi: 10.1128/jb.73.4.569-580.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBRISH S. A., MARR A. G. Location of enzymes in Azotobacteragilis. J Bacteriol. 1962 Jan;83:158–168. doi: 10.1128/jb.83.1.158-168.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M. THE GENUS VEILLONELLA. I. GENERAL CULTURAL, ECOLOGICAL, AND BIOCHEMICAL CONSIDERATIONS. J Bacteriol. 1964 Jan;87:162–170. doi: 10.1128/jb.87.1.162-170.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADANA J. C., JAGANNATHAN V. Purification and properties of the hydrogenase of Desulfovibrio desulfuricans. Biochim Biophys Acta. 1956 Mar;19(3):440–452. doi: 10.1016/0006-3002(56)90467-x. [DOI] [PubMed] [Google Scholar]
- SADANA J. C., MOREY A. V. The purification of hydrogenase of Desulfovibrio desulfuricans. Biochim Biophys Acta. 1959 Apr;32:592–593. doi: 10.1016/0006-3002(59)90655-9. [DOI] [PubMed] [Google Scholar]
- SADANA J. C., RITTENBERG D. SOME OBSERVATIONS ON THE ENZYME HYDROGENASE OF DESULFOVIBRIO DESULFURICANS. Proc Natl Acad Sci U S A. 1963 Nov;50:900–904. doi: 10.1073/pnas.50.5.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZ A., BOVELL C., Jr Growth and hydrogenase activity of a new bacterium, Hydrogenomonas facilis. J Bacteriol. 1952 Jan;63(1):87–98. doi: 10.1128/jb.63.1.87-98.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C. BACTERIAL FERREDOXIN. Bacteriol Rev. 1964 Dec;28:497–517. doi: 10.1128/br.28.4.497-517.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C., BRILL W. J., SAGERS R. D. FERREDOXIN LINKED DPN REDUCTION BY PYRUVATE IN EXTRACTS OF CLOSTRIDIUM ACIDI-URICI. Biochem Biophys Res Commun. 1963 Aug 1;12:315–319. doi: 10.1016/0006-291x(63)90303-6. [DOI] [PubMed] [Google Scholar]
- WHITELEY H. R., DOUGLAS H. C. The fermentation of purines by Micrococcus lactilyticus. J Bacteriol. 1951 May;61(5):605–616. doi: 10.1128/jb.61.5.605-616.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDER M., VALENTINE R. C., AKAGI J. M. FERREDOXIN OF CLOSTRIDIUM THERMOSACCHAROLYTICUM. J Bacteriol. 1963 Oct;86:861–865. doi: 10.1128/jb.86.4.861-865.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



