Abstract
Red blood cells (RBCs) have been ascribed an essential role in matching blood flow to local metabolic demand during hypoxic vasodilation. The vasodilatory function of RBCs evidently relies on the allosteric properties of hemoglobin (Hb), which couple the conformation of Hb to tissue oxygen tension (PO2) and thereby provide a basis for the graded vasodilatory activity that is inversely proportional to Hb oxygen saturation. Although a large body of evidence indicates that the PO2-coupled allosteric transition from R (oxy)-state to T (deoxy)-state subserves the release from Hb of vasodilatory nitric oxide (NO) bioactivity, it has not yet been determined whether the NO-based signal is a necessary and sufficient source of RBC-mediated vasoactivity and it has been suggested that ATP or nitrite may also contribute. We demonstrate here by bioassay that untreated human RBCs rapidly and substantially relax thoracic aorta from both rabbit and mouse at low PO2 (≈1% O2) but not at high PO2 (≈21% O2). RBC-mediated vasorelaxation is inhibited by prior depletion of S-nitroso-Hb, potentiated by low-molecular-weight thiols, and dependent on cGMP. Furthermore, these relaxations are largely endothelium-independent and unaffected by NO synthase inhibition or nitrite. Robust relaxations by RBCs are also elicited in the absence of endothelial, neuronal or inducible NO synthase. Finally, contractions that appear following resolution of RBC-mediated relaxations are dependent on NO derived from RBCs as well as the endothelium. Our results suggest that an S-nitrosothiol—based signal originating from RBCs mediates hypoxic vasodilation by RBCs, and that vasorelaxation by RBCs dominates NO-based vasoconstriction under hypoxic conditions.
Keywords: red blood cell, hemoglobin, nitric oxide, hypoxic vasodilation
The present perspective on the mechanisms of peripheral blood flow regulation is informed by the recognition that tissue perfusion is coupled to the O2 saturation of hemoglobin (Hb), and not to the partial pressure of oxygen (PO2),1 and by a requirement that changes in blood flow can be elicited very rapidly (arteriovenous transit time is seconds or less). Blood flow is also dependent on the bioavailability of nitric oxide (NO).1 It has thus been appreciated over the past decade that red blood cells (RBCs) themselves are principal transducers of the physiological response, hypoxic vasodilation, and that this involves NO-based bioactivity: Hb desaturation in the arterial periphery, results in the release from RBCs not only of O2 but also of vasodilatory activity.1-6 Dysregulated vasodilation by RBCs has been implicated in a broad spectrum of pathophysiological conditions characterized by tissue hypoxemia7-13 and in the defect accompanying blood storage.14,15
NO bioactivity in biological systems is present in the form of NO and S-nitrosothiol derivatives (SNOs).1 NO itself cannot escape the RBC because it is rapidly sequestered and inactivated by both oxygenated and deoxygenated Hb.3,16 In contrast, SNOs are impervious to heme and thus retain the ability to elicit vasodilation in the presence of Hb.6 Substantial evidence supports a model in which an SNO derivative of a highly conserved cysteine residue within the β-chain of Hb (SNO-Hb) serves as a principal source of PO2-regulated, RBC-mediated vasoactivity.1 Intracellular SNO-Hb is in equilibrium with other SNOs, including S-nitrosoglutathione12,17,18 and its effector dipeptide metabolite S-nitroso-cysteinylglycine,18,19 which are potent vasodilators. Vasodilation by RBCs5 is faithfully recapitulated both in vitro20 and in vivo1,15 by SNO-Hb, which dilates blood vessels at low PO2 and constricts blood vessels at high PO2. Under this model, the R-state to T-state allosteric transition facilitates the release from Hb of both O2 and vasodilatory NO equivalents,3,20 thereby coupling local metabolic demand and blood flow.1,17,20 However, the nature of the RBC-derived vasoactive species and the mechanism of its delivery to the vessel wall are not fully understood,1,3 and it has been suggested that RBCs may deliver alternative agents, in particular ATP,21,22 whose vasodilatory activity is dependent on the endothelium.1,23 It has also been suggested that RBC-induced vasorelaxation is mediated by nitrite,24 but it is unclear from recent studies whether nitrite can elicit relaxations in the presence of Hb,25-27 unless it were to form SNO-Hb.28-30
We have developed an in vitro bioassay using native human RBCs that recapitulates the PO2 dependence of RBC-mediated vasodilation and vasoconstriction.5 Here, we use this assay to provide evidence that an SNO-based signal originating in a PO2-dependent fashion is the principal effector of hypoxic vasodilation by RBCs. RBCs elicit cGMP-mediated relaxations that are largely independent of endothelium, unaffected by added nitrite, and attenuated by depletion of intracellular SNO-Hb.
Materials and Methods
Red Blood Cells
Human venous blood was drawn from an antecubital vein and RBCs, collected by centrifugation at 1000g, were rinsed 3 times in PBS at 4°C, stored in PBS on ice at 50% hematocrit, and used within 4 hours. Blood sampling from human subjects was approved by the Institutional Review Board of the Duke University Medical Center. Depletion of SNO-Hb (by ≈80%) was induced by storage of RBCs for 24 hours, as described recently.15
Animals and Preparation of Aortic Ring Segments
New Zealand White rabbits (5 to 6 lbs), wild-type C57BL/6J mice, endothelial NO synthase—null (eNOS-/-) mice, inducible NO synthase—null (iNOS-/-) mice, and neuronal NO synthase—null (nNOS-/-) mice (The Jackson Laboratory, 8 to 16 weeks of age) were euthanized by CO2 inhalation. A segment of thoracic aorta was excised, placed in Krebs solution, and cleaned of adventitial tissue and adherent blood cells before division into rings of 2 to 3 mm in length. All experimental procedures were approved by the Animal Care and Use Committee of Duke University Medical Center.
Bioassay of RBC Vasoactivity
Aortic rings were attached to isometric force transducers (Grass) and suspended in jacketed organ chambers containing Krebs-bicarbonate buffer (pH 7.4, 37°C) gassed with either 21% O2/5% CO2/74% nitrogen or 1% O2/5% CO2/94% nitrogen (measured PO2≈5 to 12 mm Hg). Resting tension was maintained at 2 or 1 g for rabbit and mouse aortic segments, respectively. Contraction elicited by potassium chloride (80 mmol/L) was evaluated initially, and active tension (50% to 80% of the KCl-induced tension) was evoked by phenylephrine before administration of RBCs (larger amounts of phenylephrine were required to maintain tension under hypoxia versus normoxia). RBCs were added to produce a bath hematocrit of 0.4% (higher hematocrit resulted in excessive hemolysis), and rings were rinsed 3 times following each measurement. Hypoxia (1% O2) was maintained for 5 minutes before adding RBCs.
Denudation of Rings and Pharmacological Inhibitors
Endothelium was removed from rings by gentle abrasion with a cotton swab and/or the smooth surface of a 27-gauge hypodermic needle. Relaxation to acetylcholine (10-7 mol/L) was assessed to confirm the presence or absence of intact endothelium in both denuded and control rings. When used, Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME) (Sigma) or N-(3-(aminomethyl)benzyl)acetamidine (1400W) (Cayman) was added to baths 1 hour before assessment of RBC vasoactivity, Nω-methyl-L-arginine acetate (L-NMMA) (Sigma) 30 minutes before assessment, 1H[1,2,4]oxadiazolo[4,3a]quinoxalin-1-one (ODQ) (Sigma) 20 minutes before assessment, and nitrite (VWR) 2 minutes before assessment.
Assay Design and Data Analysis
Responses were assessed separately for intact and denuded mouse rings; responses were assessed in intact rabbit segments before denudation and reassay of the same segments. Rings exhibiting no relaxation to RBCs (≈10% of experiments) or acetylcholine were not included in the data analysis. Relaxation or contraction is expressed as a percentage of the (equal) initial, active (phenylephrine-induced) tension. Data are expressed as means±SE, and n is the number of rings tested. Statistical significance was evaluated with Student t test and P<0.05.
Results
RBC-Mediated Vasoactivity
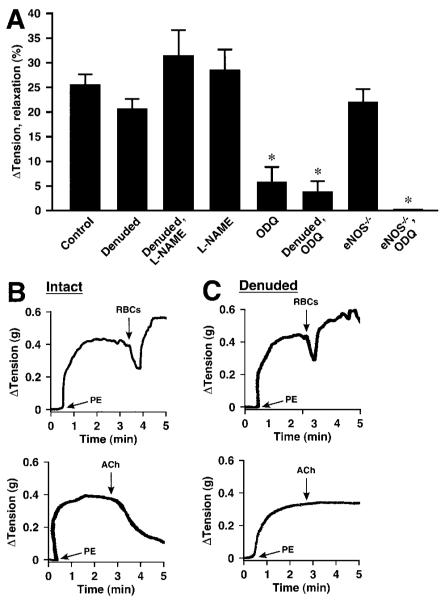
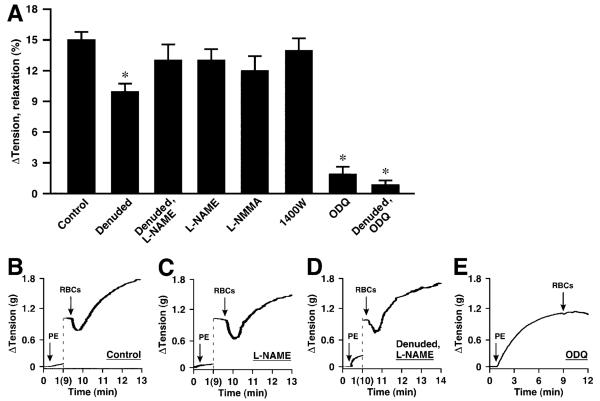
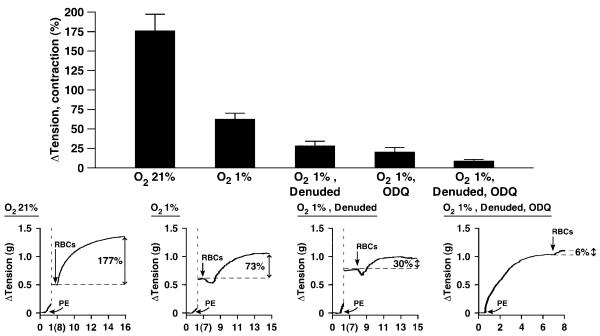
At ≈1% O2, representative of tissue PO2 during moderate activity,31,32 administration of human RBCs rapidly elicited relaxation of aortic rings with latencies of ≈8 to 12 seconds and durations of ≈0.5 to 1.5 minutes (return to starting tension; for reference, arteriovenous transit times are seconds or less) (Figures 1B, 1C, and 2B through 2D). The magnitudes of relaxation (means±SEM) averaged 26±2% (range 10% to 38%; n=34) and 15.1±0.8% (range 8% to 41%; n=110) for mouse and rabbit rings, respectively (Figures 1A and 2A). RBC-induced relaxations were followed by contraction. The magnitude of contraction was PO2-dependent, as detailed below (Figure 5). In contrast, at 21% O2, RBCs induced immediate contraction (latencies of contraction at 21% O2 were equivalent to latencies of relaxation at 1% O2). It has been shown that the amplitude of contraction progressively diminishes as O2 concentrations are lowered below 9% (PO2≈60 mm Hg), ultimately converting to overt relaxations.5 Thus, both vasorelaxation and vasoconstriction by RBCs are coupled to PO2.
Figure 1.
Hypoxic vasodilation (≈1% O2) by native human RBCs of mouse aortic segments, precontracted with phenylephrine (PE). A, Lack of effect of endothelial denudation or inhibition of NOS activity with L-NAME. Vasorelaxation was greatly attenuated by the guanylate cyclase inhibitor ODQ (n=9 to 34). *P<0.05. B and C, Representative examples of the elimination of acetylcholine (ACh)-induced vasorelaxation following endothelial removal versus preserved RBC-induced vasorelaxation.
Figure 2.
Hypoxic vasodilation by native human RBCs of rabbit aortic segments. A, No significant effect on vasorelaxation resulted from inhibition of NOS activity with L-NAME, L-NMMA, or 1400W. Vasorelaxation was greatly attenuated in both intact and denuded rings by treatment with ODQ (n=12 to 110) *P<0.05. B through E, Representative examples illustrate the lack of effect of NOS inhibition, the lack of effect of NOS inhibition combined with denudation, and the elimination of vasorelaxation by ODQ.
Figure 5.
PO2 dependence of vasoconstriction by native human RBCs of rabbit aortic segments. RBC-induced contraction terminated the relaxation at ≈1% O2 and exhibited latencies at ≈21% O2 (in the absence of relaxation) that were indistinguishable from the latencies of relaxation at ≈1% O2. Contractions were greater at ≈21% O2 than at ≈1% O2 and greatly attenuated by endothelium removal and by ODQ (n=9 to 36). *P<0.05. Representative examples illustrate the attenuation of vasoconstriction by hypoxia, denudation, and exposure to ODQ.
Nature of RBC-Derived Vasodilatory Signal: Role of Endothelium and eNOS
A large body of evidence supports a role for an NO-based effector in mediating the vasodilatory influence of RBCs,1,4 but the possibility has been raised of additional or alternative RBC-derived signals, including ATP,21,22 and a role for the endothelium in transducing an NO-based or other RBC-derived signal has not been elucidated fully.
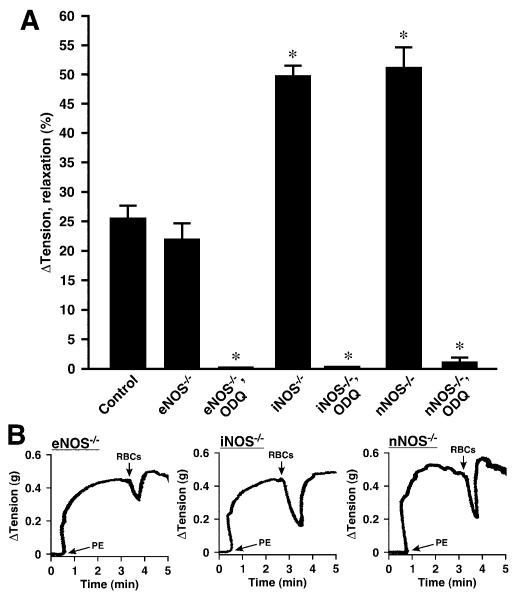
ATP-induced vasorelaxation of intact aorta is dependent on eNOS.14,23 Inhibition of NOS with any of several pharmacological inhibitors including L-NAME, L-NMMA, or 1400W had no significant effect on RBC-induced vasodilation at ≈1% O2, either alone or in combination with endothelial denudation (Figures 1A, 2A, 2C, and 2D). Thus, NOS activation by ATP, or any other RBC-derived effector, does not contribute significantly to RBC-mediated, PO2-coupled vasodilation under these conditions. This conclusion is supported further by the finding that hypoxic vasodilation by RBCs did not differ in aortic segments derived from wild-type versus eNOS-/- knockout mice (Figure 3A and 3B). In addition, eNOS is found in RBCs,33 but pretreatment of RBCs with 1 mmol/L L-NAME (1 hour) did not inhibit RBC-induced vasodilation of rabbit aortic rings (24±4%; n=8).
Figure 3.
Hypoxic vasodilation by native human RBCs of mouse aortic segments. A, Lack of effect of genetic elimination of eNOS (eNOS-/-). Elimination of iNOS (iNOS-/-), or nNOS (nNOS-/-) was associated with enhanced vasorelaxation. Vasorelaxation was greatly attenuated following treatment with the guanylate cyclase inhibitor ODQ in all cases (n=6 to 29). *P<0.05. B through D, Representative tracings illustrate the lack of effect of eNOS elimination and the enhancement of relaxation by nNOS and iNOS elimination.
By contrast, exposure of rings to the soluble guanylate cyclase inhibitor ODQ (3 μmol/L) greatly reduced the magnitude of RBC-induced hypoxic vasorelaxation in both mouse and rabbit rings (Figures 1A, 2A, 2E, 3A). Thus, RBC-mediated hypoxic vasodilation is largely dependent on enhanced cGMP production that is coupled to the delivery by RBCs to the vessel wall of an NO-based effector.
Removal of the endothelium, verified by complete loss of vasorelaxation to acetylcholine (Figure 1B and 1C), did not affect the magnitude of RBC-induced hypoxic vasodilation of mouse aortic segments (intact and denuded rings were assessed separately) (Figure 1A). Denudation of rabbit rings (which followed assessment of intact rings) resulted in a small decrease (≈33%) in some but not all studies, ie, in the absence but not presence of L-NAME (Figures 1A and 2A). Interpretation of the effects of endothelial denudation is complicated by the possibility of attendant damage to smooth muscle that might compromise import of NO bioactivity.18,19 Thus, although our results exclude a role of endothelium-derived NO (ie, relaxations were unaffected by NOS inhibition or eNOS deletion), they do not eliminate a role for the endothelium in the relay of an NO-based signal from RBCs. We, therefore, examined the possible involvement of endothelial release of prostaglandins, which may contribute to flow-induced vasodilation. However, the prostaglandin synthesis (cyclooxygenase) inhibitors indomethacin or acetylsalicylic acid had no effect on RBC-mediated hypoxic vasodilation (Figure 4A).
Figure 4.
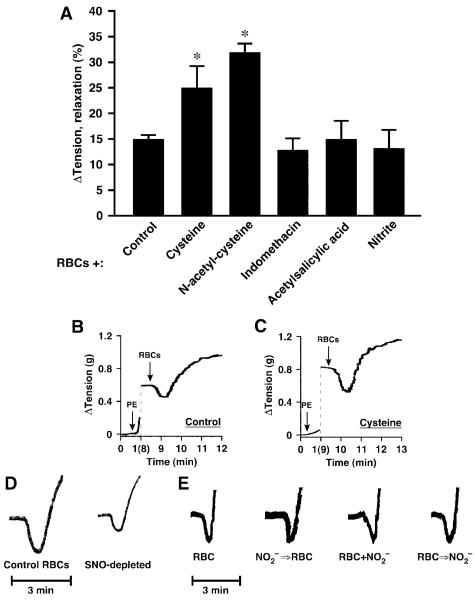
Hypoxic vasodilation by native human RBCs of rabbit aortic segments. A, RBC-induced vasorelaxation was potentiated by cysteine and by N-acetylcysteine but was unaffected by nitrite or the prostaglandin synthesis inhibitors indomethacin and aspirin (all agents added 2 minutes before RBCs) (n=5 to 11). *P<0.05. B through D, Representative tracings illustrate the augmentation of relaxation by cysteine and the attenuation of relaxation by SNO-Hb depletion (≈80% depletion). E, Addition of nitrite to the bath (even at supraphysiological concentrations of 1 μmol/L, which are higher than those used in Cosby24) before (NO2-⇒RBC) or simultaneously with the addition of RBCs (NO2-+RBC), or near the termination of RBC-induced vasorelaxation (RBC⇒NO2-) had no discernible effect on the magnitude or duration of RBC-induced hypoxic vasodilation.
It has been proposed that iNOS may be upregulated by lipopolysaccharide in bioassays,34 and nNOS present in smooth muscle may also counteract contractile responses.35,36 In addition, both nNOS and iNOS have been implicated in some hypoxic responses (bioassays are performed at 1% O2).37 Therefore, we wanted to be sure that neither iNOS nor nNOS was responsible for hypoxic relaxations by RBCs. In comparison to wild-type and eNOS-/- mice, we found it more difficult to sustain contractions under hypoxia in vessels from iNOS-/- and nNOS-/- mice (contractions began to decline spontaneously at ≈2 minutes versus ≈10 minutes in wild-type mice), and RBC-induced relaxations were enhanced in nNOS-/- (51±4%) and iNOS-/- (50±3%) aortae (Figure 3A and 3B). RBC-mediated relaxations in both strains were largely unaffected by L-NAME (data not shown) but were blocked by ODQ and, thus, dependent almost entirely on cGMP (Figure 3A). Accordingly, the NO-dependent relaxations in nNOS- and iNOS-null mice are likely enhanced (versus wild-type mice) because of increased intrinsic sensitivity of these vessels to hypoxia.
Palmer et al18 have recently characterized the effects of the thiol N-acetylcysteine (NAC) on intraerythrocytic SNO-Hb levels and on exported bioactivity as a function of PO2. NAC depletes SNO-Hb at low PO2, forming S-nitroso-NAC, as determined in vitro, ex vivo, and in vivo. Furthermore, NAC potentiated RBC bioactivity in vivo, as measured by pulmonary arterial responses.18 SNO-NAC itself was hypoxiamimetic in vivo. Collectively, these data indicate that RBCs transduce systemic and pulmonary responses to hypoxia via SNO. Additional evidence of a role for SNO-based vasodilatory activity was obtained from our finding of significant potentiation of RBC-mediated hypoxic vasodilation by NAC (1 mmol/L) or cysteine (100 μmol/L), which also readily forms S-nitrosothiols that can serve to transfer NO groups (Figure 4A through 4C).20,38 This finding is consistent with the possibility that an S-nitrosothiol may serve as an intermediate in the transfer of NO-based bioactivity from RBCs to the vessel wall in the context of hypoxic responses.
Finally, to elucidate further the origin within RBCs of NO-based vasodilatory activity that is potentiated by thiols, we manipulated levels of endogenous SNO-Hb. We have reported previously that exposure of RBCs to exogenous S-nitrosothiols,3,6 or to NO (followed by oxygenation),3 increases SNO-Hb levels and potentiates RBC-induced relaxation, whereas conditions that deplete SNO-Hb, including storage15 or maintained deoxygenation for >30 minutes,10 are associated with attenuated relaxation. Here, we show that storage (18- to 24-hour)-induced depletion of SNO-Hb in RBCs, as assessed by Hg-coupled photolysis chemiluminescence (≈80% depletion; 2.0 versus 0.25×10-3 NO/Hb tetramer),3,6,14,15 is associated with diminished relaxation (Figure 4D). It has also been suggested that generation of NO-based bioactivity by RBCs entails a nitrite reductase activity of Hb that directly releases NO.24 However, others have not interpreted the responses elicited in these nitrite bioassays as commensurate with hypoxic relaxations or with NO release,1,4,10,27,39 and it remains unclear how NO would escape the RBC to activate guanylate cyclase (let alone on timescales commensurate with arterial-venous transit).16 We, therefore, examined the effects in rabbit aorta of the addition of nitrite (1 μmol/L) ≈2 minutes before or simultaneously with the addition of RBCs or at the end of RBC-induced relaxations (on return to initial tension). There was no detectable effect on the magnitude or time course of relaxation by the addition of nitrite at any point (Figure 4A and 4E); specifically, the magnitude of RBC-induced relaxation after the prior addition of nitrite averaged 13±3.9% (n=7) versus 15% in the absence of added nitrite. Evidently, any NO produced by nitrite reduction is scavenged by Hb. Thus, under these conditions, nitrite does not appear to play a role in the export of NO bioactivity by RBCs.
RBC-Induced Vasoconstriction
RBCs produced contractions that either truncated the RBC-induced relaxation at ≈1% O2 or occurred in isolation (in the absence of relaxation) at ≈21% O2 (Figure 5). Endothelium removal, and thus loss of NO scavenging by Hb,3 attenuated but did not eliminate RBC-induced contraction (Figure 5). Notably, residual contractions of denuded rings under hypoxia were greatly attenuated by ODQ (Figures 2 and 5), consistent with evidence that NO/cGMP might exert a contractile effect.40-41 Thus, NO-based vasoactivity of RBCs reflects net relaxation versus constriction, with the former dominating at low PO2 and the latter at high PO2.
Discussion
The original discovery that RBCs can dilate blood vessels6 was followed by the demonstration that RBCs3,5 (and isolated SNO-Hb1,20) can replicate the physiological responses of hypoxic vasodilation and hyperoxic vasoconstriction across a PO2 continuum. RBCs are the only cell type known to recapitulate this physiology ex vivo. Here, we provide a detailed characterization of these standard bioassay responses. Our analysis indicates that hypoxic vasodilation by native human RBCs in bioassay is largely independent of the endothelium, potentiated by thiols and mediated by cGMP. Furthermore, we find that RBCs depleted of SNO-Hb elicit attenuated relaxations. Thus, our results confirm that native RBCs contain vasodilatory activity that is characteristic of SNO and released on the transition to low PO2. Previous work has shown that RBC vasoactivity is directly correlated with amounts of RBC SNO-Hb3,7,10,14,15 and is replicated in vitro by isolated SNO-Hb (in added presence of thiol).1,5,20 Collectively, these studies strongly support the conclusion that NO-based bioactivity is necessary and sufficient to convey this PO2-dependent vasodilatory influence. Additional physiological context for these studies is provided by the work of Gaston and colleagues,17,18 who showed that deoxygenation of RBCs results in a decline in SNO-Hb that is potentiated by thiols and paralleled by accumulation of plasma SNOs, indicating that NO groups are also exchanged between SNO-Hb and low-molecular-weight thiols in vivo.
There is also evidence that ATP is released from many cells, including endothelium, platelets, and RBCs, under hypoxia. However, whereas a role in hypoxic vasodilation for ATP release by RBCs has been thoughtfully considered,2,21,22 RBC-induced responses were never actually studied in bioassays,21 and in humans and mice, NOS inhibition has little effect on the coupling of blood flow to metabolic demand, evidently excluding a major role for ATP.1,42,43 In addition, direct measurements of ATP1,2 during (and following) hypoxic relaxations by RBCs do not support a primary role in RBC-mediated vasodilation, consistent with the lack of effects of eNOS deletion and NOS inhibitors in vivo, which do not generally affect microcirculatory flow responses to hypoxia (exercise).42,43 Most recently, 1 group has suggested that RBC-mediated relaxation is endothelium-dependent (and thus dependent on ATP),44 but the effects of eNOS (and ATP) inhibition, in fact, were no different from those reported here (attenuation of relaxation by a small percentage). Moreover, those studies are difficult to interpret because they were conducted at PO2 levels at which RBCs do not elicit overt relaxations in our hands, and basal ring tone was apparently not controlled for across different experimental conditions. Inasmuch as no mechanism has been proposed that would couple changes in PO2 (for which Hb is the only available sensor) to graded ATP release, RBC lysis (in bioassays) will need to be excluded as a likely factor. One possibility is that ATP contributes to settling points in vivo1,4 and/or increases gain of the responses to SNO-based signals acting on more rapid timescales, and may be released by RBCs to compensate for SNO deficiency (resulting from disease, storage, or preparatory losses).
Our results demonstrate an obligate role for cGMP in RBC-mediated vasodilation, as revealed by the inhibitory effect of ODQ. In contrast, a significant proportion of vasodilation by endothelially derived NO may be cGMP-independent in rabbit aortae,45,46 and a role for direct modification by NO (likely through S-nitrosylation) of charybdotoxin-sensitive Ca2+-dependent K+ channels45 and calcium ATPase47 has been proposed. It is important to note that, although the cGMP-dependence of RBC-mediated vasodilation of aortic segments identifies an NO-based mechanism, the extent to which RBC-mediated vasodilation in the microcirculation is dependent on activation of guanylate cyclase or on other, NO-based protein modifications has not been determined.
The role of nitrite in hypoxic vasodilation has been the subject of intense interest since Cosby et al reported that Hb potentiated nitrite-mediated relaxations by 3 orders of magnitude in bioassays.24 However, the same investigators48 and other groups10,26,27 have reported more recently that both Hb and RBCs in fact block nitrite-mediated relaxations,25,27,48 and thus the original studies await confirmation. As shown here, we find no evidence for a direct role of nitrite in RBC-mediated responses, in agreement with others.10,14,25 Although increases in nitrite (infusion or oral intake) elicit vasodilatory effects,24,49 these are commensurate with pharmacological rather than hypoxic vasodilation,1,4,26 and nitrite is an extremely weak vasodilator in comparison to organic nitrates or endogenous S-nitrosothiols. Moreover, “hypoxic potentiation,” characteristic of many compounds that induce vasodilation, is conflated with “hypoxic vasodilation,” in which blood flow is coupled to metabolic demand.4 With that said, nitrite may react with deoxy-Hb (venous blood) to generate Fe(III)NO, which converts to SNO following oxygenation (across the lungs),28-30 and may be converted into SNOs following acidification in the gut.50 Therefore, although nitrite may serve as a source of SNOs, a role for “nitrite reductase” in the generation of vasoactivity during hypoxic vasodilation (ie, rapid relaxations that are coupled directly to Hb deoxygenation during arteriovenous transit)24 cannot be adduced from this or other work.
Most recently, a role for nitrite in RBC-mediated hypoxic vasodilation has been inferred from studies in a Cys mutant mouse that purportedly cannot form SNO-Hb.51 However, whereas hypoxic vasodilation is a measure of blood flow coupled to PO2, neither blood flow nor PO2 was measured in any experiment. Moreover, the assessment of RBC-mediated relaxations was mistakenly performed in pulmonary arteries that normally constrict to hypoxia, and the data in fact show markedly impaired relaxations of mutant RBCs compared to normal literature controls (wild-type controls were not performed by the authors).10 Additionally, relaxations shown in that study are endothelium-dependent (presumably ATP mediated), consistent with either lysis of RBCs or absence of SNO-Hb.
Several salient aspects of RBC-mediated vasoactivity in bioassay support a role for RBCs in hypoxic vasodilation in vivo. In particular, the response to RBCs is rapid (seconds, reflecting the minimal latency in bioassays) and fairly substantial (≈15% to 50% of initial tension), even at 0.4% hematocrit. Because blood flow is a function of vessel radius to the fourth power, RBCs can probably exert substantial vasoactivity in vivo.15 Moreover, the vasodilatory response to RBCs under hypoxia occurs in the face of opposing vasoconstriction that is mediated by NO scavenging. Thus, RBC vasoactivity represents a net response that may enable RBCs to fulfill requirements of both vasodilation and vasoconstriction to match blood flow with metabolic demand. Vasodilation is evidently carried out by SNOs, which are the only endogenous species known to retain vasodilatory activity in the presence of Hb, whereas vasoconstriction is mediated partly by heme-based sequestration of endothelial NO. However, RBC-induced contraction was also reduced significantly by exposure to ODQ, implicating a role for cGMP. Constrictor effects of SNOs have been previously reported,40,52 as have contractile effects of cGMP,41 and new data suggest that such effects may result from modulation of GPCR signaling, including inhibition of desensitization by NO.53 RBC-derived NO bioactivity may have different effects from that derived in the endothelium.
Dysregulated formation of SNO-Hb is evident in a number of pathophysiological conditions characterized by tissue hypoxemia, including sickle cell disease,7 diabetes,8,9 pulmonary hypertension,10 septic shock,11,12 and congestive heart failure13; when examined by bioassay, impaired production of SNO-Hb is reflected in impaired vasodilation by RBCs.7,9,10 In addition, as shown here and previously,14,15 SNO-Hb levels decline in stored RBCs, in close concert with a loss of vasodilatory ability, providing a potential explanation for at least some part of the ischemic morbidity and mortality associated with blood transfusion. Loss of SNO during preparation of RBCs may also account for discrepancies between groups that have investigated the RBC-mediated vasodilatory response.14 Thus, identification of an NO-based signal as a necessary and sufficient RBC-mediated effector in hypoxic vasodilation supports a focus on SNO-Hb formation and NO/SNO delivery from RBCs to ameliorate deficiencies in tissue oxygenation.
Acknowledgments
Sources of Funding: This work was supported by NIH grants SP01-HL-42444 and T32-6 M-069331-04.
Footnotes
Disclosures: None.
References
- 1.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 4.Allen BW, Piantadosi CA. How do red blood cells cause hypoxic vasodilation? The SNO-hemoglobin paradigm. Am J Physiol Heart Circ Physiol. 2006;291:H1507–H1512. doi: 10.1152/ajpheart.00310.2006. [DOI] [PubMed] [Google Scholar]
- 5.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 6.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 7.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci U S A. 2005;102:2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padron J, Peiro C, Cercas E, Llergo JL, Sanchez-Ferrer CF. Enhancement of S-nitrosylation in glycosylated hemoglobin. Biochem Biophys Res Commun. 2000;271:217–221. doi: 10.1006/bbrc.2000.2617. [DOI] [PubMed] [Google Scholar]
- 9.James PE, Lang D, Tufnell-Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94:976–983. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 10.McMahon TJ, Ahearn GS, Moya MP, Gow AJ, Huang YC, Luchsinger BP, Nudelman R, Yan Y, Krichman AD, Bashore TM, Califf RM, Singel DJ, Piantadosi CA, Tapson VF, Stamler JS. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A. 2005;102:14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104:1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 13.Datta B, Tufnell-Barrett T, Bleasdale RA, Jones CJ, Beeton I, Paul V, Frenneaux M, James P. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004;109:1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 14.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsoukias NM, Popel AS. Erythrocyte consumption of nitric oxide in presence and absence of plasma-based hemoglobin. Am J Physiol Heart Circ Physiol. 2002;282:H2265–H2277. doi: 10.1152/ajpheart.01080.2001. [DOI] [PubMed] [Google Scholar]
- 17.Doctor A, Platt R, Sheram ML, Eischeid A, McMahon T, Maxey T, Doherty J, Axelrod M, Kline J, Gurka M, Gow A, Gaston B. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci U S A. 2005;102:5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer LA, Doctor A, Chhabra P, Sheram ML, Laubach VE, Karlinsey MZ, Forbes MS, Macdonald T, Gaston B. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- 20.McMahon TJ, Stone AE, Bonaventura J, Singel DJ, Stamler JS. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J Biol Chem. 2000;275:16738–16745. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- 21.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278:H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 23.Harrington LS, Evans RJ, Wray JA, Norling LV, Swales KE, Vial C, Ali F, Carrier MJ, Mitchell JA. P2X1 receptors mediate endothelial dependent vasodilatation to ATP. Mol Pharmacol. 2007;72:1132–1136. doi: 10.1124/mol.107.037325. [DOI] [PubMed] [Google Scholar]
- 24.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 25.Deem S, Min JH, Moulding JD, Eveland R, Swenson ER. Red blood cells prevent inhibition of hypoxic pulmonary vasoconstriction by nitrite in isolated, perfused rat lungs. Am J Physiol Heart Circ Physiol. 2007;292:H963–H970. doi: 10.1152/ajpheart.00812.2006. [DOI] [PubMed] [Google Scholar]
- 26.Luchsinger BP, Rich EN, Yan Y, Williams EM, Stamler JS, Singel DJ. Assessments of the chemistry and vasodilatory activity of nitrite with hemoglobin under physiologically relevant conditions. J Inorg Biochem. 2005;99:912–921. doi: 10.1016/j.jinorgbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol. 2007;292:H3072–H3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 28.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagababu E, Ramasamy S, Rifkind JM. S-nitrosohemoglobin: a mechanism for its formation in conjunction with nitrite reduction by deoxyhemoglobin. Nitric Oxide. 2006;15:20–29. doi: 10.1016/j.niox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Laterreur J, English AM. Hemoglobin S-nitrosation on oxygenation of nitrite/deoxyhemoglobin incubations is attenuated by methemoglobin. J Inorg Biochem. 2007;101:1827–1835. doi: 10.1016/j.jinorgbio.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Boegehold MA, Bohlen HG. Arteriolar diameter and tissue oxygen tension during muscle contraction in hypertensive rats. Hypertension. 1988;12:184–191. doi: 10.1161/01.hyp.12.2.184. [DOI] [PubMed] [Google Scholar]
- 32.Arakaki LS, Burns DH, Kushmerick MJ. Accurate myoglobin oxygen saturation by optical spectroscopy measured in blood-perfused rat muscle. Appl Spectrosc. 2007;61:978–985. doi: 10.1366/000370207781745928. [DOI] [PubMed] [Google Scholar]
- 33.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozuyaman B, Schnurch HG, Godecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rosen P, Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 34.Chauhan SD, Seggara G, Vo PA, Macallister RJ, Hobbs AJ, Ahluwalia A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J. 2003;17:773–775. doi: 10.1096/fj.02-0668fje. [DOI] [PubMed] [Google Scholar]
- 35.Schuh K, Quaschning T, Knauer S, Hu K, Kocak S, Roethlein N, Neyses L. Regulation of vascular tone in animals overexpressing the sarcolemmal calcium pump. J Biol Chem. 2003;278:41246–41252. doi: 10.1074/jbc.M307606200. [DOI] [PubMed] [Google Scholar]
- 36.Talukder MA, Fujiki T, Morikawa K, Motoishi M, Kubota H, Morishita T, Tsutsui M, Takeshita A, Shimokawa H. Up-regulated neuronal nitric oxide synthase compensates coronary flow response to bradykinin in endothelial nitric oxide synthase-deficient mice. J Cardiovasc Pharmacol. 2004;44:437–445. doi: 10.1097/01.fjc.0000139450.64337.cd. [DOI] [PubMed] [Google Scholar]
- 37.McLaren AT, Marsden PA, Mazer CD, Baker AJ, Stewart DJ, Tsui AK, Li X, Yucel Y, Robb M, Boyd SR, Liu E, Yu J, Hare GM. Increased expression of HIF-1α, nNOS, and VEGF in the cerebral cortex of anemic rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R403–R414. doi: 10.1152/ajpregu.00403.2006. [DOI] [PubMed] [Google Scholar]
- 38.Cooke JP, Stamler J, Andon N, Davies PF, McKinley G, Loscalzo J. Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am J Physiol. 1990;259:H804–H812. doi: 10.1152/ajpheart.1990.259.3.H804. [DOI] [PubMed] [Google Scholar]
- 39.Allen BW, Piantadosi CA. How do red blood cells dilate blood vessels? Circ Res. 2004;94:e105. [PubMed] [Google Scholar]
- 40.Sonveaux P, Kaz AM, Snyder SA, Richardson RA, Cardenas-Navia LI, Braun RD, Pawloski JR, Tozer GM, Bonaventura J, McMahon TJ, Stamler JS, Dewhirst MW. Oxygen regulation of tumor perfusion by S-nitrosohemoglobin reveals a pressor activity of nitric oxide. Circ Res. 2005;96:1119–1126. doi: 10.1161/01.RES.0000168740.04986.a7. [DOI] [PubMed] [Google Scholar]
- 41.Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, Losano G. Nitric oxide and cardiac function. Life Sci. 2007;81:779–793. doi: 10.1016/j.lfs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Frandsenn U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with N(G)-nitro-L-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 46.Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, Radomski MW, Moncada S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci U S A. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 48.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol. 2007;293:H2565–H2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 49.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 50.Bjorne HH, Petersson J, Phillipson M, Weitzberg E, Holm L, Lundberg JO. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J Clin Invest. 2004;113:106–114. doi: 10.1172/JCI200419019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med. 2008;14:773–777. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernal PJ, Leelavanichkul K, Bauer E, Cao R, Wilson A, Wasserloos KJ, Watkins SC, Pitt BR, Croix CM. Nitric oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circ Res. 2008;102:1575–1583. doi: 10.1161/CIRCRESAHA.108.171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]