Abstract
Background
We have previously demonstrated that putaminal but not caudate volumes are associated with poor outcome in patients with chronic schizophrenia. Present longitudinal study was designed to investigate progressive differences in striatal volumes among chronic schizophrenia patients with different outcomes and healthy subjects.
Method
Structural MRI scans were acquired at baseline and at follow-up four years later to evaluate volumetric changes in 26 poor-outcome schizophrenia patients, 23 good-outcome patients and 16 healthy subjects.
Results
Schizophrenia patients with different outcomes entered the study with similar volumes of the caudate nucleus and putamen. The rate of decline in volumes of the putamen was greater in patients with poor outcome than in the good-outcome group, so that their putaminal but not caudate volumes were significantly smaller at the time of follow-up. There were no differences in baseline and follow-up volumes of the putamen or in the rate of their progression among patients with schizophrenia and healthy comparison subjects. The caudate volumes were lower in schizophrenia patients than healthy subjects at baseline and follow-up, but showed no differential patterns of progression between the groups.
Conclusions
Volumes of the putamen may represent a longitudinal marker of treatment responsiveness and outcome in patients with chronic schizophrenia.
Keywords: Chronic schizophrenia, caudate nucleus, putamen, poor outcome, Kraepelinian, striatum
1. Introduction
Striatal abnormalities have been postulated and described in patients with schizophrenia, but, as attested by their recent review (Brandt and Bonelli, 2008), still remain inconclusive. While most of the first-episode schizophrenia studies reported normal volumes of the caudate nucleus, with only a third revealing volumetric reductions, findings in chronic schizophrenia patients were equally divided between its enlargement and no volumetric change. Fewer studies have assessed volumes of the putamen, but with near unanimity in reporting no changes in first-episode and putaminal enlargement in chronic schizophrenia patients. Given these indications of striatal expansion in chronic schizophrenia and the rich dopaminergic innervation of the basal ganglia, a relationship between the acute exposure to antipsychotic agents and striatal enlargement has been proposed, with differential effects from the typical and atypical antipsychotics (Shihabuddin et al., 1998). Whether typical vs. atypical affiliation of the agents plays a differential role in striatal changes in the long run has not been firmly established (Gur et al., 1998; Mamah et al., 2007; Navari and Dazzan, 2009; Zhou et al., 2003). Several short-term longitudinal studies appear to support enlargement of the caudate nucleus in first-episode and chronic schizophrenia patients following exposure to typical neuroleptic agents (Chakos et al., 1994; Keshavan et al., 1994; Lieberman et al., 2001) and atypical agents (Massana et al., 2005; Okugawa et al., 2007), others revealed no progressive changes or even decline in its density with mostly atypical agents (Glenthoj et al., 2007; Heitmiller et al., 2004; Lang et al., 2001; McClure et al., 2008; Stip et al., 2008; Tauscher-Wisniewski et al., 2005) and a typical agent (Glenthoj et al., 2007), or its enlargement with the typical and shrinkage with the atypical neuroleptization (Chakos et al., 1995; Corson et al., 1999; Lang et al., 2004; Scheepers et al., 2001). Two longer-term studies that compared schizophrenia patients and healthy subjects found no progressive changes in the caudate nucleus or decline in its density (DeLisi et al., 1997; van Haren et al., 2007) in patients over at least 4 years of follow-up. Brief exposure of schizophrenia patients to quetiapine was reported to result in decreased putaminal density (Stip et al., 2008) and to risperidone - in putaminal enlargement (Glenthoj et al., 2007), but the single longitudinal assessment of the putamen with normal control group published to date uncovered no morphometric differences between schizophrenia patients and healthy comparison subjects (Wang et al., 2008).
We previously reported larger putamens in schizophrenia patients with good outcome than poor-outcome (Kraepelinian) patients and proposed that rather than being a consequence of prolonged neuroleptic exposure putaminal enlargement may be a marker of responsiveness to antipsychotic treatment with the corrolary that its shrinkage may be associated with poor clinical outcome (Buchsbaum et al., 2003). In the present study, we followed a cohort of chronic good-outcome and poor-outcome schizophrenia patients over a four-year period in order to compare longitudinal progression of volumes of both the caudate nucleus and putamen.
2. Methods
2.1. Subjects
49 patients with schizophrenia (age at baseline scan 42.69±12.29 years) and 16 healthy subjects (age at baseline 41.63±12.23 years, t63=0.30, p=ns) were scanned twice approximately 4 years apart (4.10±0.54 years for schizophrenia patients and 4.22±0.52 years for healthy subjects, t63=0.76, p=ns; see full sample description in Mitelman et al., 2009). Schizophrenia patients and healthy subjects did not significantly differ in major demographic characteristics. Based on the predominant pattern of antipsychotic treatment over the three-year period preceding the baseline scan (available for 37 participants), schizophrenia patients were divided into those treated with predominantly typical antipsychotics (30%), atypical antipsychotics (24%), no antipsychotics (30%), and a mixture of both antipsychotic types (16%).
Patients with schizophrenia were classified into the good-outcome (n=23) and poor-outcome (n=26) subgroups based on the criteria by Keefe et al (1987). These required that poor-outcome patients for at least five years prior to study contact displayed: 1) continuous hospitalization or complete dependence on others for food, clothing, and shelter; 2) no useful employment; and 3) no evidence of symptom remission. Patients classified as poor-outcome (age 47.35±11.9 years) were outpatients, living in supervised group home setting, who were significantly older at baseline scan than patients with good outcome (37.44±10.68 years, t47=3.05, p=0.004), but did not significantly differ in length of between-scan interval (3.98±0.4 years vs. 4.24±0.65 years, respectively, t47=1.76, p=0.09, a trend level) or MMSE scores (25.79±3.56 vs. 27.32±2.36, t=1.6, p=0.12). PANSS assessments at baseline showed that patients with poor outcome, as compared to the good-outcome group, had more severe positive (22.4±6.64 vs. 15.39±4.92, t47=4.12, p=0.0002), negative (22.36±7.40 vs. 15.35±5.08, t47=3.79, p=0.0004), and general psychopathology scores (45.12±10.05 vs. 35.32±14.63, t47=2.7, p=0.01), as well as more years since initiation of antipsychotic treatment (24.77±11.71 vs. 12.29±8.78, t41=3.94, p=0.0003).
2.2. Image acquisition and analysis
T1-weighted MR images were acquired using a 1.5T Signa 5× scanner (GE Medical Systems) with a 3D-SPGR sequence (TR=24msec, TE=5msec, flip angle=40°, matrix size 256×256, field of view 23cm, slice thickness 1.2mm, total slices 128). SPGR images were resectioned to standard position using the algorithm of Woods et al (1993), a 6-parameter rigid-body transformation, and the standard MNI brain. The caudate nucleus and putamen were outlined precisely as in our previous reports (Buchsbaum et al., 2003; Shihabuddin et al., 1998). Their baseline volumes were separately entered into ANCOVAs (age at scan date as covariate) with two independent groups (schizophrenia patients vs. healthy subjects or good-outcome vs. poor-outcome patients) and repeated measures for hemisphere (right, left). Same analyses were performed for nuclear volumes at follow-up scan. For longitudinal assessments, volumes of the caudate and putamen were separately entered into a multiway ANCOVA with two independent groups (schizophrenia patients vs. healthy subjects or good-outcome vs. poor-outcome patients), repeated measures of time (baseline, follow-up) and hemisphere (right, left), with age at baseline scan as covariate. These analyses were performed with both absolute and relative (to total brain size) volumes. Three strategies were employed to estimate effects of antipsychotic treatment on longitudinal volume change. First, we ran these ANCOVAs adding years since initiation of antipsychotic treatment till scan date as the second covariate, with this variable not available for 5 subjects. Second, similar analyses were run on two groups of schizophrenia patients: those with predominantly typical or atypical antipsychotic treatment over the three-year period preceding the baseline scan. Finally, product-moment correlations between the years since initiation of antipsychotic pharmacotherapy and baseline-to-follow-up volume changes in patients were assessed.
3. Results
At baseline, there were no significant differences in absolute and relative volumes of the caudate nucleus or putamen among any of the compared groups (Table 1). There was a trend towards lower relative right caudate volumes in schizophrenia patients than in healthy subjects (F1, 60=3.87, p=0.054).
Table 1.
Volumes of the caudate nucleus and putamen (in mm3)
| Caudate nucleus | Putamen | |||
|---|---|---|---|---|
| Left | Right | Left | Right | |
| Healthy subjects | ||||
| Baseline | 4626±886 | 4891±873 | 4802±1005 | 4681±728 |
| Follow-up | 4005±890 | 4231±909 | 4364±806 | 4307±655 |
| Schizophrenia patients | ||||
| Baseline | 4272±822 | 4424±922 | 4873±846 | 4701±780 |
| Follow-up | 3518±635 | 3799±721 | 4382±603 | 4283±632 |
| Poor-outcome patients | ||||
| Baseline | 4134±714 | 4249±929 | 4846±954 | 4658±902 |
| Follow-up | 3402±666 | 3679±786 | 4179±639 | 4110±714 |
| Good-outcome patients | ||||
| Baseline | 4427±921 | 4623±893 | 4903±725 | 4750±630 |
| Follow-up | 3649±585 | 3936±630 | 4611±475 | 4477±465 |
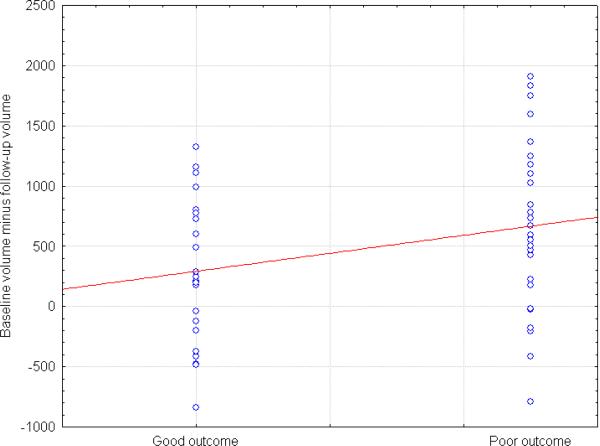
Absolute and relative volumes of the caudate nucleus and putamen dwindled over the time to follow-up in both schizophrenia patients and healthy subjects, with no significant between-group differences in the rate of progressive changes. Both absolute and relative volumes of the putamen decreased over the time to follow-up significantly more in patients with poor outcome than in the good-outcome group (diagnostic group×time interaction, F1, 46=6.31, p=0.02, and with duration of antipsychotic treatment as additional covariate, F1, 39=5.09, p=0.03; Fig. 1). Simple interactions for each hemisphere confirmed faster decline in the poor-outcome group for the left putamen (diagnostic group×time interaction, F1, 46=7.12, p=0.01, and with duration of antipsychotic treatment as additional covariate, F1, 39=5.92, p=0.02).
Figure 1.
Distribution of progressive changes in volumes (mm3) of the left putamen in schizophrenia patients with different outcomes
At follow-up (controlled for differences in duration of antipsychotic treatment), patients with poor outcome ended up with significantly smaller absolute and relative putaminal volumes in comparison to the good-outcome patient group (main effect of diagnostic group, F1, 39=5.13, p=0.03). Follow-up simple interactions confirmed significantly smaller putaminal volumes for the left hemisphere only (F1, 39=7.65, p=0.008). Relative volumes of the caudate nucleus at follow-up were smaller in schizophrenia patients than in healthy subjects (main effect of diagnostic group, F1, 62=4.71, p=0.03).
ANCOVA comparisons of schizophrenia patients divided into those predominantly treated with typical or atypical antipsychotics over the three-year period preceding the baseline scan produced no significant interactions involving the diagnostic group. No significant correlations were found between the longitudinal changes in the caudate and putaminal volumes and the number of years since initiation of neuroleptic treatment. No significant interactions between any of the measures and subjects' sex were elicited.
4. Discussion
Poor outcome in patients with chronic schizophrenia is associated with a more pronounced shrinkage of the putamen, which begins approximately two decades after the onset of the disease. We have recently demonstrated that in contrast to the deceleration of cortical gray matter decline with age in this cohort of patients with good outcome, cortical shrinkage in the poor-outcome group as they age remains unabated (Mitelman et al., 2009). Present results may suggest the dynamic spread of pathological changes, at this phase of the illness extending beyond the cortex.
Volumes of both the caudate nucleus and putamen in right and left hemispheres at baseline and follow-up were lower in schizophrenia patients than in healthy subjects and in poor-outcome patients than in patients with good outcome (Table 1). Most of the differences, however, did not reach statistical significance and may be subtle.
Poor-outcome schizophrenia is characterized by a treatment-resistant and unremitting course (Harvey et al., 1991; Mitelman et al., 2007), so that progressive decline of the putaminal volumes to the excess of that seen in patients with good outcome may be a marker of treatment resistance. We thus propose that expansion of the putamen - as reported in several cross-sectional studies - is not merely the consequence of exposure to antipsychotic agents but may specifically signify responsiveness to their therapeutic effects. Contrasting changes in putaminal volumes following a brief exposure to quetiapine (Stip et al., 2008) and risperidone (Glenthoj et al., 2007), as well as no significant changes with other typical and atypical agents (Glenthoj et al., 2007; Wang et al., 2008) may thus not be due to the pharmacological particulars of the employed antipsychotics, but rather due to the variable patient responsiveness to treatment. If confirmed, further studies should evaluate clinical utility of the putaminal volumetry in monitoring antipsychotic efficacy in patients with schizophrenia.
Acknowledgements
This work was supported by NARSAD Young Investigator Award and NIMH MH 077146 grant to Serge A. Mitelman and by NIMH grants P50 MH 66392-01, MH 60023, and MH 56489 to Monte S. Buchsbaum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brandt GN, Bonelli RM. Structural neuroimaging of the basal ganglia in schizophrenic patients: a review. Wien. Med. Wochenschr. 2008;158:84–90. doi: 10.1007/s10354-007-0478-7. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Brickman AM, Miozzo R, Prikryl R, Shaw R, Davis KL. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr. Res. 2003;64:53–62. doi: 10.1016/s0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am. J. Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Alvir J, Bilder RM, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am. J. Psychiatry. 1999;156:1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;76:131–138. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- Glenthoj A, Glenthoj BY, Mackeprang T, Pagsberg AK, Hemmingsen RP, Jernigan TL, Baaré WF. Basal ganglia volumes in drug-naïve first-episode schizophrenia patients before and after short-term treatment with either a typical of an atypical antipsychotic drug. Psychiatry Res. Neuroimaging. 2007;154:199–208. doi: 10.1016/j.pscychresns.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naïve and treated patients with schizophrenia. Am. J. Psychiatry. 1998;155:1711–1777. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Putnam KM, Davidson M, Kahn RS, Powchik P, McQueeney R, Keefe RSE, Davis KL. Brief neuroleptic discontinuation and clinical symptoms in Kraepelinian and non-Kraepelinian chronic schizophrenic patients. Psychiatry Res. 1991;38:285–292. doi: 10.1016/0165-1781(91)90018-k. [DOI] [PubMed] [Google Scholar]
- Heitmiller DR, Nopoulos PC, Andreasen NC. Changes in caudate volume after exposure to atypical neuroleptics in patients with schizophrenia may be sex-dependent. Schizophr. Res. 2004;66:137–142. doi: 10.1016/j.schres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Kendler KS, Horvath TB, Nora R, Davis KL. Characteristics of very poor outcome schizophrenia. Am. J. Psychiatry. 1987;144:889–895. doi: 10.1176/ajp.144.7.889. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Lang DJ, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Goghari VM, Honer WG. An MRI study of basal ganglia volumes in first-episode schizophrenia patients treated with risperidone. Am. J. Psychiatry. 2001;158:625–631. doi: 10.1176/appi.ajp.158.4.625. [DOI] [PubMed] [Google Scholar]
- Lang DJ, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Goghari VM, Lapointe JS, Honer WG. Reduced basal ganglia volumes after switching to olanzapine in chronically treated patients with schizophrenia. Am. J. Psychiatry. 2004;161:1829–1836. doi: 10.1176/ajp.161.10.1829. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Chakos MH, Wu H, Alvir J, Hoffman E, Robinson D, Bilder RM. Longitudinal study of brain morphology in first episode schizophrenia. Biol. Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- Mamah D, Wang LE, Barch DM, deErasquin G, Thompson P, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophr. Res. 2007;89:59–71. doi: 10.1016/j.schres.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massana G, Salgado-Pineda P, Junqué C, Pérez M, Baeza I, Pons A, Massana J, Navarro V, Blanch J, Morer A, Mercader JM, Bernardo M. Volume changes in gray matter in first-episode neuroleptic-naive schizophrenic patients treated with risperidone. J. Clin. Psychopharmacol. 2005;25:111–117. doi: 10.1097/01.jcp.0000155818.29091.53. [DOI] [PubMed] [Google Scholar]
- McClure RK, Carew K, Greeter S, Maushauer E, Steen G, Weinberger DR. Absence of regional brain volume change in schizophrenia associated with short-term atypical antipsychotic treatment. Schizophr. Res. 2008;98:29–39. doi: 10.1016/j.schres.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int. Rev. Psychiatry. 2007;19:345–357. doi: 10.1080/09540260701486563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Canfield EL, Newmark RE, Brickman AM, Torosjan Y, Chu K-W, Hazlet EA, Haznedar MM, Shihabuddin L, Buchsbaum MS. Longitudinal assessment of gray and white matter in chronic schizophrenia: A combined diffusion-tensor and magnetic resonance imaging study. Open Neuroimag. J. 2009;3:31–47. doi: 10.2174/1874440000903010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol. Med. 2009 doi: 10.1017/S0033291709005315. in press. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Takase K, Saito Y, Yoshimura M, Kinoshita T. Olanzapine increases grey and white matter volumes in the caudate nucleus of patients with schizophrenia. Neuropsychobiol. 2007;55:43–46. doi: 10.1159/000103575. [DOI] [PubMed] [Google Scholar]
- Scheepers FE, de Wied CC, Hulshoff Pol HE, van der Flier W, van der Linden JA, Kahn RS. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology. 2001;24:47–54. doi: 10.1016/S0893-133X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- Shihabuddin L, Buchsbaum MS, Hazlett EA, Haznedar MM, Harvey PD, Newman A, Schnur DB, Spiegel-Cohen J, Wei T, Machac J, Knesaurek K, Vallabhajosula S, Biren MA, Ciaravolo TM, Luu-Hsia C. Dorsal striatal size, shape, and metabolic rate in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch. Gen. Psychiatry. 1998;55:235–243. doi: 10.1001/archpsyc.55.3.235. [DOI] [PubMed] [Google Scholar]
- Stip E, Mancini-Marïe A, Fahim C, Bentaleb LA, Létourneau G, Potvin S. Decrease in basal ganglia grey matter density associated with atypical antipsychotic treatment in schizophrenia patients. Schizophr. Res. 2008;103:319–321. doi: 10.1016/j.schres.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Tauscher-Wisniewski S, Tauscher J, Christensen BK, Mikulis DJ, Zipursky RB. Volumetric MRI measurement of caudate nuclei in antipsychotic-naive patients suffering from a first episode of psychosis. J. Psychiatr. Res. 2005;39:365–370. doi: 10.1016/j.jpsychires.2004.10.001. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Brans R, Carati I, Rais M, Kahn RS. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol. Psychiatry. 2008;63:106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Wang L, Mamah D, Harms MP, Karnik M, Price JL, Gado MH, Thompson PA, Barch DM, Miller MI, Csernansky JG. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biol. Psychiatry. 2008;64:1060–1068. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J. Comput. Assist. Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, Yamashita I, Seto H, Kurachi M. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol. Psychiatry. 2003;54:427–436. doi: 10.1016/s0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]