Abstract
Objective
To relate ambulatory blood pressure (ABP) to cardiac target organ measurement in children at risk for primary hypertension (HTN).
Study design
Left ventricular mass index (LVMI) and ABP were measured concomitantly in children (6 to 18 years) at risk for hypertension using a cross-sectional study design.
Results
LVMI showed a significant positive correlation with 24-hour systolic blood pressure (SBP) load, SBP index (SBPI), and standard deviation score (SDS). When subjects were stratified by LVMI percentile, there were significant differences in SBP load, 24-hour SBPI, and 24-hour SSDS. The odds ratio (OR) of having elevated LVMI increased by 54% for each incremental increase of SDS in 24-hour SSDS after controlling for race and BMI (OR = 1.54, unit = 1 SDS, CI = 1.1, 2.15, P = .011) and increased by 88% for each increase of 0.1 in BPI (OR = 1.88, CI = 1.03, 3.45, P = .04). Subjects with stage 3 HTN had significantly greater mean LVMI compared with normal subjects (P = .002 by ANOVA; LMVI, 31.6 ± 7.9 versus 39.5 ± 10.4).
Conclusions
As systolic ABP variables increase, there is greater likelihood for increased LVMI. Staging based on ABPM allows assessment of cardiovascular risk in children with primary hypertension.
In contrast to adults with hypertension (HTN), the blood pressure level in childhood that predicts future cardiovascular disease has not been clearly defined. Generally, population-based blood pressure percentiles are applied to individual children, with acceptable levels designated as less than the 90th percentile and abnormal levels as ≥95th percentile. As the pediatric medical community strives to achieve the prevention and early detection of future cardiovascular disease in childhood, the question arises as to what level of blood pressure increases the risk for target organ damage.1
Characterization of blood pressure using the 24-hour ambulatory monitor is superior to casual measurement in predicting morbidity among adults.2 In addition, ambulatory blood pressure monitoring (ABPM) allows for identification of white coat HTN and nocturnal HTN. Among children with type I diabetes, increased systolic blood pressure (SBP) during sleep predicted the development of microalbuminuria.3 Nocturnal SBP measured by ABPM correlates with left ventricular mass (LVM) in children with established hypertension not yet receiving antihypertensive therapy and LVM correlates with 24-hour mean SBP in children with HTN.4,5 In these two studies relating LVMI to ABPM, the subjects were classified as hypertensive based on “casual” clinic blood pressure measurements and not with ABPM measures.
The aim of this study was to measure LVMI and ABP in children at risk for primary hypertension. In doing so, we wished to 1) categorize individual blood pressure based on ABPM rather than casual blood pressure; in addition to use of the blood pressure index, we wished to analyze standard deviation scores; 2) assess the likelihood for increased LVMI based upon ABPM; and 3) implement recently proposed staging in the assessment of cardiovascular risk.
METHODS
Subjects were recruited from primary care clinics and from referrals to the University of Tennessee Medical Group nephrology or cardiology clinics for evaluation of elevated blood pressure. Children ages 6 to 18 years with a casual SBP or diastolic blood pressure (DBP) ≥90th percentile or a first-degree relative on antihypertensive therapy were eligible for the study. The inclusion criteria were expected to result in the enrollment of children who probably would be defined as normotensive, based on ABPM. Children were excluded if they were taking antihypertensive medications or had a diagnosis of renal or cardiac disease. Informed consent was obtained from the parent and assent obtained from the study subject as appropriate. The research protocol was approved by the University of Tennessee Health Science Center Institutional Review Board and followed the guidelines for good clinical practice.
Study Procedures
During the 2-day study period, ABP and LVM were measured in each subject. Subject height and weight were measured by using a balance beam scale and pediatric wall-mounted stadiometer. Height percentile was calculated using the CDC NHANES III data tables by age in months.6 Body mass index (BMI) was calculated and assigned a percentile using the CDC tables from NHANES III.6 Subjects were classified on the basis of BMI according to the definitions of overweight (BMI percentile for age >95th) or at risk for overweight (BMI percentile 85th to 95th). Ethnicity was categorized as that reported by the subject.
ABP monitoring was performed using the AM5600 ambulatory blood pressure monitor (Advanced Biosensor, Columbia, SC). The monitors were programmed to take the blood pressure every 20 minutes for a 24-hour period using the auscultatory technique to detect SBP at Korotkoff phase I and DBP at Korotkoff phase V. After selection of the appropriate cuff size, the brachial artery of the nondominant arm at the antecubital fossa was located. The microphone was taped to the subject’s arm over the strongest impulse followed by placement of the cuff and electrodes. During the AM5600 “office check period,” with the subject in a seated position, a minimum of three readings were taken simultaneously in the same arm via a 3-way stopcock, with a mercury sphygmomanometer and the AM5600, using the recorder’s blood pressure cuff. The calibration readings allowed for adjustments to be made in the recorder’s microphone amplification and to establish a baseline for ABPM versus manual SBP and DBP. The office check readings were not included in the 24-hour results; casual blood pressure readings were derived from the mean of three readings by mercury sphygmomanometer at the study visit. The parent/guardian was asked to record the subject’s bedtime and time of awakening; using the reported times, the asleep period was designated by the next reading after the time of sleep and the last reading before the time recorded for awakening. After 24 hours, the cuff and monitor were removed, and the data downloaded using AMPSI software. Subjects with fewer than 30 total readings or less than 5 nocturnal readings were not analyzed.
The mean SBP and DBP were calculated separately for the 24-hour period and for awake (designated as daytime) and sleep (designated as nighttime) periods. Blood pressure load was calculated as the number of readings over the 95th percentile within each time period. The BPI for each subject was calculated by expressing the mean SBP or DBP as a ratio to the appropriate 95th percentile as reported by Soergel et al.7 When the BPI is greater than 1, the mean blood pressure is above the 95th percentile. In addition, we calculated standard deviation scores (SDS) for mean blood pressure using the method of Wuhl et al.8 An SDS >1.5 was considered to be abnormal, corresponding to the 95th percentile for blood pressure. Casual SBP index (SBPI) and DBP index (DBPI) were also calculated using the working group definitions from the 1996 Task Force report,9 as the study was initiated before the most recent report.10
White coat hypertension (WCH) was defined as a casual BPI >1 with 24-hour mean ambulatory BPI ≤1.11 An alternative definition for WCH was also used (casual BPI > 1 with daytime mean ambulatory BPI ≤1).12 Masked hypertension was defined as casual BPI <1 but a daytime mean SBPI >1.12
Intermethod reliability between the mercury sphygmomanometer and the AM5600 ABPM system was compared by using an ANOVA mixed-effects model. The Pearson correlation coefficients were 0.992 and 0.979 for SBP and DBP, respectively, with coefficients of variation of 1.5% and 3.5%. Waveform analysis to detect artifactual readings was performed by a co-investigator (P. Richey) unfamiliar with the subjects. Artifactual readings were discarded based on the waveform in the recording according to methods previously described.13 The accuracy of each reading was determined by inspection of the graphic display of the Korotkoff signal.
Measurement of Left Ventricular Mass
Echocardiography was performed according to the standard of the American Society of Echocardiography (ASE). Subjects were positioned in the partial left decubitus position. Measurements performed in M-mode of the dimensions of the interventricular septal thickness (IVS), posterior wall thickness (PWT), and left ventricular internal dimension (LVID) were taken at or just below the mitral valve tips. Left ventricular mass (LVM) was calculated using the formula by Devereux et al according to the ASE guidelines14: LV mass (g) = 0.81[1.04(IVS + PWT + LVID)3 − (LVID)3] + 0.06. Echocardiography was performed by the same technician, and all measurements were performed in triplicate by the same cardiologist, who was blinded as to the subject’s blood pressure. LV mass index (LVMI) was derived by dividing LV mass in grams by the subject’s height in meters raised to the 2.7 power.15,16 Subjects were classified into two groups on the basis of previously published criteria: increased LVMI was defined as greater than or equal to the sex-based 90th percentile: 36.69 g/ht2.7 in male subjects and 34.11 g/ht2.7 in female subjects.15,17
Statistical Analysis
SAS software was used for statistical analyses to calculate the mean, standard deviation, distribution, and normal plots of the variables studied. Univariate analysis of continuous variables was performed using Pearson’s correlation, Student’s t test, and the Wilcoxon rank sum test. Dichotomous variables were analyzed using 2 × 2 tables and the chi-square test (by the method of Cochran-Mantel-Haenzel). The sensitivity, specificity, and odds ratios (OR) of individual blood pressure variables in predicting increased LVMI were calculated. Univariate and multivariable analysis using logistic regression was performed using LVMI ≥90th percentile as a dichotomous dependent variable. Covariables included in the analysis included race (African-American [AA] versus non-AA), percent nocturnal decline, and BMI percentile.
RESULTS
Subjects
A total of 129 subjects were recruited. Eighteen subjects did not meet the criteria for adequate ABPM due to insufficient readings. Although we sought to enroll children with or at risk for primary hypertension, secondary hypertension was later identified in 5 children: 2 with neurofibromatosis who had not undergone angiography, 1 with autosomal dominant polycystic kidney disease, 1 with an upper pole, multicystic kidney, and 1 with focal segmental glomerulosclerosis. These children were not included in this analysis. The mean age for the 106 subjects whose data were adequate for analysis was 13.6 ± 2.6 (7–18) years. There was a predominance of African-American children (76 AA/29 W/1 Asian) as well as males (68 M/38 F). Based on BMI, 55.7% were categorized as overweight and 14.1% as at risk for overweight. After exclusion of erroneous readings, the mean number of 24-hour readings was 54.8 ± 10.8 (median, 55) with an average of 32 ± 9.4 daytime and 23 ± 6.1 nighttime readings.
Subjects were classified according to casual blood pressure and various ambulatory blood pressure levels (Table I). White coat HTN was present in 46.5% of subjects with abnormal casual SBP and 59% of those with abnormal diastolic blood pressure. Masked HTN was found in 14.6% of subjects with normal casual SBP and 13.5% of those with normal casual DBP.
Table I.
Classification of subjects based on various BP parameters
| N (% of total subjects, 106) | |
|---|---|
| Mean casual SBPI >1 | 58 (54.7) |
| Mean casual DBPI >1 | 17 (16) |
| Ambulatory SBPI >1 | 42 (39.6) |
| Ambulatory DBPI >1 | 22 (20.8) |
| Systolic SDS >1.5 | 41 (38.7) |
| Diastolic SDS >1.5 | 23 (21.7) |
| Systolic load ≥25% | 74 (69.8) |
| Diastolic load ≥25% | 50 (47.2) |
| White coat HTN | |
| Systolic | |
| Using 24-h SBPI | 23 (21.7) |
| Using awake SBPI | 27 (25.5) |
| Diastolic | |
| Using 24-h DBPI | 7 (6.6) |
| Using awake DBPI | 10 (9.4) |
| Masked HTN | |
| Systolic | 7 (6.6) |
| Diastolic | 12 (11.3) |
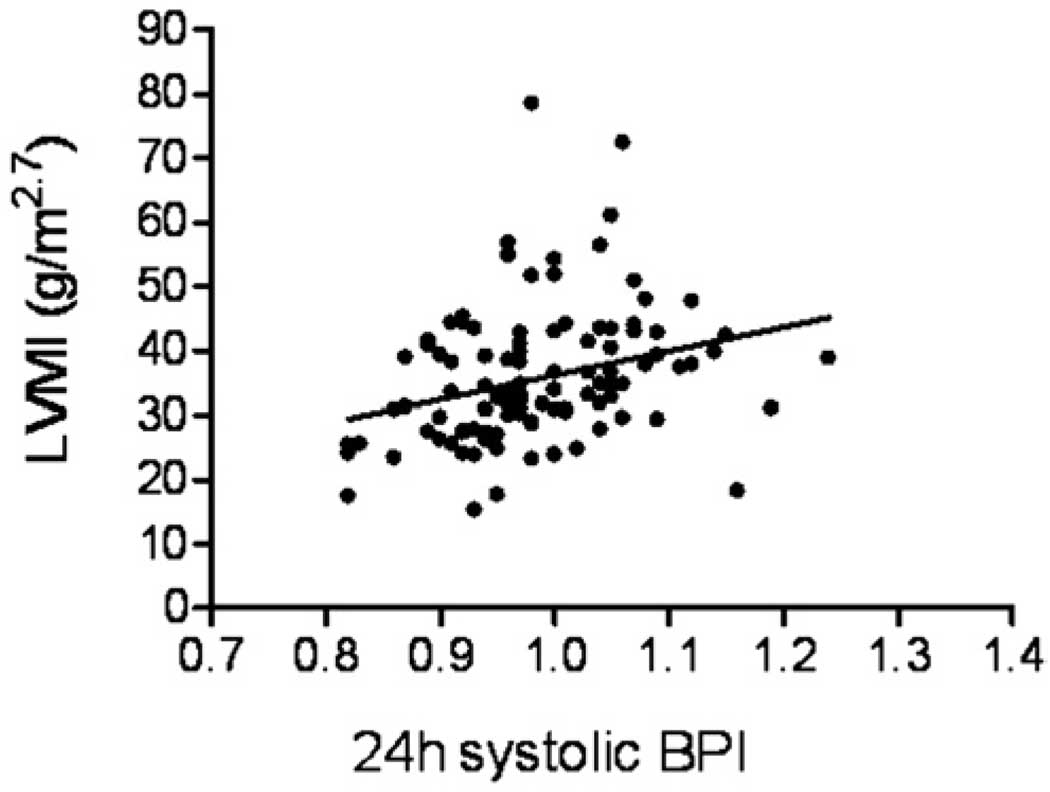
Correlation analyses demonstrated that LVMI significantly correlated with 24-hour systolic load (r = 0.29, P = 0.004), 24-hour SBPI (r = 0.31, P = .002, Figure), and 24-hour systolic SDS (r = 0.3, P = .003). In contrast, LVMI was not significantly associated with diastolic ABP, the degree of nocturnal decline, or casual BPI. Systolic blood pressure levels expressed as load, BPI, or SDS were significantly higher among those subjects with increased LVMI (Table II). Diastolic blood pressure levels were significantly different between the 2 groups for 24-hour load, 24-hour BPI, and 24-hour and daytime SDS. Analysis of a subgroup of individuals classified as hypertensive based upon casual systolic BPI >1, revealed that 24-hour SBPI was significantly different among those with normal versus abnormal LVMI: 1 ± 0.055 versus 1.03 ± 0.065, P = .036. The 24-hour systolic SDS was also greater in the subgroup with normal LVMI compared with those with increased LVM (1.49 ± 0.98 versus 2.08 ± 1.14, P = .038). Nighttime systolic blood pressure load was greater among those subjects whose casual SBP was abnormal: 51.6% ± 32.5% versus 68.3% ± 28.9%, (P = .043) for those with normal and increased LVMI, respectively. Casual blood pressure levels were not significantly different between children with LVMI ≥90th percentile versus those with LVMI <90th percentile.
Figure.
Correlation of SBPI with LVMI. Pearson correlation coefficient, 0.31, P = .002.
Table II.
Comparison of BP levels in subjects with and without increased LV mass
| LVMI <90th | LVMI ≥90th | |||
|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |
| Casual BPI | 1.01 ± 0.09 | 0.91 ± 0.1 | 1.05 ± 0.12 | 0.9 ± 0.1 |
| Nocturnal decline (%) |
12.7 ± 7.8 | 9.7 ± 6.8 | 12.7 ± 7.5 | 9.6 ± 7.2 |
| Load (%) | ||||
| 24 h | 36.7 ± 22.8 | 25.6 ± 17.2 | 50.7 ± 26.5† | 34.8 ± 25.2* |
| Daytime | 32.9 ± 26.1 | 16.6 ± 17.6 | 46.2 ± 29.9* | 24.9 ± 24.3 |
| Nighttime | 38.1 ± 33 | 30.6 ± 28.3 | 54.0 ± 35* | 47.6 ± 33. |
| BPI | ||||
| 24 h | 0.97 ± 0.07 | 0.91 ± 0.08 | 1.01 ± 0.08† | 0.95 ± 0.1* |
| Daytime | 0.96 ± 0.07 | 0.88 ± 0.08 | 1 ± 0.08† | 0.91 ± 0.1 |
| Nighttime | 0.98 ± 0.09 | 0.97 ± 0.11 | 1.03 ± 0.1* | 1.01 ± 0.13 |
| SDS | ||||
| 24 h | 0.8 ± 1.22 | 0.43 ± 1.08 | 1.64 ± 1.54† | 0.97 ± 13.4* |
| Daytime | 0.84 ± 1.25 | 0.28 ± 1.16 | 1.57 ± 1.35† | 0.82 ± 1.49* |
| Nighttime | 1.18 ± 1.19 | 1.42 ± 1.19 | 1.87 ± 1.57* | 1.76 ± 1.28 |
BPI, Blood pressure index; SDS, standard deviation score.
Values are mean ± SD, comparison between systolic and diastolic BP among subjects with LVMI <90th percentile or ≥90th percentile.
P < .05.
P < .01.
We used recently recommended definitions for abnormal blood pressure based upon ABPM.8,11 These are listed in Table III with their respective sensitivity, specificity, and odds ratios with 95% confidence intervals and P values. Systolic load for the entire 24 hours was >50% in 41 subjects (38.7%); they were more likely to have increased LV mass (P = .008). The 24-hour diastolic load was >50% in 20 subjects (18.9 %). Although a 24-hour systolic load ≥25% demonstrated 78% sensitivity and 36% specificity for prediction of elevated LVMI, load >50% decreased the sensitivity yet increased specificity. Use of BPI >1 is the most universally accepted definition of abnormally increased blood pressure; SBPI >1 had 51% sensitivity and 69% specificity for prediction of elevated LVMI. Use of the SDS for SBP >1.5 offered no advantage over SBPI. The combination of SBPI >1 and 24-hour systolic load >50% had a sensitivity of 57.5% and specificity of 66.7%. Nighttime variables were examined separately and are also found in Table III. Casual blood pressure levels were not predictive of elevated LVMI.
Table III.
Prediction of LVMI based on selected BP parameters: Sensitivity, specificity, OR with 95% confidence intervals
| Parameter | Sensitivity | Specificity | OR | CI | P |
|---|---|---|---|---|---|
| 24-h SBP load ≥25% | 78 | 36 | 1.97 | 0.82, 4.74 | .13 |
| 24-h SBP load >50% | 53 | 72 | 2.96 | 1.32, 6.65 | .008 |
| 24-h DBP load ≥25% | 51 | 56 | 1.32 | 0.61, 2.85 | .49 |
| 24-h DBP load >50% | 29 | 89 | 3.13 | 1.13, 8.67 | .024 |
| 24-h SBPI >1 | 51 | 69 | 2.31 | 1.04, 5.13 | .038 |
| 24-h DBPI >1 | 31 | 87 | 2.99 | 1.13, 7.93 | .024 |
| 24-h SSDS >1.5 | 51 | 70 | 2.5 | 1.12, 5.57 | .024 |
| 24-h DSDS >1.5 | 31 | 85 | 2.61 | 1.01, 6.73 | .043 |
| 24-h SBP load >50% and SBPI >1 | 58 | 67 | 2.71 | 1.2, 6.08 | .015 |
| Nighttime SBPI >1 | 50 | 63 | 1.72 | 0.79, 3.77 | .17 |
| Nighttime DBPI >1 | 49 | 62 | 1.55 | 0.71, 3.4 | .27 |
| Nighttime SBP load >50% | 55 | 66 | 2.34 | 1.06, 5.18 | .035 |
| Nighttime DBP load >50% | 50 | 62 | 1.61 | 0.72, 3.6 | .24 |
| Nighttime SBP load >50% and SBPI >1 | 53 | 65 | 2.14 | 0.97, 4.73 | .059 |
| Nighttime DBP load >50% and DBPI >1 | 51 | 62 | 1.75 | 0.78, 3.92 | .18 |
BPI, Blood pressure index; SDS, standard deviation score; OR, odds ratio; CI, 95% confidence intervals.
Assignment of risk groups was performed using recently published recommendations for use of ABPM.11 Stage 1 HTN was defined as mean SBP less than the 95th percentile with SBP load of 25% to 50%, stage 2 HTN was defined as mean SBP >95th percentile and load 25% to 50%, and stage 3 HTN was defined as mean SBP >95th percentile and load >50%. Using the systolic BPI and load, 31 subjects demonstrated normal ABPM, 32 were classified as having stage 1 hypertension, 2 subjects were classified with stage 2 HTN, and 40 subjects were classified as having stage 3 HTN. Therefore, among our cohort, it was unusual to have a mean SBP greater than the 95th percentile and a load <50%. The risk for increased LVMI was positively correlated with blood pressure stage (P = .014 by Kruskal-Wallis, MHχ2 = 6.1). In addition, those subjects with stage 3 HTN had significantly greater mean LVMI compared with normal subjects (P = .002 by ANOVA, LMVI 39.5 ± 10.4 versus 31.6 ± 7.9). The OR for increased LVMI among those with stage 3 HTN was 3, (CI = 1.1, 7.9, P = .027) referenced to those with normal ABP.
Using logistic regression analysis, the risk for having elevated LVMI increased by 54% for each incremental increase of 1 standard deviation in 24-hour systolic SDS after controlling for race and BMI (OR = 1.54, unit = 1 SDS, CI = 1.1, 2.15, P = .011) and increased by 88% for each incremental increase of 0.1 in the BPI (OR = 1.88, CI = 1.03, 3.45, P = .04). Analysis of systolic load as a continuous variable with units of 10% increase had an OR of 1.2 (CI = 1.04, 1.47, P = .016). Alternatively, dividing subjects by systolic load (<25%, 25% to 50%, 51% to 75%, and >75%) revealed increasing odds for elevated LVM as the load increased by increments of 25% (OR = 1.73, CI = 1.12, 2.66, P = .013). This relationship between systolic load and LVMI was maintained after controlling for race, BMI percentile, and systolic nocturnal decline.
The effect of obesity on cardiovascular risk is substantial. As expected, BMI was significantly correlated with LVMI (r = 0.47, P = .001); however SBPI did not show significant correlation with BMI (r = 0.23, P = .11). As the BMI classification increased, the prevalence of elevated LVMI increased from 19% (normal BMI) to 40% (at risk for overweight) and to 56% (overweight). Furthermore, addition of BMI percentile to logistic regression analysis had a significant impact on the OR. The OR for increased LVMI for each 0.1 increase in 24-h SBPI was 2.43 (CI = 1.3, 4.4) after adjusting for race and nocturnal decline in SBP. When BMI percentile was added, the OR decreased to 2 (CI = 1.1, 3.7). This effect of BMI percentile on the relationship between SBP and LVMI was consistently observed for all ambulatory blood pressure variables.
The impact of race on nighttime blood pressure was examined. Mean diastolic nocturnal decline was lower in AA compared with non-AA: 8.8% ± 7.1% versus 12% ± 5.9%, (P = .023). However, race did not significantly affect the relationship between blood pressure, and specifically night-time blood pressure, and LVMI. When percent nocturnal decline was added to the logistic regression model, there was no significant effect on the relationship between ambulatory blood pressure and LVMI.
DISCUSSION
In the present cross-sectional study, we characterized blood pressure using ABPM and concurrently measured LVMI in a multiethnic group of children and adolescents at risk for hypertension, either because of an elevated office blood pressure level (≥90th percentile) or a first-degree relative with a diagnosis of hypertension. We have added the SDS method to that of the BPI in the interpretation of ABPM. Using odds ratios and logistic regression, the incremental increase in risk for LVMI according to systolic blood pressure has been documented.
ABPM has been adopted as a valuable tool for the diagnosis and treatment of children and adults with hypertension. 11 The definition of normal ambulatory blood pressure was initially based on Task Force standards for casual blood pressure.4,18–20 Since the multicenter European study provided ABPM data from 1141 children,7 most investigators have adopted these as the best standards for ABP. The same data set was transformed using the LMS method [degree of skewness (L), distribution median (M), and coefficient of variation (S)] to allow calculation of SDS based on either age or height.8 This method was employed by Patzer et al,21 who found higher nocturnal blood pressure SDS in children with renal scarring and vesicoureteral reflux than in healthy control subjects. They also found a correlation between blood pressure SDS and the degree of renal scarring. Our group has used ABPM-derived SDS to characterize blood pressure patterns in pediatric renal allograft recipients.22 Experts continue to debate which ABPM variables (load versus mean blood pressure) and/or exact blood pressure levels confer risk for future cardiovascular disease or progression of renal disease in children and adolescents.23,24 There may be advantages of the LMS method for interpretation of ABPM25,26; however, our study does not indicate any advantage of SDS over BPI.
The most commonly assessed HTN-induced cardiovascular effect in pediatric studies is increased LVM. Studies have described LV changes including LVH among previously diagnosed children with hypertension; these do not attempt to correlate LVM with blood pressure level.27,28 Selected studies have correlated ABP with LVMI in children already diagnosed with HTN based on casual blood pressure.4,5 Our findings confirm those of previous investigators4,5; mean 24- hour SBP above the 95th percentile (SBPI >1) and/or 24-hour systolic load >50% are associated with increased likelihood to have abnormal LVMI after controlling for BMI, race, and degree of nocturnal blood pressure decline. Our study found a similar relationship between LVMI and SBP, as well as a lack of correlation between DBP and LVMI. Most importantly, in our cohort, categorization as hypertensive was based on the definition of hypertension using ABPM alone, not casual, clinic-based blood pressure levels. Unfortunately, the sensitivity and specificity of ABPM to indicate which children are likely to have increased LVMI are not optimal. The ability to predict which children will have increased LVMI is confounded by the enormous impact of BMI on LVMI. In addition, the cross-sectional study design fails to assess the duration of blood pressure elevation, which would likely modify LVM.
We do not wish to imply that ABPM can be used without echocardiography in the assessment of cardiovascular risk. The initial goal of this research project was to determine which ambulatory blood pressure levels would identify children with mild to moderate primary hypertension most likely to benefit from pharmacologic therapy; we wanted this to be based on an outcome measure related to cardiovascular morbidity. We did not recruit children with severe HTN or use more stringent definitions of LVH as the outcome measure because individuals with these characteristics would obviously benefit from antihypertensive therapy.
Potential limitations to the present study include the method for ABPM and the somewhat arbitrary selection of <90th percentile to designate normal LVMI. The AM5600 is an auscultatory monitor. We carefully standardized each subject’s blood pressure with a mercury manometer; this method for standardization has also been applied to oscillometric monitors. Although most pediatric centers now use oscillometric monitors due to the reduction in noise artifacts, we believe that our results may be generalized to results obtained with the alternative method. Staessen et al29 compared measurements obtained with both oscillometric and auscultatory monitors in a large group of adults and found minimal differences in mean blood pressure.
We elected to stratify LVMI at the 90th percentile based upon previous use of the 90th percentile in the literature. Assumption that normal is less than the 90th percentile has been used by other investigators.4,12,27 Among our study subjects, there were only five children who had an LVMI >51 g/ht2.7 and, therefore, met the more stringent definition of LVH.
There is a greater prevalence of hypertension among overweight children (defined as BMI greater than the 95th percentile). LVMI has also been associated with the degree of ponderosity in children and adolescents. In a school-based screening program, Sorof and Portman30 noted a marked increase in the prevalence of hypertension as BMI increased. LVH was significantly associated with increasing BMI in a retrospective cross-sectional study of children with hypertension from 3 hypertension study groups.28 Our study supports the findings of previous investigators. As BMI increased, so did the prevalence of elevated LVMI. We found a significant correlation between LVMI and BMI. However, the relationship of SBP to risk for increased LVMI persisted after controlling for BMI.
A recent review proposed to classify ABPM patterns into stages, assuming increasing risk for target organ damage with increasing stage.11 This is an attractive method to prioritize treatment strategies as well as theoretical risk for future cardiovascular disease; however, it is not evidence-based. In our cohort, there were few individuals in the stage 2 HTN category. There was a significant difference, however, in left ventricular mass and an increased risk to have elevated LVMI in those classified with stage 3 HTN.
Acknowledgments
The authors would like to acknowledge the editorial assistance of Andrea Patters and the dedication of the echocardiography technician, Sharon Myers.
Supported by a grant from the Children’s Foundation Research Center at Le Bonheur Children’s Medical Center, Memphis, Tennessee, and the University of Tennessee Health Science Center, MO1 USPHS Grant RR-00211 and NIH NHLBI #5K23HL83910-2.
Abbreviations
- ABP
Ambulatory blood pressure
- ABPM
ABP monitoring
- BMI
Body mass index
- DBP
Diastolic blood pressure
- HTN
Hypertension
- LVMI
Left ventricular mass index
- SBP
Systolic blood pressure
- SDS
Standard deviation scores
- WCH
White coat hypertension
REFERENCES
- 1.Ingelfinger JR. Pediatric antecedents of adult cardiovascular disease: awareness and intervention. N Engl J Med. 2004;350:2123–2126. doi: 10.1056/NEJMp048069. [DOI] [PubMed] [Google Scholar]
- 2.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 3.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 4.Belsha CW, Wells TG, McNiece KL, Seib PM, Plummer JK, Berry PL. Influence of diurnal blood pressure variations on target organ abnormalities in adolescents with mild essential hypertension. Am J Hypertens. 1998;11:410–417. doi: 10.1016/s0895-7061(98)00014-4. [DOI] [PubMed] [Google Scholar]
- 5.Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. 2002;39:903–908. doi: 10.1161/01.hyp.0000013266.40320.3b. [DOI] [PubMed] [Google Scholar]
- 6.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 7.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, et al. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 8.Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens. 2002;20:1995–2007. doi: 10.1097/00004872-200210000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98:649–658. [PubMed]
- 10.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed]
- 11.Lurbe E, Sorof JM, Daniels SR. Clinical and research aspects of ambulatory blood pressure monitoring in children. J Pediatr. 2004;144:7–16. doi: 10.1016/j.jpeds.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 12.Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J, et al. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension. 2005;45:493–498. doi: 10.1161/01.HYP.0000160320.39303.ab. [DOI] [PubMed] [Google Scholar]
- 13.Richey PA, Jones CL, Harshfield GA, Somes GW, Johnson KC, Bailey JE, et al. The AM5600 ambulatory blood pressure recording system. Blood Press Monit. 1997;2:193–195. [PubMed] [Google Scholar]
- 14.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 15.Daniels SR, Meyer RA, Liang YC, Bove KE. Echocardiographically determined left ventricular mass index in normal children, adolescents and young adults. J Am Coll Cardiol. 1988;12:703–708. doi: 10.1016/s0735-1097(88)80060-3. [DOI] [PubMed] [Google Scholar]
- 16.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 17.Daniels SR. Hypertension-induced cardiac damage in children and adolescents. Blood Press Monit. 1999;4:165–170. [PubMed] [Google Scholar]
- 18.Khan IA, Gajaria M, Stephens D, Balfe JW. Ambulatory blood pressure monitoring in children: a large center’s experience. Pediatr Nephrol. 2000;14:802–805. doi: 10.1007/s004679900291. [DOI] [PubMed] [Google Scholar]
- 19.Nehal US, Ingelfinger JR. Pediatric hypertension: recent literature. Curr Opin Pediatr. 2002;14:189–196. doi: 10.1097/00008480-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Flynn JT. Differentiation between primary and secondary hypertension in children using ambulatory blood pressure monitoring. Pediatrics. 2002;110:89–93. doi: 10.1542/peds.110.1.89. [DOI] [PubMed] [Google Scholar]
- 21.Patzer L, Seeman T, Luck C, Wuhl E, Janda J, Misselwitz J. Day-and night-time blood pressure elevation in children with higher grades of renal scarring. J Pediatr. 2003;142:117–122. doi: 10.1067/mpd.2003.13. [DOI] [PubMed] [Google Scholar]
- 22.McGlothan KR, Wyatt RJ, Ault BH, Hastings MC, Rogers T, DiSessa T, et al. Predominance of nocturnal hypertension in pediatric renal allograft recipients. Pediatr Transplant. 2006;10:558–564. doi: 10.1111/j.1399-3046.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy SE, Mackie FE, Rosenberg AR, Craig E, Kainer G. Agreement on reporting of ambulatory blood pressure monitoring in children. Pediatr Nephrol. 2005;20:1766–1768. doi: 10.1007/s00467-005-2066-7. [DOI] [PubMed] [Google Scholar]
- 24.Koshy S, Macarthur C, Luthra S, Gajaria M, Geary D. Ambulatory blood pressure monitoring: mean blood pressure and blood pressure load. Pediatr Nephrol. 2005;20:1484–1486. doi: 10.1007/s00467-005-2014-6. [DOI] [PubMed] [Google Scholar]
- 25.Graves JW. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens. 2002;20:1939–1940. doi: 10.1097/00004872-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Graves JW, Althaf MM. Utility of ambulatory blood pressure monitoring in children and adolescents. Pediatr Nephrol. 2006;21:1640–1652. doi: 10.1007/s00467-006-0175-6. [DOI] [PubMed] [Google Scholar]
- 27.Daniels SR, Loggie JM, Khoury P, Kimball TR. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation. 1998;97:1907–1911. doi: 10.1161/01.cir.97.19.1907. [DOI] [PubMed] [Google Scholar]
- 28.Hanevold C, Waller J, Daniels S, Portman R, Sorof J. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328–333. doi: 10.1542/peds.113.2.328. [DOI] [PubMed] [Google Scholar]
- 29.Staessen JA, Bieniaszewski L, O’Brien E, Gosse P, Hayashi H, Imai Y, et al. Nocturnal blood pressure fall on ambulatory monitoring in a large international database: the ‘Ad Hoc’ Working Group. Hypertension. 1997;29:30–39. doi: 10.1161/01.hyp.29.1.30. [DOI] [PubMed] [Google Scholar]
- 30.Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113:475–482. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]