Abstract
Background
Although ventricular assist devices (VADs) provide effective treatment for end-stage heart failure, VAD support remains associated with significant risk for adverse events (AEs). To date there has been no detailed assessment of the incidence of a full range of AEs using standardized event definitions. We sought to characterize the frequency and timing of AE onset during the first 60 days of VAD support, a period during which clinical observation suggests the risk of incident AEs is high.
Methods
A retrospective analysis was performed utilizing prospectively collected data from a single-site clinical database including 195 patients aged ≥18 receiving VADs between 1996 and 2006. AEs were coded using standardized criteria. Cumulative incidence rates were determined, controlling for competing risks (death, transplantation, recovery/wean).
Results
During the first 60 days after implantation, the most common AEs were bleeding, infection, and arrhythmias (cumulative incidence rates, 36%–48%), followed by tamponade, respiratory events, reoperations, and neurologic events (24%–31%). Other events (e.g., hemolysis, renal, hepatic events) were less common (rates <15%). Some events (e.g., bleeding, arrhythmias) showed steep onset rates early after implantation. Others (e.g., infections, neurologic events) had gradual onsets during the 60-day period. Incidence of most events did not vary by implant era (1996–2000 vs. 2001–2006) or by left ventricular vs. biventricular support.
Conclusions
Understanding differential temporal patterns of AE onset will allow preventive strategies to be targeted to the time periods when specific AE risks are greatest. The AE incidence rates provide benchmarks against which future studies of VAD-related risks may be compared.
Keywords: circulatory assist devices, heart failure, outcomes
INTRODUCTION
Ventricular assist devices (VADs) provide effective treatment for end-stage heart failure, whether as bridges to transplantation [1–3], bridges to recovery [4–6], or as destination therapy [7–11]. Once supported, patients have resolution of their heart failure state, improved survival to transplantation, and enhanced quality of life and functional status [1–3,12–18]. Continued developments in VAD technology and increasing clinical experience with VADs have resulted in improved patient outcomes over time [11,19–23]. Nevertheless, VAD support remains associated with serious adverse events (AEs) that can limit its efficacy.
While the risk of developing AEs is clinically well-recognized, the frequency and timing of important AEs are not well characterized in the current literature. The majority of studies report simple percentages of patients who experience the most common AEs, including bleeding, infection, and device malfunction [2,16,24,25]. These studies do not adjust for duration of VAD support or consider whether incidence rates vary over time during the period of support. Other studies have adjusted for duration of support, but provide little to no evidence of how AE incidence rates change over time [1,7,9,11,18,26,27]. The few reports that have rigorously examined the timing of event onset have focused on only one or two of the most prominent AEs, rather than considering the full spectrum of these events [1,28–32]. Moreover, the results from these studies are difficult to compare and thus generalize due to the lack of standard definitions for the specific AEs.
The purpose of the present study was to examine both the cumulative incidence rates and the timing of onset of the full range of AEs in a large patient cohort who received VADs over a ten-year period at our institution. Because prior studies have suggested that the risk of incident AEs is greatest in the first few months post-implantation [18,26], we focused our analysis on the first 60 days after VAD implantation. In order to enhance the potential to generalize our findings, we employed the standardized definitions of AEs now available as a result of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) [33].
PATIENTS AND METHODS
Subjects
Subjects included all patients aged ≥18 receiving a VAD for long-term left ventricular or biventricular support between January 1996 and December 2006 at the University of Pittsburgh Medical Center (UPMC). Patients receiving mechanical circulatory support devices intended for temporary (<14 day) support were excluded. Among the long-term VAD population during this time period at our center, only a few received a VAD solely for right ventricular support (n = 6) and thus they were excluded. In addition, the few patients receiving VAD support with a continuous flow device (n = 14) were excluded. Subjects in the sample received either a Novacor left ventricular assist system (WorldHeart Corp., Oakland, CA), Heartmate XVE LVAS (Thoratec Corp., Pleasanton, CA), Thoratec VAD (implanted as either a left ventricular assist device [LVAD] or biventricular assist devices [BiVAD]) (Thoratec Corp.), or Thoratec implantable ventricular assist device (IVAD) (Thoratec Corp.).
Study Design and Data Collection
This retrospective study of electronic medical records data was approved by the University of Pittsburgh Institutional Review Board. It utilized deidentified patient information that had been prospectively collected and entered into the UPMC Cardiothoracic Transplantation Program’s electronic clinical records database, the Transplant Patient Management System (TPMS). For the present study, TPMS data were reviewed in order to classify each patient’s AEs into INTERMACS [33] -defined AE categories. This was performed by two trained coders under supervision of the senior author (RLK). Occasionally imprecise descriptions of events required adjudication of these events into the proper category via consensus between both coders and the senior author. Information on the initial timing of each event was also extracted from the database, as were patients’ background demographic and clinical characteristics (described below).
Measures
Fourteen categories of clinically significant AEs were examined. AEs were defined by INTERMACS [33] definitions (Appendix). The AEs were broadly grouped as cardiac/vascular, other organ systems, and other AEs. Patient demographics, VAD type, intention to treat (e.g., as bridge therapy), and era of VAD implant (1996–2000 vs. 2001–2007) were also collected.
Appendix Table.
Definitions of clinically significant acute AEs within the first 60 days post-VAD implantation.
| Event Type | Definition |
|---|---|
| CARDIAC/VASCULAR | |
| Arrhythmia (ventricular or atrial) | Ventricular or atrial arrhythmia resulting in a clinical compromise (e.g., diminished VAD outflow, oligouria, pre-syncope or syncope) that requires hospitalization or occurs during the hospital stay. Ventricular arrhythmia: Sustained ventricular arrhythmia requiring defibrillation or cardioversion Atrial arrhythmia: Sustained supraventricular arrhythmia requiring drug treatment or cardioversion |
| Tamponade | Accumulation of fluid or clot in the pericardial space that requires surgical intervention or percutaneous catheter drainage. |
| RV Failure | Symptoms and signs of persistent right ventricular dysfunction (CVP >18mmHg with cardiac index <2.0 L/min/m2 in the absence of elevated left atrial/pulmonary capillary wedge pressure (>18mmHg) tamponade, ventricular arrhythmias or pneumothorax) requiring RVAD implantation. |
| Thromboembolism (arterial or venous) |
Arterial thromboembolism: Acute systemic arterial perfusion deficit in any non- cerebrovascular organ system confirmed by clinical and lab findings, operative or autopsy Venous thromboembolism: Evidence deep vein thrombosis or other venous thrombotic event. |
| Hemolysis | Plasma free hemoglobin >40mg/dl in association with clinical signs of hemolysis occurring within the first 72 hours post-implantation. |
| OTHER ORGAN SYSTEMS | |
| Respiratory (tracheostomy or reintubation) | Impairment of respiratory function requiring tracheostomy or reintubation, tracheostomy. |
| Neurologic (infarct or hemorrhagic CVA, or TIA) | Any new, temporary or permanent, focal or global neurological deficit ascertained by standard neurological examination. Includes transient ischemic attacks and ischemic or hemorrhagic cardiovascular accidents. |
| Renal | Acute renal dysfunction (abnormal kidney function requiring dialysis in patients who did not require this procedure before implant or a rise in serum creatinine >3 times normal baseline or >5mg/dL) and chronic renal dysfunction (an increase in serum creatinine of ≥2mg/dL above baseline or requirement of hemodialysis for ≥90 days). |
| Hepatic | An increase in any two of the following lab values (total bilirubin, AST, or ALT) to a level >3 times the upper limit of normal 14 days post-implant (or if hepatic dysfunction is the primary cause of death). |
| GI | Cholecystitis, Crohn’s disease, diverticulitis, esophagitis, GERD, hiatal hernia, ischemic bowel requiring surgical exploration, pancreatitis with abnormal amylase/lipase requiring nasogastric suction therapy, polyps, or ulcer. |
| OTHER | |
| Bleeding (coagulopathy, mediastinum or pocket, thorax, gastrointestinal) | An episode of coagulopathy, or bleeding of the mediastinum, pocket, thorax, or gastrointestinal system that results in death or the need for re-operation, hospitalization, or transfusions of red blood cells (≥4U PRBC within any 24 hr period in the first 7 days post-implant or ≥2U PRBC within any 24 hr period after 7 days post-implant). |
| Infection (driveline, blood stream, pulmonary, mediastinum or pocket) | An infection accompanied by pain, fever, drainage and/or leukocytosis that is treated by anti-microbial agents (non-prophylactic). Driveline infection: Positive culture from the skin and/or tissue surrounding the drive line or from the tissue surrounding the external pump housing, with the need for treatment, when there is clinical evidence of infection (pain, fever, drainage, leukocytosis). Blood stream infection: Evidence of systemic involvement by infection manifested by positive blood cultures and/or hypotension. Pulmonary infection: Positive culture from bronchial lavage with findings on a CT scan or x-ray of lung consolidation. Mediastinum or pocket: Positive culture from skin/tissue surrounding the external housing of an implanted pump or the mediastinum, requiring treatment, when there is clinical evidence of infection (pain, fever, drainage, leukocytosis). |
| Reoperation (bleeding, infection, wound dehiscence, wound debridement) | Return operation due to bleeding, infection or disruption of the apposed surfaces of a surgical incision requiring surgical repair. |
| Device Malfunction | Failure of one or more components of the mechanical circulatory device system which either directly causes or could potentially induce a state of inadequate circulatory support or death. This includes pump and non-pump failures. |
Statistical Analysis
Descriptive information (proportions, means, medians) on demographics and VAD-related characteristics of the sample were examined. Competing risk models [34] were estimated in order to calculate the cumulative incidence rate (i.e., time to initial onset) of each AE. Specifically, for each AE, a model was fit that included the AE as well as the hazards for death, transplant, or recovery/wean during the period of observation. A competing risk model was required because the occurrence of the three competing risks influenced the risk for an AE during the observation period [34]. Failure to take competing risks into account would result in biased estimates of AE risk. The impact of patient gender, age (<55 vs. ≥55 years, based on a median split), VAD intention to treat (bridge to transplant vs. other reasons), type of VAD (LVAD vs. BiVAD), and era of VAD implant (1996–2000 vs. 2001–2006) on risk of each type of AE was examined using Cox proportion hazards models. Race/ethnicity was not considered in the multivariate models due to insufficient variability in this characteristic in the sample.
RESULTS
Cohort Characteristics
A total of 195 patients aged ≥18 underwent LVAD or BiVAD implantation within the ten-year study period. Details of the patient cohort are listed in Table 1; the cohort is similar demographically to other VAD patient samples [1,2,9,18,25]. The majority of patients received an LVAD, and for most the intention to treat was as a bridge-to-transplant.
Table 1.
Patient demographic and clinical characteristics (N = 195).
| Characteristic | Descriptive Statistic |
|---|---|
| Male gender, % | 76.4 |
| Race/ethnicity, % | |
| European American | 88.7 |
| African American | 8.7 |
| Other | 2.6 |
| Age at Implant, years, mean | 49.7 ± 12.1 |
| median | 53 |
| Days of VAD support, mean | 136.6 ± 146.0 |
| median | 89 |
| Intention to Treat, % | |
| Bridge-to-Transplant | 84.1 |
| Postcardiotomy Failure | 6.2 |
| Bridge-to-Recovery | 6.2 |
| Destination Therapy | 3.6 |
| Device Type, % | |
| LVAD | 63.6 |
| BiVAD | 36.4 |
| Device, % | |
| Thoratec | 59.5 |
| Novacor | 24.1 |
| Heartmate XVE | 16.4 |
| Implant Era, % | |
| 1996–2000 | 47.7 |
| 2001–2006 | 52.3 |
Clinical Adverse Events in the Acute Post-Implantation Period
For the full sample of 195 patients, the cumulative incidence rates for the occurrence of any of the 14 major categories of clinically significant AE, as well as the cumulative incidence of the specific types of AEs included within each category are shown in the first three columns of Table 2. For example, the table shows that 174 of the 195 patients had at least one AE in the first 60 days post implant, with a cumulative incidence rate of 89%. The cumulative incidence estimates the probability of occurrence of an AE during the 60-day period after VAD implant, controlling for the competing risks of death, transplant or recovery/wean. The cumulative incidence rate represents the kind of risk information that is often provided to patients, i.e., if a patient were to live with a VAD for 60 days (and, for example, not receive a transplant or die or be weaned during that time), the patient would have an 89% chance of experiencing one or more AEs. The first three columns of Table 2 further show the rates for each specific type of AE. The most common types of events were bleeding, infection and arrhythmias (with incidence rates between 36% and 48%), followed by tamponade, respiratory events, neurological events, and reoperations (with rates between 24% and 31%). Remaining events were less common. The confidence intervals around each estimate indicate that the risk of each AE was significantly greater than zero with the exception of gastrointestinal (GI) events.
Table 2.
Patient risk of clinically significant adverse events (AEs): Cumulative incidence by 60 days after VAD implant.
| Total Sample (N = 195) |
LVAD patients (n = 124)b |
BiVAD patients (n = 71)b |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Event Type | No. of patients with AE |
AE rate: Cumulative incidencea |
95% Confidence Interval |
No. of patients with AE |
AE rate: Cumulative Incidencea |
95% Confidence Interval |
No. of patients with AE |
AE rate: Cumulative incidencea |
95% Confidence Interval |
| ANY ADVERSE EVENT | 174 | 89.2 | 84.0, 92.8 | 112 | 90.3 | 83.6, 94.4 | 62 | 87.3 | 77.1, 93.2 |
| CARDIAC/VASCULAR | |||||||||
| Arrhythmia, any | 71 | 36.4 | 29.7, 43.1 | 56 | 45.2 | 36.2, 53.6 | 15 | 21.1 | 12.5, 31.2 |
| Ventricular | 36 | 18.5 | 13.4, 24.2 | ||||||
| Atrial | 42 | 21.5 | 16.1, 27.5 | ||||||
| Tamponade | 55 | 28.2 | 22.1, 34.6 | 37 | 29.8 | 22.1, 38.0 | 18 | 25.3 | 16.0, 35.8 |
| RV failure | 21 | 10.8 | 6.9, 15.6 | 21 | 16.9 | 11.0, 24.0 | -- | -- | -- |
| Thromboembolism (non-CNS) | 17 | 8.7 | 5.3, 13.2 | 13 | 10.5 | 5.9, 16.6 | 4 | 5.6 | 1.8, 12.7 |
| Hemolysis | 6 | 3.1 | 1.3, 6.2 | 2 | 1.6 | 0.3, 5.2 | 4 | 5.6 | 1.8, 12.7 |
| OTHER ORGAN SYSTEMS | |||||||||
| Respiratory, any | 47 | 24.1 | 18.3, 30.3 | 24 | 19.4 | 12.9, 26.7 | 23 | 32.3 | 21.9, 43.3 |
| Tracheostomy | 24 | 12.3 | 8.2, 17.3 | ||||||
| Reintubation | 26 | 13.3 | 9.0, 18.5 | ||||||
| Neurologic | 48 | 24.7 | 18.9, 30.9 | 33 | 26.8 | 19.3, 34.8 | 15 | 21.1 | 12.5, 31.2 |
| Infarct or Hemorrhagic CVA | 30 | 15.4 | 10.7, 20.8 | ||||||
| Transient Ischemic Attack | 23 | 11.8 | 7.8, 16.8 | ||||||
| Renal | 28 | 14.4 | 9.9, 19.7 | 13 | 10.5 | 5.9, 16.6 | 15 | 21.1 | 12.5, 31.2 |
| Hepatic | 17 | 8.7 | 5.3, 13.2 | 7 | 5.6 | 2.5, 10.7 | 10 | 14.1 | 7.2, 23.2 |
| Gastrointestinal | 1 | 0.5 | 0.0, 2.6 | 1 | 0.8 | 0.1, 4.0 | 0 | -- | -- |
| OTHER | |||||||||
| Bleeding, any | 93 | 47.7 | 40.5, 54.5 | 49 | 39.5 | 30.9, 48.0 | 44 | 62.0 | 49.6, 72.1 |
| Coagulopathy | 33 | 16.9 | 12.0, 22.5 | ||||||
| Mediastinum or Pocket | 51 | 26.2 | 20.2, 32.5 | ||||||
| Thorax | 20 | 10.2 | 6.5, 15.0 | ||||||
| Gastrointestinal | 10 | 5.1 | 2.6, 8.9 | ||||||
| Infection, any | 82 | 42.1 | 35.1, 48.9 | 48 | 38.7 | 30.2, 47.2 | 34 | 47.8 | 35.9, 58.9 |
| Driveline | 34 | 17.4 | 12.5, 23.1 | ||||||
| Blood Stream | 35 | 17.9 | 12.9, 23.6 | ||||||
| Pulmonary | 35 | 17.9 | 12.9, 23.6 | ||||||
| Mediastinum or Pocket | 12 | 6.1 | 3.3, 10.1 | ||||||
| Reoperation, any | 61 | 31.3 | 24.9, 37.9 | 42 | 33.9 | 25.7, 42.2 | 19 | 26.8 | 17.2, 37.4 |
| Bleeding | 45 | 23.1 | 17.4, 29.2 | ||||||
| Infection | 12 | 6.1 | 3.3, 10.1 | ||||||
| Wound dehiscence | 3 | 1.5 | 0.4, 4.1 | ||||||
| Wound debridement | 13 | 6.7 | 3.7, 10.7 | ||||||
| Device Malfunction | 18 | 9.2 | 5.7, 13.8 | 10 | 8.1 | 4.1, 13.7 | 8 | 11.3 | 5.3, 19.8 |
adjusted for the competing hazards of death, transplantation, or recovery/wean.
see Table 4 for tests of differences in rates between LVAD vs. BiVAD patients.
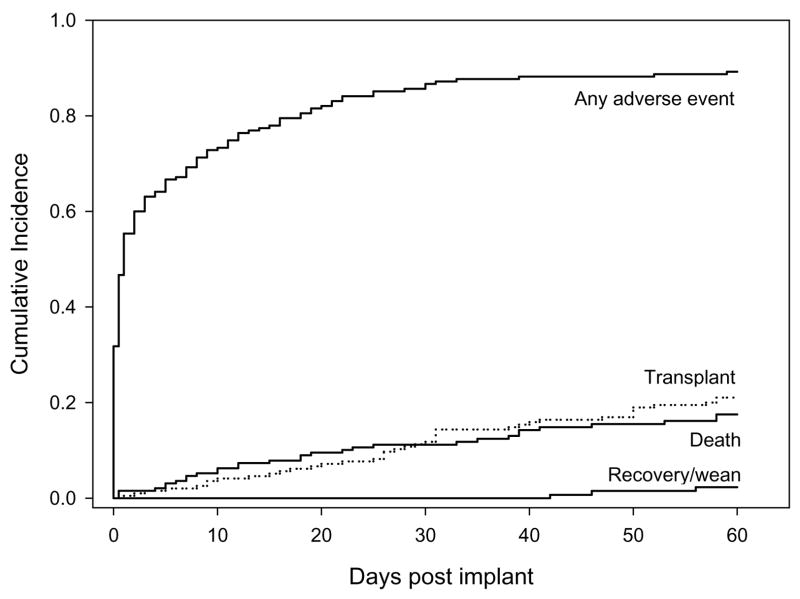
The cumulative incidence curves for the onset of any AE, as well as for the competing risks of death, transplant, and recovery/wean for the entire cohort of 195 patients are shown in Figure 1. The figure shows a steep increase in the overall incidence of AEs (without regard to specific type of AE) immediately after VAD implant, with a slower rate of increase after approximately 30 days. The incidences of death and transplant show a steady increase during the 60-day period. Thirty one of the 195 patients did not survive the 60-day period (actuarial incidence of death, 17.5%), and 41 were transplanted (actuarial incidence, 21.0%). Recovery/weaning occurred at low rate during the first 60 days after VAD implantation, beginning toward the end of the observation period. Three patients were weaned (actuarial incidence, 2.3%).
Figure 1.
Cumulative incidence of any AE during the first 60 days after VAD implant, controlling for competing risks of transplant, death, and recovery/wean.
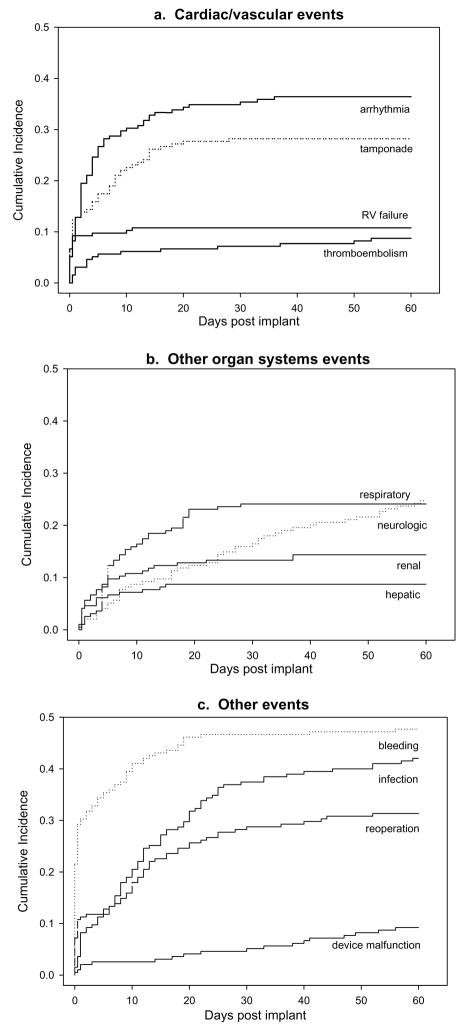
Figure 1 aggregates over all types of AEs. Analysis by specific type of AE shows that some AEs were more prominent early during the 60 day period, while others showed onsets to occur at a steady rate—or even an increased rate—as time went on. Thus, the cumulative incidence curves for each of the major categories of events are shown in Figure 2, grouped by cardiac/vascular AEs, other organ systems AEs, or other types of AEs. (Neither hemolysis nor GI AEs were included in these plots due to the very limited numbers of events.) Many AEs such as arrhythmias, tamponade, RV failure, renal and hepatic events, and bleeding show steep rates of initial onset early after VAD implant and then few, if any, new events during the remainder of the first 60 days. In contrast, AEs such as neurologic events, infection, reoperations, and device malfunction show more gradual onsets; incident events within these categories continued to occur throughout the 60-day period.
Figure 2.
Cumulative incidence of major categories of AEs during the first 60 days after VAD implant.
The incidence curves in Figures 1 and 2 display the complete profiles of the manner in which each category of AE unfolds over the 60-day period in VAD recipients. The specific rates of each AE and their incremental risks at 10, 20, and 30 days are shown in Table 3. For example, by 5 days post VAD implantation, patients had a 24.6% chance of having an arrhythmia. Between 5 and 10 days, the incremental risk was 5.1% yielding a total, cumulative incidence of arrhythmias at 10 days of 29.7%. As in Figures 1 and 2, Table 3 shows that the greatest incidence of many AEs was in the first 5–10 days post VAD implant, with progressively smaller incremental risk thereafter. Notable exceptions were that incremental risks of respiratory events and infection at 10 days and 20 days continued to be as large as initial 5-day incidence rates; the increment to risk did not diminish until after 20 days post-implant. In addition, incremental risks of neurologic events and device malfunction were steady or continued to increase during the entire period.
Table 3.
Patient risk of clinically significant adverse events at specific time points during the first 60 days after VAD implantation (N = 195).
| Adverse event rate (cumulative incidence) at 5 days | Increment to incidence by:* |
||||
|---|---|---|---|---|---|
| Event Type | 10 days | 20 days | 30 days | 60 days | |
| ANY ADVERSE EVENT CARDIAC/VASCULAR | 64.1 | 8.7 | 8.7 | 4.1 | 3.6 |
| Arrhythmia, any | 24.6 | 5.1 | 4.1 | 1.1 | 1.5 |
| Tamponade | 15.9 | 6.2 | 5.1 | 1.0 | 0.0 |
| RV failure | 9.7 | 0.0 | 1.1 | 0.0 | 0.0 |
| Thromboembolism (non-CNS) | 5.1 | 1.1 | 0.5 | 0.5 | 1.5 |
| Hemolysis | 1.5 | 0.6 | 0.0 | 0.5 | 0.5 |
| OTHER ORGAN SYSTEMS | |||||
| Respiratory | 8.7 | 7.1 | 7.2 | 1.0 | 0.0 |
| Neurologic | 4.1 | 4.6 | 3.6 | 3.6 | 8.8 |
| Renal | 8.2 | 2.6 | 2.0 | 0.5 | 1.1 |
| Hepatic | 6.1 | 1.1 | 1.5 | 0.0 | 0.0 |
| Gastrointestinal | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 |
| OTHER | |||||
| Bleeding | 34.3 | 5.2 | 6.7 | 0.5 | 1.0 |
| Infection | 11.3 | 7.7 | 10.7 | 7.7 | 4.7 |
| Reoperation | 12.0 | 5.1 | 9.7 | 3.3 | 1.2 |
| Device Malfunction | 2.6 | 0.0 | 1.5 | 0.5 | 4.6 |
Each incremental value indicates the increased risk accrued since the previous period. For example, the increase in risk of any AE by 10 days was 8.7 beyond the risk of 64.1 (i.e., cumulative incidence) at 5 days. The increase by 20 days was 8.7 over the total risk (cumulative incidence) at 10 days; the increase by 30 days was 4.1 over the total risk at 20 days; the increase by 60 days was 3.7 over the total risk at 30 days.
Associations Between Characteristics at VAD Implant and Incidence of Acute AEs
Patients’ baseline characteristics were examined in relation to AE risk by fitting a separate Cox regression model to each category of AE (excluding hemolysis and GI events due to their low frequencies). Table 4 shows the resulting hazard ratios for each category of AE as a function of gender, age, implantation era, intention to treat, and VAD type with all the major adverse event types. The type of VAD initially implanted showed the greatest associations with AEs. A patient with an LVAD (vs. a BiVAD) had a significantly decreased risk of experiencing a renal or bleeding AE (hazard ratios of .40, .56, respectively). However, an LVAD was associated with over a twofold increased risk of developing arrhythmias. Table 2 provides descriptive data to illustrate the significant differences between LVAD and BiVAD patients: for example, the cumulative incidence of arrhythmias was 45.2% in LVAD recipients but only 21.1% in BiVAD patients. Table 2 is consistent with Table 4 in showing that there were few differences between LVAD and BiVAD patients for the majority of AEs.
Table 4.
Associations of baseline characteristics at time of VAD implant and adverse event incidence: Hazard ratios (and 95% Confidence Intervals) from multivariate models (N = 195).a
| Characteristic at VAD implantation |
||||||
|---|---|---|---|---|---|---|
| Adverse Event Type | Gender (male) | Age (>54) | Implantation Era (before 2001) | Intention to Treat (Bridge to Transplant) | VAD Type (LVAD) | Model Fit, χ2 (df=5) |
| Any AE | 0.87 (0.61, 1.26) |
0.91 (0.66, 1.24) |
0.94 (0.67, 1.31) |
0.74 (0.48, 1.13) |
1.03 (0.75, 1.42) |
3.83 |
| Arrhythmia | 1.65 (0.82, 3.29) |
0.95 (0.58, 1.54) |
0.70 (0.42, 1.16) |
2.14 (0.96, 4.76) |
2.36** (1.32, 4.21) |
16.45** |
| Tamponade | 0.98 (0.50, 1.93) |
1.15 (0.66, 2.01) |
1.24 (0.70, 2.19) |
1.50 (0.62, 3.66) |
1.07 (0.60, 1.93) |
2.26 |
| RV Failure | 1.60 (0.60, 4.34) |
1.48 (0.70, 3.14) |
1.59 (0.68, 3.76) |
0.35* (0.14, 0.89) |
---b | 6.36 |
| Thromboembolism | 0.51 (0.14, 1.81) |
3.26* (1.09, 9.80) |
0.12* (0.02, 0.91) |
0.69 (0.23, 2.04) |
1.99 (0.61, 6.55) |
14.47* |
| Respiratory | 0.60 (0.31, 1.15) |
1.01 (0.55, 1.86) |
0.71 (0.36, 1.37) |
0.95 (0.44, 2.07) |
0.60 (0.33, 1.09) |
9.19 |
| Neurologic | 0.53 (0.27, 1.03) |
1.21 (0.66, 2.23) |
2.17* (1.20, 3.93) |
2.62 (0.76, 8.85) |
1.28 (0.69, 2.39) |
16.07** |
| Renal | 1.83 (0.66, 5.09) |
1.03 (0.47, 2.25) |
0.36 (0.12, 1.09) |
0.43 (0.18, 1.04) |
0.40* (0.18, 0.89) |
15.34** |
| Hepatic | 1.43 (0.42, 4.95) |
0.97 (0.35, 2.64) |
0.27 (0.06, 1.23) |
0.58 (0.19, 1.82) |
0.37 (0.13, 1.03) |
9.81 |
| Bleeding | 0.92 (0.56, 1.50) |
0.84 (0.54, 1.30) |
0.87 (0.55, 1.38) |
0.78 (0.45, 1.38) |
0.56** (0.36, 0.85) |
11.79* |
| Infection | 1.43 (0.81, 2.51) |
0.80 (0.50, 1.27) |
1.41 (0.87, 2.27) |
0.85 (0.44, 1.63) |
0.64 (0.41, 1.00) |
7.37 |
| Reoperation | 0.64 (0.36, 1.15) |
0.95 (0.56, 1.61) |
0.92 (0.52, 1.60) |
0.76 (0.39, 1.49) |
1.29 (0.74, 2.24) |
4.07 |
| Device Malfunction | 0.48 (0.16, 1.41) |
1.21 (0.47, 3.28) |
0.30 (0.09, 1.07) |
2.47 (0.54, 11.23) |
0.76 (0.29, 1.95) |
6.67 |
p<.05
p<.01
p<.001
Hazard ratios <1.00 indicate a reduced risk of an AE associated with a given characteristic. Hazard ratios >1 indicate increased risk. Time to each AE was regressed on the set of baseline characteristics.
VAD type could not be included as a predictor of RV failure because patients who received BiVADs had right ventricular support and thus could not develop new RV failure.
The regression analyses in Table 4 revealed effects for several other factors. Patients receiving VADs before 2001 had a 2.17 times greater risk of developing a neurological complication. Implantation before 2001 was also associated with an 88% decreased risk of having a thromboembolic AE. Age and gender had little to no association with AE occurrence.
COMMENT
Clinical outcomes with VADs intended for long-term use demonstrate efficacy in the setting of bridge to transplantation and, more recently, as destination therapy [2,6–8]. However to date there has been no detailed assessment of AEs after VAD implantation using standardized definitions. The present study is unique in characterizing both the frequency and timing of onset of all types of major AEs during the first 60 days after VAD implantation. Moreover, it utilized prospectively collected data from a relatively large cohort and the analyses controlled for the competing risks of death, transplantation, or recovery/wean from the VAD.
A number of clinically relevant findings emerged. Of note, the cumulative incidence of AEs overall was 89%, indicating that almost all patients experienced at least one type of major AE within the acute post-implant phase. The most frequent individual AEs were bleeding (48%), infection (42%) and arrhythmias (36%). While these types of AEs are not unexpected for a population of patients with advanced heart failure who undergo major cardiac surgery, their incidence is significant. Other events occurred less frequently but at rates higher than anticipated, including reoperation (31%), respiratory events (24%), renal events (14%), and device malfunction (9%). With respect to the latter, the majority of 18 observed device malfunction events led to the need for either pump replacement (6 cases) or controller replacement (7 cases). There were surprisingly few GI (0.5%) and hemolysis (3%) AEs. These findings, based on a variety of pulsatile devices implanted in clinical practice, provide a benchmark to which the newer generation of continuous flow VADs can be compared.
Beyond cumulative incidence rates, our findings revealed different patterns of onset for the AEs, with some (arrhythmias, tamponade, bleeding, renal AEs, hepatic AEs) showing steep rates of initial onset in the first several post-operative days. The rate of new events then slowed dramatically so that few, if any, new events occurred beyond two weeks. In contrast, neurologic events, infection, reoperations, and device malfunction had patterns of more gradual onset; incident events within these categories continued to occur throughout the 60-day period. These findings are consistent with other reports indicating that patients continue to be at risk for these types of AEs during long-term support [29,31,32]. Understanding the differential patterns of onset of the various AEs will allow for better targeting of preventive strategies to the time periods during which the risks for each are greatest. For example, designing more biocompatible devices, reducing the extent of surgical trauma, and eliminating the need for early anticoagulation may drastically reduce the early onset of bleeding. In contrast, strategies for reducing infection and neurological events, and for averting and managing device malfunctions should be sustained throughout the duration of VAD support.
These data also provided us an opportunity to examine relationships between the incidence of AEs and patient characteristics at the time of VAD implantation. Of particular note, patients supported with LVADs alone had a relatively lower risk of bleeding AEs and renal AEs. This is consistent with the clinical observation that patients receiving an LVAD tend to be less acutely ill and have a less extensive surgical procedure than those who require BiVADs. These results support the strategy of early implementation of VAD support before the development of significant right ventricular failure, as it may significantly reduce the likelihood of at least some serious AEs.
The incidence rates of a few AEs were linked to the era of VAD support. Notably, there was a twofold greater risk of neurologic AEs in patients implanted before 2001. This is likely attributable in part to the greater number of Novacor LVADS implanted in the earlier era, because these pumps have been linked with a higher incidence of neurological AEs [32,35]. Moreover, as our center has gained experience in managing VAD patients, anticoagulation therapy has become more standardized and targeted. These changes may also have contributed to the reduction of neurological events in more recent years. Indeed, despite increasing center experience, most AEs showed no significant variability in incidence rates between earlier and more recent eras. Thus, these rates may be more reflective of the specific devices or illness severity at implantation than specific post-operative management strategies. This suggests, in turn, that we would expect to find lower incidences of these AEs in the current era of devices for several reasons. The advent of smaller, continuous flow pumps has resulted in a less extensive surgical procedure which itself should affect the incidence of early postoperative AEs such as bleeding and renal dysfunction. Long-term rates of infection may be reduced with the progressively smaller diameter of drivelines, and device malfunction risks will decrease as a result of the increased durability of the current generation of VADs [1]. Lastly, a shift in strategy towards earlier implantation before the onset of substantial renal, hepatic, and right ventricular dysfunction should also favorably impact the incidence of many of these AEs.
Our study has several limitations. We examined patients from a single center and, by relying on data from an existing clinical database, the information available on the nature and timing of AEs may have been incomplete, despite our attempts to carefully review and cross-check the information with individual patient records. As is typical of the VAD population in the United States, the study population was demographically homogeneous, consisting primarily of European American men. Our sample was too small to provide fine-grained analyses of AEs categorized by the specific pump that a given patient received (e.g., Thoratec LVAD vs. Heartmate vs. Novacor). However, we were able to provide detailed information separately for LVAD vs. BiVAD recipients. Our center employs BiVAD support for a greater proportion of patients than some large volume VAD centers. This is due to our center’s experience of improved survival rates with the provision of immediate (as opposed to delayed) biventricular support when patients present with significant right ventricular dysfunction. Interestingly, our analyses showed that most AEs did not significantly differ in incidence rates by type of VAD support.
In conclusion, our study is the first systematic and detailed analysis of the cumulative incidence and timing of onset of all major AEs in patients receiving VAD support. Adverse events in the first 60 days after VAD implantation are common, but occur in distinct temporal patterns. A better understanding of these patterns of AEs may impact future VAD design and protocols for clinical care. Moreover, information about the onset of all major AEs allows physicians to better inform patients about risks associated with VAD implantation. Future work is needed to delineate the full range of factors influencing AE incidence, as well as the effect of AEs in the first 60 days on risk for later patient morbidity and mortality.
Acknowledgments
We thank Julianne Buchanan and Michelle Navoney for technical assistance with the medical records data extraction and assembly of the dataset used in the study. Preparation of this article was supported in part by a grant to Ms. Genovese from the Dean’s Summer Research Program, University of Pittsburgh School of Medicine. Dr. Dew was supported in part by NIH Grant R01 MH072718. Dr. Simon was supported in part by NIH Grant K12 RR024154.
References
- 1.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Frazier OH, Rose EA, Oz MC, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122(6):1186–95. doi: 10.1067/mtc.2001.118274. [DOI] [PubMed] [Google Scholar]
- 3.Frazier OH, Rose EA, Macmanus Q, et al. Multicenter clinical evaluation of the HeartMate 1000 IP left ventricular assist device. Ann Thorac Surg. 1992;53(6):1080–90. doi: 10.1016/0003-4975(92)90393-i. [DOI] [PubMed] [Google Scholar]
- 4.Simon MA, Kormos RL, Murali S, et al. Myocardial recovery using ventricular assist devices: prevalence, clinical characteristics, and outcomes. Circulation. 2005;112(9 Suppl):I32–6. doi: 10.1161/CIRCULATIONAHA.104.524124. [DOI] [PubMed] [Google Scholar]
- 5.Chen JM, Spanier TB, Gonzalez JJ, et al. Improved survival in patients with acute myocarditis using external pulsatile mechanical ventricular assistance. J Heart Lung Transplant. 1999;18(4):351–7. doi: 10.1016/s1053-2498(98)00054-0. [DOI] [PubMed] [Google Scholar]
- 6.Frazier OH, Myers TJ. Left ventricular assist system as a bridge to myocardial recovery. Ann Thorac Surg. 1999;68(2):734–41. doi: 10.1016/s0003-4975(99)00801-2. [DOI] [PubMed] [Google Scholar]
- 7.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345(20):1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 8.Rogers JG, Butler J, Lansman SL, et al. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol. 2007;50(8):741–7. doi: 10.1016/j.jacc.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 9.Richenbacher WE, Naka Y, Raines EP, et al. Surgical management of patients in the REMATCH trial. Ann Thorac Surg. 2003;75(6 Suppl):S86–92. doi: 10.1016/s0003-4975(03)00485-5. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson LW, Miller LW, Desvigne-Nickens P, et al. Left ventricular assist device as destination for patients undergoing intravenous inotropic therapy: a subset analysis from REMATCH (Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure) Circulation. 2004;110(8):975–81. doi: 10.1161/01.CIR.0000139862.48167.23. [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, Tector A, Piccioni W, et al. Left ventricular assist devices as destination therapy: a new look at survival. J Thorac Cardiovasc Surg. 2005;129(1):9–17. doi: 10.1016/j.jtcvs.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Burnett CM, Duncan JM, Frazier OH, Sweeney MS, Vega JD, Radovancevic B. Improved multiorgan function after prolonged univentricular support. Ann Thorac Surg. 1993;55(1):65–71. doi: 10.1016/0003-4975(93)90475-w. [DOI] [PubMed] [Google Scholar]
- 13.Frazier OH, Macris MP, Myers TJ, et al. Improved survival after extended bridge to cardiac transplantation. Ann Thorac Surg. 1994;57(6):1416–22. doi: 10.1016/0003-4975(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 14.DeRose JJ, Umana JP, Argenziano M, et al. Implantable left ventricular assist devices provide an excellent outpatient bridge to transplantation and recovery. J Am Coll Cardiol. 1997;30(7):1773–7. doi: 10.1016/s0735-1097(97)00396-3. [DOI] [PubMed] [Google Scholar]
- 15.Dew MA, Kormos RL, Roth LH, et al. Life quality in the era of bridging to cardiac transplantation. Bridge patients in an outpatient setting. ASAIO Journal. 1993;39(2):145–52. [PubMed] [Google Scholar]
- 16.Frazier OH, Rose EA, McCarthy P, et al. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg. 1995;222(3):327–36. doi: 10.1097/00000658-199509000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaronson KD, Eppinger MJ, Dyke DB, Wright S, Pagani FD. Left ventricular assist device therapy improves utilization of donor hearts. J Am Coll Cardiol. 2002;39(8):1247–54. doi: 10.1016/s0735-1097(02)01751-5. [DOI] [PubMed] [Google Scholar]
- 18.Esmore D, Kaye D, Spratt P, et al. A prospective, multicenter trial of the VentrAssist left ventricular assist device for bridge to transplant: safety and efficacy. J Heart Lung Transplant. 2008;27(6):579–88. doi: 10.1016/j.healun.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Portner PM, Jansen PG, Oyer PE, Wheeldon DR, Ramasamy N. Improved outcomes with an implantable left ventricular assist system: a multicenter study. Ann Thorac Surg. 2001;71(1):205–9. doi: 10.1016/s0003-4975(00)02514-5. [DOI] [PubMed] [Google Scholar]
- 20.Strauch JT, Spielvogel D, Haldenwang PL, et al. Recent improvements in outcome with the Novacor left ventricular assist device. J Heart Lung Transplant. 2003;22(6):674–80. doi: 10.1016/s1053-2498(02)01182-8. [DOI] [PubMed] [Google Scholar]
- 21.Long JW, Kfoury AG, Slaughter MS, et al. Long-term destination therapy with the HeartMate XVE left ventricular assist device: improved outcomes since the REMATCH study. Congest Heart Failure. 2005;11(3):133–8. doi: 10.1111/j.1527-5299.2005.04540.x. [DOI] [PubMed] [Google Scholar]
- 22.Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116(5):497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 23.Long JW, Healy AH, Rasmusson BY, et al. Improving outcomes with long-term “destination” therapy using left ventricular assist devices. J Thorac Cardiovasc Surg. 2008;135(6):1353–60. doi: 10.1016/j.jtcvs.2006.09.124. [DOI] [PubMed] [Google Scholar]
- 24.Thomas CE, Jichici D, Petrucci R, Urrutia VC, Schwartzman RJ. Neurologic complications of the Novacor left ventricular assist device. Ann Thorac Surg. 2001;72(4):1311–5. doi: 10.1016/s0003-4975(01)03004-1. [DOI] [PubMed] [Google Scholar]
- 25.Morgan JA, John R, Rao V, et al. Bridging to transplant with the HeartMate left ventricular assist device: the Columbia Presbyterian 12-year experience. J Thorac Cardiovasc Surg. 2004;127(5):1309–16. doi: 10.1016/j.jtcvs.2003.07.035. [DOI] [PubMed] [Google Scholar]
- 26.Sharples LD, Cafferty F, Demitis N, et al. Evaluation of the clinical effectiveness of the Ventricular Assist Device Program in the United Kingdom (EVAD UK) J Heart Lung Transplant. 2007;26(1):9–15. doi: 10.1016/j.healun.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Deng MC, Edwards LB, Hertz MI, et al. Mechanical circulatory support device database of the International Society for Heart and Lung Transplantation: third annual report--2005. J Heart Lung Transplant. 2005;24(9):1182–7. doi: 10.1016/j.healun.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Gordon SM, Schmitt SK, Jacobs M, et al. Nosocomial bloodstream infections in patients with implantable left ventricular assist devices. Ann Thorac Surg. 2001;72(3):725–30. doi: 10.1016/s0003-4975(01)02888-0. [DOI] [PubMed] [Google Scholar]
- 29.Birks EJ, Tansley PD, Yacoub MH, et al. Incidence and clinical management of life-threatening left ventricular assist device failure. J Heart Lung Transplant. 2004;23(8):964–9. doi: 10.1016/j.healun.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Holman WL, Rayburn BK, McGiffin DC, et al. Infection in ventricular assist devices: prevention and treatment. Ann Thorac Surg. 2003;75(6 Suppl):S48–57. doi: 10.1016/s0003-4975(03)00479-x. [DOI] [PubMed] [Google Scholar]
- 31.Navia JL, McCarthy PM, Hoercher KJ, et al. Do left ventricular assist device (LVAD) bridge-to-transplantation outcomes predict the results of permanent LVAD implantation. Ann Thorac Surg. 2002;74(6):2051–62. doi: 10.1016/s0003-4975(02)04084-5. [DOI] [PubMed] [Google Scholar]
- 32.Tsukui H, Abla A, Teuteberg JJ, et al. Cerebrovascular accidents in patients with a ventricular assist device. J Thorac Cardiovasc Surg. 2007;134(1):114–23. doi: 10.1016/j.jtcvs.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 33.Kirklin JK, Holman WL. Mechanical circulatory support therapy as a bridge to transplant or recovery (new advances) Curr Opin Cardiol. 2006;21(2):120–6. doi: 10.1097/01.hco.0000210308.64360.8d. [DOI] [PubMed] [Google Scholar]
- 34.Kalbfleisch JD, Prentice PR. The Statistical Analysis of Failure Time Data. 2. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 35.El-Banayosy A, Arusoglu L, Kizner L, et al. Novacor left ventricular assist system versus Heartmate vented electric left ventricular assist system as a long-term mechanical circulatory support device in bridging patients: a prospective study. J Thorac Cardiovasc Surg. 2000;119(3):581–7. doi: 10.1016/s0022-5223(00)70140-1. [DOI] [PubMed] [Google Scholar]