Bioorthogonal chemistry has attracted intense interest recently because, combined with genetic encoding, it provides a powerful covalent strategy to probe biomolecular dynamics and function in living systems.[1] A number of bioorthogonal reactions have been successfully developed, including CuI—catalyzed cycloaddition (‘click chemistry’),[2] strain-promoted cycloaddition,[3] Staudinger ligation,[4] photoinduced cycloaddition (‘photoclick chemistry’),[5] and tetrazine ligation.[6] In applying bioorthogonal reactions to study biomolecular dynamics, it is imperative that the reaction proceeds in a time frame shorter than the biomolecules’ half-lives, which is especially important in case of short-lived proteins, such as the endogenous tumor suppressor protein p53 with a half-life of only 40 min.[7]
We recently reported a photoinduced 1,3-dipolar cycloaddition reaction for selective functionalization of an unactivated alkene in Escherichia coli.[5b] While the reaction proceeded selectively, the cycloaddition rate was rather low (k2 = 0.00202 ± 0.00007 M−1s−1). To accelerate the concerted cycloaddition reactions, two strategies have been commonly employed in the literature: ring strain and fluorine effect,[3] both of which work by lowering the LUMO energies of the dipolarophiles. Because enzymes involved in the biosynthetic/metabolic pathways utilize unnatural substrates that are structurally close to the natural ones, it is typically easier to encode small groups such as allyl group into biomolecules than bulky ones. Therefore, it is more desirable if we can increase the reactivity of the nitrile imine dipoles in our photoclick chemistry. Since reaction rate is inversely related to free energy gap between dipole-HOMO and dipolarophile-LUMO for the type I HO-controlled 1,3-dipolar cycloaddition,[8] we envisioned that the cycloaddition reaction can be accelerated by lifting the dipole-HOMO energy. Herein, we report the identification of several simple, yet powerful tetrazole reagents for the photoclick chemistry by systematically tuning the HOMO energies of the nitrile imines, which afforded an extremely fast (< 1 min) labeling of an alkene-containing protein in E. coli.
To examine the electronic effect of the nitrile imine on the cycloaddition reaction, we synthesized two series of tetrazoles carrying substituents either on the C5-phenyl ring (X) or on the N2-phenyl ring (Y). Using water-soluble 4-penten-1-ol as a model for the unactivated alkene dipolarophile, we measured the cycloaddition rate constants (k2) in the ACN/PBS buffer (1:1) and the data are collected in Table 1.[9] In the first series (tetrazoles 1-5), switching substituents from EWG (1 and 2) to EDG (4, 5) led to substantial increases in reaction rate whereas the unsubstituted diphenyl-tetrazole 3 proceeded almost as fast as 4 and 5. Thus, we decided to leave X as H, and varied substitutions on the N2-phenyl ring and generated the second series (tetrazoles 6-16). Compared to 3, we found EDG (8-16) generally led to rate acceleration while EWG (6, 7) resulted in rate decrease (Table 1). The substituent effect is clearly position-dependent with the para-position producing the most pronounced rate enhancement (compare 10, 12, 13; or 14, 15, 16). In particular, tetrazole 11 carrying the para-NH2 group afforded the fastest rate with k2 value of 0.79 M−1s−1, nearly 200-fold faster than tetrazole 1 we used previously.5b As expected, tetrazole 11 also showed the highest yield (~50% after 75 sec) with the water-quenching product (~10%)5a and the dimerization product (~15%)[10] as the major side products. We further calculated the HOMO energies of the nitrile imines (Table 1)[9] and plotted EHOMO vs. Log(rate) to gauge the electronic effect on the nitrile imine reactivity (Figure 1). The plot exhibited a good linear fit (R2 = 0.86), suggesting that the observed rate enhancement is primarily due to the HOMO-lifting effect.
Table 1.
Photoinduced Cycloaddition Reactions of Tetrazoles with 4-Penten-1-ola
| tetrazole | X | Y | k2 (M−1s−1) | EHOMO (eV)b |
|---|---|---|---|---|
| 1 | p-CO2Me | H | 0.004 | −7.0193 |
| 2 | p-CN | H | 0.001 | −7.1419 |
| 3 | H | H | 0.13 | −6.7517 |
| 4 | p-Me | H | 0.18 | −6.6447 |
| 5 | p-OMe | H | 0.15 | −6.6529 |
| 6 | H | p-CN | 0.01 | −7.2631 |
| 7 | H | p-CO2Et | 0.02 | −7.1403 |
| 8 | H | p-Me | 0.27 | −6.5881 |
| 9 | H | p-NHAc | 0.26 | −6.4098 |
| 10 | H | p-OMe | 0.52 | −6.4735 |
| 11 | H | p-NH2 | 0.79 | −6.1994 |
| 12 | H | o-OMe | 0.29 | −6.7826 |
| 13 | H | m-OMe | 0.19 | −6.8114 |
| 14 | H | o,p-di-OMe | 0.51 | −6.6072 |
| 15 | H | m,p-di-OMe | 0.35 | −6.7184 |
| 16 | H | m,m-di-OMe | 0.11 | −6.8873 |
The reaction was performed by irradiating a 200-μL mixture of 100 μM tetrazole and 10 mM 4-penten-1-ol in a quartz test tube with a handheld UV lamp at 302 nm.
HOMO energies of the nitrile imines.
Figure 1.
Cycloaddition rates are dependent on the nitrile imine HOMO energies. The HOMO energies of the nitrile imines were calculated using Hartree-Fock 3-21G model based on the AM1 optimized geometries (see Supporting Information for details).
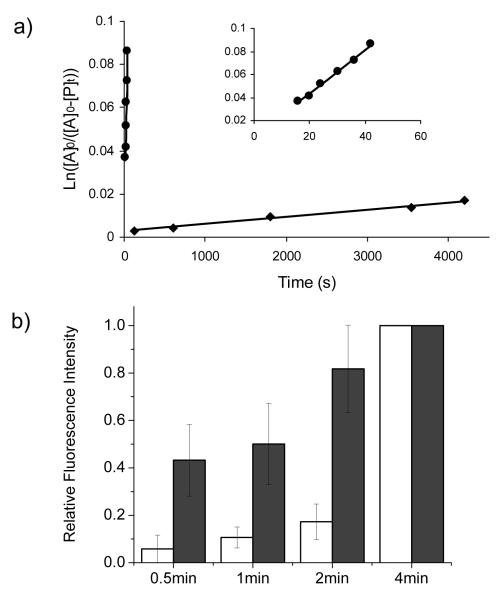
To determine whether the HOMO-lifted tetrazoles provide fast labeling of the O-allyltyrosine-encoded Z-domain protein (alkene-Z), we first compared the reaction kinetics of tetrazole 10 to that of tetrazole 1 toward allyl phenyl ether (a small-molecule substrate in place of O-allyltyrosine) in PBS buffer.[11] Tetrazole 10 exhibited robust kinetics with k2 value of 0.95 M−1s−1 (Figure 2a), 475-fold faster than tetrazole 1 (k2= 0.00202 M−1s−1).[5b] We then incubated tetrazole 10 with alkene-Z and followed the reaction progress over a 4-min period by measuring the relative fluorescence intensities of the corresponding pyrazoline-Z cycloadducts (Figure 2b). After 30 sec of photoirradiation, more than 40% cycloadduct was observed for tetrazole 10 while only 5% cycloadduct was observed for tetrazole 1 under identical condition, indicating significantly faster labeling kinetics of alkene-Z protein by tetrazole 10 in vitro.
Figure 2.
(a) Kinetic study of the cycloaddition reactions between allyl phenyl ether and tetrazole 1 (◆) and 10 (●), respectively. A close-up view of the tetrazole 10 kinetics was rendered in the insert; (b) Kinetic study of the alkene-Z labeling by either tetrazole 1 (open bar) or 10 (filled bar). The fluorescent intensities at 4 min were treated as 100% for each reaction and used in deriving the relative intensities. The concentrations of alkene-Z and tetrazoles were 10 μM and 200 μM, respectively. Error bars represent the standard deviations from three independent measurements.
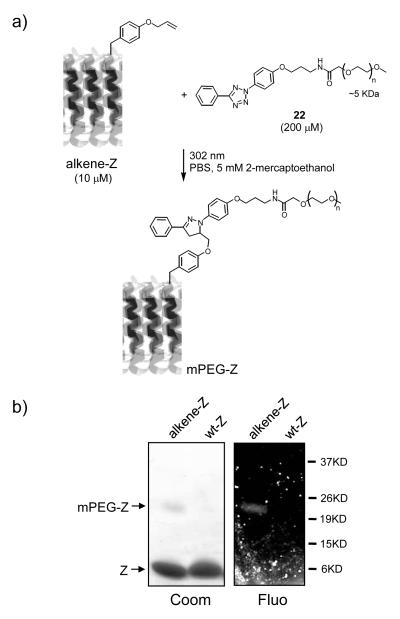
To assess the yield and selectivity of the alkene-Z labeling reaction by tetrazoles, alkene-Z and wt-Z were incubated separately with a monodisperse poly(ethylene glycol) (mPEG, MW ~5 KDa)-linked tetrazole 22[9] for 2 hr after 2-min photoirradiation (Figure 3a). A distinct higher MW band was observed prominently with alkene-Z (~20% estimated yield as measured by densitometry) over wt-Z (left panel, Figure 3b),[12] which coincides with a fluorescent band detected by in-gel fluorescence imaging of the same gel (right panel, Figure 3b), indicating both the formation of the fluorescent pyrazoline linkage in the PEGylated alkene-Z adduct (mPEG-Z)[5a] and high selectivity of the labeling reaction. Furthermore, MALDI-TOF mass spectrometry analysis of the alkene-Z reaction mixture confirmed the presence of mPEG-Z adducts with the mass envelop centered at 13,200 Da (expected ~ 13 KDa) (Figure 3c). Additionally, the labeling of alkene-Z in the bacterial cell lysates by tetrazole 10 was also found to proceed selectively, with efficiency comparable to that of the purified alkene-Z proteins.[9]
Figure 3.

Selective PEGylation of alkene-Z by mPEG-tetrazole 22: (a) reaction scheme; (b) Coomassie blue staining (left panel) and in-gel fluorescence imaging (right panel, λex = 365 nm) of alkene-Z upon photoirradiation for 2 min and additional incubation for 2 hr in the presence of mPEG-tetrazole 22. The experiment was repeated twice and similar results were obtained; (c) MALDI-TOF mass spectrum of the PEGylation mixture of the alkene-Z labeling by mPEG-tetrazole 22. A close-up view of the mass envelop representing mPEG-Z was rendered in the insert.
To examine whether fast reaction kinetics in vitro translates into faster protein labeling in vivo, we incubated BL21 (DE3) cells expressing either alkene-Z or wt-Z protein with 100 μM tetrazole 10 for 30 min at room temperature. The cells were then irradiated with a handheld UV lamp for 30 sec before immediate image acquisition via fluorescence microscopy. Since tetrazole 10 derived pyrazoline showed maximum absorption at 360 nm and a broad emission band at 450~600 nm,[9] we chose the DAPI filter set (ex 365 nm, em 445 ± 25 nm) to monitor the cycloaddition reaction in vivo. In the DAPI channel, only the alkene-Z-expressing E. coli cells showed strong fluorescence while cells expressing wt-Z did not (compare Figure 4a to 4b), indicating that the tetrazole 10 mediated cycloaddition was indeed very fast and selective toward the alkene group. By comparison, the labeling reaction employing tetrazole 1 under identical conditions did not produce fluorescent cells.[9] There was no apparent cytotoxicity associated with the tetrazole 10 treatment based on the DIC images (Figure 4c, 4d).[13]
Figure 4.
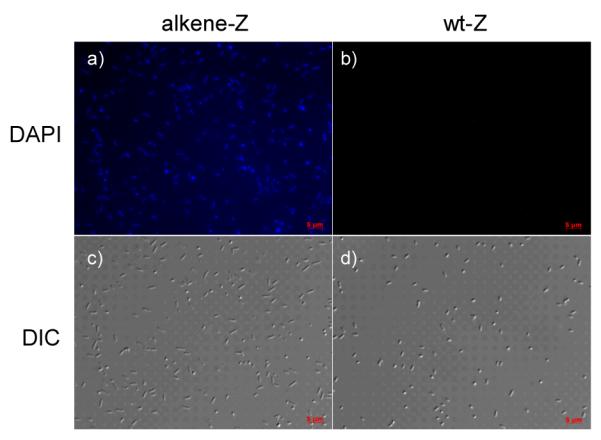
Fast, selective labeling of alkene-Z by tetrazole 10 in E. coli cells: DAPI channel (top row) and DIC channel (bottom row) images of bacterial cells expressing either alkene-Z (a, c) or wt-Z (b, d) after 30-sec photoirradiation in the presence of 100 μM tetrazole 10; scale bar = 5 μm.
In conclusion, we have demonstrated a fast photoclick chemistry by systematically tuning the HOMO energies of the nitrile imines. One of the optimized tetrazoles, compound 10, was found to label an alkene-encoded protein in less than 1 min inside E. coli cells. This dipole HOMO-lifting strategy is complementary to the existing dipolarophile LUMO-lowering strategy that when used together may provide robust reaction kinetics critical for the study of biomolecular dynamics in living systems.
Experimental Section
Live E. coli Cell Fluorescence Microscopy: E. coli cells expressing either alkene-Z or wt-Z domain proteins were sedimented by centrifugation for 5 min (6500 × g) at 4 °C. The cell pellets were washed twice with PBS, and the cells were then re-suspended in PBS buffer containing 5% glycerol and 100 μM tetrazole 10 (or control tetrazole 1). After incubation at 37°C for 30 min, 5-20 μL of the cell suspension was spotted on top of a glass slide. A glass cover slip was then placed on top of the mixture to sandwich the cells. The samples were irradiated with 302-nm UV light for 30 sec, and then immediately placed in the microscope sample chamber for image acquisition. All image acquisitions were performed under the identical conditions.
Supplementary Material
Footnotes
Q.L. is grateful to the National Institutes of Health (GM 085092) and New York State Center of Excellence in Bioinformatics and Life Sciences for financial support.
+ Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- [1] a).Prescher JA, Bertozzi CR. Nat. Chem. Biol. 2005;1:13. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]; b) Barglow KT, Cravatt BF, B. F. Nat. Methods. 2007;4:822. doi: 10.1038/nmeth1092. [DOI] [PubMed] [Google Scholar]
- [2].Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [3] a).Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2004;126:15046. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]; b) Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc. Natl. Acad. Sci. USA. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. J. Am. Chem. Soc. 2008;130:11486. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ning XH, Guo J, Wolfert MA, Boons G—J. Angew. Chem. Int. Ed. 2008;47:2253. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4] a).Saxon E, Bertozzi CR. Science. 2000;287:2007. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]; b) Hangauer MJ, Bertozzi CR. Angew. Chem. Int. Ed. 2008;47:2394. doi: 10.1002/anie.200704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5] a).Song W, Wang Y, Qu J, Madden MM, Lin Q. Angew. Chem. Int. Ed. 2008;47:2832. doi: 10.1002/anie.200705805. [DOI] [PubMed] [Google Scholar]; b) Song W, Wang Y, Qu J, Lin Q. J. Am. Chem. Soc. 2008;130:9654. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]
- [6].Blackman ML, Royzen M, Fox JM. J. Am. Chem. Soc. 2008;130:13518. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, O’ Rourke K, Koeppen H, Dixit VM. Nature. 2004;429:86. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- [8].Houk KN, Sims J, Watts CR, Luskus LJ. J. Am. Chem. Soc. 1973;95:7301. [Google Scholar]
- [9].See the Supporting Information for details.
- [10].Wang Y, Vera CI, Lin Q. Org. Lett. 2007;9:4155. doi: 10.1021/ol7017328. [DOI] [PubMed] [Google Scholar]
- [11].We did not choose tetrazole 11 for the subsequent studies because the corresponding pyrazoline was found to be unstable during the silica gel flash chromatographic purification.
- [12].The greater than 5 KDa increase in apparent MW is typically observed during electrophoresis of the PEGylation adducts, see:Deiters A, Cropp TA, Summerer D, Mukherji M, Schultz PG. Bioorg. Med. Chem. Lett. 2004;14:5743. doi: 10.1016/j.bmcl.2004.09.059. and references therein.
- [13].Subsequent culture of the treated bacterial cells, however, revealed a reduced viability (~12% based on the colony formation assay; see Supporting Information), which can be attributed exclusively to UV damage and potentially preventable by including antioxidants in the culture medium (see:Yip C—W. J. Biol. Ed. 2007;42:40.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.