Abstract
Despite the use of distal embolic protection devices (DEPs) in carotid artery (CA) stenting, an appreciable risk of stroke exists, particularly in symptomatic patients. The mechanism of embolic events is possibly related to microembolization of atherothrombotic débris that remains or forms on the stent struts. This study evaluated the safety of using thrombus-extraction catheters in the setting of CA stenting.
From August 2006 through June 2008, 43 symptomatic and asymptomatic patients with severe CA stenosis (>90%) underwent CA stenting with DEPs. After stenting and before removal of the DEP, an extraction catheter was passed through the stented segment. The extracted volume and the filtered extracted volume were visually examined for débris. The primary outcome was a composite of stroke and death at 30 days. Outcomes were compared with those in a control population of 783 patients who underwent CA stenting with a DEP, but without prophylactic thrombus aspiration. Retrospective analysis was performed on prospectively gathered data.
Substantial amounts of atherothrombotic débris were extracted from the stented segment in all 43 thrombectomy patients, none of whom died or experienced periprocedural stroke. In the control group, 3.9% of patients experienced these outcomes. Differences in primary outcome did not reach statistical significance.
We conclude that the prophylactic use of extraction catheters is safe and does not incur periprocedural events. The results of this preliminary study are encouraging, although larger, randomized trials (optimally using diffusion-weighted magnetic resonance imaging) are needed in order to evaluate this technique's potential benefits in reducing neurologic complications.
Key words: Angioplasty, balloon/instrumentation/methods; atherosclerosis/therapy; balloon dilatation/instrumentation/methods; carotid arteries/surgery; carotid stenosis/complications/therapy; catheterization; equipment design; stents; stroke/prevention & control; survival rate; treatment outcome
Advances in the endovascular treatment of carotid arterial (CA) occlusive disease have led to a reduction in treatment-related adverse events. Distal embolic protection devices (DEPs), lower-profile sheaths and stent-delivery systems, and more aggressive periprocedural antiplatelet therapy have all contributed to this improvement.1,2
Despite the improved procedural safety afforded by these technical advances, the risk of periprocedural stroke consequent to CA stenting is not negligible.3 This risk is particularly high in symptomatic patients, in octogenarians, and in subsets of patients with certain lesions, such as those with critical stenosis and ulcerated lesions.3,4 Observational data from a prospective multicenter registry that was compiled in order to assess outcomes of CA stenting revealed that 4.8% of the patients who underwent neuroprotected carotid stenting experienced a stroke.5 In addition to symptomatic strokes, subclinical microinfarctions after CA stenting range from 22% to as high as 41.5%.6–8 During intermediate-term follow-up, these microinfarctions have been associated with cognitive decline.9 Observational data also suggest that most neurologic events (77%) occur after the procedure has been completed.5 The mechanisms that underlie late occurrence of neurologic events remain undefined; however, they may be secondary to embolization of atherothrombotic débris after a DEP has been removed. Consequently, we surmised that the use of an aspiration catheter might reduce distal embolization of atherothrombotic material after stenting, via the aspiration of friable atherothrombotic débris from the stent struts while the DEP is still deployed.
We sought to investigate the safety of this novel technique by using the PRONTO® Extraction catheter (Vascular Solutions, Inc.; Minneapolis, Minn) or the QuickCat™ Extraction Catheter (Kensey Nash Corporation; Exton, Pa) as an adjunct to filter-based DEPs in the setting of CA stenting.
Patients and Methods
Study and Control Groups
For this study, we selected 43 consecutive symptomatic and asymptomatic patients with severe CA stenosis (>90%, documented angiographically) who underwent CA stenting with use of a DEP at our institution from August 2006 through June 2008. The mean age of the study group was 72 ± 10 years, with 9 octogenarians (21%). Thirty-five of the patients (81%) had chronic obstructive pulmonary disease, and 5 (12%) had symptomatic coronary artery disease. Twenty-four (56%) were symptomatic before the intervention, and 42 (98%) displayed angiographic evidence of target-lesion ulceration.
The baseline characteristics of these 43 patients were compared with those of a control population of 738 patients at our institution who had undergone CA stenting with use of a DEP during the same period of time, but without prophylactic mechanical thrombectomy. The Institutional Review Board at our institute approved the study protocol. We applied the χ2 test with use of STAT 10.8 statistical software (SAS Institute, Inc.; Cary, NC). A P value of less than 0.05 was considered statistically significant. When compared with the control group, the patients in the study group had higher rates of renal insufficiency (53% vs 30%; P = 0.001) and target-lesion ulceration (98% vs 80%, P = 0.005). Other baseline characteristics were similar in both groups (Table I). Retrospective analysis was performed on prospectively collected data.
TABLE I. Baseline Characteristics of the Patients
Aspiration Thrombectomy Preparation and Procedure
Each patient in the study group underwent independent neurologic evaluation before CA stenting was performed. All were given 300 mg of clopidogrel as a loading dose, and 75 mg daily thereafter for at least 3 days before CA stenting. They also received 325 mg of aspirin once daily for at least 3 days before stenting.
For the aspiration thrombectomy, we selected a PRONTO® V3 Extraction catheter or a QuickCat™ Extraction Catheter. Both devices are monorail catheters with a distal aspiration port that is connected at its proximal end to an aspiration syringe, which can be locked at the time of aspiration to create a negative pressure state. Aspiration is controlled by a 3-way stopcock that is opened to the syringe in order to trigger suction. The aspiration port of the PRONTO device faces the side of the catheter, whereas the QuickCat is an end-hole catheter.
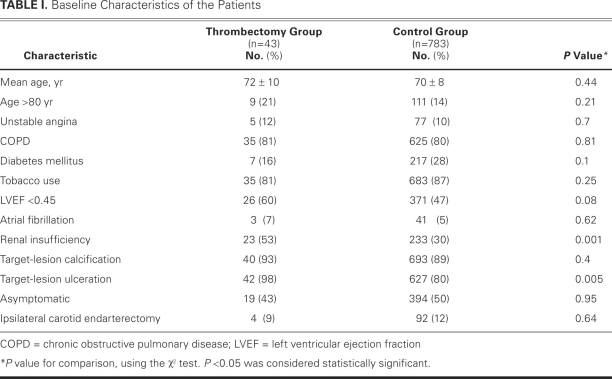
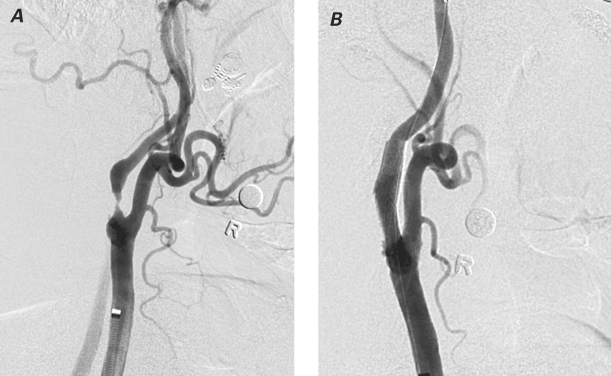
A 6F, 90-cm-long Shuttle® Select™ guiding sheath (Cook Medical Incorporated; Bloomington, Ind) was advanced into the thoracic aorta via a transfemoral approach. This sheath was then passed into the common CA, usually over a Slip-Cath® selective catheter (Cook) and a super-stiff Glidewire® (Terumo Medical Corporation; Somerset, NJ). Angiography was performed in 2 projections, and quantitative angiographic measurements were obtained (Fig. 1A). An appropriately sized filter-based DEP was then advanced across the stenosis and deployed distal to it. Predilation of the stenosis was performed with a 4-mm-diameter × 20-mm-long balloon. An appropriately sized stent was then deployed in the internal CA, covering the entire length of the stenosis. Postdilation was performed with use of a balloon of appropriate size. After the completion of CA stenting and before the removal of the DEP, a PRONTO (n=41) or QuickCat (n=2) extraction catheter was slowly advanced through the stent at approximately 1 mm/sec. Aspiration was performed from the proximal to the distal part of the stent. At least 2 passes were made with the thrombectomy catheter. If resistance was met during gentle advancement of the catheter, it was withdrawn a few mm, torqued slightly in either direction, and readvanced. Postprocedural angiography was performed, and the DEP was then retrieved (Fig. 1B). Retrieval of the DEP was performed after the thrombectomy in order to reduce the risk of catheter-mediated embolic events. After aspiration, the extracted blood volume was emptied into a 50-μ filter basket, and the extracted volume and the filter volume were visually examined for débris (Fig. 2).
Fig. 1 A) Angiography of the right carotid artery shows a >90% narrowing just after the bifurcation in the internal carotid artery of a 73-year-old man. B) After stenting and aspiration thrombectomy, angiography shows minimal residual stenosis.
Fig. 2 Extracted blood, after being emptied into a 50-μ filter basket, shows visual evidence of débris.
Analysis of Outcome
All patients were admitted for observation; they were seen by a neurologist immediately after the intervention and daily while they were hospitalized. Transcranial Doppler ultrasonography was performed in 1 patient during the procedure. Each patient underwent 30-day re-evaluation by duplex ultrasonography, and a neurologic examination was performed by 1 neurologist.
The primary outcome was a composite of stroke and death at 30 days. All neurologic events were evaluated by an independent neurologist. Stroke was defined as a new neurologic deficit that lasted longer than 24 hours. The outcomes in the study group were then compared with the outcomes in the control population, via the χ2 test.
Results
Advancement of the extraction catheter through the stent was successful in all instances. Substantial atherothrombotic material was retrieved in all 43 patients regardless of their symptomatic status. No periprocedural strokes or deaths occurred in the thrombectomy group. In the control group, 31 of the patients (3.9%) experienced a periprocedural stroke (n=25) or died (n= 6). The difference between groups in the primary outcome, however, did not reach statistical significance (P = 0.18). In the patient in whom transcranial Doppler ultrasonography was performed, no high-intensity transient signals were noted during advancement of the thrombectomy catheter.
Discussion
Carotid artery stenting is preferred by many as therapy for carotid occlusive disease in patients who are at high perioperative risk.2 However, postoperative neurologic events remain a concern after CA stenting, despite the use of DEPs. Certain subsets of patients are at higher risk than are others. Furthermore, the frequency of subclinical embolic events appears to be an order of magnitude higher than that of clinical events, as has been noted by investigators who have used post-CA stenting diffusion-weighted magnetic resonance imaging (MRI).6–8 In studies of carotid endarterectomy and CA stenting, most of the embolic events that have been found upon postprocedural diffusion-weighted MRI have not caused gross neurologic deficits, and many of these lesions were not apparent upon follow-up imaging.5,6,10 However, the late consequences of microemboli that are seen on MRI may be more dangerous than has been previously noted, as was suggested in the Rotterdam scan study, wherein new lesions found on diffusion-weighted MRI were associated with a 2-fold increase in neurocognitive decline.9
There are several possible explanations for ischemic events that are observed in the setting of CA stenting. Strokes that occur during the procedure but before the deployment of a DEP are likely related to embolization of atherothrombotic débris from catheter manipulation in the aorta or to manipulation of the target lesion itself. Alternatively, embolic material could pass through or around the DEP, causing a stroke during the procedure after successful deployment of the DEP. However, in the CAPTURE registry,5 most patients (77%) experienced their symptoms after the procedure had already been completed. Of these events, 57% were observed postprocedurally and before the patients' hospital discharge. Of note, 20% of the events occurred after the patients had been discharged from the hospital.5 The mechanisms for these late events remain undefined; however, such events may be due to microembolization of atherothrombotic débris that remains or develops on the stent struts after CA stenting. Several studies have supported such an assertion. Data from patients who have undergone carotid endarterectomy clearly show that emboli detected upon transcranial Doppler during dissection and wound-closure are independent predictors of perioperative stroke.11 Angioscopy after coronary interventions has detected plaque and thrombus protruding through stent struts, and platelet aggregates on the struts.12 Platelet-rich thrombi appear on stent struts within the first 3 days after coronary stenting and peak in number during the following week.13 In regard to carotid stenting, intravascular ultrasonography has revealed that atherothrombotic material protrudes through the stent struts after stent deployment. Balloon angioplasty at the time of postdilation of the stent may cause a cheese-grater effect on the plaque, with further plaque extrusion through the stent struts.14 In addition, platelet aggregates form on the stent struts secondary to intimal injury and as a reaction to the stent. Therefore, prophylactic removal of atherothrombotic material from the stent struts before removal of the DEP may lead to fewer late embolic events and, accordingly, reduce the risk of postprocedural neurologic events.
Limitations of the Study
Our study had several limitations. The patients were not randomized, and the results are inherently subject to the usual confounding factors. The number of patients in the study group was small, especially in light of low overall clinical-event rates that led to a statistically nonsignificant result. Aspiration thrombectomy was performed from the proximal end to the distal end within the stented segment; however, it is not certain whether the extracted débris was protruding from the struts within the stented segment or from the DEP itself. Our control group included a considerably greater number of patients than did the study group. Some differences between the study group and the control group might have affected the results: for example, the study group had a higher incidence of renal insufficiency and target-lesion ulceration. The number of octogenarians was also slightly higher in the study group. Finally, we did not perform pre- and post-CA stenting diffusion-weighted MRI, which would have enabled us to quantify and compare distal microembolization with the use of both techniques.
Conclusion
Our preliminary study highlights several important points. Prophylactic use of the PRONTO or QuickCat catheter to extract atherothrombotic débris is technically feasible, and aspiration can be accomplished easily after CA stenting and before removal of the DEP. No adverse clinical events were noted in our thrombectomy patients, despite their higher risk profile in comparison with the control group. Furthermore, the absence of high-intensity transient signals during advancement of the thrombectomy catheter suggests that the catheter did not generate further emboli. Although the differences in primary outcome did not reach statistical significance, there was a trend toward a lower occurrence of periprocedural events in the thrombectomy group than in the control population. On the basis of our preliminary data, routine prophylactic aspiration thrombectomy appears to be a safe procedure after standard CA stenting with a DEP. However, it is unclear whether this technique actually reduces distal microembolization and postoperative neurologic complications. A larger-scale, randomized trial with diffusion-weighted MRI (before and after CA stenting) is needed in order to evaluate the potential benefit of catheter-aided aspiration thrombectomy in reducing ischemic neurologic complications after CA stenting.
Footnotes
Address for reprints: Zvonimir Krajcer, MD, 6624 Fannin St., Suite 2780, Houston, TX 77030 E-mail: ZvonkoMD@aol.com
References
- 1.Al-Mubarak N, Roubin GS, Vitek JJ, Iyer SS, New G, Leon MB. Effect of the distal-balloon protection system on microembolization during carotid stenting. Circulation 2001;104 (17):1999–2002. [DOI] [PubMed]
- 2.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351(15): 1493–501. [DOI] [PubMed]
- 3.Roubin GS, Iyer S, Halkin A, Vitek J, Brennan C. Realizing the potential of carotid artery stenting: proposed paradigms for patient selection and procedural technique. Circulation 2006;113(16):2021–30. [DOI] [PubMed]
- 4.Zahn R, Ischinger T, Hochadel M, Zeymer U, Schmalz W, Treese N, et al. Carotid artery stenting in octogenarians: results from the ALKK Carotid Artery Stent (CAS) Registry. Eur Heart J 2007;28(3):370–5. [DOI] [PubMed]
- 5.Fairman R, Gray WA, Scicli AP, Wilburn O, Verta P, Atkinson R, et al. The CAPTURE registry: analysis of strokes resulting from carotid artery stenting in the post approval setting: timing, location, severity, and type. Ann Surg 2007; 246(4):551–8. [DOI] [PubMed]
- 6.Pinero P, Gonzalez A, Mayol A, Martinez E, Gonzalez-Marcos JR, Boza F, et al. Silent ischemia after neuroprotected percutaneous carotid stenting: a diffusion-weighted MRI study. AJNR Am J Neuroradiol 2006;27(6):1338–45. [PMC free article] [PubMed]
- 7.Schluter M, Tubler T, Steffens JC, Mathey DG, Schofer J. Focal ischemia of the brain after neuroprotected carotid artery stenting. J Am Coll Cardiol 2003;42(6):1007–13. [DOI] [PubMed]
- 8.Maleux G, Demaerel P, Verbeken E, Daenens K, Heye S, Van Sonhoven F, et al. Cerebral ischemia after filter-protected carotid artery stenting is common and cannot be predicted by the presence of substantial amount of débris captured by the filter device. AJNR Am J Neuroradiol 2006;27(9):1830–3. [PMC free article] [PubMed]
- 9.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348(13):1215–22. [DOI] [PubMed]
- 10.El-Koussy M, Schroth G, Do DD, Gralla J, Nedeltchev K, von Bredow F, et al. Periprocedural embolic events related to carotid artery stenting detected by diffusion-weighted MRI: comparison between proximal and distal embolus protection devices. J Endovasc Ther 2007;14(3):293–303. [DOI] [PubMed]
- 11.Ackerstaff RG, Moons KG, van de Vlasakker CJ, Moll FL, Vermeulen FE, Algra A, Spencer MP. Association of intraoperative transcranial doppler monitoring variables with stroke from carotid endarterectomy. Stroke 2000;31(8):1817–23. [DOI] [PubMed]
- 12.Senneff MJ, Schatz RA, Teirstein PS. The clinical utility of angioscopy during intracoronary stent implantation. J Interv Cardiol 1994;7(2):181–6. [DOI] [PubMed]
- 13.Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, Virmani R. Pathology of acute and chronic coronary stenting in humans. Circulation 1999;99(1):44–52. [DOI] [PubMed]
- 14.Wehman JC, Holmes DR Jr, Ecker RD, Sauvageau E, Fahrbach J, Hanel RA, Hopkins LN. Intravascular ultrasound identification of intraluminal embolic plaque material during carotid angioplasty with stenting. Catheter Cardiovasc Interv 2006;68(6):853–7. [DOI] [PubMed]