Abstract
We set out to evaluate the accuracy of 64-slice multidetector row computed tomography (MDCT) in the evaluation of graft patency in 25 unselected patients who underwent off-pump revascularization with arterial conduits. A total of 73 coronary artery bypass grafts were examined by means of 64-slice MDCT. Postoperative clinical outcomes were also evaluated as indicators of early coronary bypass malfunction. Serial data from cardiac-specific biomarkers and from hemodynamic results were obtained in all patients. Two radiologists analyzed the MDCT images and reached consensus.
No patients had evidence of postoperative acute myocardial infarction. Transient postoperative reduction of 5% to 8% in left ventricular ejection fraction was detected in 4 patients, independently of elevated biomarker serum levels. A total of 73 conduits were available for the analysis. Two grafts were judged not evaluable because of poor visualization due to irregular heartbeat. The image quality was excellent in all other scanned grafts and anastomoses.
We conclude that 64-slice MDCT technology enables accurate and reliable visualization of coronary bypass grafts with arterial conduits. We believe that it can be performed in large populations of postoperative bypass patients as a realistic alternative to coronary angiography.
Key words: Coronary angiography; graft occlusion, vascular/radiography; myocardial revascularization; tomography, spiral computed; vascular patency
Coronary artery bypass grafting (CABG) performed with cardiopulmonary bypass (CPB) and cardiac arrest provides good early and late graft patency. However, CPB is believed to be a major cause of postoperative morbidity, and the resurgence of interest in surgery without CPB (“off-pump”) reflects an attempt to avoid that additional morbidity.1–3 The development of modern stabilizers and intracoronary shunts has made off-pump coronary surgery accessible and technically feasible. There is evidence that off-pump surgery, in comparison with on-pump surgery, may decrease the incidence of postoperative complications.4–6 Although the extensive use of arterial conduits in off-pump revascularization has provided anecdotal evidence of better long-term graft patency and decreased need for reintervention, there is not sufficient evidence from randomized studies to support that contention.7,8
The postoperative assessment of bypass conduits and anastomosis after CABG is important in order to evaluate surgical results. Usually, CABG patency can be evaluated only indirectly, by intraoperative flow measurement. Until recently, selective bypass graft angiography has been considered the gold standard for the evaluation of early and late bypass patency, but it is invasive and carries risks of such serious complications as myocardial infarctions, embolic events, graft dissections, arrhythmias, and strokes.9–11 Therefore, in the last 20 years, several trials have investigated the potential of computed tomography (CT) and magnetic resonance imaging as noninvasive alternatives for the visualization of CABG patency.12 Although both methods have been limited by sensitivity to motion artifacts, recent advances in scanner performance—faster tube rotation and higher numbers of detector rows—have enabled thinner slices and better spatial resolution.13–16 The 64-slice multidetector CT (MDCT) has indeed shown improved sensitivity and specificity for assessing graft patency.14,17–20 The American Heart Association Committee, in a recent statement, has definitively validated the sensitivity (93%) and specificity (96%) of 64-slice MDCT for the detection of bypass stenoses or occlusions.21
The aim of our prospective study was to investigate graft quality and patency in an unselected group of CABG patients who underwent noninvasive evaluation of their new arterial conduits by means of 64-slice MDCT.
Patients and Methods
Our policy is to perform only off-pump CABG (OP-CAB) in all coronary patients. Intraoperative conversion to CPB support occurs only when the instability of the patient calls for it, and that did not happen during the course of this study. We used only arterial conduits for revascularization of the left coronary arteries. Saphenous veins were used only for right coronary artery (RCA) revascularization: either as aortocoronary grafts or as grafts to extensions of the right internal mammary artery (IMA) pedicle when we needed a composite graft from the left IMA.
Our study was conducted at a single cardiac surgery center. We evaluated 25 unselected patients who underwent surgery for coronary disease between January and February 2008. Our exclusion criteria were emergency surgery, associated cardiac procedures, atrial fibrillation, allergy to contrast agents, previous percutaneous coronary stenting in native vessels, and all contraindications for β-blocker drugs. Patients' characteristics are listed in Table I. All patients were evaluated by preoperative coronary angiography: the indication for surgery was ≥70% stenosis. The total number of coronary lesions was the number of ≥70% stenoses in all coronary arteries. Coronary stenoses were evaluated by means of a computerized cardiovascular angiography analysis system (Centricity® Carddas Xi, GE Healthcare; Dornstadt, Germany). Coronary runoff was evaluated by means of the Thrombolysis in Myocardial Infarction (TIMI) frame-counting method, with the aid of a frame-counter on the cineviewer. The corrected TIMI frame count (CTFC) for the long left anterior descending coronary artery (LAD) was derived by dividing by 1.7, as described by Gibson and colleagues.22 The mean preoperative TIMI frame count was 21.8 ± 4.5 (range, 16–29) for the RCA and the left circumflex coronary artery, and the CTFC was 22.8 ± 3.1 (range, 19.1–29.7) for the LAD. Hemodynamic variables were recorded in all patients through a Swan-Ganz CCOmbo pulmonary artery catheter used in combination with a Vigilance CEDV monitor (both Edwards Lifesciences LLC; Irvine, Calif). Standardized operative techniques were always used, and a shared operative strategy was established before surgery. All OPCABs were performed through a median sternotomy. Heparin was administered to obtain an activated clotting time of >300 sec. At the end of surgery, the heparin effects were reversed with protamine sulfate. The number of grafts per patient varied between 1 and 5 (mean, 2.99), and the index of completeness was 0.99. We tried to achieve complete coronary revascularization and, to that end, always preferred arterial conduits. Our usual choices of conduit were the skeletonized in situ left IMA and, when indicated, the right IMA. The radial artery (RA) was used only when constructing composite grafts from the left IMA or the right IMA. Saphenous vein was used only for RCA revascularization. In all patients, the LAD was revascularized first. Graft distribution is summarized in Table II. A suction-type Octopus® mechanical stabilizer (Medtronic Inc.; Minneapolis, Minn) was used to immobilize the target coronary artery, and a Starfish™ heart positioner (Medtronic) was used to elevate the heart for the circumflex and right coronary artery approaches. All distal anastomoses were performed with 8-0 polypropylene running sutures, and intracoronary shunts (Medtronic) were used routinely. Graft flow was in all cases measured by means of a Transonic® HT323-CS transient-time flow meter (Transonic Systems Inc.; Ithaca, NY) to confirm graft patency at the end of the procedure. No distal anastomosis had to be redone because of unsatisfactory flow as measured.
TABLE I. Baseline and Intraoperative Characteristics of the 25 Patients
TABLE II. Distribution of 73 Distal Anastomoses
A standardized protocol was followed in the intensive care unit. Ticlopidine was started 48 hours after surgery, at a dose of 250 mg/dL. Other drugs were administered when indicated.
A 12-lead electrocardiogram (ECG) was obtained before surgery, upon admission to the intensive care unit and again 12 hours later, and every day thereafter until discharge. Echocardiographic studies were obtained 24 and 48 hours postoperatively. A 5% or greater postoperative reduction of left ventricular ejection fraction (LVEF) was considered significant. Blood samples for assay of the creatine kinase-MB (CK-MB) isoenzyme, troponin I, and myoglobin were collected at baseline and at 8, 12, and 24 hours after surgery, and then on every postoperative day until each patient's hospital discharge.
Postoperative acute myocardial infarction (AMI) was diagnosed when the criteria, as indicated by the European Society of Cardiology/American College of Cardiology Guidelines Joint Committee, were fulfilled.23 Therefore, we never considered the sole laboratory evidence of an elevated biomarker to be indicative of postoperative AMI, without evidence of the other criteria.24
Postoperative renal failure was defined as a rise in serum creatinine >2.5 mg/dL. Low-output syndrome was diagnosed when the cardiac index decreased to below 2.0 L/(min·m2), when the pulmonary capillary wedge pressure exceeded 15 mmHg, when the left ventricular stroke work index (LVSWI) decreased to <22 g/(min·m2), and when mixed systemic venous oxygen saturation was <60% for at least 30 minutes after correction of all electrolyte or blood-gas abnormalities and after preload optimization. Patients in need of less than 5 μg/kg of dopamine or dobutamine were not considered to have low-output syndrome.
All CT scans were performed using an Aquilion 64-slice MDCT (Toshiba Medical Systems; Tokyo, Japan), during the period from 4 to 6 months after surgery (mean, 4.9 ± 0.2 mo). Patients with a heart rate greater than 65 beats/min before examination received 2 to 4 mg of intravenous propranolol 10 minutes before the scan. In some cases, mild sedation with midazolam was very effective for heart-rate control. Contrast-enhanced CT for coronary angiography was performed at our institution as follows: a bolus of 80 mL of iopamidol (Iopamiro® 400, Bracco S.p.A.; Milan, Italy) was injected into the antecubital vein at a flow rate of 5 mL/sec, followed by a 50-mL saline bolus-flush at a flow rate of 5 mL/sec. An automated bolus-tracking system was used to synchronize the arrival of the contrast material with the initiation of the scan. The CT scanning was performed at the following settings: retrospective ECG-gated acquisition, 120 kV, 500 mA; slice collimation, 64 × 0.5 mm; gantry rotation time, 330 ms; and table feed, 6.4 mm/gantry rotation with a beam pitch of 0.2. The window of the cardiac CT scan extended from the top of the left clavicle to the cardiac apex. The field of view was adjusted according to the size of the heart. A 3-D line Vitrea® workstation (Vital Images; Plymouth, Minn) was used to reconstruct axial images retrospectively at an optimal window: a slice thickness of 0.75 mm and an increment of 0.5 mm. The image data sets were analyzed by means of multiplanar reformatted images (vertical, long-axis, and short-axis views), curved multiplanar reformatted images, thin-slab maximum- intensity projection images, and volume-rendered images, in addition to axial.
The 3-dimensional images show the lumen of each vessel filled with contrast medium and might show wall calcifications and surgical clips. When this volume rendering is active, the volume can be rotated in all directions, thus enabling evaluation of the vessels in 3 dimensions. Two-dimensional reconstructions (curved multiplanar reformation) of the coronary arteries and grafts were performed on several planes to assess patency of the grafts and anastomoses. These 2-dimensional images show the vessel's wall and lumen and all the surrounding tissue. They are reconstructed on at least 2 orthogonal planes, and continuity of contrast material throughout the graft serves as an indication of patency.
Two experienced readers, blinded to the clinical results but aware of the operative procedure, evaluated the CT images by reaching a consensus. Grafts were first classified using a 3-grade scale: (1) occluded, (2) stenosis ≥50%, and (3) stenosis <50% or no stenosis. Graft occlusion was defined as absence of contrast material along the course of the graft, from the 1st anastomosis to the distal native artery. In composite grafts, each of the several distal anastomoses was counted as a separate graft. Significant stenosis of a patent graft anastomosis was defined as a reduction of luminal diameter by 50% or more. Patients' data were expressed as mean ± SD, and categorical variables were expressed as proportions.
The study protocol was approved by the ethics committee of our institution and by the hospital's Institutional Review Board. Informed consent was obtained from each patient before enrollment in the study.
Results
No patients had evidence of postoperative AMI. Postoperative increases in troponin I, myoglobin, and CK-MB values were observed, respectively, in 55%, 62%, and 40% of patients. The mean postoperative peak values of these markers were troponin I, 0.32 ± 0.26 ng/mL; myoglobin, 98.31 ± 31 ng/mL; and CK-MB, 9.3 ± 8.1 ng/mL. At early postoperative echocardiographic evaluation, transient LVEF reductions of 5% to 8% from preoperative measured values were detected in only 4 cases over the whole population, independent of laboratory evidence of an elevated biomarker serum level and independent of graft number. Transient postoperative renal failure occurred in 1 patient. The low-output syndrome evidence was not recorded.
All patients were asymptomatic at the time of CT scanning. The 64-slice MDCT angiograms were completed in all patients without complications. The mean heart rate during data acquisition was 55.8 ± 4 beats/min, and all patients were able to hold their breaths for the required time.
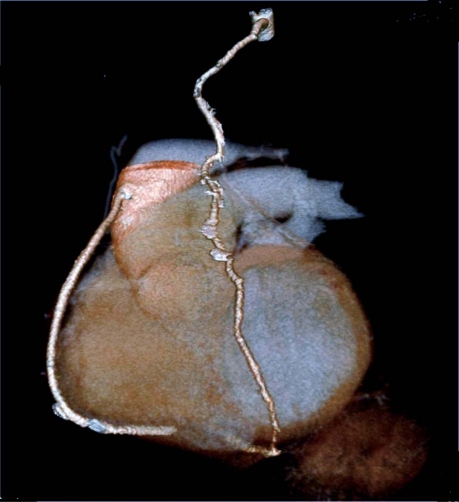
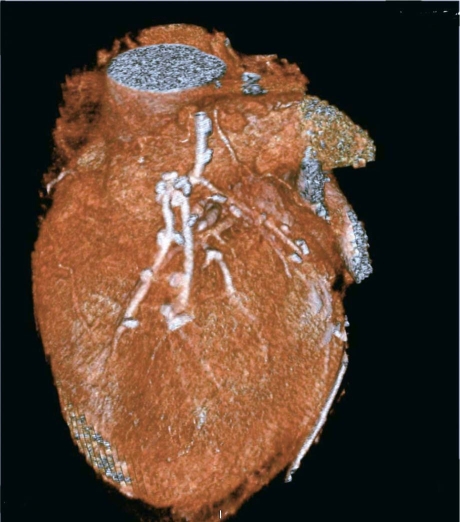
A total of 73 conduits were available for the analysis: 63 arterial grafts (including 33 in situ left and right IMAs, and 30 free right IMAs and RAs) and 10 saphenous vein grafts. There were 73 distal anastomoses and 10 proximal anastomoses of saphenous vein grafts (9 from the ascending aorta and 1 end-to-end from the right IMA. We performed 2 tangential (K-type) anastomoses between the left IMA and RA, and 26 end-to-side (Y or π type) anastomoses between the left IMA and free right IMA or RA (Table II). Among these, 2 grafts could not be visualized with diagnostic-image quality and were judged not fully evaluable (2.7%). The cause of poor visualization was the presence of relevant artifacts, due mostly to irregular heart rate during examination. Artifacts arising from metal clips never appeared in our series. Excellent graft visualization, including visualization of the anastomoses, was achieved in all the other scanned grafts. Seventy of the 71 grafts evaluated were patent on CT angiography, and the 1 remaining graft (an RA) was found to be occluded from its origin in the left IMA, with no contrast medium evident in its lumen. No significant stenoses were noted. Representative CT angiographic images are shown in Figures 1, 2, 3 and 4.
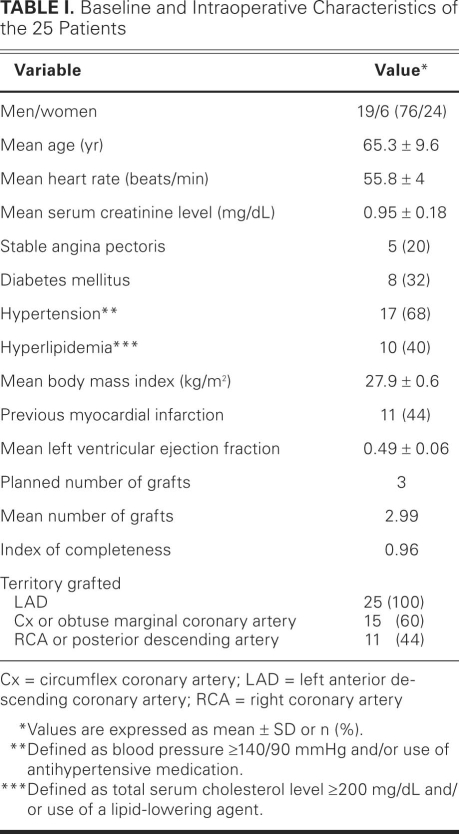
Fig. 1 Arterial graft in a 65-year-old man: a left internal mammary artery graft to the left anterior descending coronary artery.
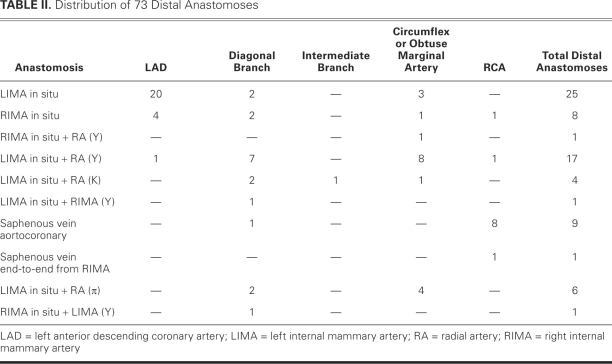
Fig. 2 Composite arterial graft in a 64-year-old man: a left internal mammary artery graft to the left anterior descending coronary artery, together with a radial artery graft that is attached proximally to the internal mammary artery in a Y-shaped anastomosis, in order to supply the obtuse marginal coronary artery.
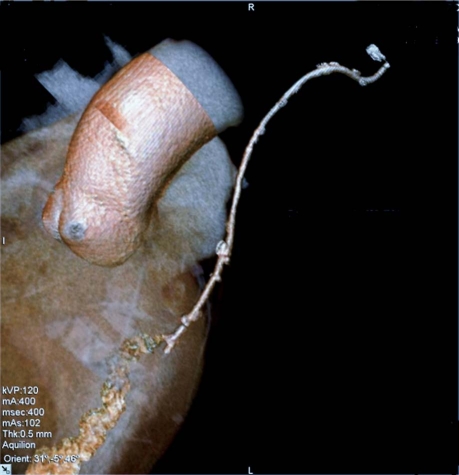
Fig. 3 Composite arterial-venous graft in a 69-year-old man: a left internal mammary artery graft to the left anterior descending coronary artery, together with a saphenous vein graft to the right coronary artery.
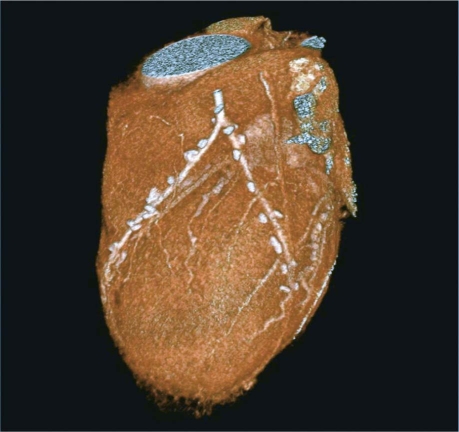
Fig. 4 Composite arterial graft in a 62-year-old man: a left internal mammary artery graft to the left anterior descending coronary artery, together with a radial artery graft that is attached proximally to the internal mammary artery in a K-shaped anastomosis, in order to supply the obtuse marginal coronary artery and the diagonal branch.
Discussion
Complete myocardial revascularization with arterial conduit is considered the most effective treatment of myocardial ischemia, and OPCAB surgery has become a widely used means of revascularization in recent years.1–3 However, limited data are available to compare the 1-year patency of bypass grafts implanted during beating-heart surgery with that of bypass grafts implanted during conventional “on-pump” surgery, and several randomized studies have provided conflicting evidence.7,8 Although OPCAB is technically more difficult than conventional CABG, the development of new devices has led to its wide practice. Therefore, the postoperative evaluation of conduits and anastomoses is important to determine the comparative quality of OPCAB.
For the evaluation of bypass conduits, conventional coronary angiography remains the gold standard; however, the limitations of angiography include a small but definable risk, the need for multiple staff members, and high costs associated with the procedure itself and with the ensuing observational period.9,10 Although useful when clinically indicated, invasive techniques are not appropriate for screening a large population of patients or for repeated measurements.21 Of the alternative noninvasive methods that have been investigated, only MDCT has provided satisfactory results. Previous studies20,25–28 of 16-channel MDCT have reported high-quality images and satisfactory accuracy in evaluating coronary artery stenosis and bypass graft patency, in comparison with coronary angiography. However, only the latest generation of 64-slice MDCT has provided a significant improvement of temporal and spatial resolution, yielding excellent diagnostic images and (with the aid of multisector reconstruction) a reduction of artifacts.17,18,21
The results of our study confirm the high sensitivity and specificity of 64-slice MDCT, as reported by the American Heart Association Committee,21 and demonstrate the potential of the recently developed 64-detector row CT scanner for the evaluation of arterial conduits after CABG. The percentage of usable bypass conduits has risen to nearly 98% (which is superior to results previously reported), and this is in combination with improved diagnostic accuracy for the detection of stenosis. The major advantages of the 64-detector row system include shorter breath-hold times, fewer respiratory and cardiac motion artifacts, faster rotation of the gantry, and a decrease in slice thickness, all of which result in better spatial resolution.
Our study examined the early results of off-pump revascularization that had been performed with arterial (mainly in situ arterial) and composite grafts, and we achieved MDCT images of satisfactory quality in all 25 patients. The high-resolution, volume-rendered, 3-dimensional scanning made the interpretation of our anatomic findings quite easy. A further advantage of MDCT over conventional coronary angiography (not demonstrated by our particular study) is its ability to show not only the lumen but the vascular wall—a property that can, in theory, enable the distinction between RA spasm and intimal hyperplasia.
For a long time, the interpretation of CT angiography has been impeded by artifacts associated with the metal hemoclips that are placed alongside bypass conduits; these artifacts are often exacerbated by motion.18 However, a number of recent studies18,29,30have reported a very low incidence of artifacts due to hemoclips, when current 64-slice MDCT is used. In the current study, the presence of clips rendered no bypass graft incapable of evaluation. This may be attributable to the high temporal and spatial resolution of 64-slice devices, as well as to our strict protocol for lowering the heart rate. Even so, 2 grafts were judged not evaluable because of poor visualization due to irregular heartbeat. In regard to the clinical aspects of our study, we can see a clear linkage between postoperative outcomes, biomarker serum levels, and the patency rate of all off-pump arterial grafts.
Conclusion
The most important findings of the present study are that 64-slice MDCT 1) enables the reliable and accurate evaluation of patency of arterial coronary bypass conduits, 2) achieves excellent image quality with fewer artifacts, and 3) is a realistic alternative to coronary angiography for postoperative early and late evaluations in a large population of patients. Finally, we are confident that our preliminary results will be confirmed in studies of larger populations of patients.
Footnotes
Address for reprints: Vito A. Mannacio, MD, Via S. Domenico 62, 80127 Naples, Italy E-mail: vitomannacio2@libero.it
References
- 1.Reston JT, Tregear SJ, Turkelson CM. Meta-analysis of short-term and mid-term outcomes following off-pump coronary artery bypass grafting. Ann Thorac Surg 2003;76(5):1510–5. [DOI] [PubMed]
- 2.Racz MJ, Hannan EL, Isom OW, Subramanian VA, Jones RH, Gold JP, et al. A comparison of short- and long-term outcomes after off-pump and on-pump coronary artery bypass graft surgery with sternotomy. J Am Coll Cardiol 2004;43(4):557–64. [DOI] [PubMed]
- 3.Hannan EL, Wu C, Smith CR, Higgins RS, Carlson RE, Culliford AT, et al. Off-pump versus on-pump coronary artery bypass graft surgery: differences in short-term outcomes and in long-term mortality and need for subsequent revascularization. Circulation 2007;116(10):1145–52. [DOI] [PubMed]
- 4.van Dijk D, Nierich AP, Jansen EW, Nathoe HM, Suyker WJ, Diephuis JC, et al. Early outcome after off-pump versus on-pump coronary bypass surgery: results from a randomized study. Circulation 2001;104(15):1761–6. [DOI] [PubMed]
- 5.Angelini GD, Taylor FC, Reeves BC, Ascione R. Early and midterm outcome after off-pump and on-pump surgery in Beating Heart Against Cardioplegic Arrest Studies (BHACAS 1 and 2): a pooled analysis of two randomised controlled trials. Lancet 2002;359(9313):1194–9. [DOI] [PubMed]
- 6.Puskas JD, Williams WH, Duke PG, Staples JR, Glas KE, Marshall JJ, et al. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: a prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003;125(4): 797–808. [DOI] [PubMed]
- 7.Khan NE, De Souza A, Mister R, Flather M, Clague J, Davies S, et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med 2004;350(1):21–8. [DOI] [PubMed]
- 8.Widimsky P, Straka Z, Stros P, Jirasek K, Dvorak J, Votava J, et al. One-year coronary bypass graft patency: a randomized comparison between off-pump and on-pump surgery angiographic results of the PRAGUE-4 trial. Circulation 2004;110 (22):3418–23. [DOI] [PubMed]
- 9.Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 1999;33(6):1756–824. [DOI] [PubMed]
- 10.Wyman RM, Safian RD, Portway V, Skillman JJ, McKay RG, Baim DS. Current complications of diagnostic and therapeutic cardiac catheterization. J Am Coll Cardiol 1988;12(6): 1400–6. [DOI] [PubMed]
- 11.Fine JJ, Hopkins CB, Ruff N, Newton FC. Comparison of accuracy of 64-slice cardiovascular computed tomography with coronary angiography in patients with suspected coronary artery disease. Am J Cardiol 2006;97(2):173–4. [DOI] [PubMed]
- 12.Budoff MJ, Achenbach S, Duerinckx A. Clinical utility of computed tomography and magnetic resonance techniques for noninvasive coronary angiography. J Am Coll Cardiol 2003; 42(11):1867–78. [DOI] [PubMed]
- 13.Yamamoto M, Kimura F, Niinami H, Suda Y, Ueno E, Takeuchi Y. Noninvasive assessment of off-pump coronary artery bypass surgery by 16-channel multidetector-row computed tomography. Ann Thorac Surg 2006;81(3):820–7. [DOI] [PubMed]
- 14.Martuscelli E, Romagnoli A, D'Eliseo A, Tomassini M, Razzini C, Sperandio M, et al. Evaluation of venous and arterial conduit patency by 16-slice spiral computed tomography. Circulation 2004;110(20):3234–8. [DOI] [PubMed]
- 15.Burgstahler C, Beck T, Kuettner A, Drosch T, Kopp AF, Heuschmid M, et al. Non-invasive evaluation of coronary artery bypass grafts using 16-row multi-slice computed tomography with 188 ms temporal resolution. Int J Cardiol 2006; 106(2):244–9. [DOI] [PubMed]
- 16.Nieman K, Pattynama PM, Rensing BJ, Van Geuns RJ, De Feyter PJ. Evaluation of patients after coronary artery bypass surgery: CT angiographic assessment of grafts and coronary arteries. Radiology 2003;229(3):749–56. [DOI] [PubMed]
- 17.Jabara R, Chronos N, Klein L, Eisenberg S, Allen R, Bradford S, Frohwein S. Comparison of multidetector 64-slice computed tomographic angiography to coronary angiography to assess the patency of coronary artery bypass grafts. Am J Cardiol 2007;99(11):1529–34. [DOI] [PubMed]
- 18.Ropers D, Pohle FK, Kuettner A, Pflederer T, Anders K, Daniel WG, et al. Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation 2006;114(22):2334–41. [DOI] [PubMed]
- 19.Schlosser T, Konorza T, Hunold P, Kuhl H, Schmermund A, Barkhausen J. Noninvasive visualization of coronary artery bypass grafts using 16-detector row computed tomography. J Am Coll Cardiol 2004;44(6):1224–9. [DOI] [PubMed]
- 20.Uva MS, Matias F, Mesquita A, Costa R, Bau J, Pedro A, Magalhaes MP. Sixteen-slice multidetector computed tomography for graft patency evaluation after coronary artery bypass surgery. J Card Surg 2008;23(1):17–22. [DOI] [PubMed]
- 21.Bluemke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, Hillis LD, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation 2008;118(5):586–606. [DOI] [PubMed]
- 22.Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 1996; 93(5):879–88. [DOI] [PubMed]
- 23.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction [published erratum appears in J Am Coll Cardiol 2001;37(3):973]. J Am Coll Cardiol 2000;36(3):959–69. [DOI] [PubMed]
- 24.Mannacio VA, Iorio D, De Amicis V, Di Lello F, Musumeci F. Effect of rosuvastatin pretreatment on myocardial damage after coronary surgery: a randomized trial. J Thorac Cardiovasc Surg 2008;136(6):1541–8. [DOI] [PubMed]
- 25.Gurevitch J, Gaspar T, Orlov B, Amar R, Dvir D, Peled N, Aravot DJ. Noninvasive evaluation of arterial grafts with newly released multidetector computed tomography. Ann Thorac Surg 2003;76(5):1523–7. [DOI] [PubMed]
- 26.Ropers D, Baum U, Pohle K, Anders K, Ulzheimer S, Ohnesorge B, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation 2003;107(5): 664–6. [DOI] [PubMed]
- 27.Dewey M, Lembcke A, Enzweiler C, Hamm B, Rogalla P. Isotropic half-millimeter angiography of coronary artery bypass grafts with 16-slice computed tomography. Ann Thorac Surg 2004;77(3):800–4. [DOI] [PubMed]
- 28.Dewey M, Laule M, Krug L, Schnapauff D, Rogalla P, Rutsch W, et al. Multisegment and halfscan reconstruction of 16-slice computed tomography for detection of coronary artery stenoses. Invest Radiol 2004;39(4):223–9. [DOI] [PubMed]
- 29.Meyer TS, Martinoff S, Hadamitzky M, Will A, Kastrati A, Schomig A, et al. Improved noninvasive assessment of coronary artery bypass grafts with 64-slice computed tomographic angiography in an unselected patient population. J Am Coll Cardiol 2007;49(9):946–50. [DOI] [PubMed]
- 30.Pache G, Saueressig U, Frydrychowicz A, Foell D, Ghanem N, Kotter E, et al. Initial experience with 64-slice cardiac CT: non-invasive visualization of coronary artery bypass grafts. Eur Heart J 2006;27(8):976–80. [DOI] [PubMed]