Primary cardiac tumors are rare: the autopsy incidence is 0.0001% to 0.0003%, or, in practical terms, about 1 in every 500 cardiac surgical cases. These primary cardiac tumors are 75% benign and 25% malignant, and, of the malignant tumors, 75% are sarcomas.1 Primary cardiac sarcomas often afflict young patients who have no predisposing factors, and these tend to have a dismal prognosis. In studies involving medical therapy alone, 90% of primary-cardiac-sarcoma patients have been dead within 9 to 12 months. Series of soft-tissue sarcomas outside of the heart have shown that complete surgical excision—or sequential resection of metastatic disease, when possible—yields the best long-term survival.
Surgical series of primary cardiac sarcoma have tended to be small, and individual surgeons (and even institutions) have not gained extensive experience with this condition. The Mayo Clinic2 reviewed its records covering a 32-year period and found 34 patients who had undergone surgical resection of primary cardiac sarcoma; the median survival time of 12 months was extended to 17 months in the event of R0 resection. Patients in this series who had an R1 resection had a median survival time of 6 months. A combined series from M.D. Anderson Cancer Center and the Texas Heart Institute3 found 21 patients over a 25-year period; those patients had an actuarial survival rate of 14% at 2 years. Those who underwent an R0 resection had a median survival time of 24 months, whereas those who underwent an R1 resection had a median survival time of 10 months, which again emphasizes the importance of complete resection in these patients.
This background information from the medical literature led our group to a systematic study of primary cardiac sarcoma, in an attempt to better define this difficult disease and to improve the poor prognosis associated with it. We classify primary cardiac sarcoma by location rather than by cell type, because we have found that cell type does not affect prognosis or treatment planning—but anatomic location does. We divide these tumors into right-heart sarcomas, left-heart sarcomas, and pulmonary artery sarcomas.4,5
Right-Heart Sarcomas
Right-heart sarcomas tend to be bulky and infiltrative, and to metastasize early. Their bulk often extends to the exterior of the heart, and it is uncommon for patients to develop right-heart failure from obstruction until very late in the course of the disease. Because these tumors usually do not present with heart failure or imminent threat of heart failure, we advise neoadjuvant chemotherapy. Neoadjuvant chemotherapy allows us to shrink these usually large tumors, to increase the possibility of achieving an R0 resection and better outcome. These tumors tend to have infiltrating “fingers” of microscopic disease extending from the periphery of the tumor, and it is hoped that neoadjuvant therapy will decrease these. We have operated on 22 patients with right-heart sarcoma: 13 men and 9 women, with a mean age of 48 years. Fifteen of these tumors were angiosarcomas. The operative mortality rate was 9% (2/22). We could achieve an R0 resection in only a third of our patients, which highlights the infiltrative nature of right-heart sarcoma. The median survival time was 27 months; the longest-surviving patient is alive at 9.5 years. We are currently completing analysis of this patient cohort for publication.
Left-Heart Sarcomas
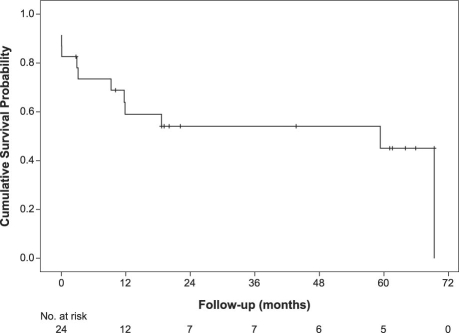
Left-heart sarcomas tend to be more solid and less infiltrative, and to metastasize later than right-heart sarcomas. Left atrial sarcomas tend to grow into the left atrial cavity, and they often present with heart failure. All left atrial sarcomas that we have seen have been resected incompletely at 1st operation under a presumed diagnosis of myxoma, and then referred to us only after aggressive recurrence. Because these tumors so often present with heart failure, neoadjuvant chemotherapy is often not an option. Complete surgical resection is the goal of therapy, but the anatomic location of left-heart sarcomas makes this a difficult surgical challenge. To enable optimal anatomic accessibility for complete resection and accurate cardiac reconstruction, our group has built upon the cardiac autotransplantation procedure introduced by Dr. Cooley for the treatment of complex left atrial tumor. We have done 24 cardiac autotransplantations for left-heart tumors.6,7 There were 18 cardiac autotransplantations alone, with no deaths, and 6 cardiac autotransplantations combined with pneumonectomies because of tumor extension. Three of these patients died, yielding a 50% mortality rate if pneumonectomy had to be combined with autotransplantation. The survival curve for left-heart sarcomas is shown in Figure 1.
Fig. 1 Survival curve for left-heart sarcomas.
Pulmonary Artery Sarcomas
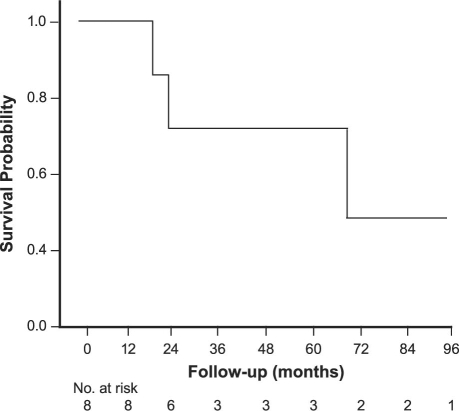
Pulmonary artery sarcoma tends to be angiosarcoma and to present with pulmonary artery obstruction and significant right-heart failure, which again precludes neoadjuvant therapy in most cases. Most arise close to the pulmonary valve, which itself is involved in about one third of cases. These tumors, interestingly, tend to grow distally along the pulmonary artery and do not grow through the artery wall itself; these characteristics aid complete resection. Aggressive complete resection is the key to survival. We have resected 8 pulmonary artery sarcomas: the median survival time has been 71 months, with a 5-year survival rate of 72.9%.8 The survival curves are shown in Figure 2.
Fig. 2 Survival curve for pulmonary artery sarcomas.
Conclusion
We believe that an aggressive surgical approach and the use of multiple methods have enabled us to improve the survival rates in this difficult disease. Due to the high probability of metastatic disease, we recommend chemotherapy after surgery in all patients, despite a current lack of supportive data.9 Future approaches in treating this deadly disease will include better biologic treatments with possible “maintenance” chemotherapy, as well as the possibility of complete cardiac excision and replacement with a mechanical device to enable radical resection and the use of cardiotoxic chemotherapy without penalty.
Footnotes
Address for reprints: Michael J. Reardon, MD, Cardiovascular Surgery Associates, 6550 Fannin St., Suite 1401, Houston, TX 77030 E-mail: mreardon@tmhs.org
Presented at the 16th International Symposium of the Denton A. Cooley Cardiovascular Surgical Society, Galveston, Texas, 4–7 June 2009.
References
- 1.Bakaeen FG, Reardon MJ, Coselli JS, Miller CC, Howell JF, Lawrie GM, et al. Surgical outcome in 85 patients with primary cardiac tumors. Am J Surg 2003;186(6):641–7. [DOI] [PubMed]
- 2.Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, Moynihan TJ. Malignant primary cardiac tumors: review of a single institution experience. Cancer 2008; 112(11): 2440–6. [DOI] [PubMed]
- 3.Putnam JB Jr, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DA. Primary cardiac sarcomas. Ann Thorac Surg 1991; 51(6):906–10. [DOI] [PubMed]
- 4.Reardon MJ, Walkes JC, Benjamin R. Therapy insight: malignant primary cardiac tumors. Nat Clin Pract Cardiovasc Med 2006;3(10):548–53. [DOI] [PubMed]
- 5.Blackmon SH, Patel A, Reardon MJ. Management of primary cardiac sarcomas. Expert Rev Cardiovasc Ther 2008;6(9): 1217–22. [DOI] [PubMed]
- 6.Reardon MJ, Malaisrie SC, Walkes JC, Vaporciyan AA, Rice DC, Smythe WR, et al. Cardiac autotransplantation for primary cardiac tumors. Ann Thorac Surg 2006;82(2):645–50. [DOI] [PubMed]
- 7.Blackmon SH, Patel AR, Bruckner BA, Beyer EA, Rice DC, Vaporciyan AA, et al. Cardiac autotransplantation for malignant or complex primary left-heart tumors. Tex Heart Inst J 2008;35(3):296–300. [PMC free article] [PubMed]
- 8.Blackmon SH, Rice DC, Correa AM, Mehran R, Putnam JB, Smythe WR, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg 2009;87(3):977–84. [DOI] [PubMed]
- 9.Bakaeen FG, Jaroszewski DE, Rice DC, Walsh GL, Vaporciyan AA, Swisher SS, et al. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg 2009;137(6):1454–60. [DOI] [PubMed]