Abstract
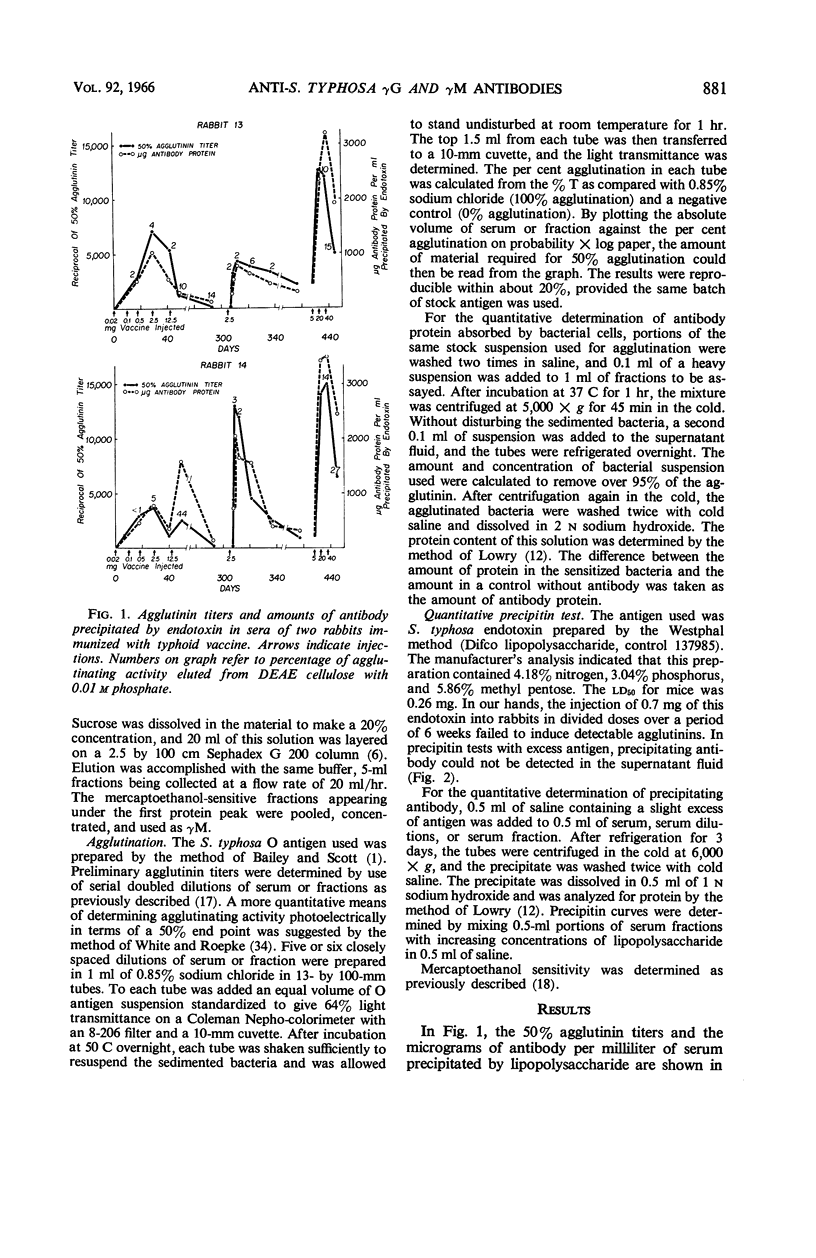
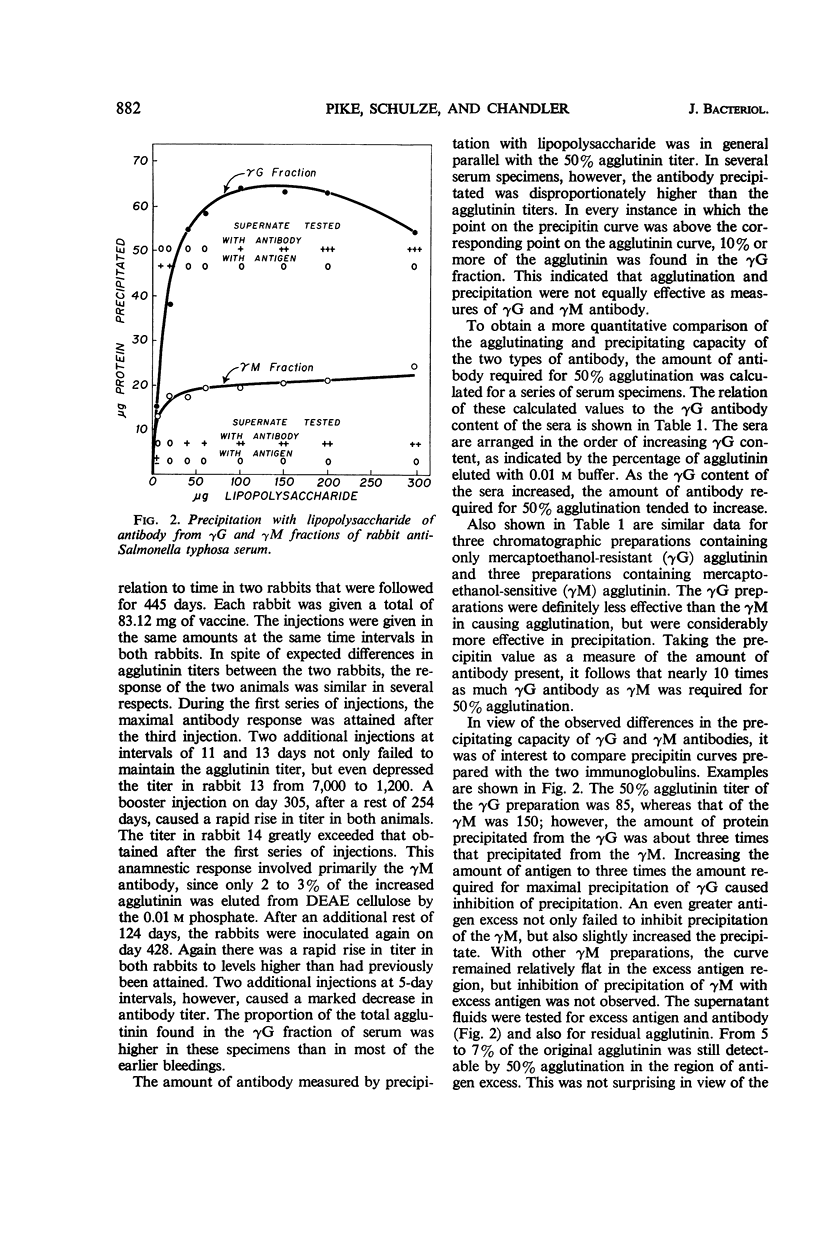
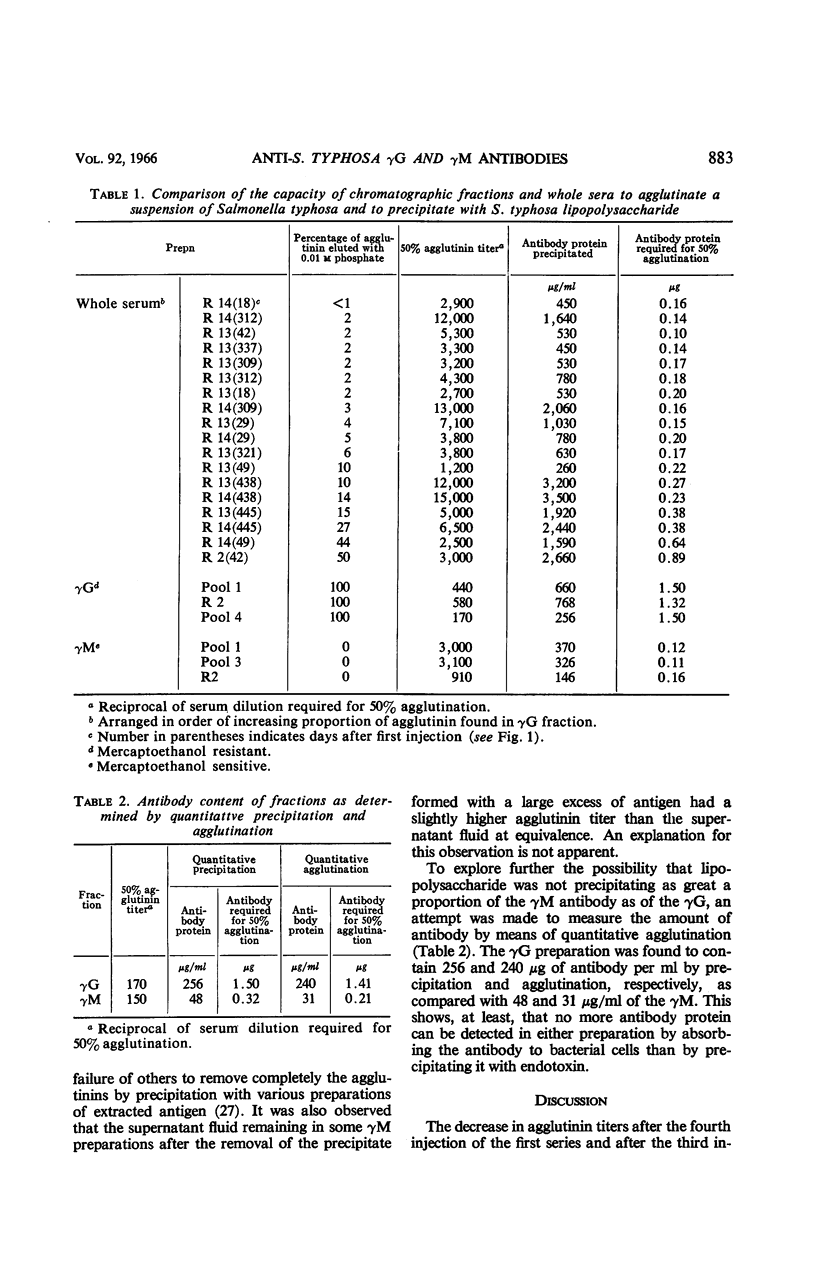
Pike, Robert M. (University of Texas Southwestern Medical School, Dallas), Mary L. Schulze, and Cleo H. Chandler. Agglutinating and precipitating capacity of rabbit anti-Salmonella typhosa γG and γM antibodies during prolonged immunization. J. Bacteriol. 92:880–886. 1966.—Antibody produced in rabbits immunized with acetone-dried typhoid bacilli was followed over a period of 445 days by agglutination and by quantitative precipitation. Repeated injections of vaccine resulted in suppression of antibody titers. Both γG and γM antibodies were rapidly increased by booster injections after rest periods during which titers had decreased to low levels. The O agglutinin titers and the amount of antibody protein, as determined by precipitation with endotoxin, generally were parallel, except in serum specimens in which unusually large proportions of the agglutinating activity were found in the γG fraction. These exceptions were explained by the greater agglutinating capacity of the γM. Endotoxin precipitated about 10 times as much antibody from γG preparations as it did from γM fractions of equivalent agglutinating strength. A much higher proportion of the serological activity, therefore, was found in the γG fractions when antibody was measured by precipitation than when agglutination was used as the measure of activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOREL Y., FAUCONNET M., MIESCHER P. A. 7S VERSUS 19S ANAMNESTIC RESPONSE IN RABBITS. Proc Soc Exp Biol Med. 1964 Nov;117:603–607. doi: 10.3181/00379727-117-29649. [DOI] [PubMed] [Google Scholar]
- DIXON F. J., MAUER P. H. Immunologic unresponsiveness induced by protein antigens. J Exp Med. 1955 Mar 1;101(3):245–257. doi: 10.1084/jem.101.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS P. R. Preparation of antisera for detection of the somatic antigens of Salmonella cultures. Public Health Rep. 1951 Jun 29;66(26):837–839. [PubMed] [Google Scholar]
- FLODIN P., KILLANDER J. Fractionation of human-serum proteins by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:402–410. doi: 10.1016/0006-3002(62)90104-x. [DOI] [PubMed] [Google Scholar]
- GREENBURY C. L., MOORE D. H., NUNN L. A. REACTION OF 7S AND 19S COMPONENTS OF IMMUNE RABBIT ANTISERA WITH HUMAN GROUP A AND AB RED CELLS. Immunology. 1963 Sep;6:421–433. [PMC free article] [PubMed] [Google Scholar]
- GREY H. M. STUDIES ON CHANGES IN THE QUALITY OF RABBIT-BOVINE SERUM ALBUMIN ANTIBODY FOLLOWING IMMUNIZATION. Immunology. 1964 Jan;7:82–90. [PMC free article] [PubMed] [Google Scholar]
- Hocker N. D., Bauer D. C. The nature of antibodies synthesized during the immune response to Leptospira biflexa. J Immunol. 1965 Nov;95(5):887–894. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Lee E. H., Fudenberg H. Immunochemical properties of human gamma-A isohemagglutinin. I. Comparisons with gamma-G and gamma-M-globulin antibodies. J Immunol. 1965 Aug;95(2):197–208. [PubMed] [Google Scholar]
- LOSPALLUTO J., MILLER W., Jr, DORWARD B., FINK C. W. The formation of macroglobulin antibodies. I. Studies on adult humans. J Clin Invest. 1962 Jul;41:1415–1421. doi: 10.1172/JCI104596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOLLER G., WIGZELL H. ANTIBODY SYNTHESIS AT THE CELLULAR LEVEL. ANTIBODY-INDUCED SUPPRESSION OF 19S AND 7S ANTIBODY RESPONSE. J Exp Med. 1965 Jun 1;121:969–989. doi: 10.1084/jem.121.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDERS M. E., ROWLEY D. A., FITCH F. W. The sustained suppression of hemolysin response in passively immunized rats. J Immunol. 1962 Jun;88:718–724. [PubMed] [Google Scholar]
- Osler A. G., Mulligan J. J., Jr, Rodriguez E. Weight estimates of rabbit anti-human serum albumin based on antigen-binding and precipitin analyses: specific hemagglutinating activities of 7 S and 19 S components. J Immunol. 1966 Feb;96(2):334–344. [PubMed] [Google Scholar]
- PIKE R. M., SCHULZE M. L. Agglutination in rheumatoid arthritis serum of sheep cells sensitized with hemolysin and infectious mononucleosis agglutinins. J Immunol. 1960 Nov;85:523–529. [PubMed] [Google Scholar]
- PIKE R. M., SCHULZE M. L. PRODUCTION OF 7S AND 19S ANTIBODIES TO THE SOMATIC ANTIGENS OF SALMONELLA TYPHOSA IN RABBITS. Proc Soc Exp Biol Med. 1964 Mar;115:829–833. doi: 10.3181/00379727-115-29050. [DOI] [PubMed] [Google Scholar]
- PIKE R. M., SCHULZE M. L. THE RELATIVE HEAT STABILITY OF ANTIBODIES IN CHROMATOGRAPHIC FRACTIONS OF RABBIT ANTISERA TO VARIOUS ANTIGENS. J Immunol. 1965 Jan;94:31–36. [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- Rosenquist G. L., Campbell G. R. Characteristics of the 19S and 7S response to bacteriophage 0X74 in the fowl. Immunology. 1966 Feb;10(2):169–178. [PMC free article] [PubMed] [Google Scholar]
- SHULMAN S., HUBLER L., WITEBSKY E. ANTIBODY RESPONSE TO IMMUNIZATION BY DIFFERENT ROUTES. Science. 1964 Aug 21;145(3634):815–817. doi: 10.1126/science.145.3634.815. [DOI] [PubMed] [Google Scholar]
- Samuelson J. S., Kraft S. C., Farr R. S. The contrasting immunosuppresant effects of acriflavine and dietary restriction. J Immunol. 1965 Dec;95(6):1013–1022. [PubMed] [Google Scholar]
- Sawaki Y., Huppert M., Bailey J. W., Yagi Y. Patterns of human antibody reactions in coccidioidomycosis. J Bacteriol. 1966 Jan;91(1):422–427. doi: 10.1128/jb.91.1.422-427.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALIAFERRO W. H., TALIAFERRO L. G. The role of the spleen in hemolysin production in rabbits receiving multiple antigen injections. J Infect Dis. 1951 Sep-Oct;89(2):143–168. doi: 10.1093/infdis/89.2.143. [DOI] [PubMed] [Google Scholar]
- TALMAGE D. W., FRETER G. G., TALIAFERRO W. H. The effect of repeated injections of sheep red cells on the hemolytic and combining capacities of rabbit antiserums. J Infect Dis. 1956 May-Jun;98(3):293–299. doi: 10.1093/infdis/98.3.293. [DOI] [PubMed] [Google Scholar]
- TURNER K. J., JENKIN C. R., ROWLEY D. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 2. ANTIBODY FORMATION DURING THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:229–236. doi: 10.1038/icb.1964.24. [DOI] [PubMed] [Google Scholar]
- Tada T., Ishizaka K. Arthus type inflammation with rabbit gammam antibody. J Immunol. 1966 Jan;96(1):112–118. [PubMed] [Google Scholar]
- WEIDANZ W. P., JACKSON A. L., LANDY M. SOME ASPECTS OF THE ANTIBODY RESPONSE OF RABBITS TO IMMUNIZATION WITH ENTEROBACTERIAL SOMATIC ANTIGENS. Proc Soc Exp Biol Med. 1964 Jul;116:832–837. doi: 10.3181/00379727-116-29386. [DOI] [PubMed] [Google Scholar]
- WHITE T. G., ROEPKE M. H. The kinetics of heat inactivation of bovine serum agglutinins for Brucella. Proc Soc Exp Biol Med. 1962 Mar;109:570–573. doi: 10.3181/00379727-109-27271. [DOI] [PubMed] [Google Scholar]
- Winter A. J. Characterization of the antibody for Vibrio fetus endotoxin in sera of normal and V. fetus infected cattle. J Immunol. 1965 Dec;95(6):1002–1012. [PubMed] [Google Scholar]



