Abstract
We evaluated the specific binding of anti-intercellular adhesion molecule 1 (ICAM-1) conjugated liposomes (immunoliposomes, or ILs) to activated human coronary artery endothelial cells (HCAEC) with the purpose of designing a computed tomographic imaging agent for early detection of atherosclerotic plaques. Covalent attachment of anti-ICAM-1 monoclonal antibodies to pre-formed liposomes stabilized with polyethylene glycol yielded ILs, with a coupling efficiency of the ICAM-1 to the liposomes of 10% to 24%. The anti-ICAM-1–labeled ILs had an average diameter of 136 nm as determined by dynamic light-scattering and cryogenic electron microscopy. The ILs' encapsulation of 5-[N-acetyl-(2,3-dihydroxypropyl)-amino)-N, N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-benzene-1,3-dicarboxamide (iohexol) was determined to be 18% to 19% by a dialysis technique coupled with ultraviolet detection of free iohexol. This encapsulation corresponded to 30 to 38 mg iodine per mL IL solution, and the ILs exhibited 91% to 98.5% iohexol retention at room temperature and under physiologic conditions. The specific binding of the ILs to cultured, activated HCAEC was measured using flow cytometry, enzyme-linked immunosorbent assays, and fluorescence microscopy. The immunosorbent assays demonstrated the specificity of binding of anti-ICAM-1 to ICAM-1 compared with control studies using nonspecific immunoglobulin G-labeled ILs. Flow cytometry and fluorescence microscopy experiments demonstrated the expression of ICAM-1 on the surface of activated HCAEC. Therefore, our iohexol-filled ILs demonstrated potential for implementation in computed tomographic angiography to noninvasively detect atherosclerotic plaques that are prone to rupture.
Key words: Atherosclerosis; binding sites, antibody; cell adhesion molecules; contrast media; endothelium, vascular; ICAM-1; immunoliposome; iohexol/diagnostic use; tomography, X-ray computed; vulnerable plaque
Atherosclerosis is the number 1 lethal disease in the United States and other industrialized countries. Atherosclerotic plaques, particularly in the coronary and carotid arteries, can rupture without warning and result in acute thrombosis, leading to myocardial infarction or stroke. The major risk factors for atherosclerosis are well understood (family history, hypertension, smoking, and high levels of low-density lipoprotein). However, new methods are needed to diagnose and locate the atherosclerotic plaques that are most likely to result in myocardial infarction or stroke. The rupture of “vulnerable” atherosclerotic plaques (plaques that have a thin fibrous cap with a large lipid core and that are not calcified) is the major cause of acute myocardial infarctions (AMIs).1,2 Furthermore, the development of new methods for detecting the individual components of these vulnerable plaques (including thin fibrotic caps, lipid pools, pulpaceous débris, cholesterol clefts, and inflammatory cells) is of great interest and importance.2
The current conventional imaging methods used for the detection of coronary atherosclerosis are intravascular ultrasound (IVUS), magnetic resonance imaging (MRI), and computed tomography (CT).3 Intravascular ultrasound can be used to identify and characterize atherosclerotic plaque in the vicinity of the ultrasound catheter, but this method involves considerable procedural risk because of its invasive nature.4 By contrast, MRI is a powerful noninvasive tool for the detection of atherosclerotic plaque, but its long image-acquisition time hinders the consistent imaging of moving structures such as coronary vessels. Computed tomography, which is sufficiently faster and considerably less expensive than MRI, provides adequate resolution to noninvasively evaluate luminal narrowing and calcific deposits in coronary atherosclerotic lesions.4 However, present CT angiographic methods cannot sufficiently visualize nonstenotic (<70% stenosis) lipid-rich lesions —which are most likely to rupture and which lead to more than half of all AMIs.
Currently, CT angiography for coronary imaging is conducted using bolus injections of iodinated contrast media, a method that successfully reveals symptomatic coronary blockages but fails to identify lipid-rich lesions within the arterial wall. The current radiographic contrast agents used for CT, such as iohexol (Omnipaque 350), diatrizoate (Hypaque 50), iopamidol (Isovue 370), and iodixanol (Visipaque 320), are limited in their applicability because of their short residence time (a few seconds) and high renal toxicity.5,6 A promising alternative to bolus injections of contrast media is the implementation of contrast agents that have been encapsulated in liposomes, which are highly biocompatible. Liposomes have been extensively studied over the past few years and have proved to be good carriers of certain drugs and contrast agents, because they reduce the toxic effects of the encapsulated materials and enhance the therapeutic effect of certain drugs.7 Recent reports8,9 have explored the possibility of using liposomes as carriers of contrast agents for CT and MRI imaging. These studies evaluated the in vivo capability of contrast-agent-loaded liposomes to circulate in the bloodstream, compared the half-lives of the free contrast agent to those of the encapsulated contrast agent, and evaluated CT and MRI signal enhancement due to liposomal formulations.
Our study addresses itself to the development of contrast-enhancing immunoliposomes (ILs), which are antibody-coupled liposomes that selectively target atherosclerotic plaque by binding to inflamed endothelium, one of the most consistent hallmarks of atherosclerosis. When loaded with radiographic-attenuating substances such as iodinated contrast media, ILs can potentially be used as molecular imaging agents in conjunction with ultrafast, multidetector CT scanners for identifying atherosclerotic plaques that are at high risk of rupture and subsequent myocardial infarction. Immunoliposomes have been used previously for noninvasive, diagnostic MRI imaging of various diseases10,11 and for IVUS imaging of atheroma components.12,13 However, the development of ILs for use in CT angiography, particularly ILs containing contrast-enhancing agents, has not been studied nearly so extensively.
The aim of the present study was to develop an IL capable of encapsulating iodinated contrast agents for use in CT imaging of atheromatous plaques. Herein we describe the preparation of iohexol-filled liposomes that are covalently attached to antibodies against intercellular adhesion molecule 1 (ICAM-1), which is expressed preferentially on the surface of inflamed endothelial cells during the early stages of atherosclerosis.13,14 It has been shown that ICAM-1 expression can be used to predict cardiovascular risk (myocardial infarction).15 Following the preparation of the ILs, we characterized them in terms of diameter, long-term stability, and antibody coupling efficiency. Dialysis and ultraviolet spectroscopy results indicated that the ILs' iohexol encapsulation yield was sufficient to detect vulnerable plaques by CT. The anti-ICAM-1 ILs' specificity toward purified ICAM-1 protein was revealed through enzyme-linked immunosorbent assays (ELISA). We then evaluated the contrast-enhanced ILs' suitability for use in CT imaging of atherosclerotic plaques through in vitro investigations with activated human coronary artery endothelial cells (HCAEC). Our targeted ILs bound to the surface of activated HCAEC with high specificity, as determined by a series of ELISA, flow cytometry, and fluorescence microscopy experiments. On the basis of these results, our iodinated ILs show much promise for use in CT imaging of vulnerable atherosclerotic plaques.
Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)2000] (DSPE-PEG(2000)-COOH), and 1,2-diastearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG(2000) were purchased from Avanti Polar Lipids, Inc. (Alabaster, Ala). N-(fluorescein-5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethaolamine (DHPE-FITC) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Molecular Probes (Invitrogen Corp; Eugene, Ore). Sepharose CL-4B, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), bovine serum albumin (BSA), immunoglobulin G (IgG), 4% paraformaldehyde, 2-(N-morpholino)ethanesulfonic acid (MES), and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, Mo). A BioRad bicinchoninic acid (BCA) protein assay kit and N-hydroxysulfosuccinimide (sulfo-NHS) were purchased from Pierce (part of Thermo Fisher Scientific, Inc.; Rockford, Ill). Purified intercellular adhesion molecule 1 protein (ICAM-1) was purchased from BenderMed Systems (Burlingame, Calif), and anti-ICAM antibody was purchased from NeoMarkers Inc. (Fremont, Calif). 5-[N-acetyl-(2,3-dihydroxypropyl)-amino]-N, N-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-benzene-1,3-dicarboxamide (iohexol; dry powder) was purchased from Fluka (part of Sigma-Aldrich Co.; St. Louis, Mo). Anti-mouse IgG 2b (γ-2b)-peroxidase and 2,2-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) were purchased from Roche Diagnostics Corp. (Indianapolis, Ind). Tween 20 was purchased from Fisher Scientific (Pittsburgh, Pa). Human coronary artery endothelial cells (HCAEC; CC-2585) and endothelial basal media supplemented with an endothelial cell growth medium (EGM-2) BulletKit and human serum were purchased from Cambrex Corp. (East Rutherford, NJ). Anti-ICAM-1 fluorescein isothiocyanate (anti-ICAM-1 FITC), interleukin-1 beta (IL1-β), and tumor necrosis factor-alpha (TNF-α) were purchased from Biosource (an Invitrogen company; Camarillo, Calif).
Methods
Preparation of Long-Circulating Liposomes
Liposomes were composed of DPPC and cholesterol in a 3:1 molar ratio. In some cases, the linker lipid DSPE-PEG(2000)-COOH was incorporated at a molar ratio of 3:1:0.3 DPPC:cholesterol:linker. The synthesized liposomes were sterically stabilized by adding a 1% solution of DSPE-PEG(2000) in chloroform. For flow cytometry and fluorescence microscopy experiments, 2 mol% of a fluorescently labeled lipid DHPE-FITC was added to the liposome solution. Liposomes were prepared by hydration of the dry lipid film as described by others.16,17 Briefly, the lipids were dissolved in chloroform, which was then carefully evaporated using a rotary evaporator. The resulting dry lipid film was hydrated at 65 °C with 100 mM, pH 5.5 MES to obtain a final phospholipid concentration of 30 mM. For encapsulation yield measurements, iohexol was added to the hydrating buffer solution as described below. Vigorous shaking of the solution formed large multilamellar vesicles of various sizes. Using an extruder (Avanti Polar Lipids), we passed the multilamellar vesicle solution 21 times through 0.2-μm polycarbonate membranes at 65 °C to yield a homogeneous solution of unilamellar vesicles with diameters of approximately 135 to 140 nm, as determined by dynamic light scattering (DLS) measurements.
Preparation of Immunoliposomes
Immunoliposomes were prepared by attaching the antibodies at the distal terminals of DSPE-PEG(2000)-COOH linker lipids, which have free carboxylic groups available for activation.18 The antibodies conjugated to the distal terminal of PEG are available for binding to antigens, and they preserve their specific activity.19
Following the dry lipid hydration and liposome isolation procedures described above, 300 μL of the 30-mM liposome solution was incubated with the activating reagents ethylcarbodiimide hydrochloride (EDC) (9 mg) and sulfo-NHS (11 mg) overnight at room temperature. The free activating reagents were then removed by size exclusion chromatography (SEC) using Sepharose CL-4B with a phosphate-buffered saline (PBS, pH= 7.4) eluent. Then, 50 μg of either anti-ICAM-1 or IgG (for nonspecific binding control studies) was added to 300 μL of the activated liposome solution and incubated overnight at room temperature. The nonattached antibodies were removed by SEC as described above.
Quantification of Antibody–Liposome Coupling Efficiency
Lipids strongly interfere with direct protein assays, such as Lowry or BCA assays.20,21 As a result, a combination of column separations, protein assays, and ELISA was performed to quantify the extent of anti-ICAM-1 or IgG antibody coupling to the surface of the liposomes. The newly formed ILs were subjected to column separation on Sepharose CL-4B, and fractions of 7 drops each were collected and analyzed for protein content with a BCA protein assay kit. The fractions containing liposomes appeared to contain proteins (that is, a false positive result was obtained) due to the lipids' interference with the assay. These fractions were then subjected to an ELISA in which purified ICAM-1 protein was used to confirm the presence of antibodies.
To quantify the extent of antibody coupling to the liposomes, we constructed an antibody calibration curve by preparing a serial dilution of antibodies in PBS and analyzing each solution for protein content, with the protein assay kit. The amount of unbound antibody that eluted from the column was quantified with the calibration curve, and the amount of antibody that was covalently coupled to the liposomes was determined by subtracting the calculated amount of unbound antibody from the initial amount of antibody that was added to the liposome solution during IL preparation. To determine the amount of IgG that was nonspecifically lost during column chromatography, IgG was mixed with plain liposomes and passed through the column. We determined that 5% to 10% of IgG was lost in this process, and this information was used in correcting measurements of the amount of free IgG that was present in samples.
Characterization of Liposomes and Immunoliposomes
The average diameter of the liposomes and ILs was measured by DLS via a Malvern Nano-ZS Zetasizer (Malvern Instruments Ltd., a Spectris company; Worcestershire, UK). The results from DLS were confirmed by both cryo-electron microscopy and negative-staining electron microscopy using a JEM-1010 transmission electron microscope (JEOL; Tokyo, Japan) and uranyl acetate negative stain.
Encapsulation and Retention of Iohexol in Immunoliposomes
Iohexol-encapsulated ILs were prepared by hydrating the dry lipid film with 100 mM MES containing enough dissolved iohexol for a 2:1 or 8:1 molar ratio of phospholipids:iohexol. The iodinated liposomes were sized by extrusion through 0.2-μm polycarbonate membranes as described above, and the nonencapsulated iohexol was removed by SEC as described above, before conjugating the liposomes with antibodies. The encapsulation yield for iohexol was determined by measuring the amount of iohexol that was encapsulated in the aqueous interior of the ILs. The iohexol-encapsulated ILs were ruptured by mixing the liposome solution with a 2% aqueous solution of Triton X-100 prepared in water (1:1 volume to volume [v/v]) and vortexing the mixture for approximately 30 seconds, thus releasing the encapsulated iohexol into solution. The absorbance of the resulting solution at 245 nm (the characteristic wavelength for iohexol) was measured with a Shimadzu UV-VIS spectrometer (Shimadzu Scientific Instruments; Columbia, Md), and the corresponding concentration of iohexol was calculated from the Beer–Lambert law. The iohexol encapsulation efficiency was then calculated using the following equation:
To determine the ILs' retention of iohexol, 500 μL of iohexol-encapsulated ILs were dialyzed (8,000 molecular-weight cutoff) against 25 mL PBS at temperatures of 4, 25, and 37 °C. Aliquots of dialysate (500 μL) were removed periodically over a period that extended anywhere from 24 hours to approximately 3.5 weeks, and the amount of iohexol in the dialysate was quantified by measuring the ultraviolet absorbance at 245 nm.
Computed Tomographic Measurements
100-μL aliquots of the iohexol-encapsulated ILs, liposomes without antibodies, water, and PBS were pipetted into a 96-well plate, and the radiographic attenuation of these samples was measured with an eXplore RS Micro CT Scanner (GE Healthcare; Piscataway, NJ). A 3 × 3 × 4-mm volume was used for measurements, and the results were reported in contrast numbers (Hounsfield Units [HU]).
In Vitro Endothelial Cell Binding Experiments
Specificity of Immunoliposome Binding to Purified ICAM-1 Protein. The specificity of IL binding to ICAM-1 protein was evaluated by means of ELISA. Briefly, a 96-well plate was coated with 8 μg/mL purified ICAM-1 protein (50 μL) in PBS and incubated overnight at 4 °C. Next, the plate was emptied and the residual liquid was tapped out. Nonspecific binding sites were then blocked with 3% BSA for 2 hours at room temperature, and then various IL samples were added (100 μL, 86 nmol lipids) and incubated for another 2 hours at room temperature. After extensive washing with PBS that contained 0.1% Tween 20 (5 times, 5-min incubation with PBS each time) to remove any unbound ILs, the wells were incubated with a secondary antibody, anti-mouse IgG 2b (γ-2b)-peroxidase (Roche), at a dilution factor of 1:2000 (secondary antibody:0.1% Tween 20 in PBS, v/v) for 1 hour at room temperature. The unbound secondary antibody was then carefully removed by repeated washings with PBS containing 0.1% Tween 20; then the substrate (ABTS) was added and incubated for 10 minutes. The extent of antibody binding to the purified ICAM-1 protein was then quantified by measuring the ABTS absorption at 405 nm.
Assessment of ICAM-1 Expression in Activated HCAEC. The HCAEC (CC-2585) were grown in endothelial basal media supplemented with an EGM-2 Bullet Kit (Cambrex) at 37 °C in 5% CO2. The ICAM-1 was expressed on the surface of the HCAEC by activating the cells with various amounts of IL1-β or TNF-α, and the extent of ICAM-1 expression was evaluated by means of ELISA and fluorescence microscopy. For ELISA, the cells were incubated with IL1-β or TNF-α for 24 hours at 37 °C and 5% CO2 in a 96-well plate. Concentrations of 0.5-, 2-, 4-, 6-, and 8-ng/mL IL1-β as well as 0.5-, 2-, 4-, 6-, and 8-ng/mL TNF-α were used. The next day, the cells were washed with PBS, fixed with formalin for 20 minutes at room temperature, incubated with 3% BSA and 0.1% Tween 20 and then incubated with anti-ICAM antibody (1:1,000 dilution in PBS, v/v) for 2 hours at 25 °C. The unbound anti-ICAM antibody was removed from the activated HCAEC by washing with PBS, and the cells were incubated with anti-mouse IgG 2b (γ-2b)-peroxidase for 1 hour at room temperature. The excess secondary antibody was washed away with PBS, and then ABTS substrate was added as described above. After 10 minutes of incubation, the absorbance at 405 nm was measured with a Tecan plate reader (Tecan Group Ltd.; Männedorf, Switzerland). Non-activated cells were also subjected to ELISA as controls.
For fluorescence microscopy measurements, cells (4 × 104 cells/chamber) were incubated in 8-chamber tissue culture slides overnight at 37 °C in 5% CO2. The next day, the cells were activated with IL1-β (8 ng/mL media) for 24 hours at 37 °C in 5% CO2. Next, the cells were washed with PBS and fixed with 4% paraformaldehyde for 20 minutes at room temperature. The cells were then washed 2 more times with PBS, and the unreacted sites were blocked with 3% BSA and 0.1% Tween 20 for 1 hour. The cells were incubated with anti-ICAM-1 FITC for 2 hours, and the unbound antibody was removed by washing with PBS before the chamber partitions were removed and the slides were dried in air. The HCAEC nuclei were labeled with DAPI, and then the images were captured with an IX71 inverted microscope (Olympus America Inc.; Center Valley, Pa) equipped with FITC and DAPI filters for epi-fluorescence measurements.
Specificity of Immunoliposome Binding to Activated HCAEC. The specificity of the ILs' binding toward activated HCAEC was evaluated by means of flow cytometry, ELISA, and fluorescence microscopy. To quantify the liposome binding to the surface of HCAEC by means of flow cytometry, activated or non-activated cells (5 × 105 cells/flask) were detached using 0.25% trypsin followed by neutralization with endothelial basal media. The cells were then washed with PBS, fixed with 4% paraformaldehyde (300 μL) at room temperature for 20 minutes, and washed again with PBS, followed by incubation with IL samples (100 μL) (1: anti-ICAM-1 ILs, 69 nmol lipid; 2: IgG ILs, 69 nmol lipid) for 2 hours at 4 °C. The cells were then washed again with PBS to remove any unbound liposomes, resuspended in PBS, and then analyzed by flow cytometry on a Cytomics FC 500 (Beckman Coulter, Inc.; Fullerton, Calif). Liposomes were fluorescently labeled with FITC, and the binding of liposomes to the surface of activated and non-activated cells was evaluated by measuring the fluorescence intensity (X-axis) against the count of events (Y-axis).
For ELISA and fluorescence microscopy, samples of anti-ICAM-1 ILs, IgG ILs, and non-activated liposomes were used. In addition, control studies using unconjugated anti-ICAM-1 antibodies and pure PBS buffer were conducted. For ELISA experiments, the same procedure as that described above for expression of ICAM-1 was used. The HCAEC were plated on a 96-well plate (at 4 × 104 cells/well) and incubated overnight at 37 °C in 5% CO2 without incubation in cytokines. The next day, the cells were activated for 24 hours with IL1-β (8 ng/mL) before being washed, fixed, and analyzed. The fluorescence microscopy experiments were conducted in the same manner as described above.
Results
Quantification of Antibody–Liposome Coupling Efficiency
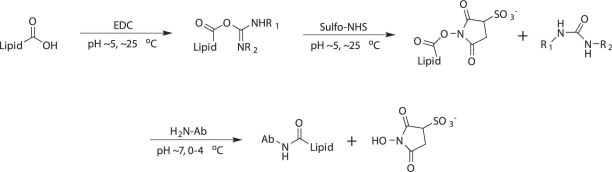
Immunoliposomes were prepared by activating the free carboxyl group of the linker lipid incorporated in pre-formed liposomes with EDC and sulfo-NHS and then by covalently conjugating antibodies to the lipids through displacement of sulfo-NHS groups by antibody amines, as depicted in Figure 1. To quantify the amount of covalently attached antibody to the surface of activated liposomes, we performed a calibration curve for the antibody, and then we analyzed the different fractions (ILs and non-covalently coupled antibody) that had been collected during the separation of free antibody for protein content, using the same protein assay kit that had been used for the calibration curve. The amount of antibody that was covalently coupled to the liposomes was quantified by subtracting the amount of free antibody from the initial amount of antibody that was added. A 10% to 24% α-ICAM coupling efficiency was observed for various IL formulations. Although anti-ICAM-1 in the IL fractions could not be quantitated by means of the calibration curve due to signal interference by the lipids, the presence of anti-ICAM-1 in these fractions was confirmed by performing ELISA with purified ICAM-1 protein.
Fig. 1 Preparation of immunoliposomes by EDC and sulfo-NHS treatment.
Ab = antibodies; EDC = ethylcarbodiimide hydrochloride; sulfo-NHS = N-hydroxysulfosuccinimide
Characterization of Liposomes and Immunoliposomes
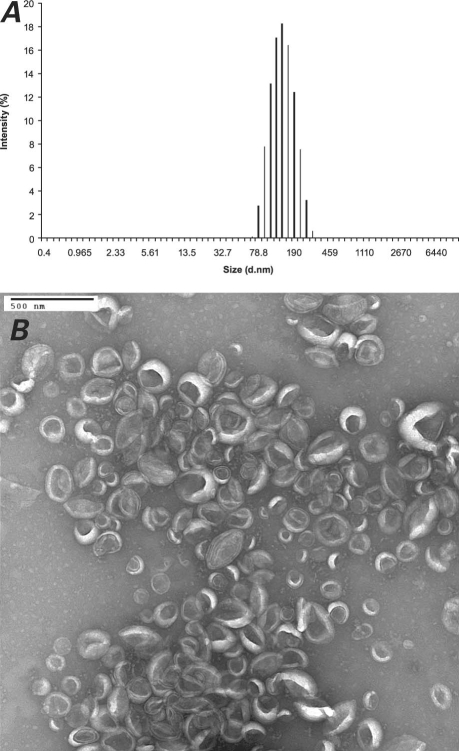
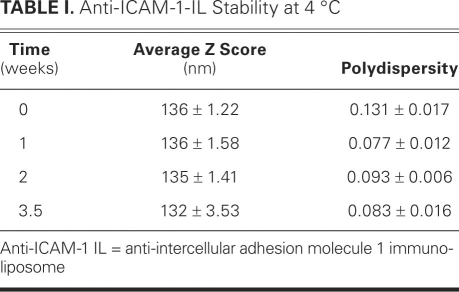
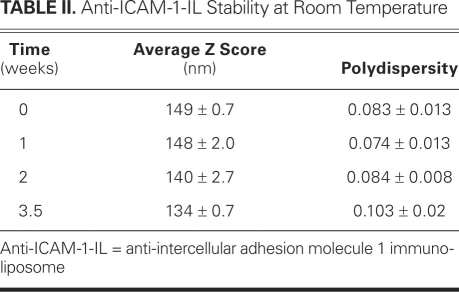
The unconjugated liposome solution was characterized by DLS and electron microscopy, as were the IgG- and anti-ICAM-1-IL solutions. The results obtained for the anti-ICAM-1 ILs are shown in Figure 2. The average diameters of the liposomes, anti-ICAM-1 ILs, and IgG ILs were 141, 136, and 141 nm, respectively. As shown in Table I, the ILs were stable at 4 °C for 3.5 weeks, exhibiting minimal variation in their average diameter and polydispersity index during this time. Table II shows that the ILs were also stable at room temperature. The polydispersity index, which indicates the variation in particle size, can vary from 0 to 1: a value of 0 indicates that the particle size does not vary and a value of 1 means that the sample is very polydisperse. Values below 0.2 indicate that a sample is monodisperse.22,23 Therefore, the polydispersity index values reported here indicate that the ILs were very monodisperse. The results presented in Tables I and II are the averages of 13 runs each, of 3 different IL preparations.
Fig. 2 A) Dynamic light scattering and B) negative-stain electron microscopy of anti-intercellular adhesion molecule 1 immunoliposomes.
TABLE I. Anti-ICAM-1-IL Stability at 4 °C
TABLE II. Anti-ICAM-1-IL Stability at Room Temperature
Encapsulation and Retention of Iohexol in Immunoliposomes and Computed Tomographic Measurements
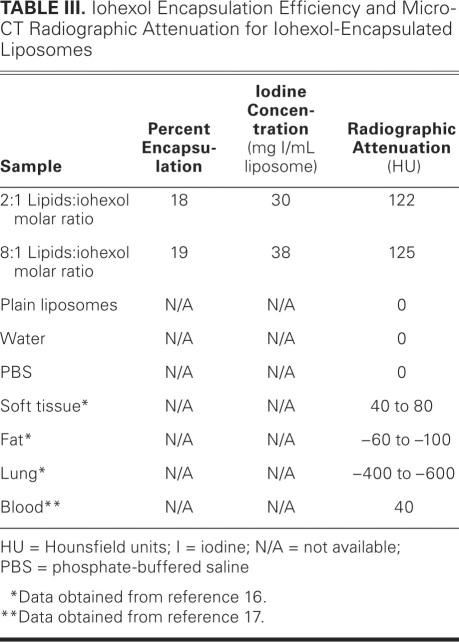
The entrapment data obtained from iohexol absorbance measurements indicated 18% to 19% encapsulation efficiency (Table III), which corresponds to an encapsulation of 30 to 38 mg of iodine per mL of IL solution. Table III also reports the results of the radiographic attenuation for iohexol-encapsulated liposomes as measured with a micro-CT scanner, as well as the radiographic attenuation for the unconjugated liposomes of the same lipid concentration as our iohexol-encapsulated ILs. For comparison, the previously reported radiographic attenuation of water, PBS, soft tissue, fat, lung, and blood are also presented.24,25 A radiographic attenuation of 122 to 125 HU was obtained for the 2 iohexol-liposome formulations.
TABLE III. Iohexol Encapsulation Efficiency and Micro-CT Radiographic Attenuation for Iohexol-Encapsulated Liposomes
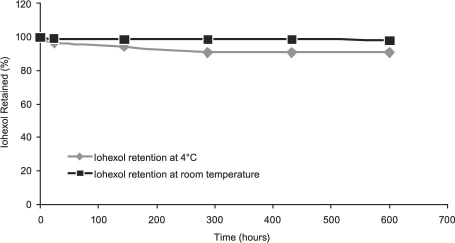
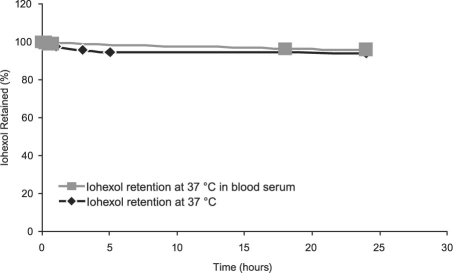
We analyzed the retention of the encapsulated iohexol in the interior of our anti-ICAM-1 ILs through dialysis for a period ranging from 24 hours to approximately 3.5 weeks. Over a period of approximately 3.5 weeks, the ILs retained 91% of the originally encapsulated iohexol at 4 °C, and they retained 98.4% of the iohexol at room temperature (25 °C) (Fig. 3). Furthermore, the ILs retained 94% and 96% of the iohexol over a period of 24 hours at 37 °C in PBS buffer and in serum, respectively (Fig. 4).
Fig. 3 Iohexol retention in anti-intercellular adhesion molecule 1 immunoliposomes at 4 °C and room temperature.
Fig. 4 Iohexol retention in anti-intercellular adhesion molecule 1 immunoliposomes at 37 °C in buffer and in blood serum.
In Vitro Endothelial Cell Binding Experiments
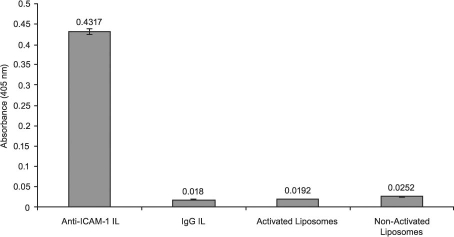
Specificity of Immunoliposome Binding to Purified ICAM-1 Protein. The specificity of binding of the ILs toward ICAM-1 protein was demonstrated by ELISA. We compared the extent of ICAM-1 protein binding of several independently prepared anti-ICAM-1-IL samples with that observed for IgG ILs—for liposomes activated by EDC and sulfo-NHS but not conjugated to antibodies, and for non-activated liposomes (liposomes that were exposed neither to coupling agents nor to antibodies). As shown in Figure 5, the anti-ICAM-1 ILs exhibited a substantially greater binding affinity toward purified ICAM-1 protein than did the nonspecific IgG ILs or either antibody-free liposome sample. The anti-ICAM-1 ILs displayed a 23-fold increase in binding specificity toward purified ICAM-1 protein relative to the IgG ILs, which showed an ABTS absorption response similar to those of the activated and non-activated liposomes (Fig. 5).
Fig. 5 ELISA results show the binding—in each case, to purified ICAM-1 protein—of specific anti-ICAM-1 ILs (100 μL, 0.86 mM lipids), nonspecific IgG ILs (100 μL, 0.86 mM lipids), liposomes after activation with ethylcarbodiimide hydrochloride (EDC) and sulfo-NHS (100 μL, 0.86 mM lipids), and liposomes before activation with EDC and sulfo-NHS (100 μL, 0.86 mM lipids). Data are representative of 3 experiments.
Anti-ICAM-1 ILs = anti-intercellular adhesion molecule 1 immunoliposomes; ELISA = enzyme-linked immunosorbent assay; ICAM-1 = intercellular adhesion molecule 1; IgG ILs = immunoglobulin G immunoliposomes; sulfo-NHS = N-hydroxysulfosuccinimide
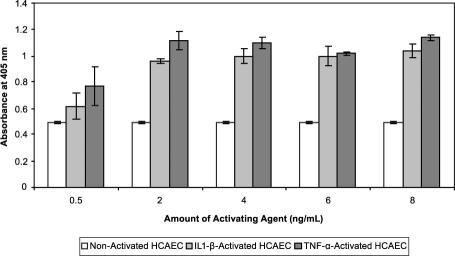
Assessment of ICAM-1 Expression in Activated HCAEC. The extent of ICAM-1 expression on the surface of IL1-β- and TNF-α-activated HCAEC was evaluated by means of ELISA and fluorescence microscopy. The ELISA results (Fig. 6) show that although the non-activated HCAEC expressed a measurable amount of ICAM-1, a more than 2-fold increase in the expression of ICAM-1 was observed after activation of the cells with IL1-β or TNF-α. As seen in Figure 6, ICAM-1 expression on the surface of HCAEC was induced upon activation with 2 to 8 ng/mL of IL1-β and TNF-α for 24 hours. Activating reagents (0.5 ng/mL) did not induce ICAM-1 expression in comparison with non-activated cells, but by increasing the amount of activating reagents, a 2.3-fold increase above the control cells was observed. The results in Figure 6 were confirmed by fluorescence microscopy (Fig. 7), in which both non-activated and IL1-β-activated HCAEC were incubated with fluorescently labeled anti-ICAM-1 (anti-ICAM-1 FITC; green fluorescence in Fig. 7). The HCAEC nuclei were stained with DAPI (blue fluorescence in Fig. 7).
Fig. 6 Intercellular adhesion molecule 1 (ICAM-1) expression in IL1-β- or TNF-α-activated HCAEC as measured by ELISA. The HCAEC were incubated for 24 hours at 37 °C and 5% CO2 with various amounts of activating agents (IL1-β or TNF-α). Pure anti-ICAM-1 antibody (1:1,000 dilution in PBS, v/v) was used to determine ICAM-1 expression in each sample. Non-activated cells were used as controls. Data are representative of 3 experiments.
Anti-ICAM-1 = anti-intercellular adhesion molecule 1; ELISA = enzyme-linked immunosorbent assay; HCAEC = human coronary artery endothelial cells; IL1-β = interleukin-1 beta; PBS = phosphate-buffered saline; TNF-α = tumor necrosis factor-alpha; v/v = volume to volume
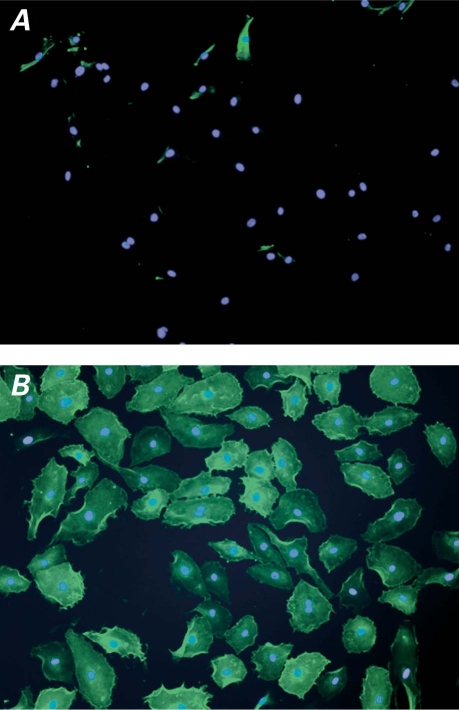
Fig. 7 Fluorescence microscopy images of anti-ICAM-1 binding at A) non-activated and B) IL1-β-activated HCAEC. In both samples, green indicates anti-ICAM-1-FITC incubation, and blue indicates nuclei labeled with DAPI.
Anti-ICAM-1 = anti-intercellular adhesion molecule 1; DAPI = 4′,6-diamidino-2-phenylindole; FITC = fluorescein-5-thiocarbamoyl; HCAEC = human coronary artery endothelial cells; IL1-β = interleukin-1 beta
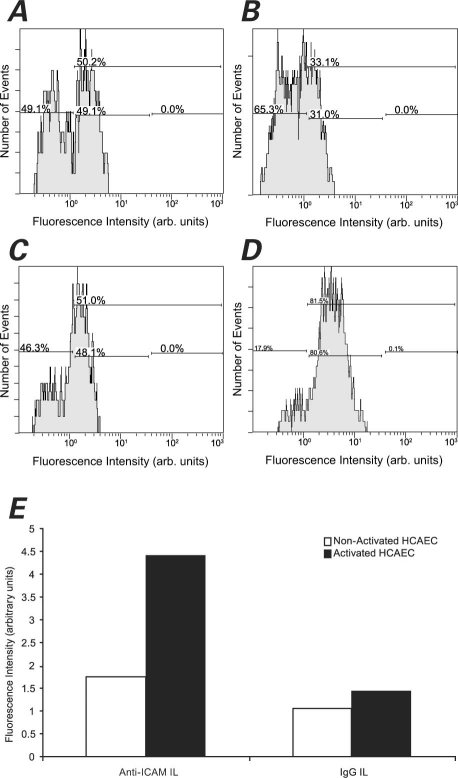
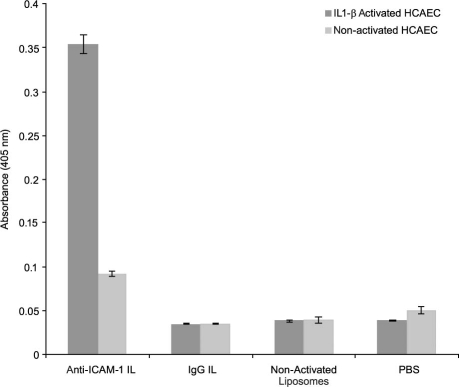
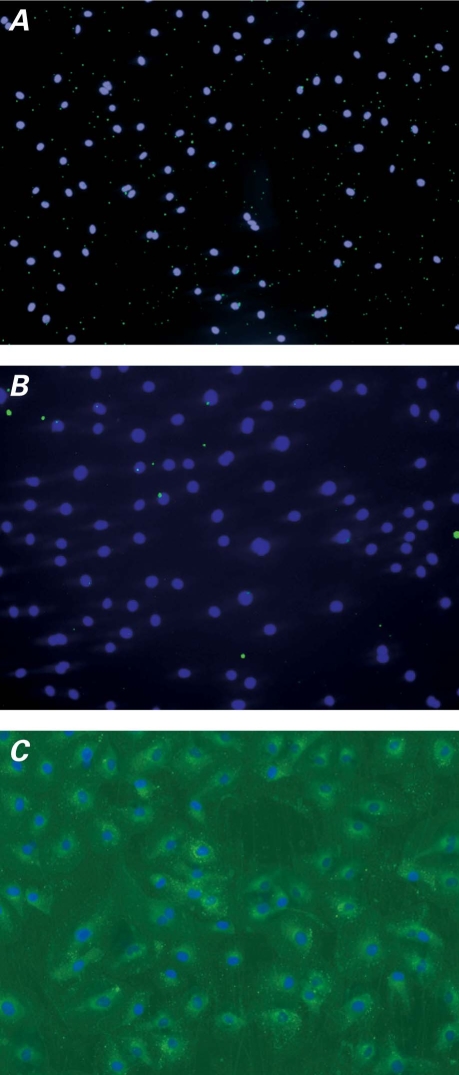
Immunoliposome Binding Specificity toward Activated HCAEC. The binding specificity of anti-ICAM-1 ILs toward IL1-β-activated HCAEC was examined by means of flow cytometry, ELISA, and fluorescence microscopy. The flow cytometry results (Fig. 8) demonstrated binding specificity of the anti-ICAM-1 ILs toward activated HCAEC. The ABTS absorbance readings from ELISA supported this finding (Fig. 9), because anti-ICAM-1-IL binding appeared preferential over that of IgG ILs, non-activated liposomes, unconjugated anti-ICAM-1, and PBS buffer. These results were further demonstrated by fluorescence microscopy (Fig. 10).
Fig. 8 A) Binding of IgG ILs to non-activated HCAEC; B) Binding of anti-ICAM-1 ILs to non-activated HCAEC; C) Binding of IgG ILs to IL1-β-activated HCAEC; D) Binding of anti-ICAM-1 ILs to IL1-β-activated HCAEC; and E) Binding specificity of IgG ILs (nonspecific) and anti-ICAM-1 ILs (specific) to IL1-β-activated HCAEC, as measured by flow cytometry.
Anti-ICAM-1 ILs = anti-intercellular adhesion molecule 1 immunoliposomes; arb. = arbitrary; HCAEC = human coronary artery endothelial cells; IgG ILs = immunoglobulin G immunoliposomes; IL1-β = interleukin-1 beta
Fig. 9 ELISA results show the binding of ILs (100 μL, 0.86 mM lipids) covalently coupled to anti-ICAM-1 (after separation of unbound anti-ICAM), ILs covalently coupled to nonspecific anti-IgG, plain liposomes, and PBS to non-activated and IL1-β (8 ng/mL, 24 hr activation at 37 °C, 5% CO2)-activated HCAEC. Data are representative of 3 experiments.
Anti-ICAM-1 = anti-intercellular adhesion molecule 1; anti-IgG = anti-immunoglobulin G; ELISA = enzyme-linked immunosorbent assay; HCAEC = human coronary artery endothelial cells; IL1-β = interleukin-1 beta; ILs = immunoliposomes; PBS = phosphate-buffered saline
Fig. 10 Fluorescence microscopy images demonstrate binding specificity to IL1-β-activated HCAEC, of A) plain liposomes, B) IgG ILs (nonspecific), and C) anti-ICAM-1 ILs (specific).
Anti-ICAM-1 ILs = anti-intercellular adhesion molecule 1 immunoliposomes; HCAEC = human coronary artery endothelial cells; IgG ILs = immunoglobulin G immunoliposomes; IL1-β = interleukin-1 beta
Discussion
Both the unconjugated liposome solution and the IL solution (after the activation of the free carboxyl group of the lipid linker and covalent attachment of antibodies) were characterized by DLS and electron microscopy. The results show very homogenous liposome formulations (very low polydispersity number of 0.08), with a narrow size distribution. The DLS results (Fig. 2A), along with the negative-staining electron microscopic images (Fig. 2B), prove that the liposomes preserve their size and shape after the chemical modification. For practical use as CT contrast agents, the synthesized ILs should remain stable for a substantially long time (>3 weeks), with no special handling or storage requirements.6 Therefore, we conducted a study of the anti-ICAM-1 ILs' stability at various temperatures to determine their optimal long-term storage conditions. The data in Tables I and II are very encouraging, for they show that indeed no special conditions are required for the long-term storage of our ILs.
The contrast agent iohexol was encapsulated in the ILs' interior during the dry-lipid hydration step as described in Methods. To determine optimal encapsulation conditions for suitable radiographic attenuation (that is, CT imaging capability), different molar ratios of phospholipids:ioxehol were analyzed. We determined that a molar ratio of less than 2:1 provided poor encapsulation efficiency for iohexol and that an iohexol saturation level of approximately 19% was reached at molar ratios of 8:1 or higher. Table III provides information for the 2:1 and 8:1 molar ratios of phospholipids:iohexol, which are the upper and lower limits of the optimum encapsulation conditions. For the detection of vulnerable plaques by means of CT, ILs must encapsulate approximately 40 to 50 mg of iodinated contrast agent per gram of liposomal lipid.6 The data in Table III indicate that our ILs of these molar ratios are capable of encapsulating the amount of iodine necessary for CT imaging. Moreover, the radiographic attenuation observed for these ILs should enable differentiation of the iohexol-encapsulated ILs from anatomic features such as soft tissue, fat, lung, or blood, as evidenced by comparison of the data in Table III.
Computed tomography contrast agents should have an adequate shelf-life (months), so the ILs should retain their encapsulated contrast agents over time. Temperatures of 4, 25, and 37 °C were chosen for dialysis studies of the ILs' performance over a period of about 3.5 weeks. Furthermore, dialysis at 37 °C in blood serum was performed in order to evaluate the ILs in an in vitro experimental environment. On the basis of the results shown in Figures 3 and 4, the ILs' contrast agent retention and overall stability for CT imaging procedures appears promising, and our ILs remained stable longer than did other recently reported iohexol-encapsulated liposome formulations.6 Furthermore, the ILs retained 96% of their encapsulated iohexol after 24 hours at 37 °C in blood plasma. Future in vivo experiments in rabbits will establish the distribution of our iohexol-encapsulated ILs in the body as well as the optimum time for the visualization of our bound ILs. Even though the optimum time for CT imaging has not yet been established for our ILs, we do believe that 24 hours is an acceptable length of time for the administration of contrast agents to patients and subsequent imaging.
Although atherosclerosis, in the early days of its investigation, was linked mainly to the accumulation of lipids in the arteries, recent studies have revealed that the outset of atherosclerosis is characterized by the inflammation of the endothelium, which is followed by the formation of atherosclerotic plaques at the sites of inflammation.26 Cell adhesion molecules (CAMs), including ICAM-1, are expressed on activated endothelial cells, thereby indicating the initiation of atherosclerotic plaques. Our ILs are configured to have relatively long-term circulation in the blood (more than 3 hr5,6), in order to enable identification, accumulation, and binding to the particular area of interest: in this case, ICAM-1. The results shown in Figures 5 and 6 suggest that our anti-ICAM-1 ILs successfully and specifically target the ICAM-1 expressed in inflamed endothelial cells. As seen in Figure 7A, the anti-ICAM-1 FITC did not bind at all to the non-activated HCAEC. In contrast, extensive anti-ICAM-1-FITC binding was observed at the IL1-β-activated HCAEC (Fig. 7B), clearly indicating the successful induction of ICAM-1 expression on the surface of activated HCAEC.
Notably, binding was observed by means of flow cytometry at non-activated HCAEC, whether incubated with anti-ICAM ILs (49% of cells were positive for anti-ICAM; Fig. 8A) or incubated with nonspecific IgG ILs (31% positive; Fig. 8B). In addition, 48% of the activated HCAEC that had been incubated with nonspecific IgG ILs was positive for anti-ICAM (Fig. 8C). Nevertheless, anti-ICAM-1 ILs showed a substantially higher degree of binding to activated HCAEC than any of these other systems: 81% of the cells tested positive for anti-ICAM in flow cytometry (Fig. 8D). These results are summarized together in Figure 8E.
The data in Figure 9 indicate that although non-activated HCAEC expressed a certain level of ICAM-1 protein capable of binding to the tested samples, the amount of anti-ICAM-1 ILs that bound to activated HCAEC was 4 times greater than the amount that was bound to the non-activated cells. In addition, a 10-fold increase in binding specificity of specific anti-ICAM-1 ILs toward activated HCAEC was observed relative to that observed for IgG ILs. Whereas non-activated liposomes (Fig. 10A) and IgG ILs (Fig. 10B) did not exhibit any noticeable binding to the activated HCAEC, the anti-ICAM-1 ILs clearly bound specifically to activated HCAEC (Fig. 10C).
After injection, free iohexol solution is immediately cleared from the body by the reticuloendothelial system. Encapsulating iohexol inside our ILs extends the residence time of iohexol, thereby enabling the ILs to bind to target ICAM-1 protein and therefore enhancing atherosclerotic plaques in CT imaging. Both the amount of iohexol encapsulated in the anti-ICAM-1 ILs and the radiographic attenuation observed here are very promising preliminary results. Studies with small-animal models will enable us to further develop our anti-ICAM-1 ILs for ultimate application in visualizing atherosclerotic plaques.
Conclusions
Computed tomographic imaging provides the best opportunity for the noninvasive detection of early plaque, but it has serious limitations in the absence of contrast agents that can distinguish between stable and unstable plaques. We have developed a targeted nanoparticle that is able to recognize inflammatory molecules and also to deliver a high concentration of contrast agent that could possibly be detected by CT imaging.
The in vitro results demonstrate the capability of our contrast agent to bind selectively to inflamed endothelium and to provide enough contrast to possibly enable the diagnosis of early atherosclerosis. The work has the potential to affect medicine by providing a noninvasive tool for the diagnosis of developing atherosclerosis. In the future, we plan to test the efficiency of this developed immunoliposome (with its encapsulated contrast agent) in the diagnosis and characterization of atherosclerotic lesions in rabbits in which atheroma has been induced. We also plan to validate our contrast agent ex vivo in explanted carotid arteries and aortas from atherosclerotic rabbits.
Acknowledgment
We acknowledge Dr. Russell M. Lebovitz of Marval Biosciences for scientific discussions and helpful suggestions.
Footnotes
Address for reprints: Jodie L. Conyers, PhD, 7000 Fannin St., Suite 795, Houston, TX 77030 E-mail: Jodie.L.Conyers@uth.tmc.edu
Funding support: Department of DefenseContract W81XWH-04-2-0035, “T5: Advanced Imaging.”
References
- 1.MacNeill BD, Lowe HC, Takano M, Fuster V, Jang IK. Intravascular modalities for detection of vulnerable plaque: current status. Arterioscler Thromb Vasc Biol 2003;23(8):1333–42. [DOI] [PubMed]
- 2.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108(14):1664–72. [DOI] [PubMed]
- 3.Kaul S, Lindner JR. Visualizing coronary atherosclerosis in vivo: thinking big, imaging small. J Am Coll Cardiol 2004; 43(3):461–3. [DOI] [PubMed]
- 4.Mohler ER III. Overview of vulnerable plaque: an overview of the characteristics, detection methods, and response to treatment of vulnerable plaque in the carotids. Endovascular Today 2006;August:79–82.
- 5.Mukundan S Jr, Ghaghada KB, Badea CT, Kao CY, Hedlund LW, Provenzale JM, et al. A liposomal nanoscale contrast agent for preclinical CT in mice. AJR Am J Roentgenol 2006; 186(2):300–7. [DOI] [PubMed]
- 6.Kao CY, Hoffman EA, Beck KC, Bellamkonda RV, Annapragada AV. Long-residence-time nano-scale liposomal iohexol for X-ray-based blood pool imaging. Acad Radiol 2003;10 (5):475–83. [DOI] [PubMed]
- 7.Nallamothu R, Wood GC, Pattillo CB, Scott RC, Kiani MF, Moore BM, Thoma LA. A tumor vasculature targeted liposome delivery system for combretastatin A4: design, characterization, and in vitro evaluation. AAPS PharmSciTech 2006; 7(2):E32. [DOI] [PubMed]
- 8.Zheng J, Liu J, Dunne M, Jaffray DA, Allen C. In vivo performance of a liposomal vascular contrast agent for CT and MR-based image guidance applications. Pharm Res 2007;24(6): 1193–201. [DOI] [PubMed]
- 9.Zheng J, Perkins G, Kirilova A, Allen C, Jaffray DA. Multimodal contrast agent for combined computed tomography and magnetic resonance imaging applications. Invest Radiol 2006;41(3):339–48. [DOI] [PubMed]
- 10.Brandwijk RJ, Mulder WJ, Nicolay K, Mayo KH, Thijssen VL, Griffioen AW. Anginex-conjugated liposomes for targeting of angiogenic endothelial cells. Bioconjug Chem 2007; 18(3):785–90. [DOI] [PubMed]
- 11.Mulder WJ, van der Schaft DW, Hautvast PA, Strijkers GJ, Koning GA, Storm G, et al. Early in vivo assessment of angiostatic therapy efficacy by molecular MRI. FASEB J 2007; 21(2):378–83. [DOI] [PubMed]
- 12.Hamilton AJ, Huang SL, Warnick D, Rabbat M, Kane B, Nagaraj A, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol 2004; 43(3):453–60. [DOI] [PubMed]
- 13.Huang SL, Hamilton AJ, Nagaraj A, Tiukinhoy SD, Klegerman ME, McPherson DD, Macdonald RC. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. J Pharm Sci 2001;90(12):1917–26. [DOI] [PubMed]
- 14.Hamilton A, Rabbat M, Jain P, Belkind N, Huang SL, Nagaraj A, et al. A physiologic flow chamber model to define intravascular ultrasound enhancement of fibrin using echogenic liposomes. Invest Radiol 2002;37(4):215–21. [DOI] [PubMed]
- 15.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res 2001;89(9):763–71. [DOI] [PubMed]
- 16.Laginha K, Mumbengegwi D, Allen T. Liposomes targeted via two different antibodies: assay, B-cell binding and cytotoxicity. Biochim Biophys Acta 2005;1711(1):25–32. [DOI] [PubMed]
- 17.Hansen CB, Kao GY, Moase EH, Zalipsky S, Allen TM. Attachment of antibodies to sterically stabilized liposomes: evaluation, comparison and optimization of coupling procedures. Biochim Biophys Acta 1995;1239(2):133–44. [DOI] [PubMed]
- 18.Maruyama K, Takizawa T, Takahashi N, Tagawa T, Nagaike K, Iwatsuru M. Targeting efficiency of PEG-immunoliposome-conjugated antibodies at PEG terminals. Advanced Drug Delivery Reviews 1997;24(2–3):235–42.
- 19.Torchilin VP, Levchenko TS, Lukyanov AN, Khaw BA, Klibanov AL, Rammohan R, et al. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim Biophys Acta 2001;1511(2):397–411. [DOI] [PubMed]
- 20.Kessler RJ, Fanestil DD. Interference by lipids in the determination of protein using bicinchoninic acid. Anal Biochem 1986;159(1):138–42. [DOI] [PubMed]
- 21.Klegerman ME, Hamilton AJ, Huang SL, Tiukinhoy SD, Khan AA, MacDonald RC, et al. Quantitative immunoblot assay for assessment of liposomal antibody conjugation efficiency. Anal Biochem 2002;300(1):46–52. [DOI] [PubMed]
- 22.Duzgunes N, editor. Liposomes. Part A. Series: Methods in enzymology. Boston: Elsevier Academic Press; 2003. p. 22.
- 23.Hengst V, Oussoren C, Kissel T, Storm G. Bone targeting potential of bisphosphonate-targeted liposomes. Preparation, characterization and hydroxyapatite binding in vitro. Int J Pharm 2007;331(2):224–7. [DOI] [PubMed]
- 24.Jackson SA, Thomas RM, editors. Cross-sectional imaging made easy. New York: Churchill Livingstone; 2004. p. 3–16.
- 25.Title RS, Harper K, Nelson E, Evans T, Tello R. Observer performance in assessing anemia on thoracic CT. AJR Am J Roentgenol 2005;185(5):1240–4. [DOI] [PubMed]
- 26.Libby P. Inflammation in atherosclerosis. Nature 2002;420 (6917):868–74. [DOI] [PubMed]