Abstract
There is increasing evidence that severe mood disorders are associated with impairment of structural plasticity and cellular resilience. Cumulative data demonstrate that mood stabilizers regulate intracellular signaling cascades, including protein kinase C (PKC), PKA, mitogen-activated protein (MAP) kinase, glycogen synthase kinase 3-β (GSK3-β) and intracellular calcium, which are signaling pathways that regulate synaptic plasticity. In this context, it is noteworthy that a growing body of data indicates that the glutamatergic system, has a major role in neuronal plasticity and cellular resilience, might be involved in the pathophysiology and treatment of mood disorders. AMPA glutamate-receptor trafficking is important in synaptic plasticity and might play crucial roles in maintaining critical neuronal circuits associated with mood. Two clinically effective, structurally dissimilar, antimanic agents, lithium and valproate (VPA), down-regulate synaptic expression of AMPA receptor subunit GluR1 in hippocampus in chronically treated rats. This reduction in synaptic GluR1 by lithium and VPA is due to attenuated phosphorylation of GluR1 at a specific PKA site (residue 845 of GluR1), which is crucial for AMPA receptor insertion. By contrast, imipramine, which can provoke mania, increases synaptic expression of GluR1 in the hippocampus in vivo. Furthermore, there is ample evidence from preclinical and clinical research that the glutamatergic system is involved in the pathophysiology of mood disorders and that many of the somatic treatments used for mood disorders including antidepressants, mood stabilizers, atypical antipsychotic drugs and electroconvulsive therapy have both direct and indirect effects on the glutamatergic system. Given these findings, further research with medications that specifically affect the glutamatergic system is warranted. Recent studies in our lab have shown that riluzole, a FDA approved medicine that regulates the glutamatergic system, shows antidepressant efficacy in unipolar and bipolar depression. These studies indicate that regulation of glutamate-mediated synaptic plasticity might play a role in the treatment of mood disorders, and raise new avenues for novel therapies for this devastating illness.
Keywords: Lithium, valproate, antidepressant, bipolar disorder, glutamatergic system
INTRODUCTION
Bipolar disorder (also known as manic–depressive illness) is a severe, often life-threatening illness, that affects 2.3 million Americans (Goodwin and Jamison, 1990). The costs associated with disability and premature death from major mood disorders represent an economic burden of tens of billions of dollars annually in the United States alone (Simon, 2003). Despite the devastating impact of bipolar disorder on millions worldwide, little is known about underlying etiology and neurobiology. Historically, the brain systems that receive the greatest attention in neurobiological studies of mood disorders are the monoaminergic neurotransmitter systems, which are distributed extensively throughout the network of limbic, striatal and prefrontal cortical neuronal circuits that are thought to support the behavioral and visceral manifestations of mood disorders (Drevets, 2001; Manji et al., 2001; Nestler et al., 2002a). In addition to the growing appreciation that investigation of the pathophysiology of complex mood disorders have focused excessively on monoaminergic systems, there is growing concern that progress in developing truly novel and improved medications has been limited. Recognition of the clear need for better treatments, and the lack of significant advances in our ability to develop novel, improved, therapeutics for these devastating illnesses has led to investigation of the putative roles of intracellular signaling cascades and synaptic plasticity. Recent evidence demonstrating that impairments of neuroplasticity might underlie the pathophysiology of mood disorders, and that antidepressants and mood stabilizers exert major effects on the signaling pathways that regulate cellular plasticity has generated considerable excitement among the clinical neuroscience community and is reshaping views about the neurobiological underpinnings of these disorders (Manji and Lenox, 2000a; Manji et al., 2001; D'Sa and Duman, 2002; Nestler et al., 2002a; Young, 2002).
Signaling networks regulated by mood stabilizers and antidepressants as key modulators of synaptic plasticity
Recent research into the pathophysiology and treatment of mood disorders has moved from a focus on neurotransmitters and cell surface receptors to intracellular signaling cascades. Multi-component cellular signaling pathways interact at various levels, thereby forming complex signaling networks that allow cells to receive internal and external cues, process these cues and respond to information (Fig. 1). These signaling networks enable the integration of signals across multiple time points and the generation of distinct outputs depending on input strength and duration. Moreover, these signaling networks regulate intricate feed-forward and feedback loops (Bourne and Nicoll, 1993; Bhalla and Iyengar, 1999; Weng et al., 1999) (Fig. 1). Given their widespread, crucial role in the integration and fine-tuning of physiological processes, it is not surprising that abnormalities in signaling pathways have been identified in several human diseases.
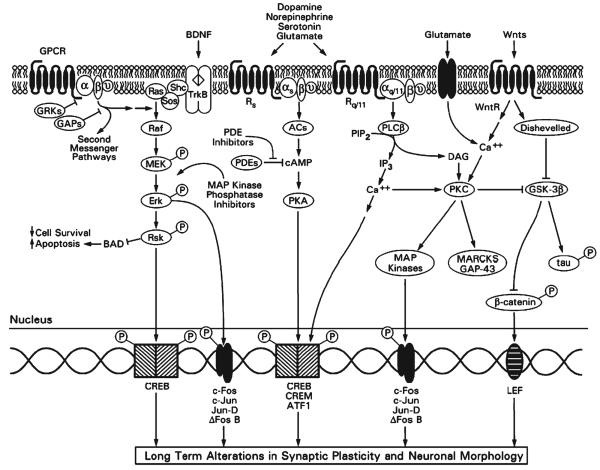
Fig. 1. Major intracellular signaling pathways in bipolar disorder.
Cell-surface receptors transduce extracellular signals such as neurotransmitters and neuropeptides into the interior of the cell. Most neurotransmitters and neuropeptides communicate with other cells by activating seven-transmembrane-spanning G-protein-coupled receptors (GPCRs). As their name implies, GPCRs activate G proteins, which are composed of α and βγ subunits. Two families of proteins turn off the GPCR signal and might, therefore, represent attractive targets for new medication development. G-protein-coupled-receptor kinases (GRKs) phosphorylate GPCRs, thereby uncoupling them from their respective G proteins. GTPase-activating proteins (GAPs, also called RGS or regulators of G-protein-signaling proteins) accelerate the G-protein turn-off reaction (an intrinsic GTPase activity). Two major signaling cascades that are activated by GPCRs are the cAMP-generating second-messenger system and the PI system. cAMP activates PKA, a pathway that has been implicated in the therapeutic effects of antidepressants. Among the potential targets for the development of new antidepressants are phosphodiesterases (PDEs) that catalyze the breakdown of cAMP. Thus, PDE inhibitors are expected to sustain the cAMP signal and might represent an antidepressant-augmenting strategy. Activation of receptors coupled to PI hydrolysis results in the breakdown of phosphoinositide 4,5-biphosphate (PIP2) into two second messengers, inositol 4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP mobilizes Ca2+ from intracellular stores, whereas DAG is an endogenous activator of PKC, which is also activated directly by Ca2+. PKC, PKA and other Ca2+-dependent kinases either directly or indirectly activate several transcription factors, including CREB, CREM, ATF-1, c-Fos, c-Jun, Jun-D and ΔFos B. Endogenous growth factors such as BDNF utilize different types of signaling pathways. BDNF binds to and activates its tyrosine kinase receptor (TrkB), which facilitates the recruitment of other proteins (SHC and SOS) and results in the activation of the ERK–MAP kinase cascade (via sequential activation of Ras, Raf, MEK, Erk and Rsk). In addition to regulating several transcription factors, the ERK–MAP kinase casade, via Rsk, down-regulates BAD, a pro-apoptotic protein. Enhancing the ERK–MAP kinase cascade might have similar effects to endogenous neurotrophic factors; one potential strategy is to utilize inhibitors of MAP kinase phosphatases (to inhibit the turn-off reaction) as potential drugs with neurotrophic properties. In addition to utilizing GPCRs, many neurotransmitters (e.g. glutamate and GABA) produce their responses via ligand-gated ion channels. Although these responses are very rapid, they bring about more stable changes via regulation of gene transcription. One pathway that is gaining increasing attention in adult mammalian neurobiology is the Wnt signaling pathway. Wnts are a group of glycoproteins that are active in development and play important roles in the mature brain. Binding of Wnts to the Wnt receptor (WntR) activates an intermediary protein, Disheveled, which regulates GSK-3β. GSK-3β exerts many cellular effects; it regulates cytoskeletal proteins, including tau, and has an important role in determining cell-survival/cell-death decisions. GSK-3β has been identified recently as a target for the action of lithium. GSK-3β also regulates the phosphorylation of β-catenin, a protein that, when dephosphorylated, acts as a transcription factor at lymphoid enhancer factor (LEF) sites. Additional abbreviations: CREB, cAMP response element binding protein; Rq and Rs, extracellular GPCRs that are coupled to stimulation and inhibition of adenylate cyclases, respectively; Rq/11, GPCR coupled to activation of PLC; MARCKS, myristoylated alanine rich C kinase substrate, which is associated with several neuroplastic events; PLC, phospholipase C.
Signaling pathways also represent major targets for a number of hormones, including glucocorticoids, thyroid hormones and gonadal steroids (Manji, 1992; Speigel, 1998). These biochemical effects might play a role in mediating certain clinical manifestations of altered hormonal levels in mood-disorder subjects (e.g. the frequent onset of bipolar disorder in puberty, triggering of episodes in the postpartum period, association with depression, potentially rapid cycling with hypothyroidism, and triggering of mood episodes in response to exogenous glucocorticoids).
Complex signaling networks might be especially important in the CNS, where they balance and integrate diverse neuronal signals, and transmit them to effectors, thus, forming the basis of a complex information-processing network (Bourne and Nicoll, 1993; Bhalla and Iyengar, 1999; Weng et al., 1999). Highly complex signaling networks might be one mechanism by which neurons acquire the flexibility for generating the wide range of responses observed in the nervous system. These pathways are undoubtedly involved in regulating diverse vegetative functions such as mood, appetite and wakefulness. Therefore, they are likely to be involved in the pathophysiology of bipolar disorder. We now describe direct and indirect evidence supporting a role for signaling pathway abnormalities in the pathophysiology and treatment of bipolar disorder.
The Gs protein cAMP-generating signaling pathway
Several laboratories report abnormalities in G-protein subunits in bipolar patients. Data from postmortem studies of the brains of bipolar patients demonstrate increased levels of the stimulatory G protein (Gas), accompanied by increases in post-receptor stimulated adenylate cyclase activity (Young et al., 1993; Warsh et al., 2000a; Warsh et al., 2000b). These observations are supported by the demonstration of increased agonist-activated (Emamghoreishi et al., 1997) GTPγS binding to G-protein α subunits in the frontal cortical membranes of patients with bipolar disorder (Wang and Friedman, 1996). Several studies find elevated Gαs protein and mRNA levels in peripheral circulating cells in bipolar disorder, but how these levels depend on clinical mood state is unclear (Manji et al., 1995; Mitchell et al., 1997; Lenox and Manji, 1998; Spleiss et al., 1998; Wang and Friedman, 1999; Warsh et al., 2000a).
Recent studies from our laboratory and others show that basal activity of protein kinase A (PKA) is decreased in hippo-campal samples from animals chronically treated animals with either lithium or valproate (VPA) (Du et al. 2004a; reviewed by Gould et al., 2004b). In addition, Perez et al. looked further downstream in this signaling pathway and found higher levels of cAMP-stimulated phosphorylation of an ~22 kDa protein (subsequenty identified as Rap1) in platelets from treated, euthymic, bipolar patients compared with healthy subjects (Perez et al., 2000).
The PKC signaling pathway
The PKC family of enzymes is a group of calcium- and phospholipid-dependent enzymes that comprise a family of closely related kinase subspecies and appears to play a crucial role in regulating synaptic plasticity and various forms of learning and memory (Stabel and Parker, 1991; Nishizuka, 1992; Newton, 1995; Nishizuka, 1995). PKC is one of the major intracellular-signal mediators that is generated after external stimulation of cells via several neurotransmitter receptors (including acetylcholine M1, M3 and M5 receptors, α1 adrenoceptors, metabotropic glutamate receptors and serotonin 5HT2A receptors), which induce the hydrolysis of various membrane phospholipids.
Recent evidence indicates that alterations in PKC activity might play a significant role in mood disorders. Friedman et al. (1993) investigated PKC activity and PKC translocation in response to serotonin in platelets obtained from bipolar disorder patients before and during lithium treatment. They report that the ratio of platelet-membrane-bound:cytosolic PKC activity is elevated in manic subjects. In addition, serotonin-elicited platelet PKC translocation is enhanced in these subjects. Wang and Friedman (1996) measured PKC isozyme levels, activity and translocation in postmortem brain tissue from bipolar disorder patients. They report increased PKC activity and translocation in bipolar disorder brains compared with controls, accompanied by elevated levels of selected PKC isozymes in the cortex.
Evidence from several groups demonstrates clearly that therapeutically relevant concentrations of lithium exert significant effects on the PKC signaling cascade. Data indicate that chronic treatment with lithium attenuates PKC activity and down-regulates the expression of isozymes PKCα and PKCε in the frontal cortex and hippocampus of patients with bipolar disorder (Manji and Lenox, 1999; Manji and Lenox, 2000b). Chronic lithium also dramatically reduces the hippocampal levels of a major PKC substrate, myristoylated alanine-rich C kinase substrate (MARCKS), which has a role in regulating long-term neuroplastic events.
To validate the therapeutic relevance of these biochemical findings, it is noteworthy that the structurally-dissimilar antimanic agent VPA produces similar effects to lithium on PKC α and PKC ε isozymes and the MARCKS protein (Manji and Lenox, 1999; Manji and Lenox, 2000b). Following chronic treatment with lithium, PKC activation was significantly reduced in rat brains, measured by translocation of cytoplasmic PKC to membrane-bound PKC. Lithium and VPA might bring about their effects on the PKC signaling pathway by distinct mechanisms, consistent with clinical observations that some patients show preferential response to one of these agents, and that additive therapeutic effects are often observed in patients when the two agents are co-administered. A recent study has shown that lithium inhibits phorbol 12-myristate 13-acetate-induced PKC activity in the prefrontal cortex, which might have an impact on learning and memory (Birnbaum et al., 2004).
In view of the pivotal role of the PKC signaling pathway in regulating neuronal excitability, neurotransmitter release and long-term synaptic events (Conn and Sweatt, 1994; Hahn and Friedman, 1999), we postulated that the attenuation of PKC activity might play a role in the antimanic effects of lithium and VPA. To test this idea, we piloted a study in seven bipolar manic patients treated with tamoxifen, a non-steroidal anti-estrogen that inhibits PKC inhibitor at higher concentrations (Couldwell et al., 1993). Tamoxifen showed antimanic efficacy in this study (Bebchuk et al., 2000), but because of the small sample size, these results are considered preliminary. However, this preliminary data suggesting the involvement of the PKC signaling system in the pathophysiology of bipolar disorder indicates that PKC inhibitors might be useful in the treatment of mania, and warrant larger, double-blind, placebo-controlled studies of tamoxifen and novel, selective PKC inhibitors.
Glycogen synthase kinase
Glycogen synthase kinase 3 (GSK-3) is a crucial kinase that functions as an intermediary in many intracellular signaling pathways. Recent research indicates the importance of this enzyme in bipolar disorder (Chen et al., 1999; Gould and Manji, 2002). GSK-3 is inhibited directly by the mood stabilizer lithium in concentrations within therapeutical range (~1 mM) (Klein and Melton, 1996). GSK-3 has two, nearly identical isoforms in mammals, the a and b isoforms. GSK-3 is unique among kinases because it is generally constitutively active. Therefore, intracellular signals such as the Wnt pathway, phosphoinositide (PI) 3-kinase pathway, PKA and PKC inactivate this enzyme. Several endogenous growth factors (e.g. nerve growth factor and brain-derived growth factor; BDNF) use the PI 3-kinase signaling cascade as a major effector system. Thus, growth factors might exert many of their neurotrophic and neuroprotective effects in part by inhibiting GSK-3. GSK-3 phosphorylates, and thereby inactivates, many transcription factors, and modulates the function of cytoskeletal proteins such as the Alzheimer's disease protein tau (a previous name for GSK-3 is tau kinase). Thus, inhibition of GSK-3 releases this inhibition and activates multiple cellular targets.
Growing evidence indicates that GSK-3 plays an important role in regulating neuroplasticity and cellular resilience, including synapse formation and axonal growth. Studies indicate that changes in GSK-3-mediated phosphorylation of mitogen-activated protein-1B (a cytoskeletal protein) are associated with the loss and/or unbundling of stable axonal microtubules (Lucas et al., 1998). Furthermore, inhibition of GSK-3β results in accumulation of synapsin I, which is involved in synaptic vesicle docking and release of growth cone-like areas (Lucas and Salinas, 1997). Recent data also show that VPA protects cells from endoplasmic reticulum (ER)-stress-induced lipid accumulation and apoptosis by inhibiting GSK-3 (Kim et al., 2005).
Amphetamine-induced hyperactivity is the most established rodent model for mania; this hyperactivity is attenuated by a number of mood stabilizers including lithium, anticonvulsants, and antipsychotics. Recently, Beaulieu et al. reported that dopamine-dependent activity increases in mice are mediated via a GSK-dependent mechanism, and that lithium and other inhibitors of GSK-3 attenuate the hyperactivity in mice that lack the dopamine transporter (Beaulieu et al., 2004). We have also found that peripheral administration of a GSK-3 inhibitor decreases amphetamine-induced hyperactivity in rats (Gould et al., 2004b). Taken together, these data support the possibility that inhibition of GSK-3 might be involved in the antimanic effects of lithium.
ERK MAP kinase pathways
Studies from our lab demonstrate that therapeutically relevant concentrations of lithium and VPA activate the extracellular-signal related kinase (ERK) mitogen-activated protein (MAP) kinase cascade in human neuroblastoma SH-SY5Y cells and in crucial limbic and limbic-related areas of rodent brain (Yuan et al., 2000; Einat et al., 2003). MAP kinase is involved in neurotrophic factor signaling pathways, promoting cell survival, and might have important roles in neuroprotection and neuroplasticity in mood disorders (reviewed by Du et al., 2003).
Calcium-signaling abnormalities
Calcium ions play a crucial role in regulating the synthesis and release of neurotransmitters, neuronal excitability and long-term neuroplastic events and, therefore, it is not surprising that several studies have investigated the role of intracellular Ca2+ in peripheral cells in bipolar disorder. Intracellular Ca2+ signaling and homeostasis are maintained by an intricate array of processes that act in concert including, for example, inositol trisphosphate (IP3)- and ryanodine-stimulated release of Ca2+ from ER storage pools, voltage- and ligand-gated ion-channel-mediated Ca2+ influx, store-operated Ca2+ entry, plasma membrane Ca2+–ATPase pumps, sarcoplasmic–ER Ca2+–ATPase pumps, and mitochondrial Ca2+ uptake, storage and release (reviewed by Pisani et al., 2004). Regardless of the complexity of intracellular Ca2+ regulation, impaired regulation of Ca2+ cascades is the most reproducible biological abnormality described in bipolar disorder research. For this reason, mechanisms involved in Ca2+ regulation are postulated to underlie aspects of the pathophysiology of bipolar disorder. To date, cumulative studies consistently revealed elevations in basal intracellular Ca2+ concentrations in platelets, lymphocytes and neutrophils of patients with bipolar disorder (Emamghoreishi et al., 1997).
Post-mortem brain studies have also revealed changes that might reflect possible ‘signatures’ of abnormal Ca2+ homeostasis in bipolar disorder. These include a marked blunting of G-protein-activated PI hydrolysis (Jope et al., 1996) and altered mRNA expression of two candidate proteins that might have important roles in Ca2+ homeostasis. These are inositol monophosphatase (IMPase) type II (Yoon et al., 2001a) and a transient receptor potential channel, TRPM2 (TRPC7 in earlier nomenclature) (Yoon et al., 2001b), which is a ligand-gated plasma membrane ion channel that also mediates Ca2+ entry into cells. In addition, lithium and VPA inhibit IMPase (Hallcher and Sherman, 1980), which has been suggested to diminish overactive signaling through PI-linked second messengers and, in turn, Ca2+ (Berridge et al., 1982).
In summary, mood disorders are associated with alterations in signaling networks that might, subsequently, regulate synaptic plasticity and cell resilience of neuronal circuitry associated with affective disorders. It is noteworthy that modulation of synaptic plasticity by signaling cascades has been studied extensively in the glutamatergic system, specifically for α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor trafficking.
AMPA receptor trafficking has crucial roles in regulating forms of neural plasticity
Surprisingly, the potential role of the glutamatergic system in the pathophysiology and treatment of bipolar disorder has only recently started to be evaluated. Glutamate is the major excitatory synaptic neurotransmitter and regulates numerous physiological functions in the mammalian CNS such as synaptic plasticity, learning and memory. It is a major neurotransmitter system in the circuitry that is thought to subserve many of the symptoms of severe, recurrent, mood disorders (Drevets, 2001). Three major classes of ionotropic glutamate receptors are expressed throughout the mammalian CNS including AMPA, kainate and N-methyl-D-aspartate (NMDA) receptors (Fig. 2). AMPA receptors mediate the majority of excitatory synaptic transmission in the CNS; the AMPA R channel is composed of the combination of GluR1, GluR2, GluR3 and GluR4 subunits. The AMPA receptor is stimulated by the presence of glutamate and, characteristically, produces a fast excitatory synaptic signal that is responsible for the initial reaction to glutamate in the synapse (Fig. 2).
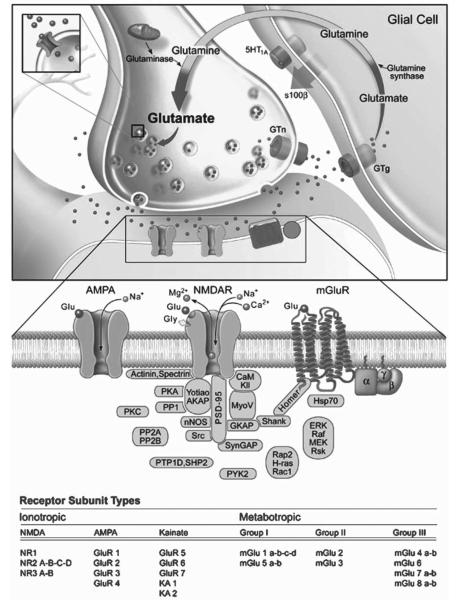
Fig. 2. The regulatory processes involved in glutamatergic neurotransmission.
The biosynthetic pathway for glutamate involves synthesis from glucose and the transamination of α-ketoglutarate; however, a small proportion of glutamate is formed more directly from glutamine by glutamine synthetase. The latter is synthesized in glia and, via an active process (requiring ATP), is transported to neurons where glutaminase converts this precursor to glutamate. In astrocytes, glutamine is oxidized to α-ketoglutarate which can also be transported to neurons and participate in glutamate synthesis. Glutamate is either metabolized or it is sequestered and stored in secretory vesicles by vesicular glutamate transporters (VGluTs). Glutamate is then released by a calcium-dependent excitotoxic process. Once released from the presynaptic terminal, glutamate binds to many excitatory amino acid (EAA) receptors, including ionotropic (e.g. AMPA and NMDA) and metabotropic receptors. Presynaptic regulation of glutamate release occurs through metabotropic glutamate receptors (mGluR2/3), which act as autoreceptors. However, these receptors are also located on the postsynaptic element. The action of glutamate is terminated in the synapse by reuptake mechanisms that utilize distinct transporters (GLUTs) which exist on presynaptic nerve terminals and astrocytes. Current data indicate that astrocytic uptake of glutamate might be more important for clearing excess glutamate, raising the possibility that astrocytic loss (as documented in mood disorders) might contribute to deleterious glutamate signaling. It is known that there several intracellular proteins alter the function of glutamate receptors (see diagram).
The NMDA receptor is activated by glutamate, a co-agonist (either glycine or D-serine) and depolarization, to open and permit the entry of Na+ and Ca2+ (Fig. 2). The NMDA receptor channel is composed of combination of NR1, NR2A, NR2B, NR2C, NR2D, NR3A and NR3B subunits (Fig. 2). It is generally believed that activation of the AMPA receptor results in neuronal depolarization sufficient to liberate the Mg2+ cation from the NMDA receptor, thereby permitting its activation. NMDA receptors play a crucial role in regulating synaptic plasticity (Malenka and Nicoll, 1999), including AMPA receptor trafficking. The best-studied forms of synaptic plasticity in the CNS are long-term potentiation (LTP) and long-term depression (LTD) of excitatory synaptic transmission. The molecular mechanisms of LTP and LTD are characterized extensively and proposed to represent cellular models of learning and memory (Malenka and Nicoll, 1999). During NMDA-receptor-dependent synaptic plasticity, Ca2+ influx through NMDA receptors can activate a wide variety of kinases and/or phosphatases that, in turn, modulate synaptic strength (Fig. 2).
Recent data indicate that AMPA receptor trafficking, including receptor insertion, internalization and delivery to synaptic sites provides an elegant mechanism for activity-dependent regulation of synaptic strength. AMPA receptor subunits undergo constitutive endocytosis and exocytosis; however, the process is highly regulated and several signal transduction cascades can produce short- and long-term changes in expression of AMPA receptor subunits at the synaptic surface (Malenka and Nicoll, 1999). Although the mechanisms of LTP and LTD have not been elucidated completely, it is widely accepted that AMPA-receptor trafficking is the key player in these phenomena.
Regulation of AMPA-receptor trafficking by signaling cascades
Most vesicle trafficking requires the ordered coating of a donor membrane, budding and fusion to form transport vesicles, transport by either passive or active delivery along microtubules and, finally, fusion with the target membrane (Antonny and Schekman, 2001). This mechanism delivers AMPA receptors to the neuronal membrane surface. Each subunit of the AMPA receptor is composed of an N-terminal extracellular domain, a membrane-spanning domain, and a C-terminal intracellular domain (Bennett and Dingledine, 1995; Wo et al., 1995). AMPA-receptor trafficking is subunit-specific, and regulated by phosphorylation of its C-terminal domain and subsequent alteration of protein–protein interactions.
PKA pathway
The GluR1 subunit appears to govern the trafficking behavior of heteromeric GluR1/GluR2 receptors, by preventing constitutive exchange and conferring inducible delivery of the heteromer (Shi et al., 2001). Phosphorylation of GluR1at the PKA site P845 facilitates the insertion of GluR1 onto the membrane and synapses, and is often associated with LTP (Lee et al., 2000). Dephosphorylation of the GluR1 by protein phosphatases (e.g. calcineurin and PP1) target GluR1 to recycling endosomes, where re-phosphorylation by PKA might occur and reinsertion of the receptors onto the membrane (Ehlers, 2000). Phosphorylation of GluR1 at the PKA site is enhanced by synapse associated protein 97 (SAP97)/protein A kinase anchoring protein (AKAP79) complex, which directs PKA to GluR1 via a PDZ-domain interaction (Colledge et al., 2000).
A growing body of data indicates the importance of this PKA phosphorylation site of GluR1 in psychiatric disorders. Very recent studies also demonstrate that chronic administration of antidepressant enhances the membrane expression of GluR1 as well as phosphorylation of GluR1 at this PKA site (P845) (Martinez-Turrillas et al., 2002; Svenningsson et al., 2002). It has also been shown that dopamine, which is a neurotransmitter with psychostimulant effects, binds to D1 receptors and enhances the surface expression of GluR1 receptor through activation of PKA at this site and AKAP79 (Mangiavacchi and Wolf, 2004).
Calcium/calmodulin-dependent kinase II (CaMKII) pathway
Numerous studies demonstrate that CaMKII is required for the proper formation of LTP in slice preparations, and in regulating learning and memory in rodents (Fink and Meyer, 2002). In response to stimulation, CaMKII translocates to postsynaptic sites, where it has two major effects on AMPA receptor activity at the post-synaptic density during the formation of LTP (Fink and Meyer, 2002). First, the AMPA single conductance is increased directly by CaMKII at Ser831 of the GluR1 subunit (Derkach et al., 1999). Second, CaMKII is required for delivery of the AMPA receptor to the synapse, which lacks AMPA receptors (Shi et al., 1999; Liao et al., 2001; Shi et al., 2001). Enhancement of synaptic GluR1 levels by activation of CaMKII requires an intact C-terminal domain of GluR1, and might involve interaction with SAP97 (Hayashi et al., 2000). Protein phosphatase 1 (PP1), an important modulator of learning and memory, can dephosphorylate the phosphorylation by CaMKII of the P831 site of GluR1 (Genoux et al., 2002). It is noteworthy that this CaMKII site of GluR1 is phosphorylated after antidepressant treatment (Svenningsson et al., 2002) and CaMKII might also be involved in the pathophysiology of mood disorders (Du et al., 2004b).
ERK MAP kinase pathway
A recent study reported that the small GTPases Ras and Rap are involved in AMPA-receptor trafficking through a post-synaptic signaling mechanism. Ras mediates activity evoked increases in AMPA receptors that contain GluR1 and GluR4 at the synaptic surface via a pathway that requires p42/44 MAPK activation. By contrast, Rap mediates NMDA-dependent removal of synaptic GluR2/GluR3-containing vesicles via a pathway that involves p38 MAP kinase. Regulation through Ras and Rap, which work as molecular switches, might, in turn, control the AMPA-receptor level at synapses (Zhu et al., 2002).
PKC pathways
AMPA GluR2 receptors respond to secondary signals by constitutive receptor recycling. Phosphorylation of Ser880 on GluR2 causes a switch from receptor retention at the membrane by binding to ABP/GRIP, to receptor internalization by binding to PICK 1. Therefore, phosphorylation of GluR2 at Ser880 by PKC might release the AMPA receptor from anchoring proteins and initiate the internalization of receptors (Matsuda et al., 2000; Xia et al., 2000; Kim et al., 2001; Perez et al., 2001).
The mechanism of AMPA-receptor trafficking is specific for brain region and type of neuron. For example, the endocytosis of AMPA receptors that mediate LTD is triggered by different signaling cascades in different cell types, despite the fact that a conserved cell-biological mechanism (i.e. clathrin/dynamine-dependent endocytosis) appears to be involved. Specifically, in CA1 pyramidal cells, protein phosphatases are involved in triggering LTD through dephosphorylation of GluR1. Phosphorylation of the PKA site on GluR1 is associated with LTP (Ehlers, 2000). However, in midbrain dopamine cells, activation of PKA appears to trigger LTD and endocytosis of AMPA receptors (Gutlerner et al., 2002). Most importantly for the present discussion, AMPA-receptor trafficking is highly regulated by PKA, PKC, CaMKII and MAP kinase signaling cascades (reviewed by Du et al., 2004c). These are the same signaling cascades on which mood stabilizers and antidepressants exert major effects (Gould et al., 2004a; Gould et al., 2004b; Payne et al., in press; Popoli et al., 2003). These observations have led to an extensive series of studies that demonstrate that AMPA receptor trafficking is highly regulated by anti-depressants and mood stabilizers (Gray et al., 2003; Du et al., 2004a).
Mood stabilizers modulate synaptic plasticity by regulating AMPA-receptor trafficking
In view of the crucial role of AMPA-receptor trafficking in regulating various forms of plasticity, our laboratory has sought to determine if two structurally dissimilar anti-manic agents, lithium and VPA, exert effects on AMPA-receptor trafficking. Lithium, a monovalent cation, and VPA, an eight-carbon fatty acid, are the two most commonly used agents in the treatment of mania. Because lithium and VPA require several weeks to exert their therapeutic effects, it is widely believed that adaptive changes in intracellular signaling and/or cellular physiology underlie their beneficial effects. These two agents exert robust effects on the signaling pathways that regulate AMPA-receptor trafficking. Thus, we investigated if lithium and VPA regulate synaptic plasticity and AMPA-receptor trafficking in the hippocampus, a brain region that is presumed to be involved in the circuitry of mood disorders.
We find that lithium and VPA have a common effect of down-regulating AMPA GluR1 synaptic expression in the hippocampus after prolonged treatment with therapeutically relevant concentrations both in vitro and in vivo (Fig. 3). However, total protein concentrations of GluR1 and synaptophysin remain unchanged after lithium and VPA treatment in vitro and in vivo. In hippocampal neurons in culture, lithium and VPA attenuate surface expression of GluR1 after long-term treatment (Du et al., 2004a) and phosphorylation of a specific PKA site (GluRP845) is attenuated significantly by lithium and VPA treatment (by 52 and 33%, respectively). Sp-cAMP treatment reversed the attenuation of phosphorylation by lithium and VPA and returned GluR1s to the surface, which indicates that phosphorylation of GluRP845 is involved in the mechanism of GluR1 surface attenuation (Du et al., 2004a). To support the therapeutic relevance of these findings, we found the antidepressant imipramine, which provokes mania, has an opposite effect as it up-regulates AMPA synaptic strength in the hippocampus (Fig. 4).
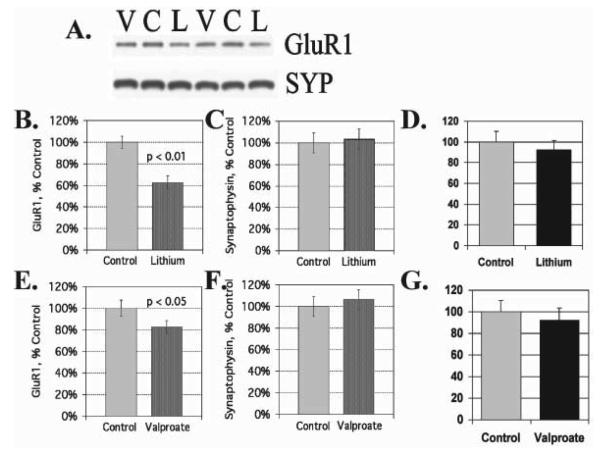
Fig. 3. Attenuation of AMPA receptor subunit GluR1 in synaptosomal preparations from animals treated long-term with lithium and VPA.
(A) Western blot analysis of hippocampal synaptosomal preparation from animals treated with either lithium or VPA with anti-GluR1 and anti-synaptophysin antibodies. (B) Quantification of GluR1 in hippocampal synaptosomes from lithium-treated (n = 5) and control animals (n = 6) (T-test, P < 0.01). (C) Quantification of synaptophysin in hippocampal synaptosomal preparations from lithium-treated and control animals. (D) Total GluR1 concentration in hippocampal tissue homogenates from lithium-treated and control animals. (E) GluR1 protein in hippocampal synaptosomes from VPA-treated (n = 8) and control animals (n = 7) (T-test, P < 0.05). (F) Synaptophysin in hippocampal synaptosomal preparations from VPA-treated and control animals. (G) Total GluR1 concentrations in hippocampal tissue homogenates from VPA-treated and control animals.
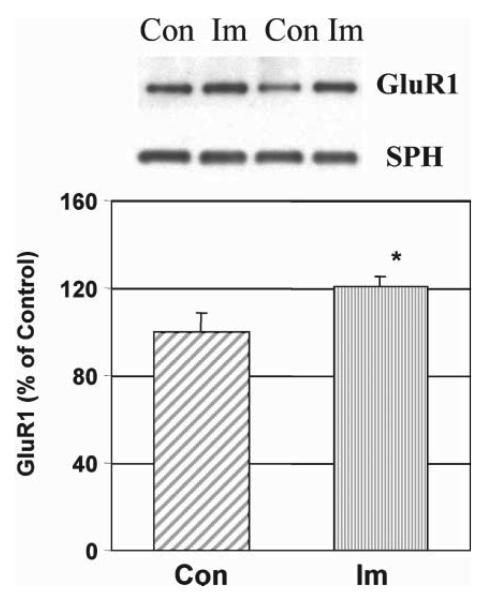
Fig. 4. Imipramine increases synaptic GluR1 in vivo.
Rats were treated with imipramine (Im; 10 mg kg−1, twice daily, intraperitoneal injection) for 4 weeks and hippocampal tissue isolated and synaptosome preparations obtained using Ficoll gradients. Equal amount of proteins were separated by gel electrophoresis and analyzed with anti-GluR1 antibodies and anti-synaptophysin (SPH) antibodies. The bands were analyzed by Kodak imaging system. n = 8, T-test, P < 0.05.
Further supporting our data are recent studies showing that AMPA receptor antagonists attenuate several ‘manic like’ behaviors produced by amphetamine administration. Thus, AMPA antagonists attenuate psychostimulant-induced development or expression of sensitization and hedonic behavior without affecting spontaneous locomotion. Additionally, some studies demonstrate that AMPA receptor antagonists reduce amphetamine/cocaine-induced hyperactivity (Burns et al., 1994; Li et al., 1997; Mead and Stephens, 1998; Hotsenpiller et al., 2001; Backstrom and Hyytia, 2003; Tzschentke, 2003). The need for caution in the appropriate application of animal models to complex neuropsychiatric disorders is well articulated, and it is unlikely we will ever develop rodent models that display the full range of symptoms that are observed clinically in humans (Nestler et al., 2002b ; Einat et al., 2003). However, one current model of mania that is used extensively and has reasonable heuristic value in the study of mood disorders involves the use of psychostimulants in appropriate paradigms. Thus, psychostimulants like amphetamine and cocaine induce manic-like symptoms in healthy volunteers, and trigger frank manic episodes in individuals with bipolar disorder (Goodwin and Jamison, 1990). Thus, the best-established animals of mania utilize the administration of either amphetamine or cocaine to produce hyperactivity, risk-taking behavior and increased hedonic drive, which are all important facets of the clinical condition of mania in humans. Moreover, these psychostimulant-induced behavioral changes are attenuated by the administration of chronic lithium in a therapeutically relevant time-frame. Thus, the fact that AMPA receptor antagonists can attenuate psychostimulant-induced sensitization, hyperactivity and hedonic behavior (Burns et al., 1994; Li et al., 1997; Mead and Stephens, 1998; Hotsenpiller et al., 2001; Backstrom and Hyytia, 2003; Tzschentke, 2003) provides compelling behavioral support for our contention that AMPA receptors have important roles in regulating affective behavior. Taken together, the biochemical and behavioral studies that investigate the effects of antimanic (lithium and VPA) and pro-manic (antidepressants, cocaine and amphetamine) agents on GluR1 indicate strongly that AMPA-receptor trafficking is an important target in the pathogenesis and treatment of some facets of bipolar disorder.
Role of the glutamatergic system in treatment of mood disorders
The noradrenergic and serotonergic hypothesis of mood disorders, which was developed from the pharmacological effects of early drugs, no longer provides satisfactory explanations of the mode of action of all antidepressant agents and the underlying pathophysiology of depression. Several agents that are associated with glutamatergic systems demonstrate antidepressant efficacy in clinical studies. In the 1950s, Dcycloserine (DCS), which is a partial agonist at the NMDA receptor glycine site and used as a part of multidrug antituberculosis treatment, was reported to have mood elevating effects (Crane, 1959; Crane, 1961; Heresco-Levy and Javitt, 1998). Subsequent studies with DCS have reported cases of euphoria and amnestic effects in humans that resemble the effects of subperceptual doses of non-competitive NMDA antagonists (Krystal et al., 1994). Early evidence that NMDA antagonism might be important for antidepressant effects in humans comes from case series and blinded trials conducted with the non-competitive NMDA antagonist amantadine. Amantadine has antidepressant effects in patients with Parkinson's disease, and in unipolar and bipolar patients (Parkes et al., 1970; Vale et al., 1971; Rizzo and Morselli, 1972) (Fig. 5). Recently, Berman et al. (2000) reported the first placebo-controlled, double-blind trial to assess the effects of a single dose of the NMDA receptor antagonist ketamine in seven patients with depression (Fig. 5). Ketamine is a high-affinity, NMDA-receptor antagonist with lower, but potentially relevant, affinity for the μ opiate receptor and weak antagonist activity at the dopamine receptor (Wong et al., 1996) (Fig. 5). Perhaps the greatest evidence that glutamate modulation might be important in the pathophysiology of mood disorders comes from the clinical use of the anticonvulsant lamotrigine. Recently, several double-blind, placebo-controlled studies, report that lamotrigine is effective in bipolar depression (Calabrese et al., 1999). Although the exact mechanism of action of lamotrigine is unknown, inhibition of excessive release of glutamate is postulated as a likely mechanism of action (Leach et al., 1986; Calabresi et al., 1996; Wang et al., 1996).
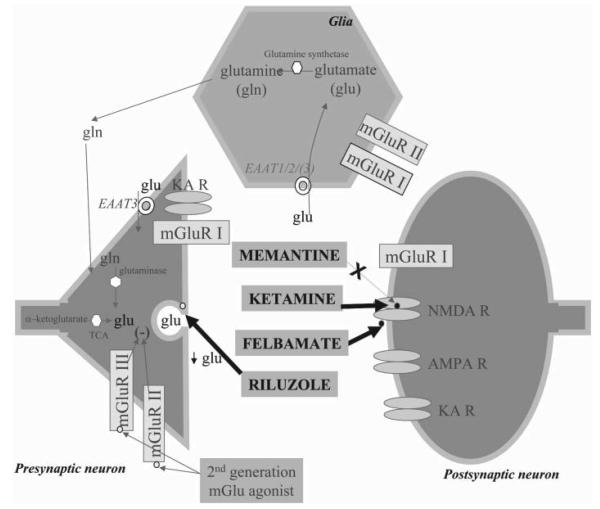
Fig. 5. Antiglutamatergic agents as putative treatments for mood disorders.
Activation of the AMPA receptor (AMPA R) by glutamate depolarizes the membrane. When glutamate and glycine are present, depolarization results in the release of magnesium from the NMDA receptor (NMDA R) channel. Calcium also enters through the NMDA R pore. In addition, interchange of cations occurs via NMDA R and kainate receptors (KA R). Activation of group I metabotropic glutamate receptors (mGlu I), which are couped to a G protein (Gq/11), activates phospholipase C-β (PLC-β). Activation of group II metabotropic glutamate receptors (mGlu II), which are coupled to either Gi or Go, inhibit adenylate cyclase (AC) and opening of potassium channels (not shown), respectively. Glutamate is synthesized in neuron from α-ketoglutarate through the tricarboxylic acid cycle (TCA). After release, glutamate is sequestered by glutamate transporters (EAAT1/2/3), shown in glia and a presynaptic neuron (EAAT 3). In glia, the enzyme glutamine synthetase catabolizes glutamate to glutamine, which diffuses to neurons and is metabolized to glutamate through the enzyme glutaminase. The different glutamate receptors and the presumed antiglutamatergic drug site of action is presented. Memantine is a non-competitive antagonist at the NMDA receptor. Felbamate is an anticonvulsant, a non-competitive NMDA receptor antagonist (NR1/NR2B-specific), and inhibits the release of glutamate (acting through blockade of Ca2+ and Na+ voltage-dependant channels). Riluzole inhibits glutamate release through blockade of Ca2+ and Na+ voltage-dependant channels. Zinc is an endogenous modulator of ligand- and voltage-gated ion channels. The second generation of mGlu group II and III receptors agonist is also depicted.
In addition, a recent study has also suggested the potential of AMPA-receptor modulation in the treatment of mood disorder because an AMPA-receptor potentiator has anti-depressant effects in animal models (Zarate et al., 2003; Du et al., 2004c). As discussed in this review, the mood stabilizers lithium and VPA appear to attenuate glutamatergic function through multiple mechanisms. Repeated administration of lithium, the prototype mood stabilizer, might promote the uptake of glutamate from the synapse (Dixon and Hokin, 1998), attenuate the function of glutamate receptors (Nonaka et al., 1998; Du et al., 2004a) and reduce the function of intracellular signaling cascades that are activated by the binding of glutamate to its receptors (Manji and Lenox, 1999).
Given the preclinical and clinical data that supports a role for the glutamatergic system in the pathophysiology of mood disorders, several therapeutic agents that act on this system should be explored as potential treatments for mood disorders. These agents include riluzole, memantine, felbamate and zinc. Riluzole is a neuroprotective agent with anticonvulsant properties that easily crosses the blood–brain barrier (Benavides et al., 1985). It inhibits the release of glutamate rather than acting as an NMDA antagonist and is one of the few antiglutamatergic agents available for use in humans. Currently, it is used in patients with amyotrophic lateral sclerosis, a severe, debilitating condition, and has a favorable side-effect profile. These characteristics make it an ideal candidate to test in patients with mood disorders (Zarate et al., 2003). A recent study from our laboratory has demonstrated that riluzole in combination with lithium is effective in bipolar depression (Fig. 6) (Zarate et al., 2005), which indicates that riluzole might have antidepressant efficacy in subjects with bipolar depression. Memantine is a potent, non-competitive, voltage-dependent NMDA antagonist that easily crosses the blood–brain barrier. It has a receptor effect that is comparable to MK801 (Bormann, 1989) and inhibits (Jope et al., 1996) the binding of MK801 to human hippocampal NMDA receptors (Berger et al., 1994). Memantine is one of the few NMDA antagonists available for use in humans and is ideal for testing in mood disorders because it has been used clinically for many years with minimal side-effects (Kornhuber et al., 1994) and has a favorable pharmacological profile. Felbamate (2-phenyl-1, 3-propanediol dicarbamate) is a unique, broad-spectrum anticonvulsant (Brown and Aiken, 1998) (Fig. 5). Kass reported substantial improvement with felbamate in a patient with severe bipolar disorder (Kass, 1994) (Fig. 5). However, the use of felbamate is limited by the increased risk of aplastic anemia. Zinc (ZnSO4) is a potent inhibitor of the NMDA-receptor complex; it plays an important role in a wide range of biochemical processes (Harrison and Gibbons, 1994) and might have a role in the treatment of mood disorders.
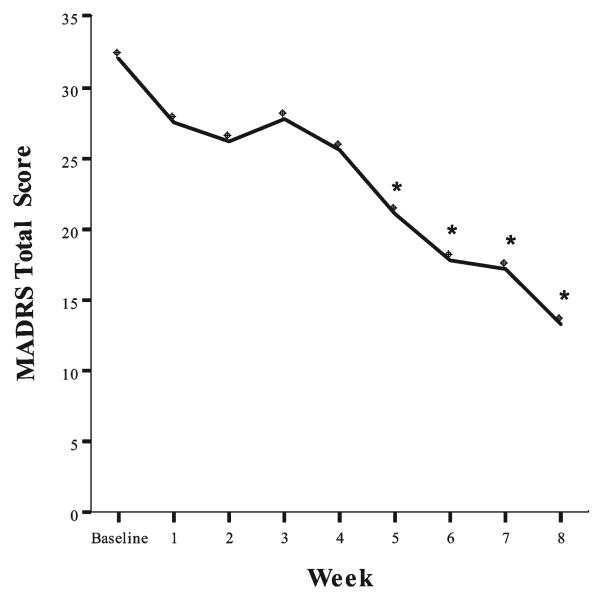
Fig. 6. Effect of riluzole in patients with treatment-resistant bipolar depression.
After open treatment with lithium for a minimum of 4 weeks, subjects who continued to have a Montgomery-Asberg Depression Rating Scale (MADRS) rating scale score >20, received riluzole (50–200 mg day−1) for 8 weeks. In all, 14 bipolar depressed patients entered the study. The linear mixed models for total MADRS showed a significant treatment effect (n = 14, *P < 0.05 compared to baseline). No switch into hypomania or mania was observed and riluzole was well tolerated overall.
Concluding remarks
For many years, a considerable body of data has supported the notion that the mono-amine neurotransmitter systems are dysfunctional in bipolar disorder. This explains why these systems are a common target for pharmacological interventions. However, conceptual and experimental evidence indicate that abnormalities in the regulation of signal-transduction cascades and neuroplasticity might be more primary causes of the pathophysiology of bipolar disorder. Thus, it is noteworthy that growing evidence from preclinical and clinical research implicates the glutamatergic system in the pathophysiology and treatment of mood disorders. For a complex disease like bipolar disorder, it is not surprising that many molecules involved in network of signaling cascades and synaptic plasticity are regulated. The finding that mood stabilizers regulate synaptic AMPA receptors in therapeutically meaningful paradigms opens potential avenues for the development of new drugs to regulate glutamatergic synaptic strength in crucial neuronal circuits. The development of new modulators of glutamatergic neurotransmission might lead to improved therapeutics to treat these devastating mood disorders.
ACKNOWLEDGEMENTS
We would like to thank Patricia J. Williams and Mrs Holly Giesen for outstanding assistance.
REFERENCES
- Antonny B, Schekman R. ER export: public transportation by the COPII coach. Current Opinion in Cell Biology. 2001;13:438–443. doi: 10.1016/s0955-0674(00)00234-9. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Attenuation of cocaine-seeking behaviour by the AMPA/kainate receptor antagonist CNQX in rats. Psychopharmacology (Berl) 2003;166:69–76. doi: 10.1007/s00213-002-1312-y. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proceedings of the National Academy of Sciences of the U.S.A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebchuk JM, Arfken CL, Dolan-Manji S, Murphy J, Hasanat K, Manji HK. A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Archives of General Psychiatry. 2000;57:95–97. doi: 10.1001/archpsyc.57.1.95. [DOI] [PubMed] [Google Scholar]
- Benavides J, Camelin JC, Mitrani N, Flamand F, Uzan A, Legrand JJ. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission–II. Biochemical properties. Neuropharmacology. 1985;24:1085–1092. doi: 10.1016/0028-3908(85)90196-0. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995;14:373–384. doi: 10.1016/0896-6273(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Berger W, Deckert J, Hartmann J, Krotzer C, Kornhuber J, Ransmayr G. Memantine inhibits [3H]MK-801 binding to human hippocampal NMDA receptors. Neuroreport. 1994;5:1237–1240. doi: 10.1097/00001756-199406020-00020. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biological Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochemical Journal. 1982;206:587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla US, Iyengar R. Emergent properties of networks of biological signaling pathways. Science. 1999;283:381–387. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Bormann J. Memantine is a potent blocker of N-methyl-D-aspartate (NMDA) receptor channels. European Journal of Pharmacology. 1989;166:591–592. doi: 10.1016/0014-2999(89)90385-3. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Nicoll R. Molecular machines integrate coincident synaptic signals. Cell. 1993;72(Suppl):65–75. doi: 10.1016/s0092-8674(05)80029-7. [DOI] [PubMed] [Google Scholar]
- Brown WM, Aiken SP. Felbamate: clinical and molecular aspects of a unique antiepileptic drug. Critical Reviews in Neurobiology. 1998;12:205–222. doi: 10.1615/critrevneurobiol.v12.i3.30. [DOI] [PubMed] [Google Scholar]
- Burns LH, Everitt BJ, Kelley AE, Robbins TW. Glutamate-dopamine interactions in the ventral striatum: role in locomotor activity and responding with conditioned reinforcement. Psychopharmacology (Berl) 1994;115:516–528. doi: 10.1007/BF02245576. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. Journal of Clinical Psychiatry. 1999;60:79–88. doi: 10.4088/jcp.v60n0203. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Siniscalchi A, Pisani A, Stefani A, Mercuri NB, Bernardi G. A field potential analysis on the effects of lamotrigine, GP 47779, and felbamate in neocortical slices. Neurology. 1996;47:557–562. doi: 10.1212/wnl.47.2.557. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. Journal of Neurochemistry. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Conn P, Sweatt J. Protein Kinase C in the Nervous System. In: Kuo J, editor. Protein Kinase C. Oxford University Press; 1994. pp. 199–235. [Google Scholar]
- Couldwell WT, Weiss MH, DeGiorgio CM, Weiner LP, Hinton DR, Ehresmann GR, et al. Clinical and radiographic response in a minority of patients with recurrent malignant gliomas treated with high-dose tamoxifen. Neurosurgery. 1993;32:485–489. doi: 10.1227/00006123-199303000-00034. discussion 489–490. [DOI] [PubMed] [Google Scholar]
- Crane G. Cycloserine as an antidepressant agent. American Journal of Psychiatry. 1959;115:1025–1026. doi: 10.1176/ajp.115.11.1025. [DOI] [PubMed] [Google Scholar]
- Crane G. The psychotropic effect of cycloserine: a new use of an antibiotic. Comprehensive Psychiatry. 1961;2:51–59. [Google Scholar]
- D'Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disorders. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulinkinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proceedings of the National Academy of Sciences of the U.S.A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JF, Hokin LE. Lithium acutely inhibits and chronically up-regulates and stabilizes glutamate uptake by presynaptic nerve endings in mouse cerebral cortex. Proceedings of the National Academy of Sciences of the U.S.A. 1998;95:8363–8368. doi: 10.1073/pnas.95.14.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Du J, Gould T, Manji HK. Neurotrophic signaling in mood disorders. In: Finkel T, Gutkind JS, editors. Signal Transduction and Human Disease. John Wiley and Sons; 2003. pp. 411–446. [Google Scholar]
- Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. Journal of Neuroscience. 2004a;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Szabo ST, Gray NA, Manji HK. Focus on CaMKII: a molecular switch in the pathophysiology and treatment of mood and anxiety disorders. International Journal of Neuropsychopharmacology. 2004b;7:243–248. doi: 10.1017/S1461145704004432. [DOI] [PubMed] [Google Scholar]
- Du J, Gray NA, Gould TD, Zerate C, Manji HK. Regulation of cellular plasticity and resilience by mood stabilizers: the role of AMPA receptor trafficking. Dialogues in Clinical Neuroscience. 2004c;5:143–155. doi: 10.31887/DCNS.2004.6.2/jdu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. Journal of Neuroscience. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamghoreishi M, Schlichter L, Li PP, Parikh S, Sen J, Kamble A, et al. High intracellular calcium concentrations in transformed lymphoblasts from subjects with bipolar I disorder. American Journal of Psychiatry. 1997;154:976–982. doi: 10.1176/ajp.154.7.976. [DOI] [PubMed] [Google Scholar]
- Fink CC, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Current Opinion in Neurobiology. 2002;12:293–299. doi: 10.1016/s0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- Friedman E, Hoau Yan W, Levinson D, Connell TA, Singh H. Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biological Psychiatry. 1993;33:520–525. doi: 10.1016/0006-3223(93)90006-y. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness. Oxford University Press; 1990. [Google Scholar]
- Gould TD, Chen G, Manji HK. In Vivo Evidence in the Brain for Lithium Inhibition of Glycogen Synthase Kinase-3. Neuropsychopharmacology. 2004a;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. Signaling networks in the pathophysiology and treatment of mood disorders. Journal of Psychosomatic Research. 2002;53:687–697. doi: 10.1016/s0022-3999(02)00426-9. [DOI] [PubMed] [Google Scholar]
- Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Molecular Psychiatry. 2004b;9:734–755. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- Gray N, Du J, Falke C, Yuan P, Manji H. Lithium regulates total and synaptic expression of the AMPA Glutamate Receptor GluR2 in vitro and in vivo. Annals of the New York Academy of Science. 2003;1003:402–404. doi: 10.1196/annals.1300.036. [DOI] [PubMed] [Google Scholar]
- Gutlerner JL, Penick EC, Snyder EM, Kauer JA. Novel protein kinase A-dependent long-term depression of excitatory synapses. Neuron. 2002;36:921–931. doi: 10.1016/s0896-6273(02)01051-6. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Friedman E. Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disorders. 1999;1:81–86. doi: 10.1034/j.1399-5618.1999.010204.x. [DOI] [PubMed] [Google Scholar]
- Hallcher LM, Sherman WR. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. Journal of Biological Chemistry. 1980;255:10896–10901. [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC. The role of N-methyl-Daspartate (NMDA) receptor-mediated neurotransmission in the pathophysiology and therapeutics of psychiatric syndromes. European Neuropsychopharmacology. 1998;8:141–152. doi: 10.1016/s0924-977x(97)00050-3. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. European Journal of Neuroscience. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Jope RS, Song L, Li PP, Young LT, Kish SJ, Pacheco MA, et al. The phosphoinositide signal transduction system is impaired in bipolar affective disorder brain. Journal of Neurochemistry. 1996;66:2402–2409. doi: 10.1046/j.1471-4159.1996.66062402.x. [DOI] [PubMed] [Google Scholar]
- Kass D. Differential response to anticonvulsants among bipolar patients. Curr Affect Illness. 1994:13. [Google Scholar]
- Kim AJ, Shi Y, Austin RC, Werstuck GH. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. Journal of Cell Science. 2005;118:89–99. doi: 10.1242/jcs.01562. [DOI] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proceedings of the National Academy of Sciences of the U.S.A. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences of the U.S.A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Weller M, Schoppmeyer K, Riederer P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. Journal of Neural Transmission. Supplementum. 1994;43:91–104. [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia. 1986;27:490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lenox R, Manji H. Drugs for treatment of Bipolar Disorder: Lithium. In: Nemeroff CB, editor. Textbook of Psychopharmacology. 2nd edn. American Psychiatry Press; 1998. pp. 379–429. [Google Scholar]
- Li Y, Vartanian AJ, White FJ, Xue CJ, Wolf ME. Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacology (Berl) 1997;134:266–276. doi: 10.1007/s002130050449. [DOI] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. Journal of Neuroscience. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. Journal of Cell Science. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Developmental Biology. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. Journal of Neurochemistry. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Manji HK. G proteins: implications for psychiatry. American Journal of Psychiatry. 1992;149:746–760. doi: 10.1176/ajp.149.6.746. [DOI] [PubMed] [Google Scholar]
- Manji HK, Chen G, Shimon H, Hsiao JK, Potter WZ, Belmaker RH. Guanine nucleotide-binding proteins in bipolar affective disorder. Effects of long-term lithium treatment. Archives of General Psychiatry. 1995;52:135–144. doi: 10.1001/archpsyc.1995.03950140053007. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nature Medicine. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biological Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. The nature of bipolar disorder. Journal of Clinical Psychiatry. 2000a;61(Supp 13):42–57. [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biological Psychiatry. 2000b;48:518–530. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]
- Martinez-Turrillas R, Frechilla D, Del Rio J. Chronic antide-pressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology. 2002;43:1230–1237. doi: 10.1016/s0028-3908(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO Journal. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. AMPA-receptors are involved in the expression of amphetamine-induced behavioural sensitisation, but not in the expression of amphetamine-induced conditioned activity in mice. Neuropharmacology. 1998;37:1131–1138. doi: 10.1016/s0028-3908(98)00101-4. [DOI] [PubMed] [Google Scholar]
- Mitchell PB, Manji HK, Chen G, Jolkovsky L, Smith-Jackson E, Denicoff K, et al. High levels of Gs alpha in platelets of euthymic patients with bipolar affective disorder. American Journal of Psychiatry. 1997;154:218–223. doi: 10.1176/ajp.154.2.218. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002a;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. Preclinical models: status of basic research in depression. Biological Psychiatry. 2002b;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. Journal of Biological Chemistry. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB Journal. 1995;9:484–496. [PubMed] [Google Scholar]
- Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proceedings of the National Academy of Sciences of the U.S.A. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes JD, Calver DM, Zilkha KJ, Knill-Jones RP. Controlled trial of amantadine hydrochloride in Parkinson's disease. Lancet. 1970;1:259–262. doi: 10.1016/s0140-6736(70)90634-3. [DOI] [PubMed] [Google Scholar]
- Payne JL, Quiroz JA, Gould TG, Zarate CA, Manji HK. Nestler E, editor. Neurobiology of Bipolar Disorder. Neuro-biology of Mental Illness. in press. [Google Scholar]
- Perez J, Tardito D, Mori S, Racagni G, Smeraldi E, Zanardi R. Abnormalities of cAMP signaling in affective disorders: implication for pathophysiology and treatment. Bipolar Disorders. 2000;2:27–36. doi: 10.1034/j.1399-5618.2000.020104.x. [DOI] [PubMed] [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. Journal of Neuroscience. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Martella G, De Persis C, Costa C, Pisani F, et al. Intracellular calcium increase in epileptiform activity: modulation by levetiracetam and lamotrigine. Epilepsia. 2004;45:719–728. doi: 10.1111/j.0013-9580.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Popoli P, Frank C, Tebano MT, Potenza RL, Pintor A, Domenici MR, et al. Modulation of glutamate release and excitotoxicity by adenosine A(2A) receptors. Neurology. 2003;61:S69–S71. doi: 10.1212/01.wnl.0000095216.89483.a2. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Morselli PL. Amantadine-induced aggressiveness. British Medical Journal. 1972;3:50. doi: 10.1136/bmj.3.5817.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Simon GE. Social and economic burden of mood disorders. Biological Psychiatry. 2003;54:208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- Spiegel A. G Proteins, receptors, and disease. Humana Press; 1998. [Google Scholar]
- Spleiss O, van Calker D, Scharer L, Adamovic K, Berger M, Gebicke-Haerter PJ. Abnormal G protein alpha(s) – and alpha(i2)-subunit mRNA expression in bipolar affective disorder. Molecular Psychiatry. 1998;3:512–520. doi: 10.1038/sj.mp.4000393. [DOI] [PubMed] [Google Scholar]
- Stabel S, Parker PJ. Protein kinase C. Pharmacology and Therapeutics. 1991;51:71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, Nomikos GG, Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proceedings of the National Academy of Sciences of the U.S.A. 2002;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ST, Gould TD, Manji HK. Neurotransmitters, receptors, signal transduction, and second messengers in psychiatric disorders. In: Nemeroff CB, editor. The American Psychiatric Publishing Textbook of Psychopharmacology. American Psychiatric Publishing Inc; 2003. pp. 3–52. [Google Scholar]
- Tzschentke TM. Reassessment of buprenorphine in conditioned place preference: temporal and pharmacological considerations. Psychopharmacology (Berl) 2003 doi: 10.1007/s00213-003-1626-4. Published on line: Nov 13. [DOI] [PubMed] [Google Scholar]
- Vale S, Espejel MA, Dominguez JC. Amantadine in depression. Lancet. 1971;2:437. doi: 10.1016/s0140-6736(71)90153-x. [DOI] [PubMed] [Google Scholar]
- Wang HY, Friedman E. Enhanced protein kinase C activity and translocation in bipolar affective disorder brains. Biological Psychiatry. 1996;40:568–575. doi: 10.1016/0006-3223(95)00611-7. [DOI] [PubMed] [Google Scholar]
- Wang HY, Friedman E. Effects of lithium on receptor-mediated activation of G proteins in rat brain cortical membranes. Neuropharmacology. 1999;38:403–414. doi: 10.1016/s0028-3908(98)00197-x. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Huang CC, Hsu KS, Tsai JJ, Gean PW. Presynaptic inhibition of excitatory neurotransmission by lamotrigine in the rat amygdalar neurons. Synapse. 1996;24:248–255. doi: 10.1002/(SICI)1098-2396(199611)24:3<248::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Warsh J, Young L, Li P. Guanine nucleotide binding (G) proteindisturbances. In: Belmaker R, editor. Bipolar Affective Disorder in Bipolar Medications: Mechanisms of Action. American Psychiatric Press; 2000a. pp. 299–329. [Google Scholar]
- Warsh JJ, Young LT, Li PP. Guanine nucleotide binding (G) protein disturbances. In: Belmaker R, editor. Bipolar Affective Disorder in Bipolar Medications: Mechanisms of Action. American Psychiatric Press; 2000b. pp. 299–329. [Google Scholar]
- Weng G, Bhalla US, Iyengar R. Complexity in biological signaling systems. Science. 1999;284:92–96. doi: 10.1126/science.284.5411.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wo ZG, Bian ZC, Oswald RE. Asn-265 of frog kainate binding protein is a functional glycosylation site: implications for the transmembrane topology of glutamate receptors. FEBS Letters. 1995;368:230–234. doi: 10.1016/0014-5793(95)00655-s. [DOI] [PubMed] [Google Scholar]
- Wong CS, Cherng CH, Luk HN, Ho ST, Tung CS. Effects of NMDA receptor antagonists on inhibition of morphine tolerance in rats: binding at mu-opioid receptors. European Journal of Pharmacology. 1996;297:27–33. doi: 10.1016/0014-2999(95)00728-8. [DOI] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Yoon IS, Li PP, Siu KP, Kennedy JL, Cooke RG, Parikh SV, et al. Altered IMPA2 gene expression and calcium homeostastis in bipolar disorder. Molecular Psychiatry. 2001a;6:678–683. doi: 10.1038/sj.mp.4000901. [DOI] [PubMed] [Google Scholar]
- Yoon IS, Li PP, Siu KP, Kennedy Macciardi F, Cooke RG, et al. Altered TRPC7 gene expression in bipolar-1 disorder. Biological Psychiatry. 2001b;50:620–626. doi: 10.1016/s0006-3223(01)01077-0. [DOI] [PubMed] [Google Scholar]
- Young LT. Neuroprotective effects of antidepressant and mood stabilizing drugs. Journal of Psychiatry and Neuroscience. 2002;27:8–9. [PMC free article] [PubMed] [Google Scholar]
- Young LT, Li PP, Kish SJ, Siu KP, Kamble A, Hornykiewicz O, et al. Cerebral cortex Gs alpha protein levels and forskolin-stimulated cyclic AMP formation are increased in bipolar affective disorder. Journal of Neurochemistry. 1993;61:890–898. doi: 10.1111/j.1471-4159.1993.tb03600.x. [DOI] [PubMed] [Google Scholar]
- Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. Journal of Biological Chemistry. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, et al. An open-label trial of the glutamate modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biological Psychiatry. 2005 doi: 10.1016/j.biopsych.2004.11.023. in press. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Du J, Quiroz J, Gray NA, Denicoff KD, Singh J, et al. Regulation of cellular plasticity cascades in the patho-physiology and treatment of mood disorders: role of the glutamatergic system. Annals of the New York Academy of Science. 2003;1003:273–291. doi: 10.1196/annals.1300.017. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]