Abstract
Objectives
To describe the methods used for, correlates of cooperation with, and validity of in-home salivary specimens collected from older adults.
Methods
Salivary specimens were collected between 2005 and 2006 during in-home interviews with a probability sample of 3,005 U.S. men and women, ages 57–85 years. Sex hormone levels were assessed by enzyme-linked immunoassay conducted at Salimetrics, LLC (State College, PA). Mean salivary sex hormone concentrations were compared by gender and in relation to medication use and health conditions.
Results
Self-collected saliva specimens were provided by 2,722 (90.6%) individuals; 95.8% of these were adequate for analysis. Black participants were significantly less likely than individuals of other racial/ethnic groups to provide a salivary specimen; age, gender, education, and self-rated health were not associated with participation. Mean testosterone levels were higher in men compared with women, and estradiol levels were higher in women using estrogens. Salivary hormone measurements obtained in the National Social Life, Health, and Aging Project (NSHAP) and other studies are of similar magnitude.
Conclusion
NSHAP is the first large, population-based study of older adults to measure salivary estradiol, progesterone, dehydroepiandrosterone (DHEA), and, in women, testosterone. These data demonstrate a high cooperation rate with in-home salivary specimen collection from older adults and good validity of sex hormone measurements.
Keywords: Sex hormone, Aging, Salivary, Saliva collection, Estrogen, Testosterone, Progesterone, DHEA, Estradiol, Older, Validity
THE National Social Life, Health, and Aging Project (NSHAP) aims to decipher biological mechanisms through which social, including sexual, relationships affect health at older ages. Although population-based studies of aging are increasingly collecting biological measures to explore social-physiological hypotheses about health (Lindau & McDade, 2007), NSHAP is unique in its collection of sex hormonal data for this purpose. Conducted between July 2005 and March 2006, the first wave of NSHAP included self-administered salivary specimen collection to begin to explore the relationship between sex hormone status, relationships, and health in later life.
Sex hormonal evaluation in the clinical setting is typically performed on a serum or plasma specimen (Kaufman & Vermeulen, 2005) and can provide information about total and free circulating sex hormones. This involves an invasive procedure (venipuncture). Most steroid hormones in blood are bound to specific proteins (e.g., sex hormone–binding globulin [SHBG]). A tiny fraction (less than 5%) of nonbound or free fraction of sex hormone accounts for actual sex hormone activity at the tissue level. Free sex hormone molecules are small relative to their bound, protein–steroid complex; when free, these molecules can penetrate small membranes such as those found in the salivary gland. Salivary sex hormone measures quantify free, or bioactive, sex hormone concentration (Lu, Bentley, Gann, Hodges, & Chatterton, 1999; Worthman, Stallings, & Hofman, 1990). Salivary measures offer a relatively convenient and minimally invasive approach for obtaining sex hormone data (Granger, Shirtcliff, Booth, Kivlighan, & Schwartz, 2004; E. Kaufman & Lamster, 2002; Worthman et al., 1990)—an advantage when working with older, infirm, and/or population-based health study participants. Saliva specimens can be self-collected and safely handled by lay personnel (Granger et al., 2007).
The methods used for, correlates of cooperation with, and validity of in-home salivary specimens collected from older adults in NSHAP are described.
BACKGROUND
Sex hormones (estrogen, progesterone, testosterone, DHEA) derive from the precursor cholesterol (Greenspan & Gardner, 2001) and play an important role in human physiology throughout the life course. Estrogens are more prevalent in women of reproductive age compared with men and are responsible for development of female secondary sex characteristics. Three major estrogens are found naturally in the human body: estrone (E1), estradiol (E2), and estriol (E3), with the weak estrogen. estriol. being the most abundant (Greenspan & Gardner, 2001; Speroff, Glass, & Kase, 1999). Estradiol (17-beta-estradiol), the most potent bioactive estrogen, is primarily synthesized from testosterone in the ovarian follicles in females whereas in males, it is produced by the testes and extraglandular conversion of androgens (Salimetrics, 2006; Tivis, Richardson, Peddi, & Arjmandi, 2005). Estrogen exerts central (brain) (Rehman & Masson, 2005) and peripheral (reproductive tract, mammary gland, skeletal, cardiovascular) effects (Hall, Couse, & Korach, 2001).
Estrogen metabolism and synthesis in men appear to remain relatively stable across the life course (Kaufman & Vermeulen, 2005). In contrast, major changes in sex hormone metabolism occur in women around natural menopause, most notably due to a decline in ovarian estrogen production (Manly et al., 2000). Although the human lifespan is increasing, the mean age of menopause has not changed as rapidly. Life expectancy at birth for American women increased from 56.2 years for women born in 1910 to 69.4 years for women born in 1940 (158 month increase) (Bell & Miller, 2002). At the same time, the mean age at natural menopause for U.S. women born in 1915 versus 1939 increased from 49.1 to 50.5 years (16.8 month increase) (Nichols et al., 2006). Women are therefore living increasingly longer in a menopausal state.
Relative estrogen depletion in women in later life has been associated with a variety of age-related changes in physical and cognitive function as well as disease. Among men and women, serum estradiol concentration has been associated with bone turnover and risk for osteoporotic fractures (lower estrogen is associated with skeletal vulnerability) (Kuchuk et al., 2007). Likewise, estradiol concentration has been associated with cognition (Carlson et al., 2001; Maki & Resnick, 2000; Tivis et al., 2005), mood, and memory (Tivis et al., 2005) in women and, in combination with testosterone levels and other factors, with preservation of memory and cognitive function in men (Barrett-Connor, Goodman-Gruen, & Patay, 1999; Carlson & Sherwin, 2000). A number of studies have linked higher levels of serum estradiol to increased risk for developing breast cancer (Chlebowski et al., 2003; Clemons & Goss, 2001; Tivis et al., 2005) and coronary problems (Chlebowski et al., 2003; Clemons & Goss, 2001; Manson et al., 2003; Tivis et al., 2005) in women. Much less is known about the role of estrogen in social functioning in general or in later life.
Estrogen depletion in menopause and sustained, low levels of circulating estrogen throughout the postmenopausal lifespan significantly affect the condition and function of the female genital tract. Estrogen is important for maintaining skin, subcutaneous, mucosal (vaginal, bladder, and rectal) and musculoskeletal integrity, the vaginal microenvironment (pH balance and microflora), vascular flow to the vagina and clitoris, and sensory perception. Over time, in the genital tract, low estrogen results in vaginal dryness, loss of epithelial cell glycogen, shortening of the vagina, narrowing of the introitus, thinning of the labia, and diminution of the fat pad underlying the mons pubis. Though the role of estrogen in female sexuality is not fully understood, it may influence sexual desire (Dennerstein, Gotts, et al., 1994; Meston & Frohlich, 2000). Estrogen replacement therapy may indirectly enhance female sexual performance, by restoring vaginal lubrication (Meston & Frohlich) and promoting positive body image and an overall positive sense of well-being (Bachmann & Leiblum, 2004). Additionally, sexual activity and other forms of physical contact may mitigate estrogen-related decline in sensory perception and function with age and may play a role in female attractiveness. Largely due to highly publicized findings in 2002 from the randomized, controlled hormone therapy trials of the U.S. Women's Health Initiative Study (comparing use of equine estrogen alone or in combination with medroxyprogesterone to placebo among post menopausal women ages 50–79 years at baseline) (Rossouw et al., 2002), a minority of women currently initiate estrogen therapy for treatment of menopausal symptoms or preservation of sexual function (Newton et al., 2008).
Progesterone is found in both women and men, although the physiological role in men is poorly understood (Andersen & Tufik, 2006). In reproductive age females, the primary function of progesterone involves preparation and maintenance of the endometrium for implantation of fertilized oocytes (Speroff et al., 1999). In women, progesterone is synthesized from pregnenolone in the adrenal cortex, corpus luteum of the ovary, in the brain, and during pregnancy, by the placenta. Due to decline in ovarian production, progesterone production is markedly decreased following menopause. This decline may result in diminished sexual function and desire (Dennerstein, Alexander, & Kotz, 2003). Increased levels of progesterone have been found in states of stress and anxiety in men and women; this may relate to its sedative or stress-counteracting effects (Wirth, Meier, Fredrickson, & Shcultheiss, 2007; Wirth & Schultheiss, 2006). Exogenous progesterone is used in older women primarily in combination with estrogen therapy to protect against estrogen's growth-promoting effects on the uterine endometrial lining and to treat pathological processes of the uterine endometrium. In men, exogenous progesterone has been used to reduce sexual activity, a mechanism of action that may relate to its antiandrogenic properties (Andersen & Tufik, 2006).
Testosterone is the defining male sex hormone, is more abundant in males than females, but appears to play an important physiological role in sexual functioning of both. Testosterone is synthesized in the male testes, the female ovaries, and the adrenal glands in both sexes (Davis & Tran, 2001; Guyton, 1991). During the aging process, testosterone levels gradually decline in both sexes (Davis & Tran; Ellison et al., 2002; Feldman et al., 2002; Lobo, 2001; Tenover, 1997). Because the ovaries are a primary, and relatively stable, source of female testosterone production throughout the life course (Davis & Tran, 2001), premenopausal bilateral oophorectomy (surgical removal of both ovaries prior to onset of natural menopause) results in abrupt and irreversible loss of the major source of testosterone in women (Hendrix, 2005; Laughlin, Barrett-Connor, Kritz-Silverstein, & von Muhlen, 2000). About 22 million women undergo surgical menopause each year in the United States (Keshavarz, Hillis, Kieke, & Marchbanks, 2002). Because estrogen synthesis largely derives from ovarian testosterone postmenopausally, surgical removal of the ovaries also exacerbates the estrogen deficit in these women.
In both sexes, decline in testosterone levels leads to bone density loss (Davis & Tran, 2001; Jassal, Barrett-Connor, & Edelstein, 1995; Lobo, 2001; Tenover, 1997) and decline in muscle mass (Ellison et al., 2002; Lobo, 2001). Testosterone, along with dehydroepiandrosterone (DHEA), plays an important role in female libido, arousal, genital sensation, and orgasm. Loss of testosterone can exacerbate vaginal mucosal atrophy, thinning of public hair, and may compromise an older woman's sense of general well-being. Low levels of testosterone have been correlated with lower coital frequency and loss of sexual desire in men and women (Davis & Tran, 2001), although the relationship between female sexual interest in later life and testosterone levels is controversial (Davis & Tran, 2001; Dennerstein, Smith, Morse, & Burger, 1994). Testosterone administration may improve mood and well-being in both sexes, especially among men (Ellison et al., 2001; Grinspoon et al., 2000; Jassal et al., 1995). Circulating testosterone in women is a strong correlate of ovarian function and androgen production, though approximately one third of circulating levels are derived via precursors from the adrenal gland (Lobo, 2001).
DHEA and its sulfate (DHEA-S) are produced by the cortex of the adrenal gland (Williams & Wilson, 1998) and act as precursors to approximately half of the active androgen concentration in adult men (Regelson, Loria, & Kalimi, 1994). DHEA production and concentration decline is significant and steady during human aging (Hackbert & Heiman, 2002; Ravaglia et al., 1996; Speroff et al., 1999; Villareal & Holloszy, 2004) in both males and females (Labrie, Belanger, Cusan, Gomez, & Candas, 1997; Panjari & Davis, 2007) and, therefore, has been suggested as a putative biomarker of physiologic aging (Johnson, Bebb, & Sirrs, 2002). Low levels of endogenous DHEA concentration are associated with cardiovascular disease (Alexandersen, Haarbo, & Christiansen, 1996), progression of HIV infection (Christeff et al., 1996), symptomatic progression of systemic lupus erythematosus (Spark, 2002), presence of rheumatoid arthritis among postmenopausal women (Masi, 1995), bone resorption, and skin pigmentation (Spark, 2002). DHEA has been studied for its role in mood and well-being (Hackbert & Heiman, 2002; Spark, 2002), sexual physiology, and treatment of sexual problems (Spark, 2002).
METHODS
Details of the NSHAP sample design and participant characteristics have been previously described (Lindau et al., 2007). The survey yielded 3,005 respondents (1,455 men and 1,550 women) ages 57–84 years. The weighted sample response rate was 75.5%. Here, we describe the collection of saliva specimens and details of the sex hormone assays. In addition to assays for DHEA, testosterone, progesterone, and estradiol, an assay for cotinine (a primary metabolite of nicotine) was performed. The salivary cotinine measure is described in detail elsewhere (Drum, Shiovitz-Ezra, Gaumer, & Lindau, in press).
Saliva Specimen Collection and Processing
Details of the salivary assays collected in the NSHAP study have been previously described (Mendoza, Curran, & Lindau, 2007a, 2007b, 2007c; Nallanathan, Mendoza, Curran, & Lindau, 2007). Approximately midway through the in-home interview, salivary specimens were collected from all willing respondents. Following procedures developed by Granger and colleagues (2007), whole unstimulated saliva was collected by passive drool. Approximately 2 mililiters of saliva was donated into a small, code-labeled polypropylene collection vial via a 5 centimeter section of a household plastic straw. The time of last food consumption and time of the saliva specimen collection were recorded. To minimize the effects of room temperature on salivary analyte levels (Whembolua, Granger, Kivlighan, Marguin, & Singer, 2006), samples were transported on cold packs immediately after collection, from the interview site to a residential-grade freezer (−20 °C) where they were stored until shipped via overnight delivery on dry ice to Salimetrics laboratories (State College, PA). Upon receipt, specimens were inventoried and then archived at −80 °C until the day of assay.
Hormone Assays
The salivary enzyme immunoassays were conducted at Salimetrics, LLC. On the day of assay, specimens were thawed completely, vortexed, and centrifuged at 1,500 × g (3,000 rpm) for 15 minutes. Clear samples were pipetted in duplicate into test wells using a 96-well plate format. To minimize the effects of freeze thaw cycles on assay results, the analyses were scheduled in the following priority order: (a) estradiol, (b) progesterone, (c) DHEA, (d) testosterone, and (e) cotinine. All assays were conducted using commercially available immunoassay kits without modification to the manufacturer's recommended protocol (Salimetrics). Assays were performed in duplicate with the average of the duplicate tests used in all analyses. The criteria for repeated testing was variation greater than 20% between duplicates, and on average, the assay protocols had intra- and interassay coefficients of variation less than 10% and 15%, respectively. Hormone values were measured in picograms per milliliter and cotinine in nanograms per milliliter. As expected, hormonal therapies (oral, transdermal, topical) cross-react with the detection antibodies used in these assays, resulting in high salivary hormone measurements. Sex hormone measurements that exceeded the upper boundary of assay and results below the assay limit of sensitivity are flagged in the publicly available NSHAP data set and summarized in Table 1. More details on the quality control issues regarding each sex hormone assay can be found in previously published NSHAP Technical Reports (Mendoza et al., 2007a, 2007b, 2007c; Nallanathan et al., 2007).
Table 1.
Lower and Upper Boundaries for Salivary Sex Hormone Measurements Performed by Salimetricsa
| Sex Hormone Test | Lower Limit of Sensitivity (pg/ml) | None Detected Reported if Value (pg/ml) | Interference Likely if Value (pg/ml) |
| Estradiol | 1 | <0.5 | >320 |
| Progesterone | 5 | <2 | >12,150 |
| DHEA | 5 | ≤2 | >5,000 |
| Testosterone | 1 | ≤0.5 | >3,000 L |
Note: aAdapted from technical reports on salivary specimen collection for the National Social Life, Health, and Aging Project (Mendoza et al., 2007a, 2007b, 2007c; Nallanathan et al., 2007).
Values for each duplicate assay are included in the public-use data set. Missing codes and flags describe problems encountered in obtaining an adequate specimen for sex hormone analysis (e.g., insufficient sample quantity, equipment problems).
Variables Used in the Analysis
Data are presented for three age strata to correspond with the age structure of the NSHAP sample design: 57–64, 65–74 years, and 75–85 years (O’Muircheartaigh, eckman, & smith, in review). Gender was assessed by field staff observation. Race and ethnicity were self-reported and categorized as White, Black, Hispanic, and other. Education level was dichotomized as “high school and below” and “some college and higher.” Respondents were asked to rate their physical health using the standard 5-point scale with responses “excellent,” “very good,” “good,” “fair,” and “poor.” For these analyses, we use three self-rated health categories: “very good/excellent,” “good,” and “fair/poor.”
Statistical Analysis
All analyses were performed using the Stata statistical software package, release 9 (StataCorp, 2005). Logistic regression (Hosmer & Lemeshow, 1989) was used to model the likelihood of participating in the saliva collection. This model included age group, gender, race/ethnicity, education, and self-rated health as covariates. Results are presented as odds ratios together with 95% confidence intervals. To account for the unequal probabilities of selection, we performed weighted analyses using the probability weights with a poststratification adjustment based on age and urbanicity. Inference was conducted using design-based variance estimates, obtained via the linearization method (Binder, 1983) as implemented in Stata (StataCorp, 2005). Differences between means were evaluated using the adjusted Wald test. All p values reported are two sided from the adjusted Wald test.
Assessment of Internal and External Validity
Internal validity of sex hormone measurements was first assessed by gender comparison of saliva testosterone levels, which are expected to be higher in men compared with women (Shirtcliff, Granger, & Likos, 2002). Internal validity of the saliva sex hormone measurements was further assessed by evaluation of association between specific sex hormone concentrations and corresponding health conditions or behaviors, including use of exogenous sex hormones or hormone blockers, obesity, and oophorectomy. History of ovary removal (oophorectomy) was determined on the basis of self-report. Medication data were collected during the in-home interview by direct observation using a computer-based medication log. Usage of hormones included hormone replacement therapy and oral contraceptives by women and androgens and anabolic steroids (predominantly for stimulation of muscle growth) by men and women. Antiandrogenic medication use by men was also logged. Obesity was defined as body mass index of 30 kg/m2 or higher (U.S. Department of Health and Human Services, 2000).
External validation of sex hormone measurements in the NSHAP sample was conducted using comparison with published data on salivary levels of estradiol, progesterone, DHEA, and testosterone among older men and women (when data on older age groups were available).
RESULTS
Participant Cooperation in Saliva Collection
Self-collected saliva specimens were provided by 2,722 (90.6%, unweighted) individuals. Table 2 shows the associations of individual sociodemographic and health characteristics with the likelihood of participation in the saliva collection. Blacks were significantly less likely to participate in saliva collection than whites. Other characteristics of respondents, including age, gender, and self-reported health, were not significantly associated with participation in the saliva protocol.
Table 2.
Association of Respondent Characteristics With Cooperation in the National Social Life, Health, and Aging Project Saliva Protocol
| Characteristics | Proportion (SE) | OR (95% Confidence Interval)a |
| Age (years) | ||
| 57–64 | 0.91 (0.01) | 1 (reference) |
| 65–74 | 0.91 (0.01) | 1.07 (0.72–1.61) |
| 75–85 | 0.91 (0.01) | 1.07 (0.70–1.63) |
| Gender | ||
| Men | 0.92 (0.01) | 1 (reference) |
| Women | 0.90 (0.01) | 0.81 (0.55–1.21) |
| Race/ethnicity | ||
| White | 0.92 (0.01) | 1 (reference) |
| Black | 0.82 (0.03) | 0.43* (0.23–0.81) |
| Hispanicb | 0.92 (0.03) | 1.12 (0.45–2.80) |
| Other | 0.95 (0.02) | 1.88 (0.85–4.14) |
| Education | ||
| Higher secondary and below | 0.90 (0.01) | 1 (reference) |
| Some college and higher | 0.91 (0.01) | 0.98 (0.75–1.29) |
| Self-rated health | ||
| Poor/fair | 0.90 (0.01) | 1 (reference) |
| Good | 0.89 (0.01) | 0.92 (0.62–1.35) |
| Very good/excellent | 0.92 (0.01) | 1.19 (0.85–1.67) |
Notes: aOdds ratio of giving consent for saliva collection.bBased on the questions “Do you consider yourself primarily White or Caucasian, Black or African American, American Indian, Asian, or something else?” and “Do you consider yourself Hispanic or Latino?” Six respondents who reported being both Hispanic and Black or African American were included in the Black group.
*p < .01.
Explanation of Missing Data
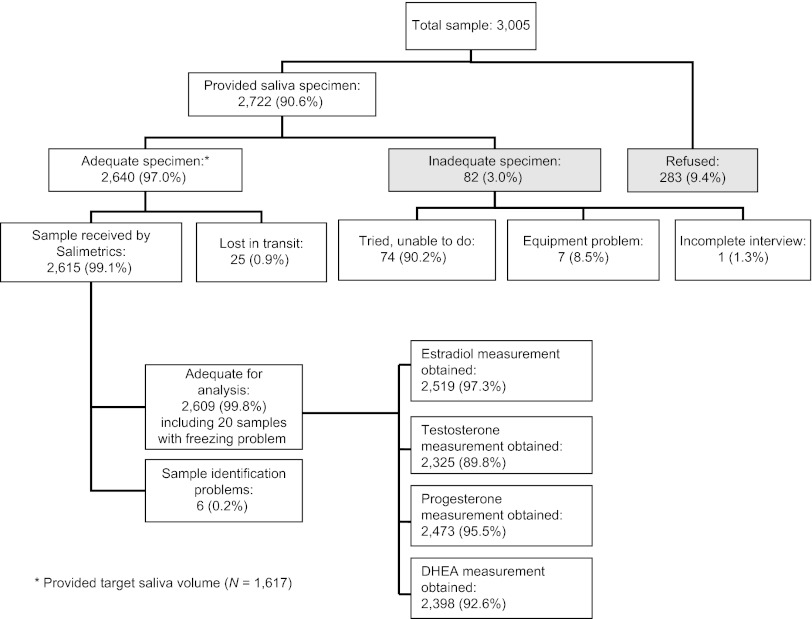
Of 2,722 respondents who agreed to provide a saliva sample, 82 (3.0%) specimens were inadequate (mainly due to inability to produce a sufficient amount of saliva). Figure 1 shows the disposition of specimens at various stages of collection, transportation, and measurement. In the process of saliva collection and transportation, freezing was compromised for 20 samples although these samples were still adequate for sex hormone measurements; these were included in the hormone measurement protocol. Inadequate freezing of these samples is flagged in the public-use data set. Twenty samples with reported freezing problems during saliva transportation and storage were excluded from further analyses of sex hormone levels in this study.
Figure 1.
Disposition of specimens at various stages of collection, transportation, and measurement.
Interference with both measures for each hormone assay, due to food or drink consumption (mean time since consumption 3.44 hr, SD = 3.08) or other factors, was likely for 4.1% of estradiol assays, 1.0% of progesterone assays, 1.3% of DHEA assays, and 0.08% of testosterone assays. The proportion of assays where both measurements were below the boundary of sensitivity was 0.46% for estradiol, 0.08% for progesterone, 0.04% for DHEA, and 0.15% for testosterone. Overall, the vast majority of specimens processed for sex hormone analysis were adequate: estradiol (97%), progesterone (96%), DHEA (93%), and testosterone (90%). The declining adequacy rate corresponds with the priority order in which the assays were performed.
Internal and External Validity of Sex Steroid Hormone Measurement
Mean and median values of sex hormone measurements, stratified by gender, are presented in Table 3.
Table 3.
Mean and Median Salivary Sex Hormone Values in Picograms Per Milliliter by Gender in a Probability Sample of Older Adult Participants in the National Social Life, Health, and Aging Project
| Men (N = 1,287a) |
Women (N = 1,302a) |
|||
| Sex Hormone | Mean Value, SEM | Median Value (range) | Mean Value, SEM | Median Value (range) |
| Estradiol | 10.65 ± −0.56 | 6.24 (0.5–303.49) | 10.03 ± 0.56 | 6.73 (0.5–320) |
| Testosteroneb | 99.50 ± −6.54 | 77.56 (3.81–4,024.2) | 46.34 ± 0.76 | 42.93 (0.5–217.43) |
| Progesterone | 49.52 ± −6.25 | 29.79 (2.0–2,438.3) | 60.82 ± 15.16 | 24.60 (2.0–12,150) |
| DHEAb | 61.22 ± 4.70 | 39.69 (2.0–5,000) | 45.92 ± 1.68 | 33.41 (2.0–449.7) |
Notes: DHEA = dehydroepiandrosterone.
This number reflects the actual number of samples appropriate for salivary hormone measurements (after data for samples having freezing problems were removed from the analysis).
Statistically significant gender differences in mean values (p < .01)
Men demonstrated higher levels of testosterone. The mean testosterone level for men (99.5 pg/ml) was about twice that of women (46.3 pg/ml). The mean salivary estradiol levels were nearly equivalent for men and women.
Table 4 reports salivary sex hormone concentrations in women in relation to exogenous sex hormone use. Of women using exogenous sex hormones (n = 160, 12.3% overall), 158 were using estrogen. Of these, 24 were using estrogen in combination with a progestin and 6 were using estrogen in combination with an androgen product. One woman used only androgen and one woman used only progestin supplementation. Women taking estrogen alone or in combination therapy (n = 158, 10.2% overall) exhibited significantly higher levels of salivary estradiol compared with nonusers (see Table 4).
Table 4.
Mean and Median Values of Four Sex Hormones in Saliva, by Usage of Exogenous Sex Hormones in Women. National Social Life, Health, and Aging Project Study
| Nonusers of Exogenous Sex Hormones (N = 1,153a) |
Users of Exogenous Sex Hormones (N = 149a) |
|||
| Sex Hormone in Saliva | Mean Value (pg/ml) | Median Value (Range) | Mean Value (pg/ml) | Median Value (Range) |
| Estradiolb | 8.90 ± 0.43 | 6.22 (0.5–320) | 17.23 ± 2.61 | 8.99 (0.5–320) |
| Testosterone | 46.58 ± 0.74 | 43.35 (0.5–193.33) | 44.89 ± 2.81 | 37.83 (2.3–217.43) |
| Progesterone | 62.10 ± 17.57 | 24.50 (2–12,150) | 52.60 ± 14.88 | 28.30 (2–2,029.57) |
| DHEA | 45.70 ± 1.66 | 33.37 (2–432.88) | 47.29 ± 6.10 | 33.61 (2–449.74) |
Notes: DHEA = dehydroepiandrosterone.
This number reflects the actual number of cases appropriate for salivary hormone measurements (after data for samples having freezing problems were removed from the analysis).
Statistically significant differences in mean values between nonusers and users of exogenous hormone therapy (p < .01)
Internal validity of the saliva sex hormone measurements was further assessed by evaluation of sex hormone levels in obese men and women, men taking drugs with antiandrogenic and androgenic activity, and women with a history of bilateral oophorectomy (Table 5).
Table 5.
Mean Salivary Sex Hormone Measurements in Relation to Medication Use and Health Conditions Where Changes in Sex Hormone Levels Are Expected. National Social Life, Health, and Aging Project Study
| Group | Na | Estradiol Level (95% CI), pg/ml | Testosterone Level (95% CI), pg/ml |
| Men | |||
| All men in the sample | 1,287 | 10.65 (9.52–11.78) | 99.50 (86.35–112.64) |
| Users of 5-alpha-reductase inhibitors | 27 | 9.02 (6.24–11.81) | 90.58 (80.87–100.29) |
| Users of androgens and anabolic steroids | 4 | 12.60 (5.71–19.49) | 1,461.62 (178.25–3,000)b |
| Obese men | 449 | 9.84 (8.24–11.43) | 106.05 (76.41–135.68) |
| Women | |||
| All women in the sample | 1,302 | 10.03 (8.89–11.16) | 46.34 (44.82–47.87) |
| Estrogen users | 147 | 17.40c (12.07–22.73) | 43.50 (38.61–48.40) |
| Ovariectomized women | 335 | 11.23 (8.19–14.28) | 44.23 (41.56–46.90) |
| Ovariectomized women using androgens or anabolic steroids | 6 | 9.92 (4.76–15.09) | 50.80 (38.45–63.15) |
| Obese women | 474 | 9.73 (8.29–11.16) | 49.26d (46.54–51.98) |
Notes: CI = confidence interval.
The numbers reflect the actual numbers of samples appropriate for salivary hormone measurements (after samples having freezing problems were removed from the analysis).
Indicates range (due to small number of observations, calculations of confidence intervals for testosterone values in this group was not feasible).
Significantly higher mean values compared with nonusers (p = .003, adjusted Wald test).
Significantly higher mean values compared with nonobese women (p = .009, adjusted Wald test).
Few men (n = 39, 2.5% overall) reported exogenous sex hormone use or use of other medications affecting sex hormone metabolism. Of these, 32 were using 5-alpha-reductase inhibitors. These drugs reduce circulating androgens by reducing conversion of testosterone to dihydrotestosterone and are used most commonly in the treatment of benign prostatic hyperplasia. Six men used androgens or anabolic steroids and one man reported progestin use. Although the associations were not statistically significant (the subgroups were very small), men taking 5-alpha-reductase inhibitors exhibit slightly lower concentrations of salivary testosterone compared with nonusers of this drug. Likewise, men taking androgens or anabolic steroids exhibit higher levels of testosterone (again, this association was not statistically significant due to the small number of men taking anabolic steroids).
Bilateral oophorectomy was reported by 392 women in the sample. Ovaries are the predominant source of androgen production in postmenopausal women. Testosterone levels were lower in ovariectomized women (44.23 ± 1.33 pg/ml) as compared with women with at least one ovary (47.03 ± 0.94 pg/ml), but this difference was not statistically significant. Only 6 of 392 ovariectomized women used androgen replacement therapy. Salivary testosterone concentration in these women was only slightly higher as compared with those not using androgen supplementation (50.8 ± 6.08 vs. 44.1 ± 1.31 pg/ml, respectively); this difference was not statistically significant. Obese men and women demonstrate higher levels of testosterone compared with nonobese participants; testosterone levels among obese women were significantly higher than among nonobese women (Table 5).
Tables 6 and 7 compare salivary measurements of estradiol, progesterone, DHEA, and testosterone in women and men with those reported by other studies. Overall, salivary levels of sex hormones demonstrate significant variability dependent on age, gender, season of the year, and time of the day (see Tables 6 and 7). Where comparable data are available, salivary sex hormone data from NSHAP are similar to other published findings for progesterone, testosterone, and estradiol (Tables 6 and 7). The mean estradiol concentration reported by Krause and colleagues for older men (Krause, Mueller, & Mazur, 2002) is very similar to estradiol levels found in NSHAP for older women and men (Tables 6 and 7). Salivary DHEA concentrations in NSHAP are lower than those reported in the literature for younger people.
Table 6.
Reported Sex Hormone Measurements in Saliva of Women as Compared with NSHAP Data
| Sample | N | Age | Mean Value (SEM, range, or SD), pg/ml | Source |
| Estradiol | ||||
| Postmenopausal women, nonusers of HRT | 1,153 | 57–85 | 8.90 ± 0.43 | NSHAP study |
| Premenopausal women (mid-cycle) | 18 | 22.5 (mean) | 2.6 ± 0.3 | Schultheiss et al. (2003) |
| Adult women | 227 | 19–39 | 9–15 (variation of mean values) | Nunez-De La Mora et al. (2008) |
| Postmenopausal women | 12 | 50–66 | 1.39 (0.54–4.78) | Tivis et al. (2005) |
| Adult women | 204 | 30.7 (mean) | 4.9a (SD = 2.4) | Emaus et al. (2008) |
| Progesterone | ||||
| Postmenopausal women | 1,302 | 57–85 | 60.82 ± 15.16 | NSHAP study |
| Premenopausal women (mid-cycle) | 18 | 22.5 (mean) | 26.4 ± 2.1 | Schultheiss et al. (2003) |
| Premenopausal women (luteal phase) | 22 | 23–39 | 27.1–103.6a (seasonal variation of mean values) | Jasienska and Jasienski (2008) |
| Postmenopausal women | 11 | 50–70 | 58.90 (SD = 29.7) | Nallanathan et al. (2007) |
| DHEA | ||||
| Postmenopausal women | 1,302 | 57–85 | 45.92 ± 1.68 | NSHAP study |
| Adult women | 16 | 30–34 | 374.0 ± 35.4 | Granger et al. (1999) |
| 16 | 35–39 | 299.4 ± 30.9 | Granger et al. (1999) | |
| 16 | 40–45 | 207.6 ± 32.7 | Granger et al. (1999) | |
| Adult women, 8 a.m. | 116 | 38.5 | 320 (SD = 150) | Harris et al. (2000) |
| Adult women, 8 p.m. | 116 | (range 23–58) | 190 (SD = 90) | Harris et al. (2000) |
| Testosterone | ||||
| Postmenopausal women | 1,302 | 57–85 | 46.34 ± 0.76 | NSHAP study |
| Premenopausal women | 20 | 18–23 | 60.86 ± 7.81 | Shirtcliff et al. (2002) |
| Young women | 174 | 18–28 | 230 (35–860) | Flegr et al. (2008) |
Notes: NSHAP = National Social Life, Health, and Aging Project; HRT = hormone replacement therapy; DHEA = dehydroepiandrosterone.
Converted from nanomoles per liter for better comparison.
Table 7.
Reported Sex Hormone Measurements in Saliva of Men as Compared with NSHAP Data
| Sample | N | Age | Mean Value (SEM or SD), pg/ml | Source |
| Estradiol | ||||
| Older men | 1,287 | 57–84 | 10.65 ± −0.56 | NSHAP study |
| Older men | 48 | 50–80 | 6.88 ± −8.35 | Krause et al. (2002) |
| Young men | 19 | 20–35 | 8.36 ± 3.42 | Rantala et al. (2006) |
| Young men | 18 | 23 (mean) | 2.6 ± 0.3 | Schultheiss et al. (2003) |
| Progesterone | ||||
| Older men | 1,287 | 57–85 | 49.52 ± −6.25 | NSHAP study |
| Young men | 18 | 23 (mean) | 22.6 (SD = 1.3) | Schultheiss et al. (2003) |
| Young men | 177 | 18–30 | 15.5–25.2 (diurnal variation of mean values) | Wirth et al. (2007) |
| Young men | 5 | 20–21 | 37.5 ± −16.0a | Celec et al. (2006) |
| DHEA | ||||
| Older men | 1,287 | 57–85 | 61.22 ± 4.70 | NSHAP study |
| Older men | ||||
| Morning | 26 | 60–69 | 180 (SD = 80) | van Niekerk et al. (2001) |
| Evening | 26 | 60–69 | 120 (SD = 60) | van Niekerk et al. (2001) |
| Morning | 15 | 70–76 | 120 (SD = 60) | van Niekerk et al. (2001) |
| Evening | 15 | 70–76 | 90 (SD = 50) | van Niekerk et al. (2001) |
| Adult men | 8 | 35–39 | 464.0 (54.3) | Granger et al. (1999) |
| Adult men | 8 | 40–45 | 351.3 (102.7) | Granger et al. (1999) |
| Testosterone | ||||
| Older men | 1,287 | 57–84 | 99.50 ± −6.54 | NSHAP study |
| Older men | 48 | 50–80 | 70 ± 15 | Krause et al. (2002) |
| Young men | 20 | 18–23 | 163.81 ± 10.24 | Shirtcliff et al. (2002) |
| Young men | 5 | 18–30 | 163.9–274.2 (seasonal variation of mean values) | Brown et al. (2008) |
Notes: NSHAP = National Social Life, Health, and Aging Project; DHEA = dehydroepiandrosterone.
Converted from nanomoles per liter for better comparison.
DISCUSSION
The NSHAP study provides the first population-representative data on salivary sex hormone concentrations in a national, population-based sample of older community-residing adults in the United States. Use of minimally invasive methods for collection of sex hormonal data is feasible and provides information relevant to furthering an understanding of the role of sex hormones in relation to sexuality in later life, cardiovascular disease, cancer, and other chronic conditions of aging. Population data complement clinical data by providing normative information.
Although saliva collection via passive drool may be difficult for older adults due to aging-related changes in saliva production (Hodgson, Freedman, Granger, & Erno, 2004), medication side effects causing dry mouth, and manual manipulation of the small collection devices, overall cooperation with the home-based protocol was high in this population-based sample of older adults. Blacks were less likely to provide saliva samples during the interview. The National Health and Nutrition Examination Survey (NHANES) has also reported relatively lower participation of this racial group in biological measure protocols, specifically collection of DNA from blood. In that study, removal of language in the consent process that linked blood collection to genetic research increased participation in every demographic category (McQuillan, Pan, & Porter, 2006). Cooperation with the NSHAP saliva collection protocol was higher than that accomplished for HIV testing, blood spots, or vaginal swab collection in the NSHAP study (Lindau et al., in press; Williams & McDade, in press). Although, in the case of vaginal swab sampling, differences in cooperation were found by age and education (older age, less educated were less likely to participate) but not by race/ethnicity (Lindau et al., in press). This may be due to the relative invasiveness of the collection protocols or other factors and requires more investigation.
Internal validity of the salivary sex hormone measurements is suggested by the expected findings of higher testosterone concentrations in men versus women (Shirtcliff et al., 2002) and similar estradiol concentrations in women and men (Greenblatt, Oettinger, & Bohler, 1976; Shirtcliff et al., 2002). The proportion of individuals using exogenous hormones was small in the NSHAP sample, as is true in the population as a whole (Wysowski, Golden, & Burke, 1995). Salivary testosterone concentrations in ovariectomized women and in men using androgens were also shifted in the expected direction (Adashi, 1994; Cappola et al., 2007). Estrogen use in women was significantly correlated with higher salivary estradiol concentrations as reported by other authors (Tivis et al., 2005). Obesity is associated with lower production of sex hormone binding globulin (SHBG) in both women and men; consistent with this, and as has been observed previously (Cappola et al., 2007; Lukanova et al., 2004; Schilling et al., 2007), obese women in NSHAP exhibit higher levels of free salivary testosterone. The expected relationship between obesity and free testosterone in men is less clear, and in NSHAP, obese men do not demonstrate significantly different levels of free testosterone than non-obese men. This may be because both SHBG and total testosterone are lower in obese as compared with other men (Allan, Strauss, Burger, Forbes, & McLachlan, 2006; Derby, Zilber, Brambilla, Morales, & McKinlay, 2006; Giagulli, Kaufman, & Vermeulen, 1994).
Normative data on salivary sex hormone concentrations in later life are scarce. As a result, external validation of NSHAP data is particularly difficult for progesterone and DHEA in men and estradiol, DHEA, and testosterone in women. Most existing data derive from small, clinical populations of young volunteers. One population-based study collected salivary specimens from 48 “randomly selected,” healthy, heterosexual, cohabiting men ages 50–80 years, from Mannheim, Germany, and 50 similar men from State College, Pennsylvania (Krause et al., 2002). In that study, salivary measures of DHEA-S (but not DHEA), testosterone, and estradiol were obtained (see Table 7). The concentrations of testosterone and estradiol are similar to those obtained in the NSHAP study. Some studies report lower levels of estradiol (Schultheiss, Dargel, & Rohde, 2003; Tivis et al., 2005) than those obtained in the NSHAP study, although other authors report similar levels of salivary estradiol (Emaus et al., 2008; Nunez-De La Mora, Bentley, Choudhury, Napolitano, & Chatterton, 2008; Rantala, Enksson, Vainikka, & Kortet, 2006). Estradiol measurements in the NSHAP study contain a small number of cases with very high values. For this reason, median (rather than mean) values of salivary estradiol in the NSHAP study show better agreement with the reported data from other studies (see Tables 3, 6, and 7).
Mean progesterone values for women in NSHAP are similar to those reported by Salimetrics for postmenopausal women using the same assay procedures (Nallanathan et al., 2007). As in the case of estradiol, progesterone measurements contain outliers, so median levels of salivary progesterone in the NSHAP study (29.8 pg/ml for men and 24.6 pg/ml for women) demonstrate better agreement with the reported data from other studies (Celec et al., 2006; Jasienska & Jasienski, 2008; Wirth et al., 2007) compared with mean values (see Tables 6 and 7).
Salivary DHEA measurements reported for younger adults by other studies (Granger, Schwartz, Booth, Curran, & Zakaria, 1999; Harris et al., 2000; van Niekerk, Huppert, & Herbert, 2001) are higher than found in the NSHAP study, as expected due to age-related decline of DHEA (Ahn, Lee, Choi, Kwon, & Chun, 2007; Granger et al., 1999). DHEA concentrations measured in serum have generally been reported to be similar in men and women, although DHEA levels in men are nearly always higher than in women (Rehman & Carr, 2004). In the NSHAP sample, men exhibit consistently higher concentrations of DHEA compared with women. This may be related to more rapid decline of DHEA levels with age in women than in men, although more studies are needed to clarify this gender difference.
Compared with younger adults (Brown et al., 2008; Flegr, Lindova, & Kodym, 2008; Shirtcliff et al., 2002), testosterone levels obtained in the NSHAP study are lower. For older men, the levels of salivary testosterone obtained in the NSHAP study and in another population-based study (Krause et al., 2002) are very similar (see Tables 6 and 7). Overall, due to varying assay techniques, study populations and study conditions, salivary hormone measurements obtained across studies are difficult to compare; those reported here are of similar magnitude to findings in the most comparable studies.
Minimally invasive, valid, and reliable methods of sex hormone measurements in saliva can enhance the application of population-based aging research to understanding of disease and function in later life. For example, low levels of endogenous DHEA and testosterone in men are related to decreased survival (Khaw et al., 2007; Maggio et al., 2007; Shores, Matsumoto, Sloan, & Kivlahan, 2006), higher risk for cardiovascular diseases (Choi & McLaughlin, 2007; Jankowska et al., 2006; Khaw et al., 2007), and depression (Morsink et al., 2007). Overall, circulating endogenous sex hormones including estrogens have a neutral or beneficial effect on cardiovascular disease in men (Muller, van der Schouw, Thijssen, & Grobbee, 2003). High estrogen levels in women may be linked to increased risk of breast (Dorgan et al., 2002; Hankinson & Eliassen, 2007) and uterine (Akhmedkhanov, Zeleniuch-Jacquotte, & Toniolo, 2001) cancer. The association between estradiol and cognitive function is complex. Although in vitro studies demonstrated beneficial effects of estrogen on the brain (Ravaglia et al., 2007), recent longitudinal studies showed positive association of endogenous estradiol and risk for vascular dementia (Geerlings et al., 2003) and Alzheimer’s disease (Ravaglia et al., 2007) in women but not in men. The NSHAP data set can be used to further explore the relationships between disease, function, and sex hormones in later life. For example, our recent study using NSHAP data shows that higher levels of some sex hormones are correlated with better sexual performance in older women (Lindau & Gavrilova, 2008).
CONCLUSION
Experience from the NSHAP study demonstrates that collection of salivary specimens from older men and women in an in-home, population-based study using nonmedical field staff is feasible and that the data are internally and externally valid. The cooperation rate for this minimally invasive procedure is high. Saliva specimens provide novel sex hormone data of relevance to studying health and illness in later life.
FUNDING
The National Health, Social Life, and Aging Project (NSHAP) is supported by the National Institutes of Health, including the National Institute on Aging, the Office of Research on Women's Health, the Office of AIDS Research, and the Office of Behavioral and Social Sciences Research (5R01AG021487). NSHAP is also supported by NORC, whose staff was responsible for the data collection. Research assistants were supported, in part, by the Population-Based Integrated Health Research Biomarker Core at the University of Chicago (1 U54 RR023560-01A1).The National Institutes of Health, National Institute on Aging University of Chicago–NORC Center on Demography and Economics of Aging Core on Biomarkers in Population-Based Health and Aging Research (5 P30 AG 012857) supported N.G.'s effort and a portion of S.T.L.'s effort for this article.
Acknowledgments
Donors of supplies include Orasure, (Bethlehem, PA), Sunbeam Corporation (Boca Raton, FL), A&D Lifesource (San Jose, CA), Wilmer Eye Institute at the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD), Schleicher & Schuell Bioscience (Keene, NH), Biomerieux (Durham, NC), Roche Diagnostics (Indianapolis, IN), Digene Corporation (Gaithersburg, MD), Richard Williams (Longboat Key, FL). We are grateful to Mary Curran at Salimetrics for coordinating salivary testing and analysis and Dr. Douglas Granger for valuable comments on the final version of manuscript. We gratefully acknowledge the assistance of Jessica Schwartz, Bhairavi Nallanathan, Andreea Mihai, and Karl Mendoza during the preparation of the manuscript. N.G. and S.T.L. originated and designed the study. N.G. conducted statistical analyses and calculations. Both authors interpreted the results and commented on drafts of the article.
References
- Adashi EY. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertility and Sterility. 1994;62:20–27. doi: 10.1016/s0015-0282(16)56810-1. [DOI] [PubMed] [Google Scholar]
- Ahn RS, Lee YJ, Choi JY, Kwon HB, Chun SI. Salivary cortisol and DHEA levels in the Korean population: Age-related differences, diurnal rhythm, and correlations with serum levels. Yonsei Medical Journal. 2007;48:379–388. doi: 10.3349/ymj.2007.48.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmedkhanov A, Zeleniuch-Jacquotte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer—Review of the evidence and research perspectives. In: Bulletti C, de Ziegler D, Guller S, LevitzHuman M, editors. 2001. Fertility and Reproduction: the Oocyte, the Embryo, and the Uterus, Annals of the New York Academy of Sciences. 943, 296–315. [DOI] [PubMed] [Google Scholar]
- Alexandersen P, Haarbo J, Christiansen C. The relationship of natural androgens to coronary heart disease in males: A review. Atherosclerosis. 1996;125:1–13. doi: 10.1016/0021-9150(96)05864-9. [DOI] [PubMed] [Google Scholar]
- Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. The association between obesity and the diagnosis of androgen deficiency in symptomatic ageing men. Medical Journal of Australia. 2006;185:424–427. doi: 10.5694/j.1326-5377.2006.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Tufik S. Does male sexual behavior require progesterone? Brain Research. Brain Research Reviews. 2006;51:136–143. doi: 10.1016/j.brainresrev.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Bachmann G, Leiblum S. The impact of hormones on menopausal sexuality: A literature review. Menopause. 2004;11:120–130. doi: 10.1097/01.GME.0000075502.60230.28. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. Journal of Clinical Endocrinology and Metabolism. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- Bell FC, Miller ML. Life tables for the United States social security area 1900–2100. Baltimore: Social Security Administration, Office of Chief Actuary; 2002. [Google Scholar]
- Binder D. On the variances of asymptotically normal estimators from complex surveys. Statistical Review. 1983;51:279–292. [Google Scholar]
- Brown GL, McGarvey EL, Shirtcliff EA, Keller A, Granger DA, Flavin K. Salivary cortisol, dehydroepiandrosterone, and testosterone interrelationships in healthy young males: A pilot study with implications for studies of aggressive behavior. Psychiatry Research. 2008;159:67–76. doi: 10.1016/j.psychres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Cappola AR, Ratcliffe SJ, Bhasin S, Blackman MR, Cauley J, Robbins J, Zmuda JM, Harris T, Fried LP. Determinants of serum total and free testosterone levels in women over the age of 65 years. Journal of Clinical Endocrinology and Metabolism. 2007;92(2):509–516. doi: 10.1210/jc.2006-1399. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB. Higher levels of plasma estradiol and testosterone in healthy elderly men compared with age-matched women may protect aspects of explicit memory. Menopause. 2000;7:168–177. doi: 10.1097/00042192-200007030-00007. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Zandi PP, Plassman BL, Tschanz JT, Welsh-Bohmer KA, Steffens DC, Bastian LA, Mehta KM, Breitner JC. Hormone replacement therapy and reduced cognitive decline in older women: The cache county study. Neurology. 2001;57(12):2210–2216. doi: 10.1212/wnl.57.12.2210. [DOI] [PubMed] [Google Scholar]
- Celec P, Ostatnikova D, Hodosy J, Skoknova M, Putz Z, Kudela M. Infradian rhythmic variations of salivary estradiol and progesterone in healthy men. Biological Rhythm Research. 2006;37:37–44. [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: The women's health initiative randomized trial. Journal of the American Medical Association. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Choi BG, McLaughlin MA. Why men's hearts break: Cardiovascular effects of sex steroids. Endocrinology and Metabolism Clinics of North America. 2007;36:365–377. doi: 10.1016/j.ecl.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Christeff N, Lortholary O, Casassus P, Thobie N, Veyssier P, Torri O, Guillevin L, Nunez EA. Relationship between sex steroid hormone levels and CD4 lymphocytes in HIV infected men. Experimental and Clinical Endocrinology & Diabetes. 1996;104:130–136. doi: 10.1055/s-0029-1211434. [DOI] [PubMed] [Google Scholar]
- Clemons M, Goss P. Estrogen and the risk of breast cancer. New England Journal of Medicine. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- Davis S, Tran J. What are “normal” testosterone levels for women? Journal of Clinical Endocrinology and Metabolism. 2001;86:1842–1844. doi: 10.1210/jcem.86.4.7436-10. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: A review of the population-based studies. Annual Review of Sex Research. 2003;14:64–82. [PubMed] [Google Scholar]
- Dennerstein L, Gotts G, Brown JB, Morse CA, Farley TM, Pinol A. The relationship between the menstrual cycle and female sexual interest in women with PMS complaints and volunteers. Psychoneuroendocrinology. 1994;19:293–304. doi: 10.1016/0306-4530(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Smith AM, Morse CA, Burger HG. Sexuality and the menopause. Journal of Psychosomatic Obstetrics and Gynaecology. 1994;15:59–66. doi: 10.3109/01674829409025630. [DOI] [PubMed] [Google Scholar]
- Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: The Massachusetts Male Ageing Study. Clinical Endocrinology. 2006;65:125–131. doi: 10.1111/j.1365-2265.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- Dorgan JF, Longcope C, Franz C, Stanczyk FZ, Chang LC, Stephenson HE, Falk RT, Kahle L, Miller R, Tangrea JA. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. Journal of the National Cancer Institute. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Drum ML, Shiovitz-Ezra S, Gaumer E, Lindau ST. Assessment of health-related behaviors and health care utilization in the National Social Life, Health, and Aging Project (NSHAP) Journals of Gerontology: Social Sciences. 2009 doi: 10.1093/geronb/gbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, Panter-Brick C, Hill K. Population variation in age-related decline in male salivary testosterone. Human Reproduction. 2002;17:3251–3253. doi: 10.1093/humrep/17.12.3251. [DOI] [PubMed] [Google Scholar]
- Emaus A, Veierod MB, Furberg AS, Espetvedt S, Friedenreich C, Ellison PT, et al. Physical activity, heart rate, metabolic profile, and estradiol in premenopausal women. Medicine and Science in Sports and Exercise. 2008;40:1022–1030. doi: 10.1249/MSS.0b013e318167411f. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay J. B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. Journal of Clinical Endocrinology and Metabolism. 2002;87(2):589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Flegr J, Lindova J, Kodym P. Sex-dependent toxoplasmosis-associated differences in testosterone concentration in humans. Parasitology. 2008;135:427–431. doi: 10.1017/S0031182007004064. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Launer LJ, de Jong FH, Ruitenberg A, Stijnen T, van Swieten JC, Hofman A, Witteman JCM, Pols HAP, Breteler MMB. Endogenous estradiol and risk of dementia in women and men: The rotterdam study. Annals of Neurology. 2003;53(5):607–615. doi: 10.1002/ana.10521. [DOI] [PubMed] [Google Scholar]
- Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. Journal of Clinical Endocrinology and Metabolism. 1994;79:997–1000. doi: 10.1210/jcem.79.4.7962311. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua G-L. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology and Behavior. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Granger DA, Schwartz EB, Booth A, Curran M, Zakaria D. Assessing dehydroepiandrosterone in saliva: A simple radioimmunoassay for use in studies of children, adolescents and adults. Psychoneuroendocrinology. 1999;24:567–579. doi: 10.1016/s0306-4530(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Greenblatt RB, Oettinger M, Bohler CSS. Estrogen-androgen levels in aging men and women: Therapeutic considerations. Journal of the American Geriatrics Society. 1976;24:173–178. doi: 10.1111/j.1532-5415.1976.tb04294.x. [DOI] [PubMed] [Google Scholar]
- Greenspan FS, Gardner DG. Basic & clinical endocrinology. 6th ed. New York: Lange Medical Books/McGraw-Hill; 2001. [Google Scholar]
- Grinspoon S, Corcoran C, Stanley T, Baaj A, Basgoz N, Klibanski A. Effects of hypogonadism and testosterone administration on depression indices in HIV-infected men. Journal of Clinical Endocrinology and Metabolism. 2000;85:60–65. doi: 10.1210/jcem.85.1.6224. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Textbook of medical physiology. 8th ed. Philadelphia: Saunders; 1991. [Google Scholar]
- Hackbert L, Heiman JR. Acute dehydroepiandrosterone (DHEA) effects on sexual arousal in postmenopausal women. Journal of Womens Health Gender Based Medicine. 2002;11:155–162. doi: 10.1089/152460902753645290. [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. Journal of Biological Chemistry. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. Journal of Steroid Biochemistry and Molecular Biology. 2007;106:24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TO, Borsanyi S, Messari S, Stanford K, Cleary SE, Shiers HM, Brown GW, Herbert J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. British Journal of Psychiatry. 2000;177:505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Hendrix SL. Bilateral oophorectomy and premature menopause. American Journal of Medicine. 2005;118(Suppl. 12B):131–135. doi: 10.1016/j.amjmed.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Hodgson N, Freedman VA, Granger DA, Erno A. Biobehavioral correlates of relocation in the frail elderly: Salivary cortisol, affect, and cognitive function. Journal of the American Geriatrics Society. 2004;52(11):1856–1862. doi: 10.1111/j.1532-5415.2004.52505.x. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M, Anker SD, Banasiak W, Poole-Wilson PA, Ponikowski P. Anabolic deficiency in men with chronic heart failure: Prevalence and detrimental impact on survival. Circulation. 2006;114(17):1829–1837. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- Jasienska G, Jasienski M. Interpopulation, interindividual, intercycle, and intracycle natural variation in progesterone levels: A quantitative assessment and implications for population studies. American Journal of Human Biology. 2008;20:35–42. doi: 10.1002/ajhb.20686. [DOI] [PubMed] [Google Scholar]
- Jassal SK, Barrett-Connor E, Edelstein SL. Low bioavailable testosterone levels predict future height loss in postmenopausal women. Journal of Bone and Mineral Research. 1995;10:650–654. doi: 10.1002/jbmr.5650100419. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Bebb RA, Sirrs SM. Uses of DHEA in aging and other disease states. Ageing Research Reviews. 2002;1:29–41. doi: 10.1016/s0047-6374(01)00369-4. [DOI] [PubMed] [Google Scholar]
- Kaufman E, Lamster IB. The diagnostic applications of saliva—a review. Critical Reviews in Oral Biology and Medicine. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocrine Reviews. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance—United States, 1994–1999. Morbidity and Mortality Weekly Report. CDC Surveillance Summaries. 2002;51(SS05):1–8. [PubMed] [Google Scholar]
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- Krause W, Mueller U, Mazur A. Measurement of steroid levels in saliva in a population-based survey of lifestyle, medical conditions, marriage, sex life and hormone status in aging men: A feasibility study. The Aging Male. 2002;5:203–215. [PubMed] [Google Scholar]
- Kuchuk N, van Schoor N, Pluijm S, Smit J, de Ronde W, Lips P. The association of sex hormone levels with quantitative ultrasound, bone mineral density, bone turnover and osteoporotic fractures in older men and women. Clinical Endocrinology. 2007;67:295–303. doi: 10.1111/j.1365-2265.2007.02882.x. [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. Journal of Clinical Endocrinology and Metabolism. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: The rancho bernardo study. Journal of Clinical Endocrinology and Metabolism. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- Lindau ST, Gavrilova NS. Understanding overall and sexual health in older women using saliva sex hormone measurements. Population Association of America April 2008 Annual Meeting. Final Program and Abstracts. 2008 (p. 226). Silver Spring, MD: Population Association of America. [Google Scholar]
- Lindau ST, Hoffmann JN, Lundeen K, Jaszczak A, McClintock MK, Jordan JA. Vaginal self-swab specimen collection in a home-based survey of older women: Methods and applications. Journals of Gerontology: Social Sciences. 2009 doi: 10.1093/geronb/gbn021. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau ST, McDade TW. Minimally invasive and innovative methods for biomeasure collection in population-based research. In: Weinstein James W, Vaupel Maxine, Wachter Kenneth W., editors. Biosocial Surveys. Washington, DC: The National Academies Press; 2007. pp. 251–277. [Google Scholar]
- Lindau ST, Schumm P, Laumann EO, Levinson W, O’Muircheartaigh C, Waite L. A national study of sexuality and health among older adults in the U.S. New England Journal of Medicine. 2007;357:22–34. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo RA. Androgens in postmenopausal women: Production, possible role, and replacement options. Obstetrical & Gynecological Survey. 2001;56:361–376. doi: 10.1097/00006254-200106000-00022. [DOI] [PubMed] [Google Scholar]
- Lu Y, Bentley GR, Gann PH, Hodges KR, Chatterton RT. Salivary estradiol and progesterone levels in conception and nonconception cycles in women: Evaluation of a new assay for salivary estradiol. Fertility and Sterility. 1999;71:863–868. doi: 10.1016/s0015-0282(99)00093-x. [DOI] [PubMed] [Google Scholar]
- Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: A cross-sectional study in healthy women. European Journal of Endocrinology. 2004;150:161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Ling SM, Metter EJ, Artoni A, Carassale L, Cazzato A, Ceresini G, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men The aging in the Chianti area (InCHIANTI) study. Archives of Internal Medicine. 2007;167(20):2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiology of Aging. 2000;21:373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Merchant CA, Jacobs DM, Small SA, Bell K, Ferin M, Mayeux R. Endogenous estrogen levels and Alzheimer's disease among postmenopausal women. Neurology. 2000;54:833–837. doi: 10.1212/wnl.54.4.833. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R. Estrogen plus progestin and the risk of coronary heart disease. New England Journal of Medicine. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- Masi AT. Sex-hormones and rheumatoid-arthritis: Cause or effect relationships in a complex pathophysiology. Clinical and Experimental Rheumatology. 1995;13:227–240. [PubMed] [Google Scholar]
- McQuillan G, Pan Q, Porter K. Consent for genetic research in a general population: An update on the National Health and Nutrition Examination Survey experience. Genetics in Medicine. 2006;8:354–360. doi: 10.1097/01.gim.0000223552.70393.08. [DOI] [PubMed] [Google Scholar]
- Mendoza K, Curran MJ, Lindau ST. Salivary DHEA measurement in wave I of the National Social Life, Health & Aging Project (NSHAP) Chicago: NORC and the University of Chicago; 2007a. [Google Scholar]
- Mendoza K, Curran MJ, Lindau ST. Salivary estradiol measurement in wave I of the National Social Life, Health & Aging Project (NSHAP) Chicago: NORC and the University of Chicago; 2007b. [Google Scholar]
- Mendoza K, Curran MJ, Lindau ST. Salivary testosterone measurement in wave I of the National Social Life, Health & Aging Project (NSHAP) Chicago: NORC and the University of Chicago; 2007c. [Google Scholar]
- Meston CM, Frohlich PF. The neurobiology of sexual function. Archives of General Psychiatry. 2000;57:1012–1030. doi: 10.1001/archpsyc.57.11.1012. [DOI] [PubMed] [Google Scholar]
- Morsink LFJ, Vogelzangs N, Nicklas BJ, Beekman ATF, Satterfield S, Rubin SM, Yaffe K, Simonsick E, Newman AB, Kritchevsky SB, et al. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: Results from the Health ABC study. Psychoneuroendocrinology. 2007;32(8–10):874–883. doi: 10.1016/j.psyneuen.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Muller M, van der Schouw YT, Thijssen JHH, Grobbee DE. Endogenous sex hormones and cardiovascular disease in men. Journal of Clinical Endocrinology and Metabolism. 2003;88:5076–5086. doi: 10.1210/jc.2003-030611. [DOI] [PubMed] [Google Scholar]
- Nallanathan B, Mendoza KM, Curran MJ, Lindau ST. Salivary progesterone measurement in wave I of the National Social Life, Health & Aging Project (NSHAP) Chicago: NORC and the University of Chicago; 2007. [Google Scholar]
- Newton KM, Buist DSM, Yu O, Hartsfield CL, Andrade SE, Wei F, Connelly MT, Chan KA. Hormone therapy initiation after the women's health initiative. Menopause: The Journal of the North American Menopause Society. 2008;15(3):487–493. doi: 10.1097/gme.0b013e318154b9a5. [DOI] [PubMed] [Google Scholar]
- Nichols HB, Trentham-Dietz A, Hampton JM, Titus-Ernstoff L, Egan KM, Willett WC, Newcomb PA. From menarche to menopause: Trends among US women born from 1912 to 1969. American Journal of Epidemiology. 2006;164(10):1003–1011. doi: 10.1093/aje/kwj282. [DOI] [PubMed] [Google Scholar]
- Nunez-De La Mora A, Bentley GR, Choudhury OA, Napolitano DA, Chatterton RT. The impact of developmental conditions on adult salivary estradiol levels: Why this differs from progesterone? American Journal of Human Biology. 2008;20:2–14. doi: 10.1002/ajhb.20698. [DOI] [PubMed] [Google Scholar]
- O’Muircheartaigh C, Eckman S, Smith S. Statistical design and estimation for the National Social Life, Health, and Aging Project (NSHAP) Journal of Gerontology: Social Sciences. 2009 doi: 10.1093/geronb/gbp045. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjari M, Davis S. DHEA therapy for women: Effect on sexual function and wellbeing. Human Reproduction Update. 2007;13:239–248. doi: 10.1093/humupd/dml055. [DOI] [PubMed] [Google Scholar]
- Rantala MJ, Enksson CJP, Vainikka A, Kortet R. Male steroid hormones and female preference for male body odor. Evolution and Human Behavior. 2006;27:259–269. [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Bastagli L, Montesi F, Pisacane N, Chiappelli M, Licastro F, Patterson C, et al. Endogenous sex hormones as risk factors for dementia in elderly men and women. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(9):1035–1041. doi: 10.1093/gerona/62.9.1035. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Boschi F, Bernardi M, Pratelli L, Pizzoferrato A, Gasbarrini G. The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine-metabolic parameters and functional status in the oldest-old. Results from an Italian study on healthy free-living over-ninety-year-olds. Journal of Clinical Endocrinology and Metabolism. 1996;81(3):1173–1178. doi: 10.1210/jcem.81.3.8772596. [DOI] [PubMed] [Google Scholar]
- Regelson W, Loria R, Kalimi M. Dehydroepiandrosterone (Dhea)—the mother steroid. 1. Immunological action. Aging Clock. 1994;719:553–563. doi: 10.1111/j.1749-6632.1994.tb56859.x. [DOI] [PubMed] [Google Scholar]
- Rehman HU, Masson EA. Neuroendocrinology of female aging. Gender Medicine. 2005;2:41–56. doi: 10.1016/s1550-8579(05)80008-7. [DOI] [PubMed] [Google Scholar]
- Rehman KS, Carr BR. Sex differences in adrenal androgens. Seminars in Reproductive Medicine. 2004;22:349–360. doi: 10.1055/s-2004-861551. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SAA, Howard BV, Johnson K, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. Journal of the American Medical Association. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Salimetrics. High sensitivity salivary estradiol enzyme immunoassay kit. State College, PA: Salimetrics LLC; 2006. [Google Scholar]
- Schilling C, Gallicchio L, Miller SR, Langenberg P, Zacur H, Flaws JA. Relation of body mass and sex steroid hormone levels to hot flushes in a sample of mid-life women. Climacteric. 2007;10:27–37. doi: 10.1080/13697130601164755. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Dargel A, Rohde W. Implicit motives and gonadal steroid hormones: Effects of menstrual cycle phase, oral contraceptive use, and relationship status. Hormones and Behaviour. 2003;43:293–301. doi: 10.1016/s0018-506x(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Likos A. Gender differences in the validity of testosterone measured in saliva by immunoassay. Hormones and Behaviour. 2002;42:62–69. doi: 10.1006/hbeh.2002.1798. [DOI] [PubMed] [Google Scholar]
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Archives of Internal Medicine. 2006;166:1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- Spark RF. Dehydroepiandrosterone: A springboard hormone for female sexuality. Fertility and Sterility. 2002;77(Suppl. 4):S19–S25. doi: 10.1016/s0015-0282(02)02987-4. [DOI] [PubMed] [Google Scholar]
- Speroff L, Glass RH, Kase NG. Clinical gynecologic endocrinology and infertility. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- StataCorp. College Station, TX: StataCorp LP; 2005. Stata statistical software: Release 9. [Google Scholar]
- Tenover JL. Testosterone and the aging male. Journal of Andrology. 1997;18:103–106. [PubMed] [Google Scholar]
- Tivis LJ, Richardson MD, Peddi E, Arjmandi B. Saliva versus serum estradiol: Implications for research studies using postmenopausal women. Progess in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29:727–732. doi: 10.1016/j.pnpbp.2005.04.029. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report [document 98–4083] Bethesda, MD: NHLBI; 2000. [Google Scholar]
- van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: Association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26:591–612. doi: 10.1016/s0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: A randomized controlled trial. Journal of the American Medical Association. 2004;292:2243–2248. doi: 10.1001/jama.292.18.2243. [DOI] [PubMed] [Google Scholar]
- Whembolua G-L, Granger DA, Kivlighan KT, Marguin JA, Singer S. Bacteria in the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Hormones and Behavior. 2006;49:478–483. doi: 10.1016/j.yhbeh.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Williams RH, Wilson JD. Williams textbook of endocrinology. 9th ed. Philadelphia: Saunders; 1998. [Google Scholar]
- Williams SR, McDade TW. The use of dried blood spot sampling in the National Social Life, Health, and Aging Project. Journal of Gerontology: Social Sciences. 2009 doi: 10.1093/geronb/gbn022. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MM, Meier EA, Fredrickson BL, Schultheiss OC. Relationship between salivary cortisol and progesterone levels in humans. Biological Psychology. 2007;74:104–107. doi: 10.1016/j.biopsycho.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Effects of affiliation arousal (hope of closeness) and affiliation stress (fear of rejection) on progesterone and cortisol. Hormones and Behavior. 2006;50:786–795. doi: 10.1016/j.yhbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF, Hofman LF. Sensitive salivary estradiol assay for monitoring ovarian function. Clinical Chemistry. 1990;36:1769–1773. [PubMed] [Google Scholar]
- Wysowski DK, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982–1992. Obstetrics & Gynecology. 1995;85:6–10. doi: 10.1016/0029-7844(94)00339-f. [DOI] [PubMed] [Google Scholar]