Abstract
Background
In asymptomatic patients with severe isolated mitral regurgitation (MR), identifying the onset of early left ventricular (LV) dysfunction can guide the timing of surgical intervention. We hypothesized that changes in LV transmural myocardial strain represent an early marker of LV dysfunction in an ovine chronic MR model.
Methods and Results
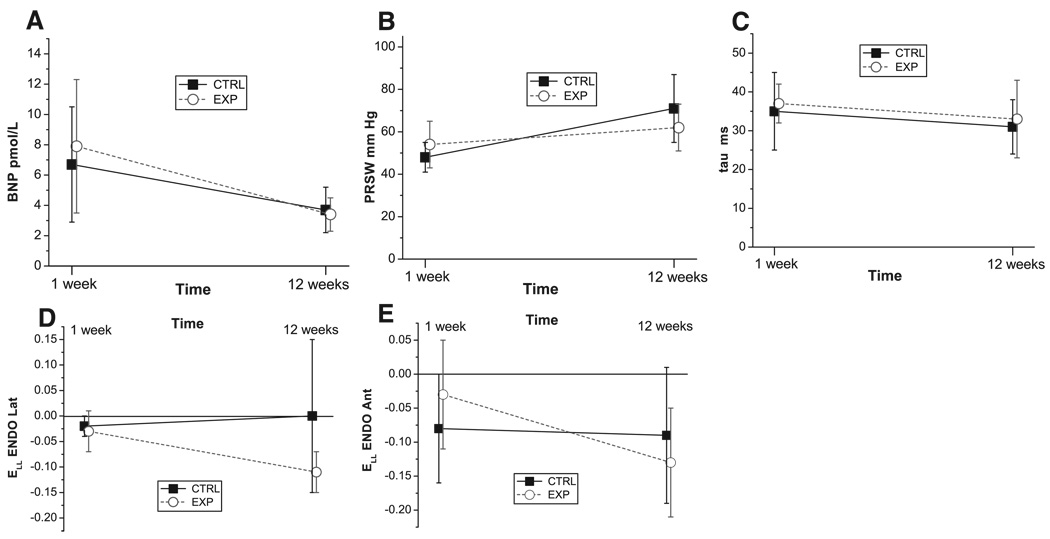
Sheep were randomized to control (CTRL, n=8) or experimental (EXP, n=12) groups. In EXP, a 3.5- or 4.8-mm hole was created in the posterior mitral leaflet to generate “pure” MR. Transmural beadsets were inserted into the lateral and anterior LV wall to radiographically measure 3-dimensional transmural strains during systole and diastolic filling, at 1 and 12 weeks postoperatively. MR grade was higher in EXP than CTRL at 1 and 12 weeks (3.0 [2–4] versus 0.5 [0–2]; 3.0 [1–4] versus 0.5 [0–1], respectively, both P<0.001). At 12 weeks, LV mass index was greater in EXP than CTRL (201±18 versus 173±17 g/m2; P<0.01). LVEDVI increased in EXP from 1 to 12 weeks (P=0.015). Between the 1 and 12 week values, the change in BNP (−4.5±4.4 versus −3.0±3.6 pmol/L), PRSW (9±13 versus 23±18 mm Hg), tau (−3±11 versus −4±7 ms), and systolic strains was similar between EXP and CTRL. The changes in longitudinal diastolic filling strains between 1 and 12 weeks, however, were greater in EXP versus CTRL in the subendocardium (lateral: −0.08±0.05 versus 0.02±0.14; anterior: −0.10±0.05 versus −0.02±0.07, both P<0.01).
Conclusions
Twelve weeks of ovine “pure” MR caused LV remodeling with early changes in LV function detected by alterations in transmural myocardial strain, but not by changes in BNP, PRSW, or tau.
Keywords: LV dysfunction, mitral regurgitation, timing of surgery, transmural strain
Degenerative mitral valve disease (valve prolapse or flail leaflets) is the most common cause of nonfunctional mitral regurgitation (MR) in Western countries.1 The prevalence of this disorder increases with age, and, if left untreated, results in disability and premature death.2 The management of asymptomatic patients with severe MR remains controversial, particularly with respect to the timing of surgical intervention.2,3 Because of adaptive remodeling of the left ventricle (LV) and atrium, patients can remain asymptomatic or minimally symptomatic for prolonged periods, despite the presence of severe MR. MR is a progressive disorder, however, leading eventually to the development of LV dysfunction, which may be irreversible. Once LV function is impaired, outcomes after surgical intervention are suboptimal.4 Hence, in patients with asymptomatic severe MR, a key is to identify the onset of early LV dysfunction so that surgical repair can be performed expeditiously.4 Traditional empirical markers indicating consideration of valve repair are an end-systolic LV dimension ≥4.0 cm or an effective regurgitant orifice >40 mm2.2,5 These thresholds may occasionally not be crossed early enough in the natural history of the disease, however, such that some patients have poor postoperative outcome.6
Perturbations in myocardial deformation putatively are implicated in triggering LV remodeling; therefore, alterations in myocardial strain may precede the onset of irreversible LV dysfunction.7–9 Accordingly, we hypothesized that changes in 3-dimensional (3-D) transmural LV myocardial strain represent the onset of latent LV dysfunction in an ovine chronic pure MR model of low pressure LV volume overload.
Methods
All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals, prepared by the National Academy of Sciences and published by the National Institutes of Health (Publication No. 85-23, Revised 1996). This study was approved by the Stanford Medical Center Laboratory Research Animal Review Committee and conducted according to Stanford University policy.
Surgical Preparation
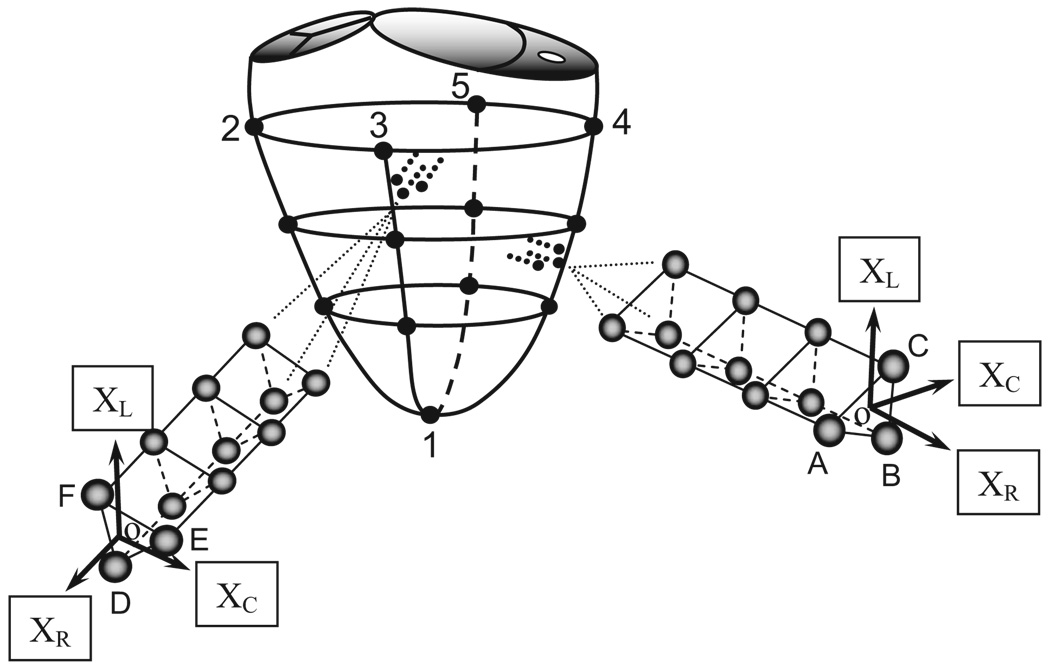
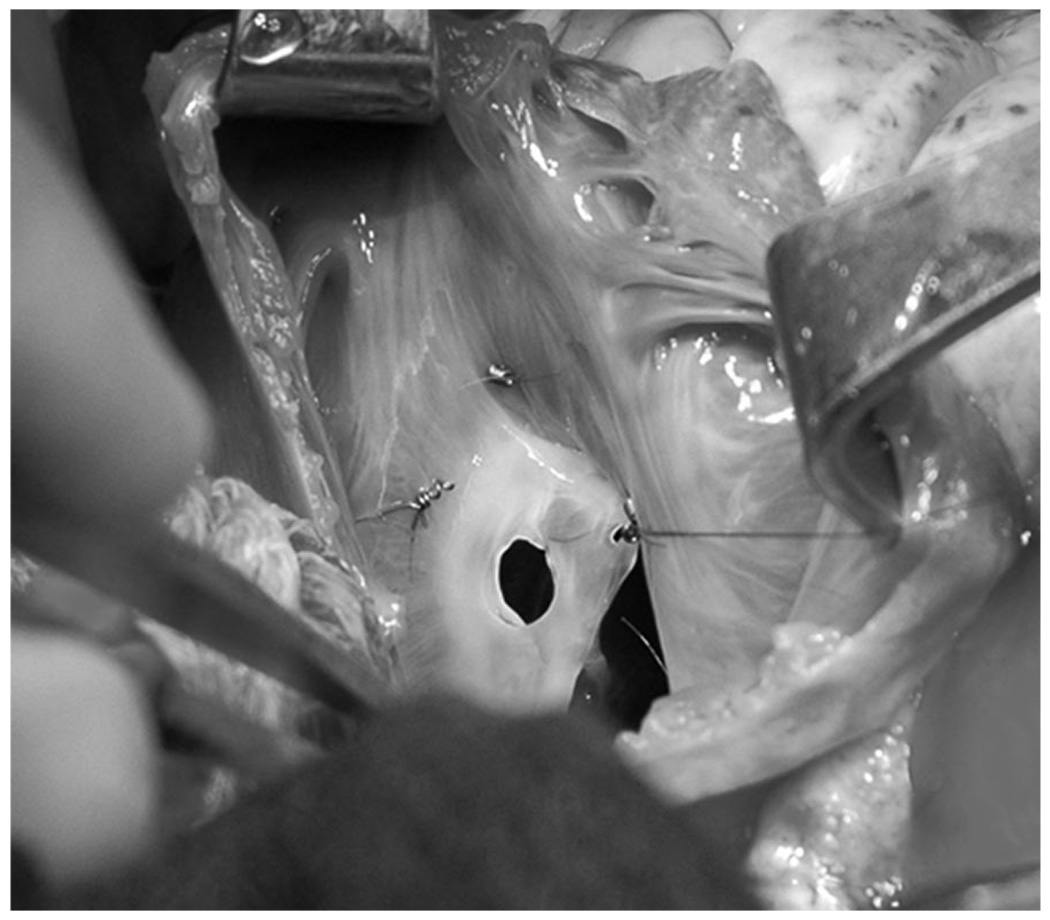
Adult male (Dorsett-hybrid) sheep were randomized to either control (CTRL, n=8) or experimental (EXP, n=12) groups. Some of these animals have also been included in a recently presented, but presently unpublished, analysis of the effect of pure MR on mitral annular geometry and dynamics (Nguyen TC, et al, unpublished data, 2007). The animals were premedicated with ketamine (25 mg/kg, i.m.), anesthetized with sodium thiopental (6.8 mg/kg, i.v.), intubated, and mechanically ventilated (Servo Ventilator 900C, Siemens-Elema). General anesthesia was maintained with inhalational isoflurane (1.5 to 2.0%) and supplemental oxygen. A left thoracotomy was performed, and epicardial echocardiography (Sonos 5500, Hewlett-Packard) was used to measure the wall thickness of a segment of the lateral equatorial LV wall between the papillary muscles, and a site in the anterior LV wall basal to the anterior papillary muscle, for placement of two transmural radiopaque bead sets. Three transmural columns of four beads each (Figure 1) were then inserted into each region using techniques similar to those described previously.10 Three 0.7 mm diameter beads in each column were evenly spaced between endo- and epicardium by means of a depth-adjustable bead insertion trocar. A fourth 1.7-mm diameter bead was then sewn onto the epicardial surface above each column. In addition, 13 miniature tantalum radiopaque markers were inserted into the LV wall subepicardium and septum to silhouette the chamber (Figure 1).11 While on cardiopulmonary bypass (CPB) with the heart arrested, the left atrium was opened. In EXP, depending on the size of the animal, a 3.5- or 4.8-mm diameter hole (Figure 2) was created in the middle scallop of the posterior mitral leaflet (PML) using an aortic hole punch to generate pure MR. The left atrium was closed and the animal was weaned from CPB. An implantable micromanometer pressure transducer (PA4.5-X6; Konigsberg Instrument Inc) was placed in the LV chamber through the apex and exteriorized through the skin between the scapulae. The chest was closed, hydromorphone hydrochloride (Dilaudid, 1 mg, i.m.) was given as needed to minimize incisional discomfort, and the animal recovered in the ovine intensive care unit.
Figure 1.
Locations of LV subepicardial markers and LV lateral equatorial and anterobasal wall transmural beadsets. XC, XL, and XR are the local circumferential, longitudinal, and radial axes, respectively.
Figure 2.
Intraoperative photograph as viewed from the left atrium of the mitral valve with a 4.8-mm diameter hole in the posterior middle leaflet.
Data Acquisition
At 1 week and again at 12 weeks postoperatively, each closed-chest animal was taken to the cardiac catheterization laboratory for data acquisition. The animals were premedicated with ketamine, intubated, mechanically ventilated, and sedation was maintained with inhalational isoflurane. A micromanometer catheter (Millar Instruments Inc) was introduced through a sheath in the left carotid artery and advanced to the ascending aorta for aortic pressure measurement. Videofluoroscopic images of all radiopaque markers and beads were acquired during steady-state baseline conditions with the heart in normal sinus rhythm and ventilation transiently arrested at end expiration.11 Images were acquired with animals in the right lateral position using a biplane videofluoroscopy system (Philips Medical Systems, North America Company). Data from the two 2-D views were digitized and then merged to yield the 3-D coordinates of the centroid of each marker every 16.7 ms using custom-designed software.12 The accuracy of these 3-D reconstructions from biplane videograms of length measurements, compared with known marker-to-marker 3-D lengths, was previously shown to be 0.1±0.3 mm.12 Aortic pressure, LV pressure (LVP), and ECG voltage signals were digitized and recorded simultaneously during marker data acquisition. All animals were followed for clinical signs of heart failure (tachypnea, lethargy, and anorexia). MR data were echocardiographically acquired at baseline (epicardial), at the 1-week study (transthoracic, TTE), and then weekly until the 12-week study (TTE). After the final catheterization study, the heart was explanted in situ. The right ventricle and both atria were subsequently excised, leaving behind only the LV, including the mitral valve apparatus, which was then weighed.
Cardiac Cycle Timing and Hemodynamics
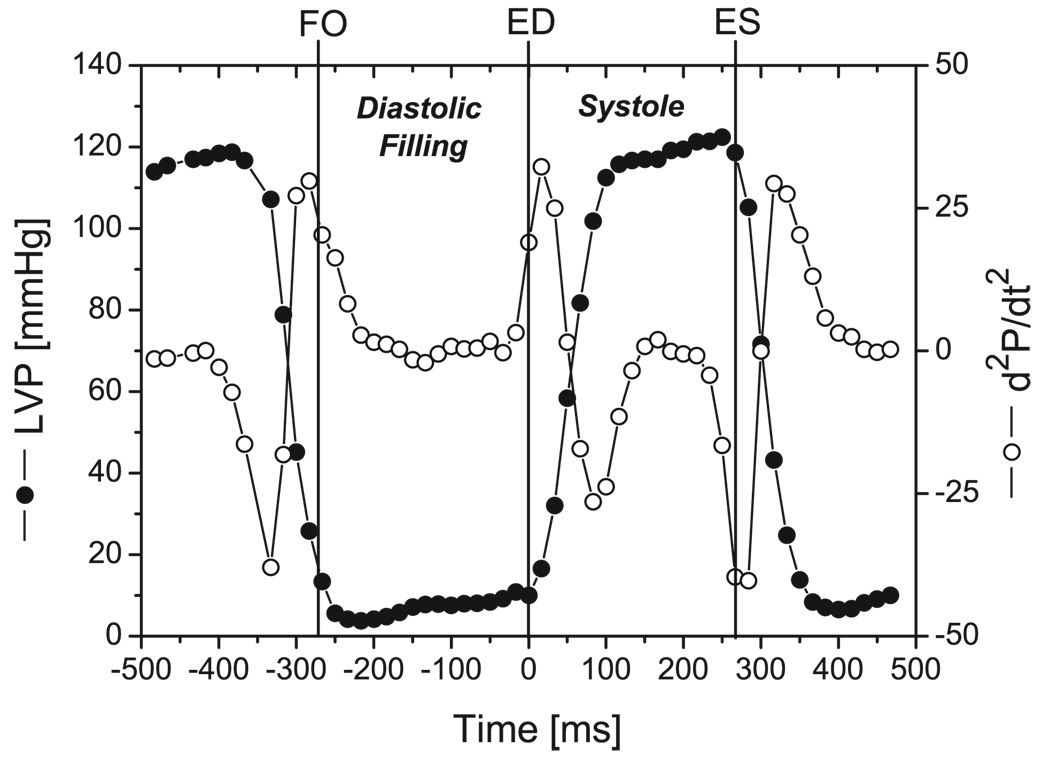
For each cardiac cycle, the time of filling onset (FO, Figure 3) was defined as the videographic frame immediately after LVP=0.1*(LVPivrmax − LVPivrmin)+LVPivrmin, where LVPivrmax and LVPivrmin were the maximum and minimum values of LVP during isovolumic relaxation (IVR), respectively.13 End-diastole (ED) was defined as the frame immediately before the frame containing the greatest second derivative of LVP with respect to time. End-systole (ES) was defined as the frame immediately before the frame containing the minimum second derivative of LVP with respect to time. Three consecutive beats in sinus rhythm were selected for analysis from each study. LV volume (LVV) was calculated every 16.7 ms from the 3-D coordinates of the LV subepicardial markers by summing the volumes of multiple space-filling tetrahedra. This epicardial LVV includes both LV chamber volume and LV myocardium volume. Previous studies have shown that the change in this LVV is an accurate measurement of the change in LV chamber volume.14 MR from all data recordings was graded (0–4) by a blinded expert echocardiographer (D.L.) on the basis of color Doppler regurgitant jet extent and width.15
Figure 3.
Cardiac cycle timing showing typical data from one heart. Filling onset (FO), end-diastole (ED), and end-systole (ES) are indicated (vertical solid lines). LVP, LV pressure (open circles); d2P/dt2, second derivative of LVP (solid circles).
BNP, PRSW, and Tau
Whole blood samples were collected from the external jugular vein, before surgery, 1 week and 12 weeks postoperatively, in EDTA tubes and centrifuged at 4 degrees Celsius at 2000g for 15 minutes. The plasma was pipetted into 6 mL plastic storage vials and stored at −80 degrees Celsius. Samples were then assayed for B-type natriuretic peptide (BNP) according to standard procedures.16 All samples were assayed together to avoid interassay variability, and in a blinded manner without knowledge of the study groups. LV preload recruitable stroke work (PRSW) was computed as the slope of the linear regression of SW on ED volume during vena caval occlusion, where SW was calculated as the integral of LVP (P) multiplied by change in volume (dV) as: SW=∫P · dV over each cardiac cycle. LV relaxation time constant (tau, τ) was computed as the negative reciprocal of the slope of the following relationship: ln(P−PB)=lnP0 = t/τ, where P0 is LVP at maximum negative time derivative of LV pressure (−dP/dtmax), P(t) is LVP at any time, t, during IVR from P0 to onset of LV filling and PB is pressure asymptote.
Cardiac Strains
Placement of the trans-mural beadsets allowed assessment of transmural 3-D myocardial deformations in the 2 regions of the LV wall (Figure 1). Detailed strain analysis methodology has been described previously.10 In each videographic frame, local orthogonal cardiac coordinates with circumferential (XC), longitudinal (XL), and radial (XR) axes were defined at the position of the bead array. A positive LV long axis was defined from the apex marker (#1, Figure 1) to the centroid of the 4 basal level markers (#2 to 5, Figure 1). The epicardial tangent plane for each bead set was defined as containing the 3 1.7-mm epicardial surface beads atop the bead columns (A–C and D–F, respectively, Figure 1). A local origin was defined at the centroid of these epicardial surface beads, and XR was defined normal to this epicardial tangent plane with positive direction away from the LV chamber. XC was defined normal to the LV long-axis and XR. XL was normal to XR and XC. In cardiac coordinates, the 3 normal strain components measure local myocardial stretch or shortening along the circumferential (ECC), longitudinal (ELL), and radial (ERR) cardiac axes. The 3 shear strains, circumferential-longitudinal (ECL), circumferential-radial (ECR), and longitudinal-radial (ELR), represent angle changes between pairs of the originally orthogonal coordinate axes. All strains were calculated, with ED as the reference configuration, for each time frame as a deformed configuration during systole and diastolic filling. Diastolic filling was defined as the period beginning at filling onset (FO, Figure 3) and ending at ED,17 and systole was defined as the period beginning at ED and ending at ES (Figure 3).10 For each beat, bead positions at filling onset and ES (deformed configurations) were compared with their positions at ED.18 The 3 wall depths chosen for detailed strain analysis were defined as 20% (subepicardium), 50% (midwall), and 80% (subendocardium) of the depth of the deepest bead measured from the epicardial surface at each time instant.10
Statistical Analysis
All values are given as group mean±1SD unless otherwise specified. Three-beat averages were used to characterize the data for each animal. Data were compared using generalized Fisher exact test (MR grade ordinal data), 2-tailed Student t test for unpaired observations, and 2-way repeated measures ANOVA with the Bonferroni correction for multiple comparisons (Sigmastat 3.5, Systat Software Inc). The statistical analysis is explained in more detail in each table legend. Statistical significance was set at P<0.05 unless otherwise specified. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
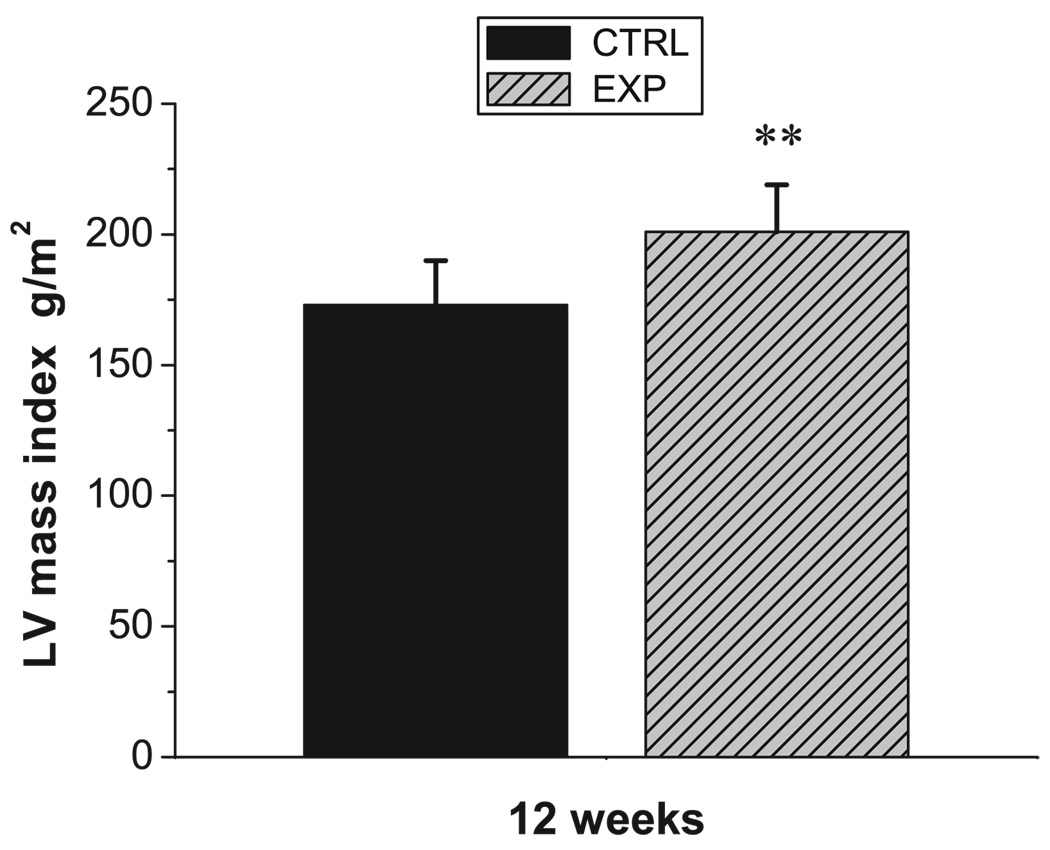
Hemodynamic data for EXP and CTRL groups are shown in Table 1. MR grade was higher in EXP versus CTRL at both 1 and 12 weeks (3.0 [2–4] versus 0.5 [0–2] and 3.0 [1–4] versus 0.5 [0–1] both P<0.001). The same findings in MR grade, ie, moderate to severe in EXP and trace in CTRL, were also observed weekly from 1 to 12 weeks. LV mass index was greater in EXP than CTRL at 12 weeks (201±18 versus 173±17 g/m2, P<0.01; Figure 4). From 1 to 12 weeks, LV ED volume index increased in EXP (115±17 to 134±31 mL/m2, P=0.015), without a change in CTRL over the same period. There was no difference in LV ES volume index, ejection fraction (EF), heart rate, and body weight between groups at 1 and 12 weeks or within groups over time. Also, there were no overt signs of heart failure observed in either group throughout the study.
Table 1.
Hemodynamic Data
| 1 Week | 1 Week | |||
|---|---|---|---|---|
| CTRL n=8 |
EXP n=12 |
CTRL n=8 |
EXP n=12 |
|
| MR, grade | 0.5 (0–2) | 3.0 (2–4)††† | 0.5 (0–1) | 3.0 (1–4)††† |
| HR, min−1 | 105±15 | 109±11 | 103±14 | 106±18 |
| Body weight, kg |
58±10 | 57±10 | 60±10 | 58±6 |
| LV massI, g/m2 |
… | … | 173±17 | 201±18** |
| LVEDVI, ml/m2 | 110±28 | 115±17 | 116±38 | 134±31‡ |
| LVESVI, ml/m2 | 87±22 | 89±13 | 91±30 | 100±22 |
| LVEF, % | 21±4 | 23±3 | 22±7 | 25±4 |
| LVPmax, mmHg | 93±10 | 93±10 | 106±15 | 102±16 |
Group mean±SD and median (range). EDVI indicates end-diastolic volume index; EF, ejection fraction; ESVI, end-systolic volume index; HR, heart rate; LV, left ventricular; massI, mass index; MR, mitral regurgitation; Pmax, maximum systolic pressure.
P<0.01 vs CTRL by t test for unpaired observations.
P<0.001 vs CTRL using generalized Fisher’s exact test.
P=0.015 vs 1 week, using 2-way repeated measures ANOVA with the Bonferroni correction (level of significance adjusted to P<0.05/3).
Figure 4.
LV mass index in CTRL (black) and EXP (shaded gray) at 12 weeks (mean±SD). **P<0.01 vs CTRL.
The BNP, PRSW, and Tau results are summarized in Table 2. There was no difference in BNP between CTRL and EXP at 1 and 12 weeks. From 1 to 12 weeks BNP decreased in EXP (P<0.01), and a similar trend was also noted in CTRL. However, from 1 to 12 weeks, there was no difference in change between EXP and CTRL for BNP (Figure 5A). The BNP level before surgery was EXP: 2.6±0.5; CTRL: 2.6±0.4 pmol/L, (NS). No difference was seen in LV contractility index (PRSW) between CTRL and EXP at 1 and 12 weeks. From 1 to 12 weeks PRSW increased in CTRL (P<0.001). Nonetheless, from 1 to 12 weeks, there was no difference in change between EXP and CTRL for PRSW (Figure 5B). Moreover, no difference was observed in the time constant of LV relaxation, tau, between groups at 1 and 12 weeks or within groups over time. From 1 to 12 weeks, there was no difference in change between EXP and CTRL for tau (Figure 5C).
Table 2.
Change in BNP, PRSW, and Tau From 1 to 12 Weeks
| CTRL (n=8) | EXP (n=12) | |||||
|---|---|---|---|---|---|---|
| 1 Week | 12 Weeks | Δ | 1 Week | 12 Weeks | Δ | |
| BNP, pmol/L | 6.7±3.8 | 3.7±1.5 | −3.0±3.6 | 7.9±4.4 | 3.4±1.1** | −4.5±4.4 |
| PRSW, mm Hg | 48±7 | 71±16*** | 23±18 | 54±11 | 62±11 | 9±13 |
| Tau, ms | 35±10 | 31±7 | −4±7 | 37±6 | 33±10 | −3±11 |
Group mean±SD. Δ indicates change from 1 to 12 weeks; BNP, B-type natriuretic peptide; PRSW, preload recruitable stroke work; tau, LV relaxation time constant. Difference in change was analyzed by t test for unpaired observations.
P<0.01 and
P<0.001 vs 1 week, using 2-way repeated measures ANOVA with the Bonferroni correction (level of significance adjusted to P<0.05/3).
Figure 5.
A through E, Change from 1 to 12 weeks in CTRL (solid squares) and EXP (open circles) for: A, B-type natriuretic peptide (BNP); B, Preload recruitable stroke work (PRSW); C, time constant of LV relaxation (tau); and, longitudinal strain (ELL) during diastolic filling in the D, lateral equatorial and E, anterobasal LV subendocardium (ENDO), respectively, (mean±SD). *P<0.01 vs CTRL at 12 weeks.
From 1 to 12 weeks, there was no difference in change between EXP and CTRL for any of the systolic normal strain components, irrespective of LV region, orientation or transmurality (supplemental Table IA and IB, available online at http://circ.ahajournals.org). All diastolic filling normal strain components are listed in Table 3 and Table 4. From 1 to 12 weeks, there was a difference in change between EXP and CTRL for longitudinal strain (ELL) in the subendocardium (lateral: −0.08±0.05 versus 0.02±0.14; anterior: −0.10±0.05 versus −0.02±0.07, respectively, both P<0.01; Figure 5D and E). Noteworthy, ELL in the lateral LV subendocardium was different between EXP and CTRL at 12 weeks (−0.11±0.04 versus 0.00±0.15, P<0.01; Figure 5D), without difference between groups at 1 week (−0.03±0.04 versus −0.02±0.02, NS). There was no difference between EXP and CTRL in any of the shear strain components.
Table 3.
Strain Change From 1 to 12 Weeks in the Lateral Equatorial LV Wall During Diastolic Filling
| CTRL (n=8) | EXP (n=12) | |||||
|---|---|---|---|---|---|---|
| 1 Week | 12 Weeks | Δ Strain | 1 Week | 12 Weeks | Δ Strain | |
| ECCEPI | −0.03±0.04 | −0.05±0.04 | −0.02±0.04 | −0.04±0.03 | −0.09±0.04 | −0.05±0.07 |
| ECCMID | −0.06±0.05 | −0.08±0.06 | −0.02±0.04 | −0.07±0.03 | −0.12±0.05 | −0.05±0.06 |
| ECCENDO | −0.11±0.04 | −0.12±0.04 | −0.01±0.04 | −0.12±0.03 | −0.15±0.05 | −0.04±0.06 |
| ELLEPI | −0.01±0.02 | −0.04±0.05 | −0.04±0.05 | −0.00±0.03 | −0.05±0.03 | −0.05±0.04 |
| ELLMID | −0.02±0.03 | −0.05±0.06 | −0.02±0.05 | −0.01±0.05 | −0.08±0.05 | −0.07±0.05 |
| ELLENDO | −0.02±0.02 | 0.00±0.15 | 0.02±0.14 | −0.03±0.04 | −0.11±0.04 | −0.08±0.05** |
| ERREPI | 0.07±0.06 | 0.20±0.14 | 0.12±0.16 | 0.07±0.05 | 0.28±0.13 | 0.20±0.16 |
| ERRMID | 0.10±0.05 | 0.23±0.14 | 0.13±0.12 | 0.12±0.06 | 0.32±0.12 | 0.20±0.13 |
| ERRENDO | 0.15±0.11 | 0.33±0.21 | 0.17±0.23 | 0.18±0.11 | 0.41±0.18 | 0.23±0.16 |
Group mean±SD. Δ indicates change from 1 to 12 weeks; ECC, circumferential strain; ELL, longitudinal strain; ERR, radial strain; EPI, subepicardial; MID, midwall; ENDO, subendocardial.
P<0.01 vs CTRL using 2-way repeated measures ANOVA with the Bonferroni correction (level of significance adjusted to P<0.05/5).
Table 4.
Strain Change From 1 to 12 Weeks in the Anterobasal LV Wall During Diastolic Filling
| CTRL (n=8) | EXP (n=12) | |||||
|---|---|---|---|---|---|---|
| 1 Week | 12 Weeks | Δ Strain | 1 Week | 12 Weeks | Δ Strain | |
| ECCEPI | −0.06±0.05 | −0.10±0.05 | −0.04±0.06†† | −0.05±0.04 | −0.08±0.07 | −0.03±0.05 |
| ECCMID | −010±0.05 | −0.11±0.05 | −0.01±0.05 | −0.08±0.05 | −0.09±0.08 | −0.02±0.08 |
| ECCENDO | −0.14±0.05 | −0.11±0.05 | −0.03±0.04 | −0.09±0.04 | −0.13±0.04 | −0.03±0.05 |
| ELLEPI | −0.08±0.05 | −0.09±0.08 | −0.01±0.06 | −0.03±0.04 | −0.09±0.07 | −0.07±0.08 |
| ELLMID | −0.09±0.06 | −0.11±0.09 | −0.02±0.05 | −0.02±0.06 | −0.11±0.07 | −0.09±0.07 |
| ELLENDO | −0.08±0.08 | −0.09±0.10 | −0.02±0.07 | −0.03±0.08 | −0.13±0.08 | −0.10±0.05** |
| ERREPI | 0.15±0.12 | 0.30±0.15 | 0.15±0.18 | 0.07±0.05 | 0.42±0.30 | 0.34±0.31 |
| ERRMID | 0.22±0.12 | 0.37±0.11 | 0.14±0.17 | 0.11±0.06 | 0.36±0.16 | 0.25±0.15 |
| ERRENDO | 0.33±0.15 | 0.51±0.20 | 0.18±0.27 | 0.19±0.13 | 0.38±0.23 | 0.19±0.22 |
Group mean±SD. Δ indicates change from 1 to 12 weeks; ECC, circumferential strain; ELL, longitudinal strain; ERR, radial strain; EPI, subepicardial; MID, midwall; ENDO, subendocardial.
P<0.01 vs CTRL,
P<0.01 vs ECCENDO using 2-way repeated measures ANOVA with the Bonferroni correction (level of significance adjusted to P<0.05/5).
Discussion
The principal findings of this study were: (1) Twelve weeks of moderate to severe “pure” MR in this ovine model caused LV remodeling; (2) Early alterations in transmural myocardial deformation, particularly longitudinal diastolic filling strains in the lateral LV subendocardium, occurred; and (3) No demonstrable changes in BNP, PRSW, or tau were detected.
The present closed-chest model allowed us to study the effect of moderate to severe pure MR on LV morphology and function. We observed that the LV mass index was greater in EXP versus CTRL at 12 weeks, and that EDV index increased in EXP over time without change in CTRL, implying that the LV myocardium underwent global remodeling. This experimental model enabled analysis and comparison of 3-D transmural LV strain with BNP and global indices of LV contractility (PRSW) and relaxation (tau) during development of LV remodeling, induced by MR of known duration. In addition, the MR was not created by disruption of the mitral subvalvular apparatus, which can directly affect LV function.19 As discussed below, our 12-week data in general represent an early phase of the disease in a potential transition from compensated to decompensated LV function, a strength of the model in relation to the purpose of the study.
Previous studies have proposed that strain imaging can detect early LV systolic dysfunction in a variety of disease states before abnormalities can be observed using traditional measures of LV function.20 This is consistent with our data. In the setting of asymptomatic severe MR, Lee et al21 demonstrated that tissue Doppler strain (total deformation) and strain rate (instantaneous deformation) was a feasible technique for detecting subclinical LV dysfunction. Moreover, Marciniak et al22 proposed that, if corrected for LV geometry, tissue Doppler based strain rate could be a sensitive tool to detect subclinical LV dysfunction in asymptomatic patients with severe MR. In a porcine chronic MR model, Neilan et al23 recently showed that tissue Doppler-derived strain and strain rate indices, which initially increased post-MR, decreased to baseline before any detected changes in global LVEF.
Derangements in myocardial strain are linked to myocyte apoptosis24 and also affect collagen turnover by activating matrix metalloproteinases.8 Thus, alterations in strain may be detected before the onset of global LV dysfunction, as perturbations in strain may trigger progressive LV remodeling. 7 Furthermore, recent findings suggest that alterations in strain adjacent to infarcted myocardium (the normally per-fused “border zone”) trigger further LV remodeling.9 After approximately 1 minute of ischemia, presumably before extracellular matrix remodeling occurred, Rodriguez et al9 observed increased shear strain adjacent to ischemic myocardium. Whether a similar time sequence between alterations in myocardial strain and LV remodeling exists in chronic isolated MR (low pressure LV volume overload) remains to be proven.
In agreement with our observations, the study of Lee et al,21 and also several other studies,25 emphasize the importance of LV longitudinal motion in the evaluation of LV function in chronic volume overload. One simple reason for this could be that tissue Doppler imaging in particular allows measurements of longitudinal LV function, and that this technique has been widely used in recent studies. But there are most likely other more complex reasons as well. A considerable proportion of longitudinally directed myocardial fibers can be found in the LV subendocardium.26,27 It is also known that chronic volume-overload induces increased LV end-diastolic wall stress, which in turn causes compensatory remodeling (eccentric hypertrophy).28 Furthermore, the sub-endocardium is probably more sensitive to abnormal physiology (eg, increased wall stress) than layers with better perfusion.26,27 Our ovine findings most likely represent a very early phase of the remodeling process, and the increase in (negative) longitudinal strain observed in the subendocardium in EXP at 12 weeks may be an earlier marker of LV dysfunction than reduced LV longitudinal motion observed in long-standing chronic MR.21
The transmural beadset method used in this study allows accurate transmural measurements of myocardial 3-D strain, and the present findings suggest possible targets for further noninvasive evaluation. An increased longitudinal diastolic filling strain, with a similar magnitude as circumferential strain, in the lateral LV subendocardium, may prove to be a useful measurement of early changes in LV function in patients with chronic isolated MR. The current findings suggest including analysis of myocardial strain also during diastole (versus systole) as a potential indicator of deranged LV function. The alterations in LV strain that we report may be considered subtle and difficult to assess clinically. With the clinical scenario of long-standing isolated MR in human hearts, however, it is likely that the disease related changes would be more extensive. Furthermore, the requirement of the spatial resolution for noninvasive imaging in human hearts may actually be lower than for the current ovine model. In humans, the LV myocardial wall is thicker, and more importantly, the magnitude of the myocardial strain is much larger than in anesthetized sheep. Among noninvasive techniques, cardiac MRI will probably be the first modality to provide these measurements clinically. Displacement encoding with stimulated echoes (DENSE)29,30 appears to be the most promising and robust technique with the potential to provide these accurate transmural measurements in the immediate future.
The cardiac neurohormone B-type natriuretic peptide (BNP) is secreted from the ventricular myocardium mainly in response to myocardial stretch and increased wall stress, in humans as well as animals.16 BNP activation aids in the diagnosis and prognosis of LV dysfunction and heart failure of various etiologies.31,32 In terms of MR, it was recently demonstrated that BNP level was higher in patients with functional than those with organic MR.32 Furthermore, it has been suggested that BNP activation in chronic organic MR reflects primarily ventricular and atrial consequences rather than degree of MR.31 In this ovine model, however, we did not observe elevated BNP levels in EXP relative to CTRL at 12 weeks. This might result from the early phase of the LV remodeling process, as well as interspecies differences in BNP activation in response to low pressure LV volume overload.
No intergroup difference in traditional indices of LV function such as PRSW, EF, or Tau were observed. Under these experimental conditions these indices do not allow detection of changes in LV function during the very early stage of the disease, and this is consistent with recent findings.23 Accordingly, a correlation analysis between changes in strain and changes in PRSW, EF or Tau, did not show any closer association between the parameters.
Limitations
Considerable caution is warranted in extrapolating these experimental findings in sheep hearts to the clinical scenario of long-standing isolated MR in human hearts. The sequela of MR in patients is a chronic and insidious phenomenon that can be tolerated clinically for years, and the 12-week follow-up in this study is insufficient to capture the complete pathophysiology of chronic MR. In this study we sought to detect very early changes in LV function, and for this purpose the actual “chronicity” of the model appears appropriate.
Insertion of myocardial markers and beads is invasive and associated with local LV wall trauma, however; this would affect the LV wall mechanics at all myocardial depths because the tunneling and placement of the beads are trans-mural in both groups of animals.
All animals underwent CPB surgery which is known to depress myocardial function. This is most likely the reason for the elevated BNP, and reduced PRSW and strain levels at 1 week relative to 12 weeks. This procedure, however, should affect both study groups equally.
Conclusions
Twelve weeks of “pure” MR in this ovine model caused signs of LV remodeling. Early changes in LV function were detected by alterations in transmural myocardial deformation, particularly in longitudinal diastolic filling strain in the subendocardium, but not by changes in BNP, PRSW, or tau. These alterations in transmural strains may prove useful in the clinical evaluation of latent LV dysfunction in asymptomatic patients with severe MR.
Supplementary Material
Acknowledgments
We appreciate the technical expertise and assistance provided by George T. Daughters, MS, Robert A. Oakes, MD, John C. Criscione, MD, PhD, Katarina Kindberg, MSc, and Mary K. Zasio, BA. We also want to acknowledge the Cardioendocrine Research Group, Christchurch School of Medicine, Christchurch, New Zealand, for providing the BNP assay.
Sources of Funding
This work was supported by Grants HL-29589 and HL-67025 from the NHLBI-NIH. Dr Carlhäll received funding from the Swedish Heart and Lung Foundation and the County Council of Östergötland, Sweden. Drs Nguyen and Itoh were Leah McConnell Cardiovascular Surgical Research Fellows. Dr Nguyen was a recipient of the Thoracic Society Foundation Research Fellowship Award. Dr Itoh received funding from the Uehara Memorial Foundation, Dr Ennis from the NHLBI-NIH (K99-HL087614), and Dr Bothe from the Deutsche Herzstiftung.
Footnotes
The online Data Supplement can be found with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.753525/DCI.
Disclosures
None.
References
- 1.Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet. 2005;365:507–518. doi: 10.1016/S0140-6736(05)17869-6. [DOI] [PubMed] [Google Scholar]
- 2.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 3.Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D, Schemper M, Maurer G, Baumgartner H. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006;113:2238–2244. doi: 10.1161/CIRCULATIONAHA.105.599175. [DOI] [PubMed] [Google Scholar]
- 4.Otto CM, Salerno CT. Timing of surgery in asymptomatic mitral regurgitation. N Engl J Med. 2005;352:928–929. doi: 10.1056/NEJMe048334. [DOI] [PubMed] [Google Scholar]
- 5.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed]
- 6.Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak TA, Bailey KR, Frye RL. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation. 1994;90:830–837. doi: 10.1161/01.cir.90.2.830. [DOI] [PubMed] [Google Scholar]
- 7.Ratcliffe MB. Nom-ischemic infarct extension: a new type of infarct enlargement and a potential therapeutic target. J Am Coll Cardiol. 2002;40:1168–1171. [Google Scholar]
- 8.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, III, Edmunds LH, Jr, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez F, Langer F, Harrington KB, Cheng A, Daughters GT, Criscione JC, Ingels NB, Miller DC. Alterations in transmural strains adjacent to ischemic myocardium during acute midcircumflex occlusion. J Thorac Cardiovasc Surg. 2005;129:791–803. doi: 10.1016/j.jtcvs.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Cheng A, Langer F, Rodriguez F, Criscione JC, Daughters GT, Miller DC, Ingels NB., Jr Transmural cardiac strains in the lateral wall of the ovine left ventricle. Am J Physiol Heart Circ Physiol. 2005;288:H1546–H1556. doi: 10.1152/ajpheart.00716.2004. [DOI] [PubMed] [Google Scholar]
- 11.Cheng A, Nguyen TC, Malinowski M, Langer F, Liang D, Daughters GT, Ingels NB, Jr, Miller DC. Passive ventricular constraint prevents transmural shear strain progression in left ventricle remodeling. Circulation. 2006;114:I79–I86. doi: 10.1161/CIRCULATIONAHA.105.001578. [DOI] [PubMed] [Google Scholar]
- 12.Daughters GT, Sanders WJ, Miller DC, Schwarzkopf A, Mead CW, Ingels NB., Jr A comparison of two analytical systems for three-dimensional reconstruction from biplane videoradiograms. IEEE Comput Cardiol. 1989:79–82. [Google Scholar]
- 13.Karlsson MO, Glasson JR, Bolger AF, Daughters GT, Komeda M, Foppiano LE, Miller DC, Ingels NB., Jr Mitral valve opening in the ovine heart. Am J Physiol. 1998;274:H552–H563. doi: 10.1152/ajpheart.1998.274.2.H552. [DOI] [PubMed] [Google Scholar]
- 14.Moon MR, Castro LJ, Derby GC, Niczyporuk MA, Daughters GT, Ingels NB, Miller DC. Calculation of biventricular volume: myocardial markers vs. sonomicrometric shell subtraction technique. Circulation. 1992;86 I-553. Abstract. [Google Scholar]
- 15.Helmcke F, de Souza A, Nanda NC, Villacosta I, Gatewood R, Jr, Colvin E, Soto B. Two-dimensional and color Doppler assessment of ventricular septal defect of congenital origin. Am J Cardiol. 1989;63:1112–1116. doi: 10.1016/0002-9149(89)90088-x. [DOI] [PubMed] [Google Scholar]
- 16.Pemberton CJ, Yandle TG, Charles CJ, Rademaker MT, Aitken GD, Espiner EA. Ovine brain natriuretic peptide in cardiac tissues and plasma: effects of cardiac hypertrophy and heart failure on tissue concentration and molecular forms. J Endocrinol. 1997;155:541–550. doi: 10.1677/joe.0.1550541. [DOI] [PubMed] [Google Scholar]
- 17.Ashikaga H, Covell JW, Omens JH. Diastolic dysfunction in volume-overload hypertrophy is associated with abnormal shearing of myolaminar sheets. Am J Physiol Heart Circ Physiol. 2005;288:H2603–H2610. doi: 10.1152/ajpheart.01276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kindberg K, Karlsson M, Ingels NB, Jr, Criscione JC. Nonhomogeneous strain from sparse marker arrays for analysis of transmural myocardial mechanics. J Biomech Eng. 2007;129:603–610. doi: 10.1115/1.2746385. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez F, Langer F, Harrington KB, Tibayan FA, Zasio MK, Cheng A, Liang D, Daughters GT, Covell JW, Criscione JC, Ingels NB, Miller DC. Importance of mitral valve second-order chordae for left ventricular geometry, wall thickening mechanics, and global systolic function. Circulation. 2004;110:II115–II122. doi: 10.1161/01.CIR.0000138580.57971.b4. [DOI] [PubMed] [Google Scholar]
- 20.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echo-cardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107:2446–2452. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 21.Lee R, Hanekom L, Marwick TH, Leano R, Wahi S. Prediction of subclinical left ventricular dysfunction with strain rate imaging in patients with asymptomatic severe mitral regurgitation. Am J Cardiol. 2004;94:1333–1337. doi: 10.1016/j.amjcard.2004.07.129. [DOI] [PubMed] [Google Scholar]
- 22.Marciniak A, Claus P, Sutherland GT, Marciniak M, Karu T, Baltabaeva A, Merli E, Bijnens B, Jahangiri M Strain rate imaging study. Changes in systolic left ventricular function in isolated mitral regurgitation. Eur Heart J. 2007 doi: 10.1093/eurheartj/ehm072. [DOI] [PubMed] [Google Scholar]
- 23.Neilan TG, Ton-Nu TT, Kawase Y, Yoneyana R, Hoshino K, Del Monte F, Hajjar RJ, Picard MH, Levine RA, Hung J. The progressive nature of chronic mitral regurgitation and the role of tissue Doppler-derived indices. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.01128.2007. In press. [DOI] [PubMed] [Google Scholar]
- 24.Cheng W, Li B, Kajstura J, Li P, Wolin MS, Sonnenblick EH, Hintze TH, Olivetti G, Anversa P. Stretch-induced programmed myocyte cell death. J Clin Invest. 1995;96:2247–2259. doi: 10.1172/JCI118280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haluska BA, Short L, Marwick TH. Relationship of ventricular longitudinal function to contractile reserve in patients with mitral regurgitation. Am Heart J. 2003;146:183–188. doi: 10.1016/S0002-8703(03)00173-X. [DOI] [PubMed] [Google Scholar]
- 26.Jones CJ, Raposo L, Gibson DG. Functional importance of the long axis dynamics of the human left ventricle. Br Heart J. 1990;63:215–220. doi: 10.1136/hrt.63.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henein MY, Gibson DG. Long axis function in disease. Heart. 1999;81:229–231. doi: 10.1136/hrt.81.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross J, Jr, Sonnenblick EH, Taylor RR, Spotnitz HM, Covell JW. Diastolic geometry and sarcomere lengths in the chronically dilated canine left ventricle. Circ Res. 1971;28:49–61. doi: 10.1161/01.res.28.1.49. [DOI] [PubMed] [Google Scholar]
- 29.Aletras AH, Ding S, Balaban RS, Wen H. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson. 1999;137:247–252. doi: 10.1006/jmre.1998.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aletras AH, Wen H. Mixed echo train acquisition displacement encoding with stimulated echoes: an optimized DENSE method for in vivo functional imaging of the human heart. Magn Reson Med. 2001;46:523–534. doi: 10.1002/mrm.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Detaint D, Messika-Zeitoun D, Avierinos JF, Scott C, Chen H, Burnett JC, Jr, Enriquez-Sarano M. B-type natriuretic peptide in organic mitral regurgitation: determinants and impact on outcome. Circulation. 2005;111:2391–2397. doi: 10.1161/01.CIR.0000164269.80908.9D. [DOI] [PubMed] [Google Scholar]
- 32.Detaint D, Messika-Zeitoun D, Chen HH, Rossi A, Avierinos JF, Scott C, Burnett JC, Jr, Enriquez-Sarano M. Association of B-type natriuretic peptide activation to left ventricular end-systolic remodeling in organic and functional mitral regurgitation. Am J Cardiol. 2006;97:1029–1034. doi: 10.1016/j.amjcard.2005.10.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.