Abstract
Background and aim
Analysis of clinical colon cancer specimens show alterations in the CD95 (Fas Ag/Fas L) pathway as tumors progress from local to metastatic disease, suggesting this pathway may play a role in invasive behavior of colon cancer. However, direct causality between these alterations and clinical disease progression has not been shown.
Methods
Surgically resected metastatic colon cancer samples were evaluated for Fas Ag/L and apoptosis. Alterations in the Fas signaling pathway found in human samples was recreated through a series of staged transfection experiments in the MC38 mouse colon cancer cell line and the effects on growth tested in vitro, and in vivo.
Results
Expression of FLICE like inhibitory protein (FLIP) confers apoptosis resistance, increasing the incidence of primary tumors through a survival advantage by avoiding apoptosis and inducing Fas mediated proliferation. Co-expression of Fas L enables colon cancer cells to metastasize to the liver from local tumors as well as from intravenous injection of cells. MC38-FasL/FLIP colon cancer cells induce apoptosis in hepatocytes via activation of type 2 Fas Ag signaling, thus creating a niche conducive to tumor growth and fueling their own growth via Fas proliferative signaling.
Conclusion
Alterations in the Fas Ag pathway which inhibit apoptosis and increase Fas mediate proliferation directly increases local colon cancer growth and enhances metastasis to the liver. Delineating points in the pathway responsible for growth and metastasis will offer targets which may be exploited for therapy.
Introduction
The machinery which allows a cell to undergo apoptosis or programmed cell death appears to be retained in all cells regardless of the level of cellular differentiation and is essential to maintain appropriate cell numbers and remove damaged cells. Cancer cells share several growth characteristics essential to sustain malignant growth (1). Central to the survival of cancer is the ability to avoid apoptosis, therefore, it is not surprising that numerous mechanisms to avoid apoptosis have been identified in cancer cells. Indeed, studies from tissue culture models, mouse models of cancer and descriptive analysis of human cancer samples support the notion that acquired apoptosis resistance is a hallmark of most, and perhaps all types of cancer (1).
One mechanism by which colon cancer cells avoid apoptosis is to acquire resistance to CD95 / Fas Ag mediated apoptosis. Similar to gastric cancer (2) colon cancer cells retain Fas receptor expression, and in some cases over express the receptor. Despite abundant receptor, colon cancer cells appear resistant to Fas mediated apoptosis (3–6) through a variety of mechanisms including the up regulation of inhibitor proteins (4,7,8) or the production of non-signaling decoy receptors which act to titrate death inducing signal away from functioning receptors (9). The fact that there is rarely loss of surface receptor suggests the Fas pathway may serve functions in addition to apoptosis and may be a beneficial pathway for the cell to retain.
Investigation of human colon cancer cell lines support the notion that loss of Fas apoptotic function correlates with progression from local to invasive tumors. Cell lines derived from local lesions or from metastatic foci demonstrate a progression from a Fas apoptosis- sensitive phenotype to a Fas apoptosis-resistant phenotype in the invasive and metastatic lesions. Additionally, paired cell lines derived from the same host demonstrate that when the primary tumor is Fas –sensitive, the metastatic tumors are Fas resistant, suggesting a pressure to acquire apoptosis resistance (10). This correlation between decreased Fas mediated apoptosis and metastatatic behavior has been analyzed in cell lines derived from rodent models as well (11) though the precise mechanism of apoptosis resistance was not established. Though a clear association has been shown, a direct causal role for the Fas pathway in the progression of colon cancer has not been established in vivo. Interestingly, total disruption of the Fas pathway using molecular based strategies does not reproduce a metastatic phenotype (10) suggesting that additional cellular changes need to be acquired in vivo to allow metastasis to occur or conversely that abrogation of the pathway in its entirety differs from selective inhibition of Fas mediated apoptosis.
Interestingly, in addition to avoidance of Fas apoptosis, colon cancer cells often express Fas L which has been suggested to function in either an “immune counter attack” manner- engaging and killing off attacking lymphocytes, or to have a role in the formation of metastasis by inducing apoptosis within endothelial cells or target organs such as the liver (12). In vivo proof for these concepts however is lacking.
Examination of normal, nonmalignant human colonic epithelium demonstrates that coexpression of Fas Ag and Fas L is rare. However, early in the adenoma to carcinoma sequence, Fas L upregulation is noted and is evident at the stage of dysplasia. This coexpression of both Fas Ag and Fas L has not been shown to result in apoptosis of the colonic cells suggesting that either cells had acquired a mechanism of apoptosis resistance or the Fas ligand and receptor were unable to interact; this distinction was not addressed (6). Metastasizing colon carcinoma cells have also been shown to upregulate Fas L expression, the significance of which has been debated. Within the liver, sinusoidal endothelial cells express Fas Ag and are sensitive to ligand induced apoptosis. In vitro, CC531s colon cancer cells expressing Fas L induce apoptosis in rat liver sinusoidal endothelial cells (13) implying that the Fas Ag/L system may be used by blood borne cells to gain access to tissues by disrupting the endothelium. These authors demonstrate sinusoidal endothelium disruption by intravenous injection of CC531s cells, but their model did not demonstrate metastasis foci formation (13).
Here we use the well described murine colon cancer cell line MC38 to address the role of Fas Ag, Fas L and Fas apoptosis resistance in the behavior of colon cancer cells. We demonstrate that Fas Ag mediated proliferative signaling is responsible for local tumor growth. Fas ligand functions to stimulate proliferative signaling in an autocrine/paracrine fashion and further aids in metastasis by creating a niche in the liver, where blood borne tumor cells can settle and propagate. These data strongly support the role of the Fas Ag/L signal transduction cascade in the invasion and metastatic behavior of colon cancer and suggests this pathway as a possible target for cancer therapy.
Results
Fas Ag/L are co-expressed in human colon cancer
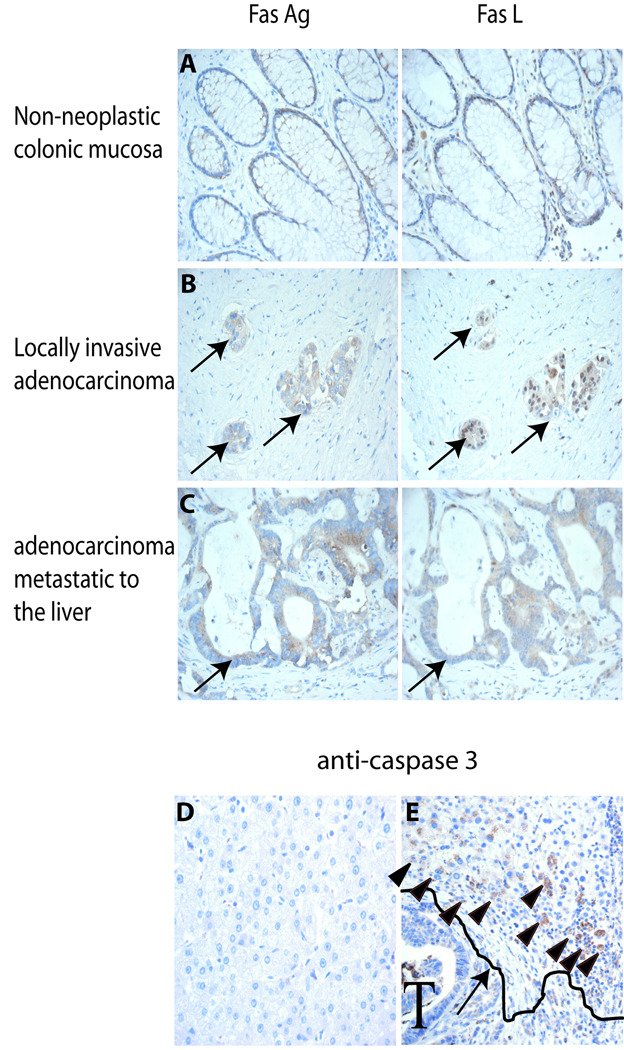
Fas Ag is expressed minimally and Fas L is not expressed in non-neoplastic colonic epithelial cells (Figure 1A, n=10) consistent with reported studies (5,6). Invasive colon adenocarcinoma expresses both Fas Ag (10/10 samples) and Fas L (6/10 samples) (Figure 1B). Metastatic colon cancer within the liver expresses high levels of both Fas Ag (14/14 samples) and Fas L (13/14 samples) within the same cells. Anti-cleaved caspase 3 IHC revealed abundant apoptotic hepatocytes surrounding Fas L expressing metastatic foci (Figure 1E), while liver at a distance from the tumor did not stain for cleaved caspase 3, and did not contain apoptotic cells (Figure 1D).
Figure 1. Immunohistochemistry to detect Fas Ag, Fas L and cleaved caspase 3 in human colon carcinoma.
Serial sections of A) histologically normal colonic mucosa B) locally invasive adenocarcinoma and C) adenocarcinoma metastatic to liver were stained with antibody directed against Fas Ag and Fas L. D) liver parenchyma distal to metastatic lesions failed to stain with anti-cleaved caspase 3 Ab. E) Areas of liver parenchyma surrounding tumor (denoted by T) contain numerous cleaved caspase 3 + hepatocytes (arrow heads). Arrow points out a drawn line to delineate the border between the tumor and the liver parenchyma. All images 60x.
Characterization of cell lines
Invasive behavior of local tumor, and apoptosis of surrounding hepatocytes supports, but does not prove a direct causal effect of the Fas signaling pathway. Therefore, we began our investigation of the Fas signaling pathway in colon cancer progression by establishing a series of colon cancer cell lines. MC38 cell line (14) was originally described as forming locally invasive and metastatic lesions; subsequent in vivo analysis in various laboratories demonstrates a more modest phenotype with fewer primary tumors and a less robust metastatic profile suggesting there may be selection in culture for an attenuated phenotype. We first characterized the FasAg and FasL expression profile of these cells, and then in order to examine the contribution of Fas Ag signaling to colon cancer growth and metastasis, we created a series of cell lines which recapitulate the staged acquisition of signaling proteins found in human colon tumors, and assayed their behavior in vitro and in vivo.
MC38 cells express surface FasAg and not FasL (Figure 2A) survivin or XIAP protein (data not shown). They express mutated p53 (15) and do not undergo spontaneous apoptosis. With the addition of 50ng/ml soluble Fas L approximately 30% of cells were apoptotic at 24 hours (Figure 2C, D) and by 72 hours, there were no viable cells identified.
Figure 2. Creation and characterization of cell lines.
The MC38 colon cancer cell line was stably transfected with plasmids expressing cFLIP and / or Fas L. A) Surface Fas Ag and Fas L expression was verified by FACS. Arrow denotes shift with appropriate antibody in cell lines expressing surface Fas Ag or Fas L protein. B) cFLIP expression was verified by Western blot. C) Fas L 50 ng/ml was added to cell cultures as indicated and subG1 population (an indication of apoptotic cells) was detected by FACS at 24 hours. Bar in subG1 area indicates the apoptotic population and is shown in graph form in D. Cell populations were grown in E) control media or F) media + 50ng/ml FasL and cell growth/death graphed. * P<0.02.
Overexpression of FLICE-like inhibitory protein (FLIP) inhibits Fas mediated apoptosis but is expected to leave other signaling pathways emanating from the DISC intact. Stable transfected cell lines were isolated and FLIP expression verified by western blot (Figure 2B). MC38 cells expressing the anti apoptotic protein FLIP (MC38/FLIP) phenotypically resemble MC38 cells, however they are relatively resistant to apoptosis induced by the addition of exogenous soluble Fas L. After 24 hours in culture with 50 ng/ml soluble Fas L only 8.5% of cells over-expressing cFLIP undergo apoptosis compared to 30% of cells not over-expressing cFLIP (Figure 2C, D).
Expression of Fas L in the absence of FLICE or other inhibitor of apoptosis protein has not been reported in colon cancer. Without a way to avoid paracrine activation of the Fas pathway, ligand expressing colon cancer cells would likely be eliminated. In order to test the effect of Fas L expression in the absence of anti-apoptotic proteins, the MC38 cells were engineered to express Fas L on the surface (MC38/L). MC38/L cells grow well when sparsely plated; however, when cultures become confluent, cell numbers begin to decline (Figure 2D). Interestingly, cells that survive in culture become apoptosis resistance (Figure 2 D, E) by one of several mechanisms including down regulation of Fas Ag receptor, down regulation of Fas L, and/or acquisition of anti-apoptotic proteins (data not shown) supporting the notion that acquiring apoptosis resistance is vital for colon cancer cell survival.
In order to further test the effects apoptosis resistance, and of non-apoptotic Fas signaling on colon cancer cell growth, we engineered MC38 cells to express FLIP (MC38FLIP), or FLIP and Fas L (MC38FLIP/L). Under normal culture conditions, MC38FLIP cells grow in a similar fashion to MC38 cells (Figure 2D). Cells resistant to apoptosis and expressing surface ligand (MC38FLIP/L) grow faster than control cells at all time points (Figure 2D). Addition of 50 ng/ml of soluble Fas ligand to cell cultures results in near elimination of MC38 cells at 3 days while cells expressing the anti-apoptotic protein FLIP are protected from apoptosis (Figure 2E).
Colon cancer cells which are sensitive to Fas mediated apoptosis are weakly tumorigenic
We next determined the local growth and metastatic ability of colon cancer cells in vivo. Five million MC38 cells injected subcutaneously formed tumors in 40% of mice by 4 weeks (n=30, Figure 3A, B). Tumors were not locally invasive. Intravenous (Figure 3A, B) or intrasplenic (data not shown) injections failed to produce gross or microscopic lung, cardiac or liver metastasis (n=10 each group).
Figure 3. In vivo effects of alterations in the Fas Ag signaling pathway.
A) MC38 or M38/FLIP/L cells were injected subcutaneously or intravenously in C57BL/6 mice. At 4 weeks local and metastatic tumor growth was evaluated. B) Tumor characteristics.
N=10 for all groups except MC38 where n=30, MC38/FLIP where n=9. All tumors were evaluated at 4 weeks except * = 3 weeks due to large tumor size).
There is a selective pressure for colon cancer cells to become apoptosis resistant
As previously stated, MC38 cells engineered to express Fas L on the cell surface grew poorly in culture. Once confluent, there is considerable apoptosis presumable due to paracrine signaling. Interestingly, subcutaneously injected MC38/L cells formed tumors, though they were unable to initiate metastatic disease when injected intravenously (Figure 3B). Examination of tumors confirmed the majority of tumor cells had lost Fas L expression, though the mechanism of this loss was not investigated (supplemental data Figure 1). Cells that retained ligand expression were found in areas of apoptosis (supplemental Figure 1) confirming activity of the Fas apoptotic pathway in these cells.
Resistance to Fas apoptosis increases local tumor growth and increases metastasis of colon cancer cells
We next tested the in vivo activity of colon cancer cells resistant to Fas mediated apoptosis. In sharp contrast to Fas sensitive colon cancer cells which form local tumors in 40% of mice, 77% of mice injected subcutaneously with the apoptosis resistant MC38/FLIP cell line formed tumors (figure 3B). The range in size of tumors was very broad (MC38; 0.5–6.2g vs MC38/FLIP; 1.5–8.3g) which may have masked any significant difference in size between the two groups. PCNA staining and mitotic body enumeration was similar (data not shown). Fas apoptosis resistance dramatically increased the number of tumors but not their size which may be because of the relative lack of Fas ligand availability within tumors. Early after injection of tumor cell, local inflammation supplies abundant Fas L which can bind to and signal the colon cancer cells. As the tumor cell mass enlarges, cells within the tumor have less exposure to inflammatory cells.
Intravenous injection of MC38 cells did not form lung, liver or cardiac tumors. When 5 million MC38/FLIP cells were injected intravenously, 44% of mice (n= 4 of 9) developed 1 – 5 metastatic lesions in the liver at 4 weeks. No lung metastases were detected (Figure 3B). Interestingly, mice that developed hepatic tumors also developed invasive right atrial intracardiac tumors.
Expression of Fas L on colon cancer cells greatly increases their local growth and ability to metastasize to the liver
Locally aggressive and metastatic human colon tumors are Fas apoptosis resistance, and express Fas L (Figure 1A–D). Therefore, we next evaluated the in vivo activity of MC38 cells engineered to express both FLIP and Fas L. Subcutaneous injection of 5 million cells formed tumors in 100% of mice (n=10). Tumors were larger and grew faster than those formed from cells not containing Fas L, necessitating euthanasia at 3 weeks (compared at 4 weeks for the other groups) (Figure 3B). Proliferation was 3 fold higher in the MC38/FLIP/FasL cell derived tumors than in tumors derived from MC38 cells (Figure 4A, B).
Figure 4. Expression of FLIP and FasL increase colon cancer proliferation and neovascularization.
MC38 and MC38/FLIP/L tumors were evaluated for tumor cell growth and vessel formation. A) mitotic bodies were evaluated in hematoxylin and eosin stained sections (arrows). B) PCNA immunohistochemistry. Positive signal is seen as brown cytoplasmic staining. C) Gross image of the tumor surface taken at necropsy demonstrates large blood vessels feeding the tumors. Arrows draw attention to the vessels originating from the overlying skin. D) Small blood vessels within the tumor are enumerated as PECAM positive channels and counted as the number of channels per high powered field. Arrows show one such channel which is shown at higher power in the boxed region. Microscopic images 20x, enlarged box in D; 60x, gross image in C; 2x.
Quite strikingly, MC38/FLIP/L tumors had a marked increase in tumor vascularity with grossly large vessels from the overlying skin feeding the tumor (Figure 4C) compared to relatively smaller and fewer vessels in the MC38 tumors. Microscopically, numerous platelet endothelial cell adhesion molecule-1 (PECAM-1) expressing vascular channels (Figure 4 C,D) were identified in the MC38/FLIP/L tumors while rare PECAM positive channels were identified in the MC38 cell derived tumors. MC38/FLIP/L subcutaneous tumors spontaneously metastasize to the liver (2/10). (Supplemental figure 2). In order to form liver metastasis, tumors must first establish an adequate blood supply, tumor cells must then migrate through the endothelium to gain access to the circulation and find a hospitable environment for anchorage and growth. Reports suggest that Fas L expressing colon cancer cells induce apoptosis in the vascular endothelium (13) and gain access to the circulation. This reported model however did not develop metastatic lesions. In order to determine the degree to which MC38/FLIP/L cells are capable of establishing lesions once in the blood stream, we used intravenous injection of cells to bypass the restrictions local tumor size has on the time of these experiments.
Intravenous injection of as few as 1 × 106 MC38/L/FLIP cells resulted in numerous large liver metastasis in 100% of the mice at 4 weeks (Figure 3A, B). All mice developed intra cardiac tumors indistinguishable from the MC38/FLIP cardiac tumors and multiple lung metastasis. All mice had greater than 20 liver lesions, with some having greater than 100. Microscopically, there was little to no inflammation surrounding lesions confirmed by specific anti-CD3 and anti-neutrophil antibody IHC (supplemental figure 3), suggesting neither lymphocytes nor neutrophils play a prominent role in this model.
Fas L creates a niche by killing Fas Ag bearing liver cells
Colon cancer cells which express Fas L have in vivo metastatic activity. It is not clear how the expression of Fas L enables cells to metastasize. We addressed the interaction between FasL expressing colon cancer cells and hepatocytes using complimentary in vivo and in vitro approaches.
We hypothesized that ligand bearing colon cancer cells would induce apoptosis in hepatocytes. We used IHC to detect cleaved caspase 3 within cells of metastatic lesions and surrounding hepatic tissue. MC38/FLIP tumors had few apoptotic tumor cells within the tumor (Figure 5 A, B arrows), and no apoptotic liver cells (figure). Metastatic liver tumors derived from MC38/FLIP/L cells showed intense apoptosis at the tumor – liver interface while cells within the tumor, and liver cells distant from the tumor-liver interface had few apoptotic cell. (Figure 5). TUNEL staining confirmed hepatic cell apoptosis (Figure 5C) suggesting the invading cancer cells created a niche within the liver by inducing apoptosis in the liver parenchyma.
Figure 5. Fas L on colon cancer cells induces hepatocyte apoptosis via type II signaling.
Liver sections containing metastatic colon cancer were evaluated by immunohistochemistry directed against cleaved caspase 3. A) MC38/FLIP tumors cells (apoptosis resistant, but not expressing Fas L ligand, and B) MC38/FLIP/L tumor cells (apoptosis resistant and expressing Fas L). T; tumor, L; liver. Boxed areas are shown in higher power to the right of the image. Arrow heads point out brown cytoplasmic staining within apoptotic cells. Apoptosis of hepatocytes was confirmed by TUNEL staining of liver tissue containing MC38/FLIP/L tumor cells. Bright field image on the left has the liver-tumor border defined by line. Fluorescent microscopy image on the right. TUNEL positive cells- red fluorescent protein (red), nuclei; DAPI (blue). Bid-/-mice, deficient in type II Fas signaling develop significantly fewer liver metastasis when injected with MC38/FLIP/L colon cancer cells than wt mice. D) Hematoxylin and eosin staining of liver. Images; low power, 10x; high power, 40x.
MC38/FLIP/L cells induce hepatocyte apoptosis in vivo and in culture via a type II signaling pathway
We did not observe any immune infiltrating cells in our tumor models, however we could not definitively exclude an interaction between the host leukocytes and the colon cancer cells to contribute to the metastatic phenotype in vivo. In order to directly address this issue, and we took advantage of the fact that hepatocytes use a type II mitochondrial dependent Fas apoptotic pathway (16) and leukocytes utilize a type I pathway which does not involve the mitochondria (16). Bid knockout (Bid KO) mice are deficient in type II signaling but maintain type I signaling allowing us to separate out MC38FLIP/L-hepatocyte and MC38/FLIP/L–leukocyte interactions in vivo (16). Bid KO mice develop subcutaneous tumors with MC38, MC38 /FLIP (not shown) and MC38FLIP/L colon cancer cells (Figure 3B). Bid KO mice do not develop spontaneous liver metastasis with MC38FLIP/L cells, and intravenous injection of 1 × 106 MC38/FLIP/L cells did not produce gross or microscopic hepatic metastasis at 4 weeks (Figure 5D). Intravenous injection of Bid KO mice with 5 × 106 MC38/FLIP/L cells resulted in few small hepatic tumors in 20% of the Bid KO mice. The incidence, size and appearance of lung metastasis did not differ between wild type mice and Bid KO mice injected with MC38/FLIP/L expressing cells suggesting lung metastasis does not require Fas-mediated apoptosis of lung parenchyma for lesions to become established.
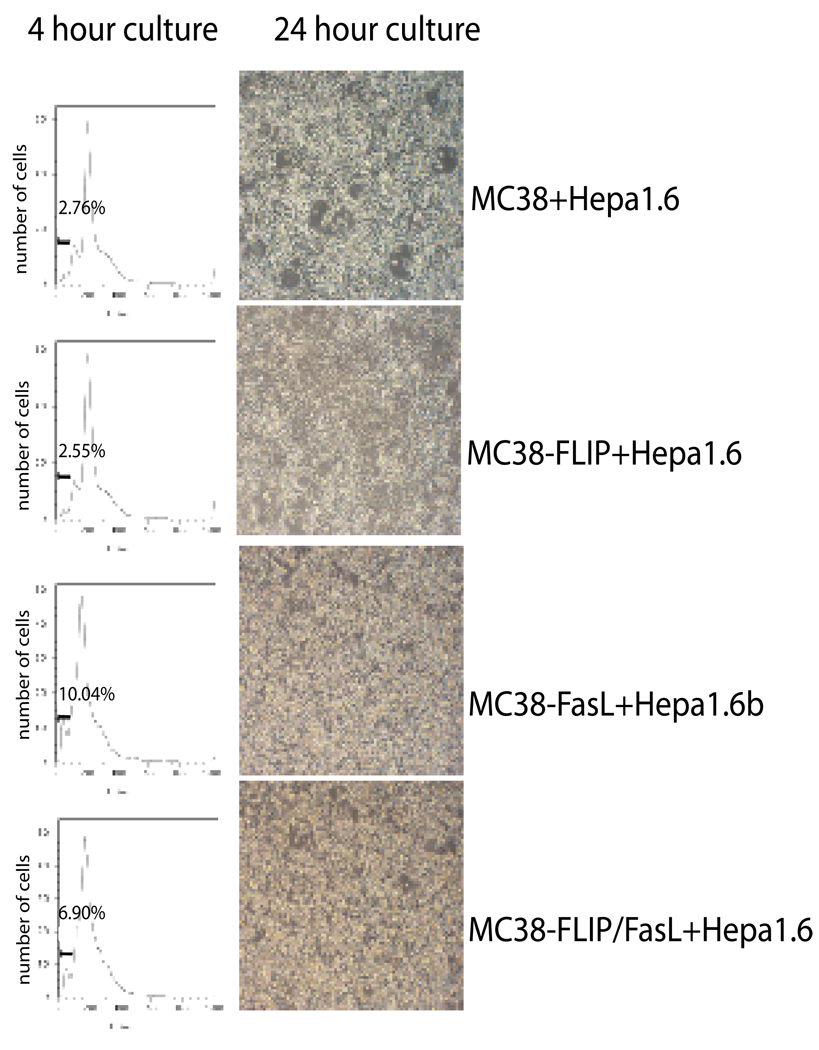
To further address signaling between Fas L bearing colon cancer cells and hepatocytes, the liver cell line, Hep1.6-EGFP was co-cultured with either MC38, MC38/FLIP, MC38/L, or MC38/FLIP/L cellsin in an over- confluent fashion to allow direct cell – cell contact for 24 hours. MC38 and MC38/FLIP / hepatocyte cultures grew together without apparent change in either cell type (figure 6A, B). Co-culture of the MC38/L and the MC38/FLIP/L cells with Hep1.6-EGFP cells resulted in significant cell death at 24 hours. (Figure 6 C, D). In order to discern which cell type was undergoing apoptosis, we collected both floating and adherent cells at 24 hours. Cells were fixed with 1% paraformaldehyde for 1 hour and separated into fluorescent (Hep1.6-EGFP) and nonfluorescent (MC38 derived cell lines) cells through fluorescence-activated cell-sorting. A standard propidium iodide staining and flow cytometry cell cycle analysis demonstrated a significant increase the level of apoptosis (sub G1 cells) in the hepatocytes cultured with the Fas ligand expressing colon cancer cells. (Figure 6). MC38/L cells also became apoptotic due to self stimulation with ligand.
Figure 6. Hep1.6 cells undergo apoptosis in contact with Fas L expressing MC38 cell lines.
After 4 hours (PI DNA content determination) and 24 hours (gross appearance of culture), Hep1.6 cells in contact with control (MC38) and apoptosis resistant colon cancer cells (MC38/FLIP) do not undergo apoptosis. Hep1.6 cells in contact with colon cancer cells expressing Fas L (MC38/FasL and MC38-FLIP/L) undergo apoptosis as early as 4 hours, with widespread apoptosis at 24 hours.
Discussion
Colon cancer is a leading cause of morbidity and mortality in the US. Local disease is controlled by surgical or endoscopic resection, however, therapies directed at metastatic disease are less effective. Therefore, understanding the signaling pathways involved in allowing malignant cells to engraft and grow in peripheral organs such as the liver will potentially provide new targets for therapy and improve the outcome of treatment.
Fas Ag/Fas L while best known as an inducer of apoptosis has roles outside of inducing cell death including proliferation (2, 16), organ regeneration (17), neuronal development, differentiation and regeneration (17–19). The signaling pathways responsible for these diverse outcomes are still under investigation and involve signal cascades directly activated by FasAg/L and influenced by other concurrently activated signaling pathways. Our laboratory has previously shown that gastric mucosal cells proliferate via direct Fas Ag/L induced Erk1/2 pathway (2) suggesting a role for Fas proliferative signaling in the luminal GI tract.
The Fas Ag/L pathway also has a “dark side” – serving a more sinister role in cancer cell growth. It is well established that most tumors inactivate the Fas Ag apoptotic pathway as they progress from local to invasive disease, and a substantial number also express Fas L. There remains however, much controversy over the exact role of Fas signaling in the evolution and survival of tumor cells as a whole. In general, avoidance of apoptosis is easy to understand from a cancer cell survival perspective; it has been less clear how gain of ligand expression confers an advantage to cancer cells. First consider Fas Ag induced apoptosis. Fas apoptotic signaling is avoided by tumor cells in a variety of ways including at the receptor level (20), through regulation of anti apoptotic proteins such as Bcl2, FLIP, IAP and survivin (21), regulation of caspase abundance or activity (22) and through the influence of other signal transduction cascades activated within the cell (23). Clinical effects likely depend upon the mechanism by which cells avoid apoptosis and the cell type involved as signaling outcomes appear to differ substantially between tissue types.
Involvement of Fas ligand in normal tissue homeostasis and in tumor growth and progression is more controversial (24, 25). Ligand expression on normal “immune privileged” cells such as in the cornea, testis and brain has been postulated to function as a form of “counter attack” by engaging and killing Fas Ag bearing immune cells. However the data supporting this claim has recently come under attack (24–26). A similar mechanism of immune cell killing by Fas L expressing cancer cells has been invoked to explain tumor cell survival based on clinical data correlating expression of ligand on tumor cells with worse clinical outcome. While appealing, direct evidence for this is sparse and the mechanism by which Fas L functions in cancer cells is still not known for certain. In its purported role of engaging and killing immune cells in an “immune counter attack” other Fas Ag bearing cells such as vascular endothelial cells and hepatocytes may also be at risk for tumor-ligand induced cell death (19). Based on these findings, enforced expression was pursued as a means to allow transplanted tissues to escape rejection. Unexpectedly, enforced expression of ligand often times results in a paradoxical increase in inflammation and death of ligand bearing cells. In line with these findings, Fas L has been reported to chemoattract neutrophils to tissues. While this may be detrimental to normal tissue, it appears to exert a paradoxical effect on tumor cells (27–30). Instead of facilitating tumor death, it appears that neutrophils secrete cytokines and growth factors which tumor cells use for proliferation. Fas ligand in soluble form, membrane bound, or in truncated form may have different physiologic effects, offering yet another mechanism by which cells regulate the pathway.
When one specifically looks at colon cancer cells, there is a graded progression of defects within the Fas Ag pathway from premalignant lesions to metastatic lesions whereby Fas Ag is first expressed coincident with one of several anti-apoptotic proteins such as FLIP. As local tumors progress in size and aggressive behavior, Fas L expression becomes prominent. Metastatic lesions almost universally have acquired Fas L (6). We provide a clear understanding of how these changes in the Fas Ag pathway initiate and enhance metastatic activity in colon cancer cells. Our data supports the notion that Fas Ag/L signaling in colon cancer is multifaceted, and includes both anti-apoptotic and proliferative signaling in tumor cells themselves and induces apoptosis in normal tissue into which the cancer cells metastasize (Figure 7). The crucial factor for colon cancer cell survival appears to be the early avoidance of apoptosis through expression of FLIP which offers the cancer cell a tremendous bonus- a previously tumor suppressor mechanism is usurped for proliferation and becomes a tumor promoter. Ligand expression is associated with more aggressive local disease and metastatic disease and appears to function in both autocrine and paracrine stimulation of Fas Ag proliferative signaling, bypassing the need for exogenous stimulation. Ligand expression by colon cancer cells also functions in the induction of apoptosis in endothelial cells and hepatocytes to establish metastasis. In our model, lymphocytes were not seen in or around tumors suggesting in this system, immune counterattack is not a prominent means of maintaining tumor survival. It is not clear if immune counter attack is a prominent function in human colon cancer. Also neutrophils were not prominent in or around tumors, and were not present in hepatic metastasis suggesting neutrophil mediated proliferation is not a central component in our system.
Figure 7. Fas signaling in metastatic colon cancer.
A) Fas Ag type II apoptotic signaling cascade. The non-apoptotic pathway is not fully defined (arrows, unknown portions of the pathway). B) Our proposed model of the interaction between apoptotic resistant colon cancer cells and hepatocytes.
It is not yet clear what pathway is utilized for Fas proliferative signaling in the colon as different cell types appear to possess unique signaling pathways (31) including Erk1/2 and NF-kappaB activation (32) inhibiting Fas receptor mobility within lipid rafts (20) and alterations in the cFLIP/FLICE ratio (2). In the liver for example, damage is associated with activation of several antiapoptotic pathways including Akt, STAT3 and NK-kappB which may act to prevent Fas mediated apoptosis, however the details of this switch are not yet fully delineated (17,33). Dissecting the signaling pathway for colon cancer -Fas proliferative signaling will provide additional potential targets for therapy.
The Fas Ag pathway has been recognized as central to the survival of many cancer types; alterations to the pathway play a pivotal role in the local growth and metastasis of tumors. Understanding the role of Fas signaling in colon cancer metastasis will offer new targets for therapy. Induction of hepatic and endothelial resistance to tumor-induced apoptosis may decrease metastatic spread of tumor, while decreasing ligand availability may serve to decrease local tumor growth. Targeting downstream signaling cascades responsible for proliferation may decrease growth and allow more effect from conventional therapy. However, it appears that restoration of Fas apoptosis sensitivity to tumor cells would be the most efficient target for therapy.
Material and Methods
For full details please see supplemental material.
Cell line, plasmids
MC38 cells (Dr. Timothy Wang) were engineered to express Fas L and/or FLIP. Expression of surface receptor, surface ligand and anti apoptotic proteins were verified by FACS and western blot.
Cell growth and apoptosis assay
Cells were seeded at equal number (10,000) into 6-well plates +/− Fas L and growth evaluated by manual cell counts in replicate plates (3 wells for each condition, repeated at least two times). Cell apoptosis was evaluated as sub-G1 population on standard propidium iodide staining and flow cytometer cell cycle analysis.
Co-culture of Hepatocyte and MC38 cells
GFP labeled Hep1.6 cells were grown in direct contact with either MC38, MC38/FLIP MC38/L, or MC38/FLIP/L cells at a ratio of 1:5. At 24 hours cells were collected fixed with 1% paraformaldehyde, and GFP positive cells sorted and evaluated for apoptosis by PI staining and cell cycle analysis.
Animals
Approval was obtained from the UMass IACUC prior to initiation of the study. Six week old male or female C57BL/6 mice (Jackson Laboratories) and Bid knock out (KO) mice (a generous gift from Dr. Stanley J. Korsmeyer) were used in these studies.
Subcutaneous and metastatic tumor model
One- five million viable cells resuspended in 500µl PBS (or PCS alone as control) were injected subcutaneously in the scapular region under light isofluorane anesthesia, or via single tail vein injection. At 3–4 weeks, tumors fixed and processed for histology, IHC or TUNEL.
Immunohistochemistry
Tissue was processed for routine histology, or immunohistochemistry using antibodies raised against: PCNA, PEcam, Fas Ag, Fas Ligand, cleaved caspase-3, CD3e, β tubulin, granulocytes. In H&E sections, mitotic bodies and PECAM were counted per 60x field. PCNA staining was scored as the percent positive cells per field. For each parameter 10–12 fields were counted per tumor to provide an average for each animal.
TUNEL staining
Apoptosis was detected using In Situ Cell Death Detection Kit, TMR red (Roche) according to the manufacturer’s protocol.
Supplementary Material
Acknowledgments
This work was supported by RO1 CA113564 to JH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human tissue samples were obtained from the UMass Cancer Center Tissue Bank (http://www.umassmed.edu/cancercenter/tissuebank/index.aspx). We would also like to thank Karen Dresser for help with immunohistochemistry.
Finincial disclosure- none to disclose.
HL, XF, CS, JL, SZ, ET, RSM, participated in the design and execution of the experiements. TCW, EKJ, SL experimental design, data interpretation, JH, study design, data interpretation, funding
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Cai X, Fan X, Moquin B, Stoicov C, Houghton J. Fas Ag-FasL coupling leads to ERK1/2-mediated proliferation of gastric mucosal cells. Am J Physiol Gastrointestintest Liver Physiol. 2008;294:G263–G275. doi: 10.1152/ajpgi.00267.2007. [DOI] [PubMed] [Google Scholar]
- 3.van der Woude CJ, Moshage H, Homan M, Kleibeuder JH, Jansen PL, Dekken H. Expression of apoptosis related proteins during malignant progression in chronic ulcerative colitis. J Clin Pathol. 2005;58:811–814. doi: 10.1136/jcp.2004.017418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao H, Song E, Chen J, Hamar P. Expression of FAP-1 by human colon adenocarcinoma: implication for resistance against Fas mediated apoptosis in cancer. British Journal of Cancer. 2004;91:1718–1725. doi: 10.1038/sj.bjc.6602136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houston A, Waldron-Lynch FD, Bennett MW, Roche D, O’Sullivan GC, Shanahan F, O’ Connell J. Fas Ligand expressed in colon cancer is not associated with increased apoptosis of tumor cells in vivo. International J of Cancer. 2003;107:209–214. doi: 10.1002/ijc.11392. [DOI] [PubMed] [Google Scholar]
- 6.Bennett MW, O’Connell J, Houston A, Kelley J, O’Sullivan GC, Collins JK, Shanahan F. Fas lignad upregulation is an early event in colonic carcinogenesis. H Clinical Path. 2001;54:598–604. doi: 10.1136/jcp.54.8.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, Boman BM. Evidence That APC Regulates Survivin Expression A Possible Mechanism Contributing to the Stem Cell Origin of Colon Cancer. Cancer Research. 2001;61:8664–8667. [PubMed] [Google Scholar]
- 8.Sarela AI, R Macadam CA, Farmery SM, Markham AF, Guillou PJ. Expression of the antiapoptosis gene, Survivin, predicts death from recurrent colorectal carcinoma. Gut. 2000;46:645–650. doi: 10.1136/gut.46.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 10.Liu K, McDuffie E, Abrams SI. Exposure of human primary colon carcinoma cells to anti-Fas interactions influences the emergence of preexisting Fas resistant metastatic populations. J Immunol. 2003;171:4164–4174. doi: 10.4049/jimmunol.171.8.4164. [DOI] [PubMed] [Google Scholar]
- 11.Velthuis JH, Gavric G, de Bont HJ, Nagelkerke JF. Impaired activation of caspases and prevention of mitochondrial dysfunction in the metastatic colon carcinoma CC531s-m2 cell line. Biochemical Pharmacology. 2005;69:463–471. doi: 10.1016/j.bcp.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell J, Bennett M, Nally K, Houston A, O’Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor-immune conflict. Annals of the New York Academy of Sciences. 2000;910:178–192. doi: 10.1111/j.1749-6632.2000.tb06708.x. [DOI] [PubMed] [Google Scholar]
- 13.Vekemans K, Timmers M, Vermijlen D, De Zanger R, Wisse E, Braet F. CC531s colon carcinoma cells induce apoptosis in rat hepatic endothelial cells by the Fas /FasL mediated pathway. Liver International. 2003;23:283–293. doi: 10.1034/j.1600-0676.2003.00840.x. [DOI] [PubMed] [Google Scholar]
- 14.Corbett TH, Griswold D, Roberts BJ, Peckham JC, Schabel FM. Tumor Induction Relationships in Development of Transplantable Cancers of the Colon in Mice for Chemotherapy Assays, with a Note on Carcinogen Structure. Cancer Res. 1975;35:2434–2439. [PubMed] [Google Scholar]
- 15.Vierboom MPM, Zwaveling S, Bos GMJ, Ooms M, Krietemeijer GM, Melief CJM, Offringa R. High Steady-State Levels of p53 Are Not a Prerequisite for Tumor Eradication by Wild-Type p53-specific Cytotoxic T Lymphocytes. Cancer Res. 2000;60:5508–5513. [PubMed] [Google Scholar]
- 16.Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 17.Desbarats J, Newell MK. Fas engagement accelerates liver regeneration after partial hepatectomy. Nature Med. 2000;8:920–923. doi: 10.1038/78688. [DOI] [PubMed] [Google Scholar]
- 18.Tamm C, et al. Differential regulation of the mitochondrial and death receptor pathways in neural stem cells. European J Neuroscience. 2004;19:2613–2621. doi: 10.1111/j.0953-816X.2004.03391.x. [DOI] [PubMed] [Google Scholar]
- 19.Zuliani C, et al. Control of neuronal branching by the death receptor CD95 (Fas/Apo-1) Cell Death & Differentiation. 2006;13:31–40. doi: 10.1038/sj.cdd.4401720. [DOI] [PubMed] [Google Scholar]
- 20.Stoicov C, Cai X, Li H, Klucevsek K, Saffari R, Houghton J. Major Histocompatibility Complex Class II inhibits Fas Antigen-mediated gastric mucosal cell apoptosis through actin-dependent inhibition of receptor aggregation. Infection and Immunity. 2005;73:6311–6321. doi: 10.1128/IAI.73.10.6311-6321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huerta S, et al. Modification of gene products involved in resistance to apoptosis in metastatic colon cancer cells: roles of Fas, Apaf-1, NFkappaB, IAPs, Smac/DIABLO, and AIF. Journal of Surgical Research. 2007;142:184–194. doi: 10.1016/j.jss.2006.12.551. [DOI] [PubMed] [Google Scholar]
- 22.Curtin JF, Cotter TG. Live and let die: regulatory mechanisms in Fas-mediated apoptosis. Cellular Signalling. 2003;15:983–992. doi: 10.1016/s0898-6568(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 23.Wajant H. The Fas Signaling Pathway: More Than a Paradigm. Science. 2002;296:1635. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 24.O’Connell JO, Houston A, Bennett MW, O’Sullivan GC, Shanahan F. Immune privilege or inflammation? insights into the Fas ligand enigma. Nature Med. 2001;7:271–274. doi: 10.1038/85395. [DOI] [PubMed] [Google Scholar]
- 25.Restifo NP. Countering the ‘counterattack” hypothesis. Nature Med. 2001;7:259. doi: 10.1038/85357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green DR, Ferguson TA. The role of Fas ligand in immune privilege. Nat Rev Mol Cell Biol. 2001;2:917–924. doi: 10.1038/35103104. [DOI] [PubMed] [Google Scholar]
- 27.Arai H, Gordon D, Nabel GJ. Gene transfer of Fas ligand induces tumor regression in vivo. Proc. Natl. Acad. Sci USA. 1997;94:13862–13867. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igney FH, Behrens CK, Krammer PH. The influence of CD95L expression on tumor rejection in mice. Eur J Immunol. 2003;33:2811–2821. doi: 10.1002/eji.200324176. [DOI] [PubMed] [Google Scholar]
- 29.Seino K, Kayagaki N, Okumura K, Yakita H. Antitumor effect of locally produced CD95 ligand. Nat Med. 1997;3:165–170. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 30.Wajant H, Pfizenmaier K, Scheurich P. Non apoptotic Fas signaling. Cytokine Growth factor Rev. 2003;14:53–66. doi: 10.1016/s1359-6101(02)00072-2. [DOI] [PubMed] [Google Scholar]
- 31.Ahn JH, Park S, Cho HS, Lee MS, Yoon JB, Vilcek J, Lee TH. Non- apoptotic signaling pathways activated by soluble Fas ligand in serum starved human fibroblasts. Mitogen activated protein kinases and NF-kappaB dependent gene expression. J Biol Chem. 2001;50:47100–47106. doi: 10.1074/jbc.M107385200. [DOI] [PubMed] [Google Scholar]
- 32.Peters ME, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.