Abstract
Background
Metabolic syndrome (MetS) is associated with incident myocardial infarction and stroke and is linked with subclinical inflammation. However, prospective data pertaining to MetS and future peripheral artery disease (PAD) are sparse with few studies examining the role of inflammation. We therefore evaluated the relationship between MetS, inflammation, and incident PAD.
Methods and Results
We conducted a prospective cohort study among 27,111 women free of baseline cardiovascular disease participating in the Women’s Health Study. Subjects were followed for incident symptomatic PAD (n=114; median cohort follow-up=13.3 years). We used Cox proportional hazards models to compare PAD risk among women with and without MetS. We also evaluated relationships between MetS and subclinical inflammation as measured by high sensitivity C-reactive protein (hsCRP) and soluble intercellular adhesion molecule-1 (sICAM-1) and adjusted for these biomarkers in multivariable models. Women with MetS had a 62% increased risk of future PAD (HR 1.62; 95% CI 1.10–2.38). After multivariable adjustment, MetS remained significantly associated with PAD (adj HR 1.48; 95% CI 1.01–2.18) with a 21% risk increase per additional MetS-defining trait (adj HR 1.21; 95% CI 1.06–1.39). Median levels of hsCRP were 4.0 versus 1.5 mg/L (p<0.0001) and 374 versus 333 ng/mL for sICAM-1(p<0.0001) in women with and without MetS, respectively. When hsCRP and sICAM-1 were added to multivariable models, risk associated with the MetS was substantially attenuated and no longer significant (HR 1.14, 95% CI 0.75–1.73).
Conclusion
MetS is associated with an increased risk of future symptomatic PAD in women. This risk appears largely mediated by the effects of inflammation and endothelial activation.
Keywords: Peripheral artery disease, metabolic syndrome, inflammation, endothelial dysfunction, women
INTRODUCTION
The metabolic syndrome (MetS), a cluster of cardiovascular risk factors associated with insulin-resistance and obesity, has been consistently associated with a moderate increase in the incidence of coronary heart disease, stroke and cardiovascular mortality 1–6. Much less information is available on the relationship between the MetS and incident lower extremity peripheral artery disease (PAD) with most data deriving from cross-sectional reports7–10. One of two previously published prospective evaluations suggested that the MetS is a predictor of end-stage PAD (lower extremity amputation or revascularization), but that this risk increase is largely attributable to the impact of diabetes alone11. Another recent analysis did not find a significant association of MetS with incident PAD, although a strong and independent relationship between the MetS and cerebrovascular disease was noted12. This potential difference in the relationship between the MetS and coronary heart disease and stroke on one hand and PAD on the other may be due to the absence of large prospective studies for incident PAD or to differences in atherogenic mechanisms affecting these distinct vascular beds 13.
Systemic inflammation, assessed through plasma levels of high sensitivity C-reactive protein (hsCRP), is an important predictor of cardiovascular morbidity and mortality 14–17 and may be an important mediator of the association between the MetS and cardiovascular disease3, 5. Furthermore, both inflammation and endothelial injury or activation (as measured by soluble cellular adhesion molecule-1, sICAM-1) have recently been shown to be strongly and independently associated with incident PAD18. These factors may therefore explain potential associations between the MetS and PAD; however, few prospective data have been available. In an attempt to clarify these issues, we assessed the relationships between the MetS, inflammation and future symptomatic PAD defined as intermittent claudication or lower extremity artery revascularization in a large cohort of initially healthy women.
METHODS
Participants
All study subjects were participants of the Women’s Health Study, a completed randomized trial evaluating the risks and benefits of low dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer. Details of the study design have been described previously 19–21.
Briefly, beginning in 1993, 39,876 female health professionals in the United States who were 45 years or older and free of cardiovascular disease, cancer or other major illnesses were randomized to receive 100 mg aspirin every other day, 600 IU vitamin E every other day, both agents or placebo. The trial initially had a beta carotene arm that was terminated early 22. Information on baseline variables was collected using mailed questionnaires. Follow-up questionnaires asking participants about study outcomes and other information were sent every six months during the first year and every 12 months thereafter.
For the purpose of this study, we excluded 11,935 participants because they did not provide a baseline blood sample, 7 participants with confirmed pre-randomization PAD, and 823 participants with missing information on body mass index (BMI), blood pressure or history of hypertension. Thus, the final study population for the present analysis consisted of 27,111 women. Written informed consent was obtained from all participants. The study was approved by the institutional review board of Brigham and Women’s Hospital, Boston, and was monitored by an external data and safety monitoring board.
Laboratory analyses
All blood analyses were performed in a core laboratory certified by the National Heart, Lung, and Blood Institute/Centers for DiseaseControl and Prevention Lipid Standardization Program. Total cholesterol, high density lipoproteincholesterol (HDL-C) and triglycerides were ascertained with direct measurement assays (Roche Diagnostics). Triglyceride levels were measured enzymatically, with correction for endogenous glycerol, using a Hitachi 917 analyzer and reagents and calibrators from Roche Diagnostics (Indianapolis, Indiana). Plasma hsCRP was measured with a validated high-sensitivity immunoturbidimetric method (Denka Seiken, Niigata, Japan). The interassay coefficients of variation using two levels of control materials ranged from 1.07 to 5.20%. sICAM-1 was measured by quantitative sandwich ELISA (R&D Systems, Minneapolis, Minn) with a reproducibility of 8.89% and 6.39% at concentrations of 171.8 and 289.1 ng/mL, respectively.
Definition of the MetS
According to the Adult Treatment Panel (ATP) III guidelines, women with three or more of the following traits are defined as having the MetS: (1) waist circumference ≥ 88 cm; (2) triglycerides ≥ 150 mg/dL; (3) HDL-C <50 mg/dL; (4) blood pressure ≥ 130/85 mm Hg; and (5) abnormal glucose metabolism as identified by a fasting blood glucose ≥ 100 mg/dL 23, 24. For the present study, we used a modified ATP III definition of the MetS previously validated in this cohort 3. Since waist circumference was not available at baseline, a BMI cutpoint for obesity of > 26.7 kg/m2 was used as a surrogate. When waist circumference was collected at the six-year follow-up, this value corresponded to the same percentile for BMI as did a waist circumference of 88 cm. In addition, prior data in the WHS have demonstrated that BMI is equivalent to waist circumference in predicting major cardiovascular events25 and a recent meta-analysis of MetS and vascular risk found no heterogeneity of effects whether waist circumference, waist-to-hip ratio or BMI was used6. Triglyceride level and HDL-C were ascertained as described above. Blood pressure at baseline was self-reported by WHS participants all of whom were female health professionals, a group in which self-report of blood pressure has proven highly accurate26. Subjects meeting the blood pressure criterion for MetS included those who reported a diagnosis of hypertension by a physician, a systolic blood pressure ≥ 130 mmHg or a diastolic blood pressure ≥ 85 mmHg at baseline. Because fasting glucose levels were not available, we used a diagnosis of diabetes at baseline or during follow-up to identify individuals with impaired glucose metabolism.
Outcome Ascertainment
Participants are surveyed annually for multiple health outcomes, including symptomatic PAD events, defined as intermittent claudication and/or peripheral artery surgery inclusive of catheter-based interventions. Case confirmation occurred by telephone interview during which the presence of vascular claudication was established by use of the Edinburgh Claudication Questionnaire. This instrument is a modified version of the World Health Organization/Rose Claudication Questionnaire, which has previously been validated in a community outpatient setting with 92% sensitivity and 99% specificity for physician-diagnosed intermittent claudication 27. In addition, medical records were obtained to assess the concordance of reported symptoms with diagnostic testing when available. Reports of peripheral arterial surgery were confirmed after review of operative notes or procedural reports in the case of peripheral angioplasty or stenting. Of 456 self-reported PAD events occurring as of November 23, 2007, 114 were confirmed utilizing these methods. Among disconfirmed events, venous disease, lower extremity arthritis, lumbar disc disease, and peripheral neuropathy were the main causes of non-ischemic leg pain. There were 73 unrefuted events in women who were unreachable for telephone interview, deceased, or unwilling to participate. Only confirmed events were considered in the analysis. Among women with confirmed PAD, there were 111 cases of intermittent claudication, 56 of these women undergoing revascularization. In addition, there were 3 cases of peripheral artery revascularization without antecedent claudication.
Statistical analyses
Baseline characteristics were compared according to the presence or absence of the MetS using Wilcoxon rank sum tests for continuous variables and chi square tests for categorical variables. Age-adjusted incidence rates were calculated using internal standardization.
The divergence of PAD incidence over time between groups with 0, 1–2 and ≥3 MetS traits was estimated using Kaplan-Meier survival curves, and the log-rank test computed to compare curves. To account for potential confounders, we constructed multivariable Cox proportional hazards models to estimate the adjusted hazard ratio (HR) of PAD for women with 1–2 and ≥3 MetS traits by creating indicator variables for these two categories and adding terms for potential confounders. Cox models were also used to compare the risk according to presence versus absence of the MetS. The HR per increasing number of MetS-defining traits was estimated by using the number of traits (ranging from 0–5) as an ordinal variable in the model. For each woman, person-years of follow-up were calculated from the date of return of the baseline questionnaire to the date of incident PAD, death or to November 23, 2007, whichever came first. All risk estimates are presented as HRs with the 95% confidence interval (CI).
Crude models were first adjusted for age and smoking status and then additionally adjusted for total cholesterol and exercise. We also assessed the effect of hormone replacement therapy on incident PAD, and as it was neither a significant predictor nor a confounder for the association between the MetS and PAD, hormone replacement therapy was not included in the final models. To assess whether the increased risk associated with the MetS is mediated by inflammation and/or endothelial activation, we included hsCRP and sICAM-1 in the model building process. Both hsCRP and sICAM-1 were log-transformed to normalize the variable distribution and better meet the assumption of linearity in risk across increasing levels of these biomarkers. We fitted separate models adjusting for each biomarker in a first step, and subsequently added both markers to the same model. Finally, to evaluate whether the risk associated with MetS is independent of baseline diabetes, we repeated the main analyses among women without established diabetes on entry into the study.
Effect modification was assessed using multiplicative interaction terms. The proportional hazards assumption was examined for all models by including a MetS by logarithm of time interaction variable into the model 28. No violation of this assumption was detected. All analyses were carried out using SAS version 9 (SAS Institute Inc, Cary, NC). A two-tailed p value <0.05 was considered to indicate statistical significance.
RESULTS
Baseline characteristics of the study population according to MetS status are shown in Table 1. Overall, 6,920 (25.5%) participants had the MetS. Compared to women without the MetS, those with MetS were significantly older (p<0.0001), more likely to smoke (p=0.009) and less likely to exercise on a regular basis (p<0.0001). Among participants with the MetS, an HDL-C <50 mg/dL was the most prevalent individual MetS-defining trait (88.0%), followed by elevated BMI (81.2%), elevated blood pressure (77.8%), elevated triglycerides (77.7%), and dysglycemia as identified by baseline or incident diabetes (28.2%).
Table 1.
Baseline Characteristics of the Study Population According to Presence or Absence of Metabolic Syndrome
| Metabolic syndrome |
|||
|---|---|---|---|
| Absent (n=20191) | Present (n=6920) | P-value | |
| Age, years | 53 (49, 58) | 54 (50, 60) | <0.0001 |
| 0.009 | |||
| Smoking, % | |||
| Current | 11.3 | 12.5 | |
| Past | 37.2 | 35.7 | |
| Never | 51.6 | 51.8 | |
| Exercise frequency in times/week, %* | <0.0001 | ||
| Rarely/never | 34.1 | 46.6 | |
| <1 | 19.1 | 20.9 | |
| 1–3 | 33.9 | 26.0 | |
| >3 | 13.0 | 6.7 | |
| Body mass index, kg/m2 | 23.7 (21.9, 25.8) | 29.9 (27.4, 33.5) | <0.0001 |
| HDL-Cholesterol, mg/dL | 56 (48, 66) | 42 (36, 47) | <0.0001 |
| Triglycerides, mg/dL | 103 (75, 140) | 193 (153, 260) | <0.0001 |
| Total cholesterol, mg/dL | 205 (182, 232) | 217 (191, 244) | <0.0001 |
| Metabolic Syndrome Traits | |||
| Body mass index >26.7 kg/m2, % | 17.1 | 81.2 | <0.0001 |
| HDL-Cholesterol <50 mg/dL, % | 29.5 | 88.0 | <0.0001 |
| Triglycerides ≥150 mg/dL, % | 20.1 | 77.7 | <0.0001 |
| Elevated blood pressure, %† | 24.0 | 77.8 | <0.0001 |
| Dysglycemia, %‡ | 1.6 | 28.2 | <0.0001 |
| History of baseline diabetes | 0.6 | 7.3 | |
| Incident diabetes | 1.0 | 20.9 | |
Data are median (interquartile range) or percentages
HDL High density lipoprotein
The following question was used to categorize exercise frequency: “How often do you engage in strenuous (aerobic) physical activity (e.g. swimming, aerobics, cycling, running)?”
Defined as blood pressure ≥130/85 mmHg or history of a physician’s diagnosis of hypertension
Dysglycemia defined by history of diabetes at baseline or incident diabetes during follow-up
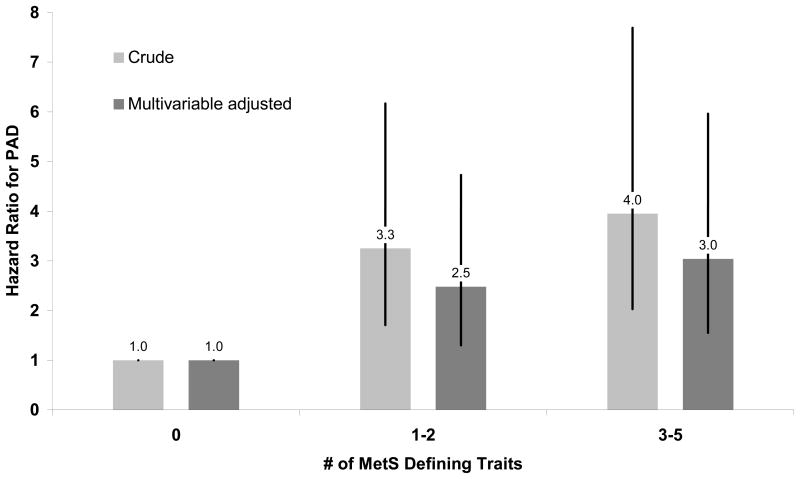
During a median (interquartile range) follow-up of 13.3 (12.5–13.8) years, 114 symptomatic PAD events occurred. There were 44 and 70 events among women with and without the MetS, respectively. Age-adjusted incidence rates in these two groups were 0.43 and 0.30 events per 1000 person-years of follow-up, respectively. Figure 1 provides the cumulative incidence curves for PAD after classifying subjects according to presence of 0, 1–2, and ≥ 3 MetS traits and shows the increasing risk of PAD with higher number of traits. Multivariable regression analysis confirmed these relationships (Figure 2). Compared to women without any MetS traits, those having one or two criteria had a 2.5-fold increased PAD risk, and women with established MetS had a 3-fold increased risk independent of age, smoking, total cholesterol, and physical activity.
Figure 1. Cumulative Incidence for PAD According to Number of MetS Traits.
Kaplan-Meier cumulative incidence curves for symptomatic PAD according to 0, 1–2, or ≥ 3 metabolic syndrome traits. MetS denotes metabolic syndrome.
Figure 2. Hazard Ratio for PAD According to Number of MetS Traits.
Data are crude (light bars) and multivariable-adjusted (dark bars) hazard ratios. Multivariable models were adjusted for age, smoking status, total cholesterol and exercise. Error bars represent 95 percent confidence intervals. MetS denotes metabolic syndrome.
When examined according to presence or absence of the MetS or per number of individual traits (Table 2), we found similar results. The hazard ratio for symptomatic PAD among women with the MetS versus those without MetS was 1.62 (95% CI 1.10–2.38) on univariable analysis and 1.48 (95% CI 1.01–2.18) after adjustment for age and smoking status. Additional adjustment for total cholesterol and exercise had virtually no effect on these risk estimates. Adjustment for low density lipoprotein cholesterol instead of total cholesterol did not change our results (data not shown). When assessed as an ordinal risk factor, after multivariable adjustment there was a 21% (95% CI 6 to 38%; p=0.004) greater hazard of PAD per additional MetS-defining trait.
Table 2.
Hazard Ratio for Incident PAD According to Presence of Metabolic Syndrome and Per Number of Metabolic Syndrome Defining Traits
| Metabolic Syndrome (Yes/No) | Per Additional Metabolic Syndrome Defining Trait | ||||
|---|---|---|---|---|---|
| No. Events | HR for PAD (95% CI) | P-Value | HR for PAD (95% CI) | P-Value | |
| Crude model (n=27111) | 114 | 1.62 (1.10–2.38) | 0.013 | 1.26 (1.12–1.43) | 0.0002 |
| Multivariable model 1* (n=27089) | 113 | 1.48 (1.01–2.18) | 0.046 | 1.21 (1.06–1.38) | 0.004 |
| Multivariable model 2† (n=27079) | 113 | 1.48 (1.00–2.19) | 0.048 | 1.21 (1.06–1.39) | 0.004 |
| Multivariable model 3‡ (n=26900) | 108 | 1.14 (0.75–1.73) | 0.55 | 1.11 (0.96–1.28) | 0.18 |
Adjusted for age and smoking status.
Additionally adjusted for total cholesterol and exercise.
Additionally adjusted for plasma levels of hsCRP and sICAM-1.
Both hsCRP and sICAM-1 were strongly associated with MetS and increasing number of MetS traits. Median levels of hsCRP were 4.0 mg/L and 1.5 mg/L (p<0.0001) among women with and without the MetS, respectively. The corresponding levels of sICAM-1 were 374 ng/mL and 333 ng/mL (p<0.0001). Among participants with 0 to 5 MetS-defining traits, median hsCRP levels gradually increased from 1.0 to 5.9 mg/L (p<0.0001) and median sICAM-1 levels from 321 to 413 ng/mL (p<0.0001).
To address whether elevated hsCRP or sICAM-1 levels might account for the relationship of MetS and incident PAD we sequentially added these variables to multivariable models (Table 2). After inclusion of hsCRP, the adjusted HR for the MetS was reduced to 1.23 (95% CI 0.82–1.85). A similar effect was observed after inclusion of sICAM-1; the adjusted HR for MetS in this model was 1.30 (95% CI 0.87–1.95). When both biomarkers were added to the same model, the association was markedly attenuated (adjusted HR 1.14; 95% CI 0.75–1.73). Findings were similar when assessed according to number of MetS-defining traits (Table 2). In analyses stratified by age, smoking status or approximate tertiles of hsCRP and sICAM-1, we found consistent results (data not shown). None of the p-values for effect modification by these factors was statistically significant (p>0.14 for all interactions tested).
Finally, we evaluated the risk associated with the individual MetS-defining traits. The HRs (95% CIs) for PAD associated with elevated BMI, elevated triglycerides, hypertension, low HDL-C and dysglycemia were 0.98 (0.65–1.46), 1.39 (0.96–2.01), 1.50 (1.02–2.19), 1.60 (1.10–1.33) and 2.05 (1.26–3.36), respectively. By comparison, the HR associated with current smoking was 12.7 (7.6–21.2). Whether MetS had an effect independent of established diabetes was evaluated among 26,364 women free of diagnosed diabetes at baseline. Among this subgroup, 105 symptomatic PAD events occurred. Similar to the main analyses, the multivariable adjusted hazard ratio for MetS was 1.43 (95% CI 0.95–2.16;p=0.09), and this risk estimate was reduced to 1.15 (95% CI 0.73–1.78) after additional adjustment for hsCRP and sICAM-1.
DISCUSSION
In this prospective study of initially healthy, middle-aged women, similar to previously published data for coronary and cerebrovascular disease, we found that the MetS is associated with a moderate increase in risk of future symptomatic PAD. This finding persisted after adjustment for age, smoking status, total cholesterol and physical activity (adj HR 1.48; 95% CI 1.01–2.18). Furthermore, the magnitude of effect was comparable in women who were non-diabetic at baseline. Risk relationships were largely attenuated after control for hsCRP and sICAM-1 suggesting that in this generally low risk population of women, the excess risk associated with MetS may be mediated through heightened inflammation and/or endothelial activation.
Most prior cross-sectional reports have demonstrated a positive association between MetS and prevalent PAD. Among participants in the National Health and Nutrition Examination Survey (NHANES), MetS was linked to a high likelihood of prevalent PAD (OR 4.8; 95% CI 2.2–34.0) and presence of PAD increased with levels of CRP 8. In another study from NHANES29, insulin resistance as measured by the homeostasis model (HOMA-IR) was also associated with prevalent PAD (OR 2.1 for highest vs. lowest HOMA-IR quartile; 95% CI 1.1–4.0) independently of traditional risk factors including other components of the MetS. However, in this population having a high prevalence of cardiovascular risk factors and established cardiovascular disease, adjustment for CRP did not attenuate this relationship. In a third cross-sectional study of patients with diagnosed vascular disease, MetS was more common in those having PAD (57%) compared to atherosclerosis affecting other vascular beds (40%, 43%, and 45% for coronary disease, cerebrovascular disease, and aortic aneurysm, respectively)7.
Despite such evidence from cross-sectional reports, few prospective comparisons of MetS and incident PAD have been available. A small prospective study in a Finnish cohort demonstrated a 2-fold increased hazard of end-stage PAD (n=57 amputations or revascularization procedures) among those with MetS. However, this relationship appeared largely due to prevalent diabetes11. After analyses adjusting for diabetes or excluding diabetic subjects, the authors conclude that MetS does not predict PAD beyond the risk associated with established diabetes. However, in this evaluation of end-stage PAD, diabetes-related chronic conditions such as neuropathic ulcers and concurrent infection rather than ischemia per se may have been major contributors as nearly 50% of events were amputations. A dominant effect of pre-existing diabetes was not confirmed in our analysis which included milder cases of PAD (intermittent claudication and limb ischemia).
The Edinburgh Artery Study did not find a significant association between the MetS and incident PAD (HR 0.89; 95% CI 0.57–1.28)12. Compared to our study population, the Edinburgh cohort was older (mean age 65 years), of mixed gender, and had a high prevalence of baseline smoking (25.3%), and asymptomatic disease (17% with enrollment ABI<0.9). Nonetheless, these null findings from a higher risk group in contrast to our positive results among low risk women invoke the intriguing possibility that MetS has a greater impact on atheroma initiation than progression in individuals with extant subclinical disease. This hypothesis requires corroboration in other cohorts but is supported by results of a recent meta-analysis of MetS and incident cardiovascular events in which MetS had a larger effect (HR 1.96 vs. 1.43; p=0.04) in studies of individuals at lower baseline cardiovascular risk (<10%)6.
As demonstrated in prior reports3–5, 12, 30, 31, our data also confirm that markers of inflammation and endothelial activation are strongly associated with MetS. We found an increase in plasma levels of hsCRP and sICAM-1 per additional MetS-defining trait such that women with the MetS had substantially higher plasma levels than those without MetS. Furthermore, the addition of either hsCRP or sICAM-1 individually to multivariable models substantially attenuated the effect of MetS on subsequent PAD, while inclusion of both markers virtually abolished this association. Our findings thus suggest that in this relatively healthy population of women, inflammation and endothelial activation may be potential mediators of the heightened PAD risk conferred by this risk factor cluster.
The present data also emphasize the importance of smoking in the pathogenesis of PAD. Smoking women had a 12.7-fold increased risk of developing PAD compared to non-smokers, and therefore smoking was by far the strongest risk factor in this population. While prior risk estimates for the association between smoking and PAD are somewhat weaker than in the current study32, our finding may reflect a heightened risk of smoking in women that requires further investigation. The relative magnitude of smoking as a risk factor compared not only to MetS but also diabetes in this study underscores the importance of abstinence from smoking for the prevention of PAD.
Our results should be interpreted in the context of several potential limitations. First, our study included women who are of predominantly Caucasian origin, and our findings may not be generalizable to other groups. Second, the use of symptomatic PAD as our primary a priori end point by definition excludes subclinical disease, which may have otherwise been detected through abnormal pulse examination or ankle-brachial index 33. However, we believe our data to be not only relevant from a mechanistic perspective but also of clinical importance for the following reasons. First, claudication and limb ischemia requiring revascularization are the principal clinical manifestations of PAD. Second, all events evaluated in this analysis were confirmed by a validated claudication questionnaire and medical record review. Third, women enrolled in this study are health professionals and are therefore less likely to encounter barriers to medical care, which may otherwise have led to underdiagnosis. Although potential misclassification resulting from atypical or occult disease may have occurred, this, if anything, would have biased our results toward the null. On the other hand, a low ankle brachial index has been shown to strongly and independently predict the occurrence of cardiovascular events independent of claudication symptoms, highlighting the importance of this endpoint 34. Nevertheless, we believe that the use of confirmed symptomatic PAD is not only valid but also represents an important clinical endpoint for this disease. Finally, a modified version of the official ATP III MetS definition was used in this analysis. The criteria for MetS have been variable and continue to evolve over time. Our use of baseline or incident type 2 diabetes instead of fasting glucose levels as a MetS defining trait likely underestimated the number of women who satisfied the MetS criteria. Again, this could have biased our results toward the null. The definition used in the current report has previously associated with incident cardiovascular disease in this cohort 3. Furthermore, it is important to note that our study was undertaken in order to describe a potential etiologic role for this risk factor cluster in the development of PAD rather than to validate the utility of the syndrome for risk prediction.
In conclusion, similar to the previously noted relationship between the MetS and incident coronary heart disease and stroke, this study demonstrated a modest positive association with future PAD in a population of otherwise low risk women. Substantially increased plasma levels of hsCRP and sICAM-1 were evident in subjects with MetS and a strong influence of these factors on the relationship between MetS and PAD was noted suggesting a possible pathophysiologic role. Prospective data from other cohorts are greatly needed not only to corroborate our results but also to further elucidate mechanistic links between risk factor clustering and onset of this disease.
Acknowledgments
FUNDING SOURCES
National Heart, Lung, and Blood Institute (HL-43851, HL-58755, HL-082740, and HL-075771), National Cancer Institute (CA-47988), and the Donald W. Reynolds Foundation. David Conen was supported by a grant from the Swiss National Science Foundation (PASMA 118586/1).
DISCLOSURES
Dr. Conen reports no disclosures; Dr. Rexrode reports no disclosures. Dr. Creager reports having research support from Sanofi-Aventis and Merck and is a consultant for BioMarin and Genzyme. Dr Ridker reports having received research funding support from multiple not-for-profit entities including the National Heart, Lung, and Blood Institute, the National Cancer Institute, the American Heart Association, the Doris Duke Charitable Foundation, the Leducq Foundation, the Donald W Reynolds Foundation, and the James and Polly Annenberg La Vea Charitable Trusts. Dr Ridker also reports having received investigator-initiated research support from multiple for-profit entities including Astra-Zeneca, Novartis, Pharmacia, Roche, Sanofi-Aventis, and Abbott, as well as non-financial research support from Amgen. Dr Ridker is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease that have been licensed to Siemens and AstraZeneca, and has served as a research consultant to Schering-Plough, Sanofi/Aventis, AstraZeneca, Isis, Dade, Merck, Novartis, and Vascular Biogenics. Dr. Pradhan reports having received research support from Sanofi-Aventis.
Footnotes
Clinical Summary
Metabolic syndrome (MetS) is associated with myocardial infarction and stroke and is linked with subclinical inflammation. We conducted a prospective cohort study among 27,111 women free of baseline cardiovascular disease to evaluate the relationship between Metabolic Syndrome (MetS), inflammation, and incident peripheral artery disease (PAD). We found that MetS was associated with significantly increased risk of PAD after multivariable adjustment (HR 1.48 (95% confidence interval 1.01–2.18), similar to previously documented associations between the MetS and coronary disease or stroke. However, when inflammatory biomarkers were added to multivariable models, risk associated with the MetS was attenuated and no longer significant. Our findings were comparable in women who were non-diabetic at baseline. Taken together, our data provide evidence that the MetS confers an increased risk of incident PAD among initially healthy women and that this increased risk is largely mediated through the effects of inflammation and endothelial activation.
References
- 1.Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 2.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 4.Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Sr, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110:380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 5.Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 6.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Olijhoek JK, van der Graaf Y, Banga JD, Algra A, Rabelink TJ, Visseren FL. The metabolic syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur Heart J. 2004;25:342–348. doi: 10.1016/j.ehj.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Vu JD, Vu JB, Pio JR, Malik S, Franklin SS, Chen RS, Wong ND. Impact of C-reactive protein on the likelihood of peripheral arterial disease in United States adults with the metabolic syndrome, diabetes mellitus, and preexisting cardiovascular disease. Am J Cardiol. 2005;96:655–658. doi: 10.1016/j.amjcard.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Jiang B, Wang J, Feng K, Chang Q, Fan L, Li X, Hu FB. Prevalence of the metabolic syndrome and its relation to cardiovascular disease in an elderly Chinese population. J Am Coll Cardiol. 2006;47:1588–1594. doi: 10.1016/j.jacc.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 10.Lahoz C, Vicente I, Laguna F, Garcia-Iglesias MF, Taboada M, Mostaza JM. Metabolic syndrome and asymptomatic peripheral artery disease in subjects over 60 years of age. Diabetes Care. 2006;29:148–150. doi: 10.2337/diacare.29.1.148. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Ruotsalainen S, Moilanen L, Lepisto P, Laakso M, Kuusisto J. Metabolic syndrome and incident end-stage peripheral vascular disease: a 14-year follow-up study in elderly Finns. Diabetes Care. 2007;30:3099–3104. doi: 10.2337/dc07-0985. [DOI] [PubMed] [Google Scholar]
- 12.Wild SH, Byrne CD, Tzoulaki I, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Metabolic syndrome, haemostatic and inflammatory markers, cerebrovascular and peripheral arterial disease: The Edinburgh Artery Study. Atherosclerosis. 2009;203:604–609. doi: 10.1016/j.atherosclerosis.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP, Robertson RM, Loscalzo J. Atherosclerotic Vascular Disease Conference: Writing Group III: pathophysiology. Circulation. 2004;109:2617–2625. doi: 10.1161/01.CIR.0000128520.37674.EF. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 15.Koenig W, Lowel H, Baumert J, Meisinger C. C-Reactive Protein Modulates Risk Prediction Based on the Framingham Score: Implications for Future Risk Assessment: Results From a Large Cohort Study in Southern Germany. Circulation. 2004;109:1349–1353. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-Reactive Protein and Low-Density Lipoprotein Cholesterol Levels in the Prediction of First Cardiovascular Events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 18.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117:823–831. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 19.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 22.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women's Health Study. J Natl Cancer Inst. 1999;91:2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 23.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 25.Gelber RP, Gaziano JM, Orav EJ, Manson JE, Buring JE, Kurth T. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol. 2008;52:605–615. doi: 10.1016/j.jacc.2008.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conen D, Ridker PM, Buring JE, Glynn RJ. Risk of cardiovascular events among women with high normal blood pressure or blood pressure progression: prospective cohort study. BMJ. 2007;335:432. doi: 10.1136/bmj.39269.672188.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101–1109. doi: 10.1016/0895-4356(92)90150-l. [DOI] [PubMed] [Google Scholar]
- 28.Cox DR. Regression models and life tables. J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 29.Pande RL, Perlstein TS, Beckman JA, Creager MA. Association of insulin resistance and inflammation with peripheral arterial disease: the National Health and Nutrition Examination Survey, 1999 to 2004. Circulation. 2008;118:33–41. doi: 10.1161/CIRCULATIONAHA.107.721878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco-Colio LM, Martin-Ventura JL, de Teresa E, Farsang C, Gaw A, Gensini G, Leiter LA, Langer A, Martineau P, Egido J. Elevated ICAM-1 and MCP-1 plasma levels in subjects at high cardiovascular risk are diminished by atorvastatin treatment. Atorvastatin on Inflammatory Markers study: a substudy of Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration. Am Heart J. 2007;153:881–888. doi: 10.1016/j.ahj.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Bacha F, Gungor N, Arslanian S. Comparison of different definitions of pediatric metabolic syndrome: relation to abdominal adiposity, insulin resistance, adiponectin, and inflammatory biomarkers. J Pediatr. 2008;152:177–184. doi: 10.1016/j.jpeds.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 32.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral artery disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 33.Orchard TJ, Strandness E. Assessment of peripheral vascular disease in diabetes. Report and recommendation of an international workshop sponsored by the American Diabetes Association and the American Heart Association September 18–20. 1992 New Orleans, Louisiana. Circulation. 1993;88:819–828. doi: 10.1161/01.cir.88.2.819. [DOI] [PubMed] [Google Scholar]
- 34.Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality. A meta analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]