Abstract
The sphingolipid metabolite, sphingosine-1-phosphate (S1P), has emerged as a critical player in a number of fundamental biological processes and is important in cancer, angiogenesis, wound healing, cardiovascular function, atherosclerosis, immunity and asthma, among others. Activation of sphingosine kinases, enzymes that catalyze the phosphorylation of sphingosine to S1P, by a variety of agonists, including growth factors, cytokines, hormones, and antigen, increases intracellular S1P. Many of the biological effects of S1P are mediated by its binding to five specific G protein-coupled receptors located on the cell surface in an autocrine and/or paracrine manner. Therefore, understanding the mechanism by which intracellularly generated S1P is released out of cells is both interesting and important. In this review, we will discuss how S1P is formed and released. We will focus particularly on the current knowledge of how the S1P gradient between tissues and blood is maintained, and the role of ABC transporters in S1P release.
Keywords: ABC transporter, Sphingosine-1-phosphate, Sphingosine kinase, Release
1. Introduction: what is S1P?
Sphingosine-1-phosphate (S1P) has drawn considerable attention during the last fifteen years, not merely as an end metabolite of sphingolipids, but as a bioactive signaling molecule [1–3]. Sphingo-lipids are structural components of all eukaryotic plasma membranes and are commonly believed to protect the cell surface by forming the mechanically stable and chemically resistant outer leaflet of the lipid bilayer. Discoveries that S1P regulates cell growth [4,5] and suppresses apoptosis [6] inspired numerous researchers to investigate S1P as a bioactive lipid mediator. These research efforts have led to numerous publications linking S1P to multiple essential cellular processes, including cell growth and survival, regulation of cell motility and invasion, angiogenesis and vascular maturation, lymphocyte trafficking and immune regulation. Further, increased S1P production has been implicated in various pathophysiological processes such as cancer, allergy, atherosclerosis, and autoimmune diseases such as multiple sclerosis (reviewed in [1,7,8]).
Growth factors, hormones, and angiogenic factors important for cancer, such as estradiol, epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF) [9], all stimulate sphingosine kinase (SphK), the enzyme that phosphorylates sphingosine to produce S1P. Two isotypes of SphK have been characterized, SphK1 and SphK2. While multiple reports support the importance of SphK1 in cancer tumorigenesis and progression, at the present time, much less is known of the functions of SphK2 [1,10]. S1P levels are tightly regulated by the balance between synthesis by SphKs, reversible conversion to sphingosine by specific S1P phosphatases (SPP1 and SPP2) and other lipid phosphate phosphohydrolases, and degradation by S1P lyase [1,2]. In contrast to S1P, which is associated with growth and survival, its precursors, sphingosine and ceramide, are associated with cell growth arrest and apoptosis [11,12]. Hence, it has been proposed that the balance between these interconvertible sphingolipid metabolites, ceramide and sphingosine versus S1P, functions as a “rheostat” that regulates cellular growth and survival in response to cellular and environmental clues [2,6,11,12]. SphK1 is a critical regulator of this rheostat, as it produces the pro-growth and anti-apoptotic S1P, and also reduces levels of pro-apoptotic ceramide and sphingosine [10]. Treatment of MCF-7 breast cancer cells with doxorubicin, a commonly used and highly potent chemotherapeutic agent against breast cancer, increases ceramide and sphingosine levels and induces apoptosis [13]. In contrast, overexpression of SphK1 in MCF-7 cells increases S1P levels, enhances cell proliferation, expedites the G1/S transition of the cell cycle, and increases DNA synthesis [14,15]. Further, SphK1 over-expression promotes breast cancer cell growth in soft agar and tumorigenesis in mice [15–17]. In addition, oophorectomized nude mice that had been implanted with estrogen pellets and subsequently received subcutaneous injection of SphK1-overexpressing MCF-7 cells formed larger and more tumors than vector transfectants [15]. These mice also developed tumors with higher microvessel density in their periphery. Moreover, it has been reported that sphingolipid metabolism is often found to be dysregulated in cancer [10], which is in agreement with the concept that the sphingolipid rheostat plays an important role in regulating cell fate. SphK1 is significantly over-expressed in multiple types of cancers (brain, breast, colon, lung, ovary, stomach, uterus, kidney, rectum, and small intestine) compared with their healthy tissue counterparts [18–20]. SphK1 has also been shown to be associated with tumor angiogenesis and resistance to radiation and chemotherapy. Recently, microarray analyses of 1269 breast tumor samples revealed a worse outcome for patients with high SphK1 expression, further supporting the notion that overproduction of S1P results in a worse prognosis for cancer patients [21].
2. S1P receptors: why is extracellular S1P important?
One explanation why S1P, a relatively simple molecule, can evoke such diverse biological functions is its well-established role as a ligand for five ubiquitously expressed and specific cell surface receptors, designated S1P1–5 [2,22]. S1P can also act intracellularly as a second messenger [23,24], through still unidentified targets.
S1P receptors display tissue-specific expression patterns and are coupled to various G proteins, enabling them to regulate a range of downstream signaling pathways, leading to regulation of numerous physiological processes. Interestingly, activation of SphK1 involves its translocation to the plasma membrane where its substrate sphingosine is located [25,26]. This results in spatially restricted formation of S1P that, in turn, activates specific S1P receptors. Thus, intracellularly generated S1P can signal “inside-out” through its cell surface receptors in an autocrine or paracrine manner (Fig. 1), which is critical for directed cell movement of fibroblasts and smooth muscle cells toward platelet-derived growth factor (PDGF) [27,28], breast cancer cells toward EGF [29,30], and melanoma cells toward heregulin [31]. Because S1P is probably generated on the inner leaflet of the plasma membrane, it is logical to assume that to reach its cell surface receptors, S1P must be either flipped or secreted. Activation of S1P1 and downstream signaling is also crucial for the migration of mast cells toward antigen [32], which, together with the observations that S1P provokes airway smooth muscle contraction and its levels are significantly elevated in bronchoalveolar lavage fluid of asthmatics after allergen challenge, suggests that extracellular S1P perpetuates inflammation and allergic responses [32,33].
Fig. 1.
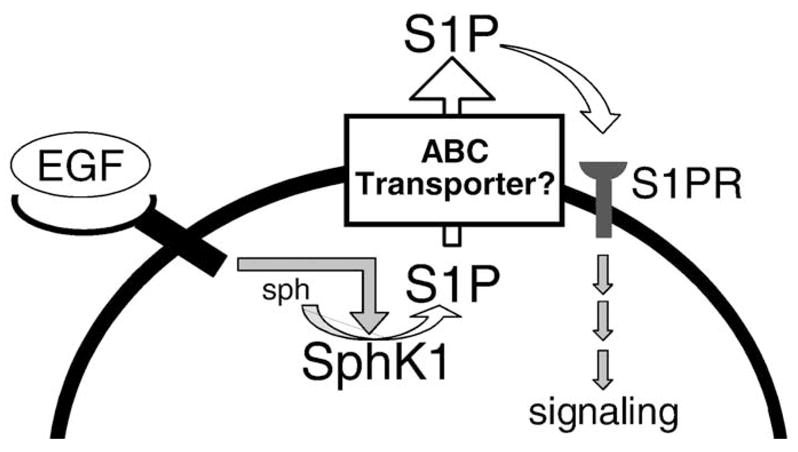
Formation, secretion, and actions of S1P. Many growth factors, including EGF, bind to a tyrosine kinase receptor, to stimulate and translocate SphK1 to the plasma membrane where its substrate sphingosine (sph) resides. This leads to spatially restricted formation of S1P that can be exported out of cells by ABC transporter family members. S1P can then bind to its receptors on the same or neighboring cells (S1PR) to stimulate G-protein regulated signaling pathways.
Because S1P receptor genes are expressed in vascular tissues, it is reasonable to assume that S1P may play an important role in angiogenesis. Indeed, homozygous S1P1−/− mice exhibited embryonic hemorrhage followed by intrauterine death at 12.5–14.5 days of gestation due to incomplete coverage of blood vessels by smooth muscle cells [34]. S1P1 expression is strongly induced in tumor vessels and injection of S1P1 small interfering RNA into xenograft tumors in mice suppresses vascular stabilization, angiogenesis and tumor growth in vivo [35]. Therefore, S1P released outside the cell appears to play a significant role in cancer progression, inflammation and allergic responses.
Strong evidence that S1P receptors play a major role in auto-immune disease, such as multiple sclerosis, emerged from studies with the immunosuppressant drug FTY720. Multiple sclerosis is the most common non-traumatic cause of neurological disability in young adults, in which autoreactive T-cells migrate across the blood–brain barrier and attack myelin sheaths, leading to demyelination and axonal damage. FTY720 is a pro-drug that is phosphorylated in vivo by SphK2 to a S1P mimetic that induces internalization and degradation of the S1P1 receptor and induces its prolonged down-regulation [36]. Thus, FTY720 deprives thymocytes and lymphocytes of a S1P signal necessary for their egress from secondary lymphoid tissues [36–38]. The results of a Phase II, double-blind, randomized, placebo-controlled clinical trial evaluating the efficacy and safety of FTY720 for treating relapsing multiple sclerosis showed that the annualized relapse rate of the FTY720 group was significantly lower than the placebo group [39]. These promising results are awaiting confirmation in the ongoing Phase III clinical trials and might spur the development of a new class of S1P mimetics for treatment of multiple sclerosis.
3. Blood, a major reservoir of extracellular S1P: what is its source?
S1P receptor expression is not the sole factor that determines S1P activity. Availability of S1P is equally as important. Interestingly, a significant concentration gradient of S1P exists between plasma and interstitial fluids: tissue levels of S1P are generally low [40,41], whereas plasma S1P levels are high and many-fold above the KD for S1P receptors [42,43]. The physiological significance of this S1P gradient is now becoming clear but how it is maintained is an active area of investigation.
The S1P gradient is important for homing of immune cells to lymphoid organs and regulating their egress into blood and lymph. S1P receptors on immune cells (for example, S1P1 on T-cells) sense the S1P gradient to enable their egress from lymphoid tissues. When levels of S1P are increased in lymphoid tissues by inflammation, inhibition of tissue S1P lyase, or after treatment with S1P mimetics, such as FTY720-phosphate, aberrant immune cell trafficking occurs. As this important area has been extensively discussed in several recent reviews [8,44,45], it will not be covered here.
Serum S1P levels are always higher than plasma levels [46], which is explained by the abundance of S1P in platelets and its extracellular release upon stimulation with thrombin, a product of the coagulation cascade, as well as shear stress during blood clotting [46–48]. It has been suggested that in platelets, sphingosine, the precursor for S1P, is supplied either by generation in plasma and subsequent uptake, or formation at the outer leaflet of the plasma membrane initiated by cell surface sphingomyelin degradation [49].
Platelets were assumed to be the major source of S1P in plasma for many years as they express a high activity of SphK1 and lack the S1P lyase that irreversibly degrades S1P [46,50]. However, recent reports have challenged this assumption, suggesting that red blood cells (RBC) and vascular endothelial cells (EC) may be the major cellular sources of S1P in plasma [51]. Pappu et al. demonstrated that transcription factor NF-E2-deficient mice, which virtually lack circulating platelets, maintained normal plasma S1P levels [52]. They also showed that the reduction of plasma S1P levels due to elimination of hematopoietic cells by lethal whole-body irradiation of conditional SphK1/2-double knockout mice was restored by infusion of wild type RBCs alone. RBCs have lower SphK activity than platelets, but lack both S1P degrading enzymes (S1P lyase and S1P phosphatases), a characteristic unique to RBCs [53]. Hanel et al. have suggested that RBCs efficiently incorporate S1P from other tissue sources, act as a plasma store, and make S1P available for future release [54]. Although platelets contain a much greater amount of S1P than erythrocytes, since erythrocytes are much more abundant in blood than platelets, the total S1P provided by erythrocytes is much greater [53].
However, Hla et al. have recently concluded that RBCs are not solely responsible for maintenance of the plasma S1P level. They demonstrated that neither elimination of hematopoietic cells by lethal whole-body irradiation of wild type and SphK deficient mice, nor chemically-induced anemia that removed more than two thirds of RBCs, had an effect on plasma S1P levels [55]. They also showed that the turnover time of plasma S1P in vivo is very rapid with a half-life of 15 min, which suggests that very active synthetic and degradative pathways of S1P metabolism exist in vivo, and that vascular endothelium might be a major contributor to plasma S1P, particularly under shear stress [55]. It is known that S1P in plasma is tightly associated with albumin and lipoproteins, particularly high-density lipoprotein (HDL) [56,57]. The interaction of S1P with lipoproteins reduces its bioactivity and active concentration, suggesting that this may prevent full activation of S1P receptors in the vascular wall. On the other hand, it has also been shown that the half-life of HDL-associated S1P is approximately fourfold that of albumin-associated S1P when examined with human umbilical vein endothelial cells [58]. This suggests that binding to lipoproteins may protect S1P from degradation by ectoenzymes, such as lipid phosphate phosphohydrolases. This may allow a stable reservoir of S1P in blood and modulate its delivery to S1P receptors. Furthermore, HDL-associated S1P demonstrated a much weaker response in short-term actions, such as intracellular calcium mobilization, but had a sustained response in long-term functions, including cell survival and proliferation [57,58]. Therefore, it has been speculated that the anti-atherogenic effects of HDL may, in part, be due to S1P bound to this lipoprotein [59].
4. Mechanism of S1P release for “inside-out” signaling: the role of ABC transporters
Intracellularly generated S1P is unable to move through hydrophobic mammalian cell plasma membranes since it possesses a polar head group. The fact that S1P levels in blood and body fluids are maintained high compared to tissues, suggests that there must be mechanisms that facilitate release of S1P out of cells. Blood cells, including platelets, RBCs, neutrophils, and mononuclear cells have all been shown to release S1P. S1P is released from platelets following stimulation with thrombin [47] and shear stress [48], which appears to closely correlate with activation of protein kinase C [47]. RBCs, on the other hand, release S1P even without stimulation [60].
Although the mechanism of release of S1P from cells is not completely understood, recent studies have drawn attention to the involvement of the ATP-binding cassette (ABC) family of transporters [61–64]. Studies originally related to multi-drug resistance in cancer cells and in yeast identified several ABC transporters that, in addition to amphiphilic drugs, catalyze the transport of lipids from the inner to the outer leaflet of the plasma membrane [65]. They contain two transmembrane domains with six membrane spanning α-helices that form a channel for substrate transport through membranes, and two cytosolic ATP-binding cassettes. Based on phylogenetic analysis and amino acid sequence alignment, the 49 human ABC genes can be grouped into seven major subfamilies: ABCA-through-ABCG. These subfamilies each have several subgroups and will be covered extensively elsewhere in this volume, and only a few examples related to sphingolipid export are mentioned. For example, ABCB1 (previously called multi-drug resistance-1 (MDR1) or P-glycoprotein (Pgp)) translocates a broad variety of phospholipids and platelet-activating factor from the cytosol to the extracellular environment [66–68], while MDR3 (ABCB4) [66] translocates phosphatidylcholine specifically. ABCC1 (also known as multi-drug resistant protein-1 (MRP1)) can also transport short-chain analogues of glucosylceramide and sphingomyelin [69].
Previous studies from our lab have shown that S1P secreted by mast cells is important for mast cell functions and their migration toward antigen [32]. In addition, S1P provokes human airway smooth muscle contraction and may promote inflammation and airway remodeling in asthma [70]. Because S1P levels are increased in bronchoalveolar lavage fluid of asthmatics after allergen challenge, we have suggested that release of S1P from mast cells perpetuates inflammation and allergic responses [33]. Thus, mast cells are an excellent model to study the mechanism of S1P release. We found that S1P is exported from mast cells independently of their degranulation and demonstrated that it is mediated by ABC transporters [61]. Antigen-stimulated S1P release was inhibited by MK571, an inhibitor of ABCC1, but not by inhibitors of ABCB1. In agreement, down-regulation of ABCC1 reduced S1P export from both rodent and human mast cells [61]. Discovery of an active transport system for mast cell secretion of S1P further supports the notion that S1P release may regulate migration of mast cells toward antigen and their arrival at sites of inflammation.
It has been reported that S1P is stored in the inner leaflet of the platelet plasma membrane and released via transporters, and not by exocytosis [62]. These authors suggested that thrombin-stimulated S1P release from platelets is mediated by an ABC transporter, as it was inhibited by glyburide, an ABCA1 inhibitor. However, neither the ABCB1 inhibitors verapamil and cyclosporin A, nor the ABCC1 inhibitor MK571 diminished stimuli-dependent S1P release, indicating that ABCA1, rather than ABCB1 or ABCC1, is involved in this process [62]. In addition, the existence of another ATP- and stimuli-independent but calcium-dependent transporter of S1P has been suggested in platelets [62].
Utilizing immobilized metal affinity chromatography coupled with quantitative high-performance liquid chromatography analysis, Lee et al. reported that human and mouse endothelial cells synthesize and release endogenous S1P more efficiently than fibroblasts and colon cancer cells, suggesting cellular specificity for release of S1P [63]. Further, using various inhibitors of ABC transporters, such as glyburide, verapamil and MK571, led them to suggest that ABCA1 and ABCC1 are involved in the release of S1P from endothelial cells. They also examined plasma from Abca1, Abca7, and Abcc1/Mrp1 single null mice and found no significant differences in S1P levels compared to their wild type counterparts. These data suggest that either these transporters are not required for the maintenance of plasma S1P, or that other ABC transporters may compensate for the loss of these specific transporters.
It is well known that S1P exerts a wide range of actions in the nervous system [71]. Since S1P receptors are particularly expressed in cerebellum and S1P metabolism occurs efficiently in cerebellar granule cells and astrocytes in primary culture, Anelli et al. investigated S1P release from these cells [72]. Sato et al. further investigated the mechanism of the release of S1P from astrocytes, which appears to be highly dependent on the ABCA1 transporter, based on inhibition by glyburide or by down-regulation of ABCA1 with specific siRNA [64]. Moreover, S1P release from astrocytes through ABCA1 was coupled with HDL formation [64]. Interestingly, SphK1 and its product S1P stimulate ABCB1 expression and transport activity in endothelial cells from brain capillaries, which suggests that S1P itself may regulate its own secretion [73].
Collectively, these reports suggest that members of the large family of ABC transporters may be important for the export of S1P out of cells. The observation that S1P can be secreted by ABC transporters has important implications that are not limited to blood cells, mast cells, and central nervous system cells, but perhaps for other physiological and pathological processes regulated by S1P, such as tumorigenesis. ABC transporters were originally characterized as multi-drug resistance gene, and have been shown to be overexpressed in a range of solid and hematological cancers and, in some cases, their expression correlated with negative responses to treatment and poor outcome [74]. This might explain why a monoclonal antibody that binds and neutralizes S1P with extremely high affinity and specificity significantly slows tumor progression and associated angiogenesis in several animal models of human cancer [75]. The ability of S1P to act in an autocrine or paracrine manner, and its actions on angiogenesis and vascular maturation, are critical for tumor progression. This suggests that increased release of S1P by cancer cells, perhaps by ABC transporters, caused by up-regulation of SphK1 or the transporter could contribute to tumorigenesis.
Two recent studies have demonstrated that the Zebrafish two of hearts is a S1P transporter which is required for S1P functions in migration of Zebrafish myocardial precursors [76,77]. In an earlier study, it was reported that mutations in the Zebrafish gene encoding the S1P2 homologue known as miles apart, disrupt the formation of the primitive heart tube [78]. It has now been found that mutations of two of hearts cause exactly the same cardia bifida (two hearts) phenotype. Two of hearts is a homologue of a multipass transmembrane protein known as spinster homologue 2 (Spns2), a member of the Spinster-like family of putative transmembrane transporters. It was shown that the export of S1P from cells requires Spns2 and that introduction of the spns2 gene [76] or injection of S1P [77] prevented the cardiac defect in the mutant. However, the biological functions of Spns2 are still not completely understood, as the phenotype of the Drosophila spinster mutant has altered endosomal/lysosomal function and a deficit in presynaptic release [79].
Export of S1P out of cells is not only the beginning of a fascinating story, it will surely be the focus of much future research. It will be important to determine the functions of Spns2, whether it is involved in secretion of S1P in response to environmental clues, and the relationship between Spns2 and other ABC transporters, including ABCC1 and ABCA1 that have been shown to export S1P out of cells.
Acknowledgments
This work was supported by K12HD055881 (K.T.), R01AI50094 (S.S.), 1U19AI077435-018690 (S.S.) R01CA61774 (S.S.) and R37GM043880 (S.S.).
Abbreviations
- S1P
sphingosine-1-phosphate
- SphK
sphingosine kinase
- SPL
S1P lyase
- SPP
S1P phosphohydrolase
- GPCR
G protein-coupled receptor
- ABC transporters
ATP-binding cassette transporters
- MDR
multi-drug resistance
- Pgp
P-glycoprotein
References
- 1.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 3.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olivera A, Spiegel S. Sphingosine-1-phosphate as a second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 6.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 7.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Cuvillier O, Nava VE, Murthy SK, Edsall LC, Levade T, Milstien S, Spiegel S. Sphingosine generation, cytochrome c release, and activation of caspase-7 in doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells. Cell Death Differ. 2001;8:162–171. doi: 10.1038/sj.cdd.4400793. [DOI] [PubMed] [Google Scholar]
- 14.Olivera A, Kohama T, Edsall LC, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res. 2002;281:115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- 16.Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D’Andrea RJ, Vadas MA. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- 17.Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 19.Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- 20.Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 21.Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grosch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- 22.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids-receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 23.Olivera A, Spiegel S. Sphingosine kinase: a mediator of vital cellular functions. Prostaglandins. 2001;64:123–134. doi: 10.1016/s0090-6980(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 24.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, Ai Y, Ristimaki AP, Fyrst H, Sano H, Rosenberg D, Saba JD, Proia RL, Hla T. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate. J Biol Chem. 2002;277:35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 26.Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 28.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 30.Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, Spiegel S. Role of sphingosine kinase 2 in cell migration towards epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 31.Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol Cell Biol. 2008;28:5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by Fc {epsilon}RI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA., Jr Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114:1082–1089. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 37.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 38.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 39.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 40.Edsall LC, Spiegel S. Enzymatic measurement of sphingosine 1-phosphate. Anal Biochem. 1999;272:80–86. doi: 10.1006/abio.1999.4157. [DOI] [PubMed] [Google Scholar]
- 41.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 42.Caligan TB, Peters K, Ou J, Wang E, Saba J, Merrill AH., Jr A high-performance liquid chromatographic method to measure sphingosine 1-phosphate and related compounds from sphingosine kinase assays and other biological samples. Anal Biochem. 2000;281:36–44. doi: 10.1006/abio.2000.4555. [DOI] [PubMed] [Google Scholar]
- 43.Berdyshev EV, Gorshkova IA, NGJG, Natarajan V, Hubbard WC. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal Biochem. 2005;339:129–136. doi: 10.1016/j.ab.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 45.Davis MD, Kehrl JH. The influence of sphingosine-1-phosphate receptor signaling on lymphocyte trafficking: how a bioactive lipid mediator grew up from an “immature” vascular maturation factor to a “mature” mediator of lymphocyte behavior and function. Immunol Res. doi: 10.1007/s12026-008-8066-5. (in press) [DOI] [PubMed] [Google Scholar]
- 46.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 47.Yatomi Y, Yamamura S, Ruan F, Igarashi Y. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid. J Biol Chem. 1997;272:5291–5297. doi: 10.1074/jbc.272.8.5291. [DOI] [PubMed] [Google Scholar]
- 48.Aoki S, Osada M, Kaneko M, Ozaki Y, Yatomi Y. Fluid shear stress enhances the sphingosine 1-phosphate responses in cell–cell interactions between platelets and endothelial cells. Biochem Biophys Res Commun. 2007;358:1054–1057. doi: 10.1016/j.bbrc.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 49.Tani M, Sano T, Ito M, Igarashi Y. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J Lipid Res. 2005;46:2458–2467. doi: 10.1194/jlr.M500268-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Jessup W. Lipid metabolism: sources and stability of plasma sphingosine-1-phosphate. Curr Opin Lipidol. 2008;19:543–544. doi: 10.1097/MOL.0b013e32830f4a90. [DOI] [PubMed] [Google Scholar]
- 52.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 53.Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 54.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 55.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ui M, Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352:809–815. [PMC free article] [PubMed] [Google Scholar]
- 57.Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 58.Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, Ui M, Okajima F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–31785. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 59.Okajima F, Sato K, Kimura T. Anti-atherogenic actions of high-density lipoprotein through sphingosine 1-phosphate receptors and scavenger receptor class B type I. Endocr J. doi: 10.1507/endocrj.k08e-228. (in press) [DOI] [PubMed] [Google Scholar]
- 60.Yang L, Yatomi Y, Miura Y, Satoh K, Ozaki Y. Metabolism and functional effects of sphingolipids in blood cells. Br J Haematol. 1999;107:282–293. doi: 10.1046/j.1365-2141.1999.01697.x. [DOI] [PubMed] [Google Scholar]
- 61.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, Yamaguchi A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, Yoshimoto Y, Kuwabara A, Okajima F. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem. doi: 10.1111/j.1471-4159.2007.04958.x. (in press) [DOI] [PubMed] [Google Scholar]
- 65.van Meer G, Lisman Q. Sphingolipid transport: rafts and translocators. J Biol Chem. 2002;277:25855–25858. doi: 10.1074/jbc.R200010200. [DOI] [PubMed] [Google Scholar]
- 66.van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 67.Raggers RJ, Vogels I, van Meer G. Multidrug-resistance P-glycoprotein (MDR1) secretes platelet-activating factor. Biochem J. 2001;357:859–865. doi: 10.1042/0264-6021:3570859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pohl A, Lage H, Muller P, Pomorski T, Herrmann A. Transport of phosphatidylserine via MDR1 (multidrug resistance 1)P-glycoprotein in a human gastric carcinoma cell line. Biochem J. 2002;365:259–268. doi: 10.1042/BJ20011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raggers RJ, van Helvoort A, Evers R, van Meer G. The human multidrug resistance protein MRP1 translocates sphingolipid analogs across the plasma membrane. J Cell Sci. 1999;112:415–422. doi: 10.1242/jcs.112.3.415. [DOI] [PubMed] [Google Scholar]
- 70.Ryan JJ, Spiegel S. The role of sphingosine-1-phosphate and its receptors in asthma. Drug News Perspect. 2008;21:89–96. doi: 10.1358/dnp.2008.21.2.1188195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bryan L, Kordula T, Spiegel S, Milstien S. Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta. 2008;1781:459–466. doi: 10.1016/j.bbalip.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anelli V, Bassi R, Tettamanti G, Viani P, Riboni L. Extracellular release of newly synthesized sphingosine-1-phosphate by cerebellar granule cells and astrocytes. J Neurochem. 2005;92:1204–1215. doi: 10.1111/j.1471-4159.2004.02955.x. [DOI] [PubMed] [Google Scholar]
- 73.Pilorget A, Demeule M, Barakat S, Marvaldi J, Luis J, Beliveau R. Modulation of P-glycoprotein function by sphingosine kinase-1 in brain endothelial cells. J Neurochem. 2007;100:1203–1210. doi: 10.1111/j.1471-4159.2006.04295.x. [DOI] [PubMed] [Google Scholar]
- 74.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 75.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 76.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter Spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 77.Osborne N, Brand-Arzamendi K, Ober EA, Jin SW, Verkade H, Holtzman NG, Yelon D, Stainier DY. The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr Biol. 2008;18:1882–1888. doi: 10.1016/j.cub.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- 79.Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster—a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]


