Abstract
Lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) are lysophospholipid mediators of diverse cellular processes important for cancer progression. S1P is produced by two sphingosine kinases, SphK1 and SphK2. Expression of SphK1 is elevated in many cancers. Here, we report that LPA markedly enhanced SphK1 mRNA and protein in gastric cancer MKN1 cells but had no effect on SphK2. LPA also up-regulated SphK1 expression in other human cancer cells that endogenously express the LPA1 receptor, such as DLD1 colon cancer cells and MDA-MB-231 breast cancer cells, but not in HT29 colon cancer cells or MDA-MB-453 breast cancer cells, which do not express the LPA1 receptor. An LPA1 receptor antagonist or down-regulation of its expression prevented SphK1 and S1P3 receptor up-regulation by LPA. LPA transactivated the epidermal growth factor receptor (EGFR) in these cells, and the EGFR inhibitor AG1478 attenuated the increased SphK1 and S1P3 expression induced by LPA. Moreover, down-regulation of SphK1 attenuated LPA-stimulated migration and invasion of MNK1 cells yet had no effect on expression of neovascularizing factors, such as interleukin (IL)-8, IL-6, urokinase-type plasminogen activator (uPA), or uPA receptor induced by LPA. Finally, down-regulation of S1P3, but not S1P1, also reduced LPA-stimulated migration and invasion of MKN1 cells. Collectively, our results suggest that SphK1 is a convergence point of multiple cell surface receptors for three different ligands, LPA, EGF, and S1P, which have all been implicated in regulation of motility and invasiveness of cancer cells.
Introduction
Two simple lysophospholipids, lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P), have recently been implicated in the etiology of cancer due to their involvement in tumor growth, angiogenesis, and metastatic potential (1, 2). The biological effects of these serum-borne lipids are mainly mediated by a family of G protein—coupled receptors (GPCR), five specific for LPA and five specific for S1P, termed LPA1-5 and S1P1-5, respectively (3). Several lines of evidence support the importance of these lysolipids in gastric cancer, one of most common causes of cancer-related deaths worldwide (4). Gastric cancer with ulcers is often associated with local bleeding, subjecting the cancer cells to high concentrations of platelet-derived mediators, including LPA and S1P. Many gastric cancer cell lines have been shown to express at least one LPA receptor. Differentiated carcinomas preferentially express LPA2, whereas LPA1 is most abundant in undifferentiated carcinomas, which are thought to be more aggressive (5). Moreover, LPA markedly increased cell migration of LPA1-expressing gastric cancer cells but not those expressing LPA2 (5). Although binding of LPA to LPA2 in gastric cancer cells did not induce their migration, it enhanced their migratory responses to hepatocyte growth factor (HGF) by transactivating the HGF receptor c-Met (5). These results suggest that the expression pattern of LPA receptors may determine the metastatic behavior of gastric cancer. Although LPA2 expression has been linked to the production of neovascularizing factors, including interleukin (IL)-8, IL-6, and vascular endothelial growth factor (6), and expression of LPA3 has been associated with cell survival (7), it has been suggested that each of the LPA receptors has the ability to mediate the major functions of LPA, with the relative efficiency determined by the spectrum of receptors activated (1).
In contrast to LPA, less is known of the functions of S1P in gastric cancer, although both LPA and S1P can transactivate the epidermal growth factor receptor (EGFR), c-Met, and ErbB-2 in gastric cancer cells (8, 9), which all have been suggested to be prognostic markers of gastric cancer that correlate with poor clinical outcome (10). Moreover, one of the kinases that produce S1P, sphingosine kinase type 1 (SphK1), which has been proposed to be oncogenic (11), is up-regulated in many types of human cancers, including colon and gastric cancers (12). Similarly, in rat colon tumors induced by azoxymethane, expression of SphK1 is up-regulated (13).
A recent study examined the role of SphK1 in intestinal tumorigenesis in the Min mouse in which intestinal adenomas develop spontaneously (14). Deletion of the Sphk1 gene in these mice resulted in reduction of adenoma size. Concomitantly, epithelial cell proliferation in the polyps was attenuated, suggesting that SphK1 regulates adenoma progression (14). Interestingly, neutralizing S1P with a specific monoclonal antibody was remarkably effective in slowing progression of multidrug-resistant cancers, such as lung, colon, breast, melanoma, and ovarian cancers, in murine xenograft and allograft models (15). A critical question raised by these observations is how neutralization of this simple lysophospholipid can have such dramatic effects on tumor progression. An intriguing possibility is that many growth and angiogenic factors involved in tumorigenesis may use S1P as their downstream signal transducer through SphK1 activation (16). Because both LPA and EGF have been implicated in progression of gastric cancer, it was of interest to examine the involvement of SphK1. We found that LPA markedly up-regulated expression of SphK1 and the S1P receptor (S1PR) S1P3 in MKN1 gastric cancer cells via the LPA1 receptor and EGFR transactivation. Moreover, SphK1 and S1P3 were critical for LPA- and EGF-induced chemotaxis and invasion in these cells. These findings provide evidence for cross-talk between the two lysolipids, LPA and S1P, and reveal a key role for SphK1 in integrating events downstream of LPA receptors and EGFRs.
Materials and Methods
Reagents
1-Oleoyl-LPA, Ki16425, an antagonist of LPA1 and LPA3 receptors, and mouse monoclonal anti-human phosphotyrosine antibody (PY20) were purchased from Sigma-Aldrich. Recombinant human EGF was from PeproTech. Antibodies against phospho-EGFR (Tyr1068) and β-tubulin were from Cell Signaling. Rabbit polyclonal anti-human EGFR antibody was from Santa Cruz Biotechnology. Mouse monoclonal anti-human phosphospecific SphK1 antibody was purchased from ECM Biosciences. Polyclonal rabbit anti-SphK1 antibody was described previously (17).
Cell culture and transfection
The MKN1 human gastric cancer cell line was obtained from the Riken Cell Bank and maintained in DMEM supplemented with 10% FCS (Biofluids) as described previously (18). For down-regulation with small interfering RNAs (siRNA), cells were plated in six-well plates at a density of 1 × 105 per well. ON-TARGETplus SMARTpool siRNA targeting SphK1 (5′-CGACGAGGACUUUGUGCUA-3′, 5′-GAUGGGGAAUUGAUGGUUA-3′, 5′-GAAAUCUCCUUCACGCUGA-3′, and 5′-GGAAAGGUGUGUUUGCAGU-3′), LPA1, LPA2, LPA3, S1P1, and S1P3 or control siRNA (Dharmacon) was transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. In some experiments, to confirm lack of off-target effects, cells were transfected with siRNAs targeted to different human SphK1 (5′-AAGGGCAAGGCCTTGCAGCTC-3′, siSphK1 #2;Qiagen) and S1P3 (5′-GCACUUGACAAUGAUCAAA-3′, siS1P3 #2; Ambion) sequences and appropriate control siRNAs.
Sphingosine kinase activity
SphK1 and SphK2 activities were measured as described previously (17). In brief, SphK1 activity was determined in the presence of 50 μmol/L sphingosine in 0.25% Triton X-100, which inhibits SphK2, whereas SphK2 activity was determined with sphingosine added as a complex with 4 mg/mL bovine serum albumin (BSA) in the presence of 1 mol/L KCl, conditions in which SphK2 activity is optimal and SphK1 is strongly inhibited (17). [32P]S1P was extracted and then separated by TLC on silica gel G60 with chloroform/acetone/methanol/acetic acid/H2O (10:4:3:2:1, v/v) as solvent. Radioactive bands corresponding to S1P were quantified with a FX Molecular Imager (Bio-Rad). SphK-specific activity is expressed as pmol S1P formed per min per mg protein.
Quantitative real-time PCR
Total RNA was isolated with Trizol reagent (Invitrogen) and reverse transcribed with the High-Capacity cDNA Archive kit (Applied Biosystems). Real-time PCRs were performed with an ABI Prism 7900HT (Applied Biosystems) using Taqman premixed, validated primer-probe sets, and Taqman Universal PCR Master Mix, with the following profile: 50°C for 2 min, 95°C for 10 min, 40 cycles at 95°C for 15 s and 60°C for 1 min. The ABI Prism software constructed a calibration curve by plotting the threshold cycle (CT) versus the logarithm of the calibrator concentration. Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Western blot analysis
Equal amounts of proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad). Blots were blocked with 5% nonfat dry milk for 1 h at room temperature and then incubated with primary antibodies overnight, stripped, and reprobed with anti-tubulin or anti-EGFR antibodies as loading controls. Appropriate horseradish peroxidase—conjugated secondary antibodies (Jackson ImmunoResearch) were added in TBS containing 5% nonfat milk. Immunoreactive signals were visualized by enhanced chemiluminescence using SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Invasion assay
Boyden chamber invasion assays were carried out essentially as described previously (19). Briefly, polycarbonate filters with 8-μm pores (Neuro Probes) were coated with Matrigel (BD Biosciences), a murine tumor extract rich in basement membrane components (particularly laminin, collagen type IV, and heparan sulfate proteoglycan) overnight at 4°C, rinsed once with PBS, and then placed into the lower chamber. LPA and S1P, prepared in DMEM containing 0.1% fatty acid—free BSA (Sigma-Aldrich), were placed in the lower chamber as chemoattractants. Cells (2.5 × 104 in 50 μL) were added to the upper chamber. The chambers were incubated in a humidified incubator at 37°C in 5% CO2/95% air for 6 h. Cells that traversed the Matrigel and spread on the lower surface of the filter were fixed in methanol and stained with HEMA3 (Fisher). Nonmigratory cells on the upper membrane surface were removed with a cotton swab. The number of cells that migrated to the lower side of the filter was enumerated by light microscopy with a 10× objective. Each data point is the average number of cells in four random fields.
Chemotaxis assays
Chemotaxis was measured as described above for the invasion assay with the exception that filter surfaces were coated with fibronectin instead of Matrigel. Fibronectin coatings promote uniform attachment to and migration across the filter, without formation of a barrier. In some experiments, random migration of cells in response to LPA was measured in wound-healing assays as previously described (20).
Statistical analysis
Experiments were repeated at least thrice with consistent results. For each experiment, data from triplicate samples were calculated and expressed as mean ± SD. Statistics were performed by twotailed ANOVA, and P < 0.01 was considered significant.
Results
LPA markedly increases SphK1 expression and activity
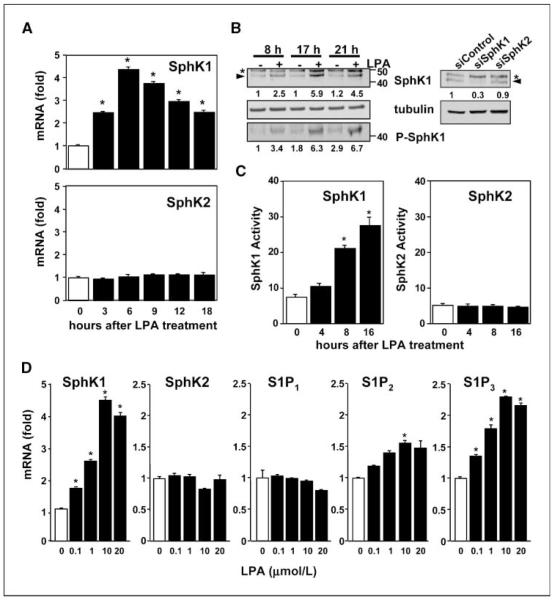
Both LPA and S1P have been shown to regulate motility and invasiveness of gastric cancer cells, including MKN1 cells (5, 9). Interestingly, we found that treatment of MKN1 cells with LPA markedly stimulated expression of one of the kinases that produce S1P, SphK1, which is up-regulated in many cancers (1). A significant increase was detected within 3 h, reached a maximum of 4.4-fold within 6 h, and declined thereafter (Fig. 1A). In contrast, LPA did not affect expression of SphK2, the other sphingosine kinase isoenzyme (Fig. 1A). LPA also increased SphK1 protein levels in a time-dependent manner as determined by immunoblotting with a specific SphK1 antibody. As shown in Fig. 1B, LPA induced increased expression of the 46-kDa SphK1 polypeptide, the major splice form of SphK1. The identity of the band was confirmed by its specific disappearance when cells were transfected with siRNA targeted to SphK1 but not with control siRNA or siRNA against SphK2 (Fig. 1B). Moreover, LPA provoked a concomitant increase in the level of SphK1 phosphorylated on Ser225 (Fig. 1B), thought to be important for its activation (21). In agreement, LPA treatment markedly stimulated SphK1 activity in a time-dependent manner (Fig. 1C), as determined by measuring sphingosine phosphorylation in the presence of Triton X-100, which inhibits SphK2 (17). In contrast, LPA did not alter SphK2 activity, determined when the substrate sphingosine was added as a BSA complex in the presence of 1 mol/L KCl, which strongly decreases SphK1 activity (17). The increase of SphK1 but not SphK2 activity by LPA (Fig. 1C) was commensurate with its effects on their mRNA levels (Fig. 1A).
Figure 1.
LPA up-regulates SphK1 and S1P3 expression. A, MKN1 cells were stimulated with LPA (10 μmol/L) for the indicated times. RNA was isolated and reverse transcribed, and SphK1, SphK2, and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold change after normalization to GAPDH. B, left, MKN1 cells were stimulated without or with LPA (10 μmol/L) for the indicated times, cell lysates were prepared, and equal amounts of protein were separated by SDS-PAGE and analyzed by immunoblotting with antibodies against SphK1 and phospho-SphK1 (P-SphK1). Blots were stripped and reprobed with anti-tubulin to insure equal loading and transfer. Asterisks, nonspecific immunostained bands; arrowheads, SphK1. Right, MKN1 cells transfected with siControl, siSphK1, or siSphK2 were lysed, and equal amounts of proteins were immunoblotted with anti-SphK1 or anti-tubulin antibodies. SphK1 and phospho-SphK1 levels were normalized to tubulin, and the ratios relative to untreated control (left) or siControl (right) are indicated. C, MKN1 cells were stimulated without or with LPA (10 μmol/L) for the indicated times and SphK1 and SphK2 activities were measured in whole-cell lysates with isozyme-specific assays as described in Materials and Methods. Columns, mean; bars, SD. Data are expressed as pmol S1P produced per mg protein per min. Similar results were obtained in two additional experiments. *, P ≤ 0.01. D, MKN1 cells were stimulated with the indicated concentrations of LPA for 6 h. RNA was isolated and reverse transcribed, and SphK1, SphK2, S1P1-3, and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold change after normalization to GAPDH.
LPA up-regulates SphK1 expression via the LPA1 receptor
LPA significantly increased SphK1 mRNA levels in MNK1 cells at a concentration as low as 0.1 μmol/L (1.61 ± 0.01–fold increase) and maximum induction was observed at 10 μmol/L LPA (Fig. 1D). In contrast, SphK2 mRNA levels were not altered by LPA even at a concentration of 20 μmol/L (Fig. 1D). Because it is well established that SphK1 produces S1P that in turn can act through its cell surface receptors, it was of interest to examine whether expression of S1PRs in MNK1 cells was also regulated by LPA. Quantitative PCR indicated that S1P3 is the predominant S1PR and the mRNA ratio for S1P3:S1P2:S1P1 is 8:3:1. S1P4 and S1P5 mRNAs were not detected, consistent with previous Northern blot analysis (18). S1P3 mRNA expression was also increased by LPA (2.3 ± 0.01–fold increase at 10 μmol/L LPA), whereas LPA had only marginal effects on S1P1 and S1P2 levels (Fig. 1D).
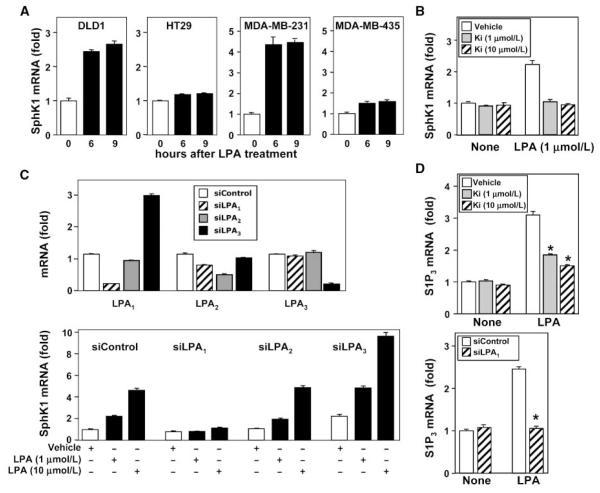
To examine whether the increased expression of SphK1 induced by LPA is cell type specific, we next examined several other cancer cell lines known to respond to LPA. LPA also markedly up-regulated SphK1 message in DLD1 colon cancer cells (2.6 ± 0.11–fold increase) and in MDA-MB-231 breast cancer cells (8.5 ± 1.6–fold increase;Fig. 2A), both of which highly express LPA1 and LPA2 (22, 23). In contrast, no significant effects on SphK1 expression were observed in HT29 colon cancer cells or MDA-MB-453 breast cancer cells, which express LPA2 but not LPA1 (22, 23). These results suggest that LPA-induced SphK1 expression is not unique to MKN1 cells but may depend on the presence of LPA1 receptor.
Figure 2.
LPA up-regulates SphK1 and S1P3 expression via the LPA1 receptor. A, DLD1 and HT29 colon cancer cells and MDA-MB-231 and MDA-MB-453 breast cancer cells were stimulated without or with 10 μmol/L LPA for 6 or 9 h. RNA was isolated and reverse transcribed, and SphK1 and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold change after normalization to GAPDH. B, MKN1 cells were pretreated with vehicle (DMSO) or Ki16425 (Ki; 1 or 10 μmol/L) for 30 min and then stimulated without or with the indicated concentrations of LPA for 6 h. RNA was isolated and reverse transcribed, and SphK1 and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold change after normalization to GAPDH. C and D, MKN1 cells were transfected with ON-TARGETplus SMARTpool siRNA targeted to LPA1,LPA2, and LPA3 receptors or control siRNA. C, RNA was isolated and reverse transcribed, and LPA1, LPA2, LPA3, and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold change after normalization to GAPDH. Bottom, duplicate cultures were stimulated without or with the indicated concentrations of LPA for 6 h. RNA was isolated and reverse transcribed, and SphK1 and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold change after normalization to GAPDH. D, MKN1 cells were pretreated with vehicle (DMSO) or Ki16425 (1 or 10 μmol/L) for 30 min. Duplicate MKN1 cultures were transfected with control siRNA (white columns) or siRNA targeted to LPA1 (stippled columns). Cells were then stimulated without or with 10 μmol/L LPA for 6 h. RNA was isolated and S1P3 and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold change after normalization to GAPDH.
MKN1 cells express the three major LPA receptors: LPA1,LPA2, and LPA3 (5, 23). As a first approach to substantiate the involvement of LPA1, Ki16425, which acts as an LPA1 antagonist and as a less potent LPA3 antagonist (24), was used. SphK1 expression induced by 1 μmol/L LPA was almost completely abrogated by addition of 1 μmol/L Ki16425 (Fig. 2B). Ki16425 also significantly reduced the ability of higher concentrations of LPA to up-regulate SphK1 (Fig. 2B).
Because Ki16425 may also interfere with LPA3-mediated actions, we used a molecular approach to confirm the specific involvement of LPA1. To this end, LPA receptors were down-regulated by specific siRNAs targeting LPA1,LPA2,or LPA3. Efficient inhibition of LPA1-3 expression in MNK1 cells was verified by real-time PCR analysis. In agreement with another study (25), transfection with LPA1-, LPA2-, or LPA3-specific siRNA led to 80%, 70%, and 90% reduction in the corresponding target gene without decreasing mRNA levels of the other LPA receptors (Fig. 2C). Of interest, down-regulation of LPA3 unexpectedly resulted in up-regulation of LPA1 expression (Fig. 2C). Importantly, down-regulation of the LPA1 receptor completely abolished LPA-induced SphK1 mRNA, whereas down-regulation of LPA2 had no significant effect (Fig. 2D). Down-regulation of LPA3 increased basal SphK1 mRNA, in agreement with its effect on LPA1. However, the fold increase in SphK1 mRNA levels by LPA was not altered by down-regulation of LPA3 (Fig. 2C). Similarly, S1P3 expression induced by LPA was also mediated by LPA1 because the response was reduced by the LPA1 antagonist Ki16425 in a dose-dependent manner (Fig. 2D). Similarly, down-regulation of LPA1 but not LPA2 or LPA3 blocked LPA-induced S1P3 expression (Fig. 2D; data not shown). Collectively, these results suggest that induction of SphK1 and S1P3 expression by LPA is mainly mediated via LPA1.
EGFR transactivation is also involved in LPA-induced SphK1 expression
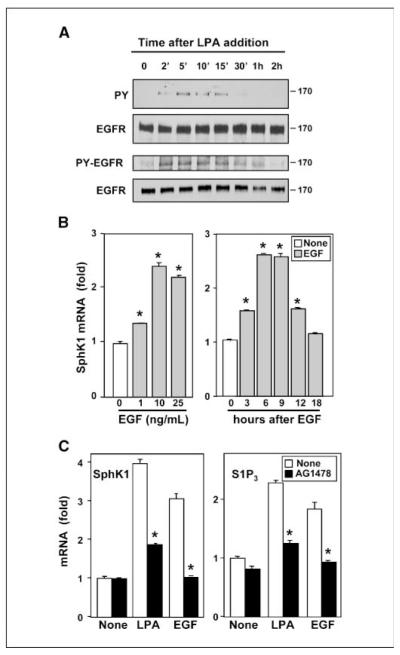
In many cancer cells, LPA transactivates EGFR to regulate diverse biological processes important for cancer progression, including proliferation, migration, and invasion (26, 27). In agreement with previous studies in gastric cancers (9, 28), LPA rapidly and transiently activated EGFR in MKN1 cells, as determined by enhanced tyrosine phosphorylation of EGFR and increased phosphorylation of Tyr1068 (Fig. 3A). Thus, it was of interest to examine whether EGF also regulated expression of SphK1 in MNK1 cells. Indeed, a significant increase in SphK1 expression was observed at 1 ng/mL EGF with a maximum of 2.5-fold at 10 ng/mL (Fig. 3B). A significant increase in SphK1 expression was detected within 3 h after exposure to EGF and reached a maximum within 6 to 9 h and declined thereafter (Fig. 3B). This was a similar time course to that observed after stimulation with LPA (Fig. 1A).
Figure 3.
Activation of EGFR is involved in LPA-induced SphK1 expression. A, MKN1 cells were stimulated with 10 μmol/L LPA for the indicated times and lysed, and equal amounts of protein were analyzed by immunoblotting with antibodies against phosphotyrosine (PY) or phospho-EGFR (Tyr1068). Blots were stripped and reprobed with EGFR antibody to confirm equal loading. B, MKN1 cells were stimulated with the indicated concentrations of EGF for 6 h or 10 ng/mL EGF for the indicated times. RNA was isolated and reverse transcribed, and SphK1 and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold changes after normalization to GAPDH. C, MKN1 cells were pretreated with vehicle (DMSO; white columns) or 0.5 μmol/L AG1478 (black columns) for 30 min and then stimulated with 10 μmol/L LPA or 10 ng/mL EGF for 6 h. RNA was isolated and reverse transcribed, and SphK1, S1P3, and GAPDH mRNA levels were measured by real-time PCR. SphK1 and S1P3 mRNA levels are expressed as fold changes after normalization to GAPDH. *, P ≥ 0.01.
To assess the involvement of transactivation of EGFR in LPA-induced SphK1 expression, we used the specific EGFR tyrosine kinase inhibitor, tyrphostin AG1478. As expected, AG1478 abolished LPA-induced tyrosine phosphorylation of EGFR (data not shown) and blocked EGF-induced SphK1 and S1P3 expression and significantly reduced LPA-induced SphK1 and S1P3 expression (Fig. 3C). These results suggest that LPA up-regulates SphK1 expression at least in part via EGFR transactivation.
Down-regulation of SphK1 attenuated LPA-induced migration, invasion,and proliferation in MKN1 cells
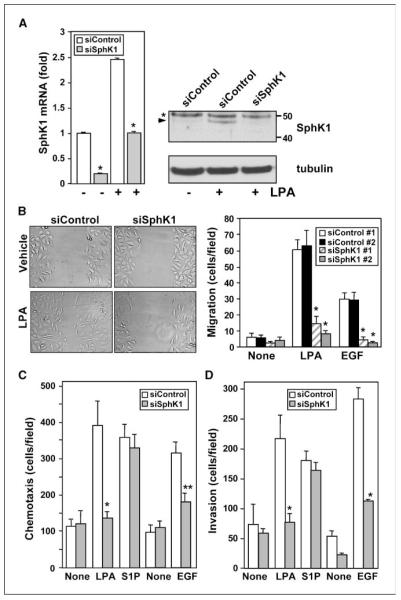
We next investigated the role of up-regulation of SphK1 in LPA-mediated biological processes important for the development or progression of gastric cancer, such as migration and invasion. For this purpose, SphK1 expression was down-regulated with siRNA. siSphK1 markedly reduced basal levels and LPA-induced SphK1 mRNA in MNK1 cells, as determined by real-time PCR (Fig. 4A), without influencing expression of SphK2 or S1PRs present in these cells (data not shown). Similarly, LPA-induced increases in SphK1 protein were abrogated by siSphK1 but not affected by control siRNA (Fig. 4A). In agreement with the well-known stimulatory effect of LPA on cancer cell motility (29), LPA stimulated random motility (chemokinesis) of MNK1 cells, determined by a wound-healing assay (Fig. 4B). This response was abolished by down-regulation of SphK1 (Fig. 4B). To exclude nonspecific off-target effects, SphK1 expression was also down-regulated with siRNA targeted to another region of the SphK1 sequence. This siSphK1 #2, but not scrambled siRNA control #2, also markedly reduced expression of SphK1 mRNA by >85% and almost completely abolished LPA- and EGF-stimulated chemokinesis (Fig. 4B).
Figure 4.
Down-regulation of SphK1 attenuates LPA-induced migration and invasion of MKN1 cells. A, MKN1 cells transfected with ON-TARGETplus SMARTpool siRNA targeted to SphK1 (gray columns) or control siRNA (white columns) were stimulated without or with 10 μmol/L LPA for 4 h, as indicated. mRNA levels of SphK1 and GAPDH were determined and data are expressed as fold change. SphK1 protein expression was analyzed by Western blotting with specific SphK1 antibodies. Blots were stripped and reprobed with anti-tubulin to insure equal loading and transfer. Asterisk, nonspecific immunostained bands; arrowhead, SphK1. B, MKN1 cells were transfected with ON-TARGETplus SMARTpool siRNA targeted to SphK1 (siSphK1 #1; hatched columns), control siRNA #1 (white columns), siSphK1 #2 (gray columns), or control siRNA #2 (black columns). Cultures were wounded and migration of cells into the wound was measured 6 h after stimulation without or with 1 μmol/L LPA or with 10 ng/mL EGF. Columns, mean of migrating cells per field; bars, SD. Left, representative images of a typical experiment. C and D, MKN1 cells transfected with control siRNA or siRNA targeted to SphK1 were allowed to migrate for 6 h through fibronectin-coated filters (C) or invade through Matrigel-coated filters (D) toward vehicle (None), 1 μmol/L LPA, 1 μmol/L S1P, or 10 ng/mL EGF. Columns, mean number of migrating cells per field; bars, SD. *, P ≥ 0.01; **, P ≥ 0.05.
Moreover, ON-TARGETplus SMARTpool siSphK1 but not siControl also markedly reduced directed motility toward LPA and EGF (chemotaxis) in Boyden chamber assays (Fig. 4C). Importantly, down-regulating SphK1 did not decrease chemotaxis toward S1P, indicating that S1P as expected can bypass the effects of down-regulation of SphK1 and further substantiating the specificity of siRNA targeted to SphK1. Similarly, siSphK1 #2 also significantly reduced chemotaxis toward LPA and EGF (Supplementary Fig. S1A). LPA and EGF also stimulated in vitro invasion of MNK1 cells (Fig. 4D), determined by their ability to invade Matrigel, a basement membrane matrix. Down-regulation of SphK1 abolished LPA- and EGF-induced invasion without affecting S1P-induced invasion (Fig. 4D; Supplementary Fig. S1B).
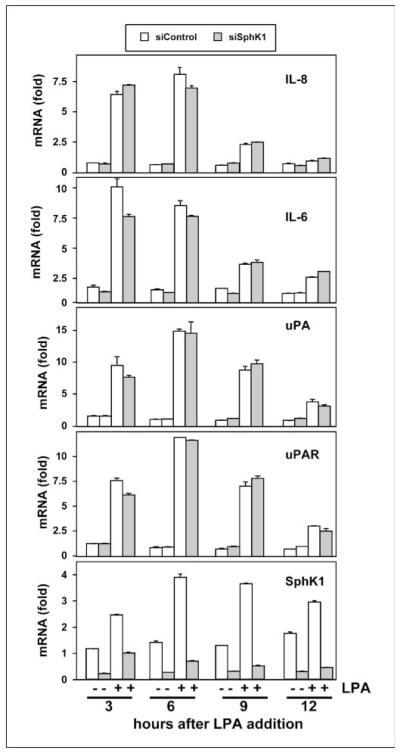
Down-regulation of SphK1 did not attenuate LPA-induced expression of IL-8, IL-6, urokinase-type plasminogen activator, or urokinase-type plasminogen activator receptor
In many cancer cells, LPA up-regulates expression of IL-8 and IL-6 (6). Because these multifunctional cytokines are elevated in gastric cancers (30) and may be involved with invasion and metastasis (31), we next examined whether LPA can enhance their expression in MNK1 cells and the potential involvement of SphK1. LPA markedly and rapidly induced expression of IL-8 and IL-6 by more than 6- and 9-fold, respectively, within 3 h (Fig. 5). LPA treatment also provoked similar increases in expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR; Fig. 5), which play important roles in matrix proteolysis facilitating invasion and metastasis and correlate with poor prognosis of gastric cancer (32). However, in contrast to inhibition of LPA-induced invasion and motility by knockdown of SphK1 (Fig. 4), its down-regulation had no statistically significant effects on LPA-induced expression of IL-6, IL-8, uPA, or uPAR (Fig. 5). These results suggest that LPA can regulate both SphK1-dependent and SphK1-independent pathways in gastric cancer cells.
Figure 5.
Down-regulation of SphK1 has no effect on LPA-induced expression of IL-8, IL-6, uPA, or uPAR. MKN1 cells transfected with control siRNA (white columns) or siRNA targeted to SphK1 (gray columns) were stimulated with 10 μmol/L LPA for the indicated times. RNA was isolated and reverse transcribed, and IL-8, IL-6, uPA, uPAR, SphK1, and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold change after normalization to GAPDH.
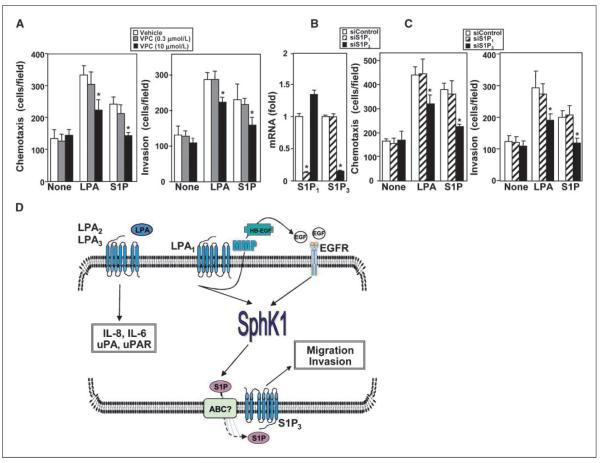
The S1PR S1P3 is involved in LPA-induced motility and invasion
The biological function of SphK1 is to produce S1P, whose most well-known actions are mediated as a ligand for the S1PR family of GPCRs (33). Therefore, we sought to determine the importance of the S1PRs expressed by MKN1 cells in events mediated by LPA that are SphK1 dependent. As a first approach, the S1P1/3 receptor antagonist VPC23019 was used (34). VPC23019 at a concentration of 0.3 μmol/L that only inhibits S1P1 had no effect on LPA- or S1P-mediated chemotaxis (Fig. 6A). However, VPC23019 at a concentration of 10 μmol/L, which has been shown to inhibit both S1P1 and S1P3 (34), significantly reduced LPA-mediated chemotaxis and invasion and completely blocked migration and invasion induced by S1P (Fig. 6A). To further confirm the involvement of S1P3, expression of S1P3 and S1P1 was down-regulated with specific siRNAs targeting these receptors (Fig. 6B). Down-regulation of S1P3 not only reduced its mRNA by 80% (Fig. 6B), it drastically inhibited S1P-induced migration and invasion and significantly decreased chemotaxis and invasion toward LPA (Fig. 6C). In contrast, down-regulation of S1P1 had no significant effects on LPA- or S1P-induced chemotaxis or invasion (Fig. 6C). Moreover, a different siRNA targeted to S1P3 also significantly reduced chemotaxis toward LPA and EGF (Supplementary Fig. S1C). Collectively, these results suggest that S1P produced by SphK1 in response to LPA can act in an autocrine/paracrine manner through S1P3 receptors to mediate the migratory and invasion responses to LPA in gastric cancer cells.
Figure 6.
Involvement of S1P3 receptor in chemotaxis toward LPA. A, MKN1 cells were pretreated for 30 min with vehicle (white columns), 0.3 μmol/L VPC23019 (VPC; gray columns), or 10 μmol/L VPC23019 (stippled columns) and then allowed to migrate for 6 h through fibronectin-coated filters or invade through Matrigel-coated filters toward vehicle (None), 1 μmol/L LPA, or 1 μmol/L S1P. Columns, mean number of migrating cells per field; bars, SD. B and C, MKN1 cells were transfected with control siRNA (white columns), siS1P1 (hatched columns), or siS1P3 (black columns). B, RNA was isolated and reverse transcribed, and S1P1, S1P3, and GAPDH mRNA levels were measured by real-time PCR. Data are expressed as fold change after normalization to GAPDH. C, cells were allowed to migrate for 6 h through fibronectin-coated filters or invade through Matrigel-coated filters toward vehicle (None), 1 μmol/L LPA, or 1 μmol/L S1P. Columns, mean number of migrating cells per field; bars, SD. *, P ≥ 0.01. D, scheme depicting the complex signaling interplay between LPA receptors and EGFRs and up-regulation of SphK1 and their roles in LPA-induced migration and invasion of gastric cancer cells. See text for more details. For simplicity, many other known signaling pathways downstream of LPA receptors and EGFRs are not shown. It is unlikely that SphK1 is the sole mechanism by which LPA induces migration. HB-EGF, heparin-binding EGF; MMP, matrix metalloproteinase.
Discussion
SphK1 is up-regulated in many types of cancers and has been suggested as a potentially new therapeutic target (16, 35, 36), yet it is not yet known what signals cancer cells use to apparently constitutively up-regulate expression of this enzyme nor is it clear why it has such a profound role in tumorigenesis. There are numerous reports of rapid and transient activation of SphK1 by growth and angiogenic factors (reviewed in refs. 37, 38) that stimulate its phosphorylation on Ser225 (21) and subsequent translocation to the plasma membrane (39) where its substrate sphingosine resides, resulting in local formation of S1P and activation of S1PRs (33). However, it is difficult to understand how such short-lived activation could be responsible for the profound involvement of SphK1 in tumorigenicity or how this relates to its up-regulation in cancer.
In this work, we have shown that the two important growth factors for gastric cancer, LPA and EGF, up-regulate the expression and activity of SphK1, which was sustained for >24 h. Similarly, EGF (40) as well as 17β-estradiol (41) and prolactin (42), hormones that may contribute to the development and progression of breast cancer, have been shown to increase SphK1 expression in MCF-7 human breast cancer cells. The pleiotropic actions of SphK1 have been attributed to its product, S1P, which functions extracellularly as a ligand of its cell surface receptors and intracellularly to regulate growth and survival (14, 33, 43). Interestingly, we found that in addition to up-regulation of SphK1, LPA and EGF also increased expression of S1P3, one of the S1PRs expressed by gastric cancer cells, thus providing an amplification loop for formation and action of S1P. Several lines of evidence suggest that these responses were mediated via LPA1: they were observed only in cancer cells expressing this receptor, including MKN1, DLD1 colon cancer cells, and MDA-MB-231 breast cancer cells, but not in HT29 colon cancer cells or MDA-MB-453 breast cancer cells, which do not express LPA1; they were inhibited by an LPA1/LPA3 antagonist; and only siRNA targeted to LPA1 and not to the other LPA receptors reduced the LPA effects on SphK1 and S1P3. It has previously been established that LPA1 is responsible for chemotaxis of gastric cancer cells toward LPA (5). This agrees with results in many other types of cancer cells where LPA1 is crucial for motility, invasion, and metastasis (44–46). Moreover, silencing the LPA1 receptor in breast and ovarian cancer cells implanted in mice significantly reduced the progression of bone metastases (47). In this study, we found that down-regulation of SphK1 abolished migration and invasion of gastric cancer cells induced by LPA. In addition, inhibition of S1P3 pharmacologically or its down-regulation indicated that LPA-induced motility was also dependent on this S1PR, consistent with the up-regulation of SphK1 and increased production of S1P. Thus, we have provided compelling evidence to suggest that an autocrine or paracrine S1P signaling loop is triggered by LPA activation of LPA1 and/or transactivation of EGFR leading to up-regulation of SphK1 expression and activity. S1P thus formed and then activates S1P3, which is also up-regulated, amplifying downstream signals that regulate motility and invasion (Fig. 6D).
Although the actions of LPA and S1P are primarily mediated via their cognate receptors, transactivation of EGFR has been identified as a key link to pathophysiologic processes in human cancer cells (22, 26, 48). A chimeric monoclonal antibody directed against EGFR improved the survival of patients with metastatic colorectal cancer, highlighting the significance of EGFR in cancer progression (49). This is particularly relevant to gastric cancer as both S1P and LPA have been shown to transactivate EGFR (8, 50). Moreover, S1P3 also transactivates EGFR in human breast cancer cells (48). Thus, it is tempting to speculate that this additional positive feedback loop could provide even more amplification and aberrant signaling and uncontrolled migration/invasion of neoplastic cells.
In summary, our study shows that SphK1 plays a coordinating role linking the two lysophospholipids, LPA and S1P, and their cognate GPCRs with the EGF growth factor tyrosine kinase receptor. Our results imply that SphK1 might be the central controller of several amplification loops that can contribute to cancer progression. Thus, targeting SphK1 is an attractive additional therapeutic strategy for treatment of gastric cancer and perhaps other cancers as well. Implementation of preclinical and clinical evaluation of SphK1 as a novel molecular target for cancer therapy is warranted.
Supplementary Material
Acknowledgments
Grant support: NIH grants CA61774 and R37GM043880 (S. Spiegel), CA102196 (X. Fang), Training Grant T32GM008695 (K. Takabe), and National Institute of Mental Health Intramural Research Program (S. Milstien).
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Murph M, Tanaka T, Liu S, et al. Of spiders and crabs: the emergence of lysophospholipids and their metabolic pathways as targets for therapy in cancer. Clin Cancer Res. 2006;12:6598–602. doi: 10.1158/1078-0432.CCR-06-1721. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–7. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 5.Shida D, Kitayama J, Yamaguchi H, et al. Dual mode regulation of migration by lysophosphatidic acid in human gastric cancer cells. Exp Cell Res. 2004;301:168–78. doi: 10.1016/j.yexcr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Fang X, Yu S, Bast RC, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279:9653–61. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 7.Ye X, Hama K, Contos JJ, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–8. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shida D, Kitayama J, Yamaguchi H, et al. Sphingosine 1-phosphate transactivates c-Met as well as epidermal growth factor receptor (EGFR) in human gastric cancer cells. FEBS Lett. 2004;577:333–38. doi: 10.1016/j.febslet.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Shida D, Kitayama J, Yamaguchi H, et al. Lysophospholipids transactivate HER2/neu (erbB-2) in human gastric cancer cells. Biochem Biophys Res Commun. 2005;327:907–14. doi: 10.1016/j.bbrc.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 10.Allgayer H, Babic R, Gruetzner KU, et al. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumorassociated protease systems. J Clin Oncol. 2000;18:2201–9. doi: 10.1200/JCO.2000.18.11.2201. [DOI] [PubMed] [Google Scholar]
- 11.Xia P, Gamble JR, Wang L, et al. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–30. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- 12.French KJ, Schrecengost RS, Lee BD, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–69. [PubMed] [Google Scholar]
- 13.Kawamori T, Osta W, Johnson KR, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–88. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 14.Kohno M, Momoi M, Oo ML, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–23. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visentin B, Vekich JA, Sibbald BJ, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–38. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Milstien S, Spiegel S. Targeting sphingosine-1-phosphate: a novel avenue for cancer therapeutics. Cancer Cell. 2006;9:148–50. doi: 10.1016/j.ccr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Hait NC, Sarkar S, Le Stunff H, et al. Role of sphingosine kinase 2 in cell migration towards epidermal growth factor. J Biol Chem. 2005;280:29462–69. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita H, Kitayama J, Shida D, et al. Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells: differential regulation on the migration and proliferation. J Surg Res. 2006;130:80–7. doi: 10.1016/j.jss.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Nohara K, Olivera A, et al. Involvement of focal adhesion kinase in inhibition of motility of human breast cancer cells by sphingosine 1-phosphate. Exp Cell Res. 1999;247:17–28. doi: 10.1006/excr.1998.4327. [DOI] [PubMed] [Google Scholar]
- 20.Bektas M, Payne SG, Liu H, et al. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J Cell Biol. 2005;169:801–11. doi: 10.1083/jcb.200407123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitson SM, Moretti PA, Zebol JR, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shida D, Watanabe T, Aoki J, et al. Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab Invest. 2004;84:1352–62. doi: 10.1038/labinvest.3700146. [DOI] [PubMed] [Google Scholar]
- 23.Kishi Y, Okudaira S, Kishi M, et al. Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. J Biol Chem. 2006;281:17492–500. doi: 10.1074/jbc.M601803200. [DOI] [PubMed] [Google Scholar]
- 24.Ohta H, Sato K, Murata N, et al. Ki16425, a subtypeselective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- 25.Lee Z, Swaby RF, Liang Y, et al. Lysophosphatidic acid is a major regulator of growth-regulated oncogene a in ovarian cancer. Cancer Res. 2006;66:2740–8. doi: 10.1158/0008-5472.CAN-05-2947. [DOI] [PubMed] [Google Scholar]
- 26.Gschwind A, Prenzel N, Ullrich A. Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res. 2002;62:6329–36. [PubMed] [Google Scholar]
- 27.Schafer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene. 2004;23:991–99. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita H, Kitayama J, Shida D, et al. Differential expression of lysophosphatidic acid receptor-2 in intestinal and diffuse type gastric cancer. J Surg Oncol. 2006;93:30–5. doi: 10.1002/jso.20397. [DOI] [PubMed] [Google Scholar]
- 29.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–91. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 30.Macri A, Versaci A, Loddo S, et al. Serum levels of interleukin 1β, interleukin 8 and tumour necrosis factor α as markers of gastric cancer. Biomarkers. 2006;11:184–93. doi: 10.1080/13547500600565677. [DOI] [PubMed] [Google Scholar]
- 31.Eck M, Schmausser B, Scheller K, et al. Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clin Exp Immunol. 2003;134:508–15. doi: 10.1111/j.1365-2249.2003.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Zhao ZS, Ru GQ, et al. Correlative studies on uPA mRNA and uPAR mRNA expression with vascular endothelial growth factor, microvessel density, progression and survival time of patients with gastric cancer. World J Gastroenterol. 2006;12:3970–6. doi: 10.3748/wjg.v12.i25.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 34.Davis MD, Clemens JJ, Macdonald TL, et al. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9633–41. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 35.Oskouian B, Saba J. Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle. 2007;6:522–7. doi: 10.4161/cc.6.5.3903. [DOI] [PubMed] [Google Scholar]
- 36.Cuvillier O. Sphingosine kinase-1—a potential therapeutic target in cancer. Anticancer Drugs. 2007;18:105–10. doi: 10.1097/CAD.0b013e328011334d. [DOI] [PubMed] [Google Scholar]
- 37.Hait NC, Oskeritzian CA, Paugh SW, et al. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–26. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–31. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- 39.Stahelin RV, Hwang JH, Kim JH, et al. The mechanism of membrane targeting of human sphingosine kinase 1. J Biol Chem. 2005;280:43030–38. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- 40.Doll F, Pfeilschifter J, Huwiler A. The epidermal growth factor stimulates sphingosine kinase-1 expression and activity in the human mammary carcinoma cell line MCF7. Biochim Biophys Acta. 2005;1738:72–81. doi: 10.1016/j.bbalip.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Sukocheva OA, Wang L, Albanese N, et al. Sphingosine kinase transmits estrogen signaling in human breast cancer cells. Mol Endocrinol. 2003;17:2002–12. doi: 10.1210/me.2003-0119. [DOI] [PubMed] [Google Scholar]
- 42.Doll F, Pfeilschifter J, Huwiler A. Prolactin upregulates sphingosine kinase-1 expression and activity in the human breast cancer cell line MCF7 and triggers enhanced proliferation and migration. Endocr Relat Cancer. 2007;14:325–35. doi: 10.1677/ERC-06-0050. [DOI] [PubMed] [Google Scholar]
- 43.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–34. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 44.Shida D, Kitayama J, Yamaguchi H, et al. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–11. [PubMed] [Google Scholar]
- 45.Yamada T, Sato K, Komachi M, et al. Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J Biol Chem. 2004;279:6595–605. doi: 10.1074/jbc.M308133200. [DOI] [PubMed] [Google Scholar]
- 46.Hama K, Aoki J, Fukaya M, et al. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004;279:17634–9. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- 47.Boucharaba A, Serre CM, Guglielmi J, et al. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A. 2006;103:9643–8. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sukocheva O, Wadham C, Holmes A, et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–10. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 50.Shida D, Kitayama J, Yamaguchi H, et al. Lysophosphatidic acid transactivates both c-Met and epidermal growth factor receptor, and induces cyclooxygenase-2 expression in human colon cancer LoVo cells. World J Gastroenterol. 2005;11:5638–43. doi: 10.3748/wjg.v11.i36.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.