Abstract
Complex regional pain syndrome is a refractory pain condition with few tested therapies. We hypothesized that botulinum toxin A (BTA) would prolong analgesia after sympathetic blocks in patients with complex regional pain syndrome. We compared the duration of standard lumbar sympathetic block (LSB) with bupivacaine to LSB with bupivacaine and BTA in nine patients with refractory complex regional pain syndrome. Median time to analgesic failure was 71 (95% confidence interval, 12–253) days after LSB with BTA compared with fewer than 10 days (95% confidence interval, 0–12) after standard LSB (log-rank, p < 0.02). BTA profoundly prolonged the analgesia from sympathetic block in this preliminary study.
Complex regional pain syndrome (CRPS) is a devastating condition characterized by pain disproportionate to an inciting injury, vasomotor changes, and occasionally trophic or motor function changes.1 It has been estimated that CRPS complicates 1 to 2% of bone fractures with an overall incidence of 20 to 26 per 100,000 people.2,3 Despite scant evidence of short- or long-term efficacy, injections to block sympathetic nerves have long been used in the treatment of CRPS.1 Unfortunately, for many patients with CRPS, the relief provided with sympathetic blockade is transient. Surgical or chemical sympathectomy has not generally been effective for prolonging analgesia in these patients and is associated with substantial morbidity such as new neuralgic pain.4
Animal and human experiments demonstrate that after trauma, injured nerves, as well as surrounding uninjured nerves, begin to express adrenergic receptors.5,6 Peripheral stimulation of these nerves by catecholamines contributes to depolarization of nociceptors, and thus augments the transduction of pain.5,7–9 These changes may constitute the molecular basis underlying sympathetically maintained pain.
Botulinum toxin type A (BTA) prevents release of acetylcholine from cholinergic nerve terminals. This inhibition is long lasting but not permanent, and it does not result in cytotoxicity or neural loss.10 Preganglionic sympathetic nerves are cholinergic,11 and animal data indicate that BTA can induce prolonged sympathetic block when placed on surgically exposed sympathetic ganglia.12
We conducted an unfunded pilot, prospective, randomized, double-blind, controlled, crossover study to test the hypothesis that a lumbar sympathetic block (LSB) with BTA would provide prolonged analgesia in patients with Sympathetically Maintained Pain (SMP) of the lower extremity caused by CRPS. In random order, patients received two lumbar sympathetic injections: one with 10ml 0.5% bupivacaine mixed with 75 units BTA, and one with 10ml 0.5% bupivacaine alone. The primary end point was duration of analgesia. This trial is registered at http://www.clinicaltrials.gov (identification number NCT00637533).
Patients and Methods
All patients met International Association for the Study of Pain criteria for CRPS type I,13 and additionally had: (1) spontaneous pain rating greater than 6 of 10; (2) duration of pain at least 6 months; (3) unsuccessful therapy with at least two nonopioid medications reported to be of benefit in patients with neuropathic pain (eg, anticonvulsants, tricyclic antidepressants); (4) perceived impairment of the lower extremity function by the patient because of pain; and (5) at least a 50% reduction in pain for greater than 5 hours but less than 2 weeks from a previous lumbar sympathetic injection. Patient characteristics are shown in Supplementary Table 1. Patients were allowed to continue current medications but were not allowed to start new therapies. Furthermore, patients were asked not to discontinue ongoing pharmacological therapies in the face of new analgesia that arose during the study.
Randomization and Blinding
Patients were randomly assigned to which injection they received first, and all physicians and patients involved in the study were blinded to which injection contained BTA. Data were not unblinded for any patient until the study was completed, and no interim analyses were performed.
Injections
LSBs were accomplished in standard fashion using intermittent fluoroscopy to place the tip of a single 6-inch 22-gauge needle at the anterolateral border of the second lumbar vertebrae.14 In one injection, the patient received a standard LSB with 10ml of 0.5% bupivacaine. In the crossover injection, the patient received an identical injection of 10ml of 0.5% bupivacaine with an added 75 units BTA. Patients were eligible for their crossover injection 1 month after they reported their pain had returned to baseline.
Measurements
Patients completed a written 10cm visual analog pain score (VAS) of pain intensity daily starting 7 days before their first injection. Patients continued to record daily VAS until they reported their pain had returned to baseline or 1 month, whichever was longer. In addition, patients were asked to record any adverse events.
Statistical Analysis
The primary end point, time to return of baseline pain (ie, “analgesic failure”), was analyzed using Kaplan–Meier analysis. The secondary end point, change in VAS over time, was analyzed using a mixed-effects model of VAS over time stratified by treatment group. All analyses were conducted on an intent-to-treat basis. No other analyses were performed.
Results
Recruitment
We initially planned to enroll 10 patients as a convenience sample. We closed the trial after enrolling nine patients with SMP and CRPS between May 2004 and March 2007. The early closure was accomplished simply to bring closure to the study whose rigid inclusion criteria made recruitment challenging. One patient (Patient 7) was censored on the day of the patient's second injection before any outcome data could be collected when it was apparent the injectate had not reached the lumbar sympathetic ganglia because of needle malpositioning. A second patient (Patient 3) returned no outcome forms and could not be included for analysis. The other seven patients experienced development of signs of immediate local anesthetic sympathetic block after each injection, manifested both as increased skin temperature and erythema compared with the unblocked leg, and completed all study forms.
Efficacy of Sympathetic Blocks
Primary End Point
The rate of pain return was significantly lower after LSB with BTA compared with local anesthetic alone (Fig 1). Median time to analgesic failure was 71 (95% confidence interval, 12–253) days after LSB with BTA compared with fewer than 10 days (95% confidence interval, 0–12) among those receiving local anesthetic alone (log-rank, p < 0.02).
Fig 1.
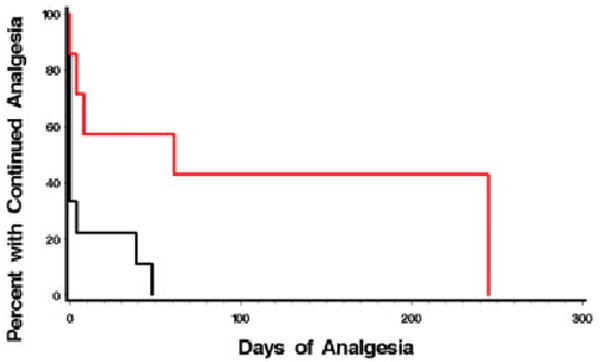
Duration of analgesia. Analgesic duration of lumbar sympathetic block is extended by botulinum toxin type A (BTA). Black line represents bupivacaine; red line represents bupivacaine plus BTA. Median time to analgesic failure was 69 days after lumbar sympathetic block with BTA compared with fewer than 8 days among those receiving local anesthetic alone. Log-rank, p < 0.02.
Secondary End Point
Botulinum toxin–enhanced sympathetic blockade was significantly more effective at reducing VAS pain scores over time than bupivacaine alone. Assignment to the BTA-enhanced LSB was associated with a mean decrease in VAS of 1.6 points (95% confidence interval, 1.2–2.0; p < 0.0001). However, among the more robust responders, the effect size was much larger (Fig 2).
Fig 2.
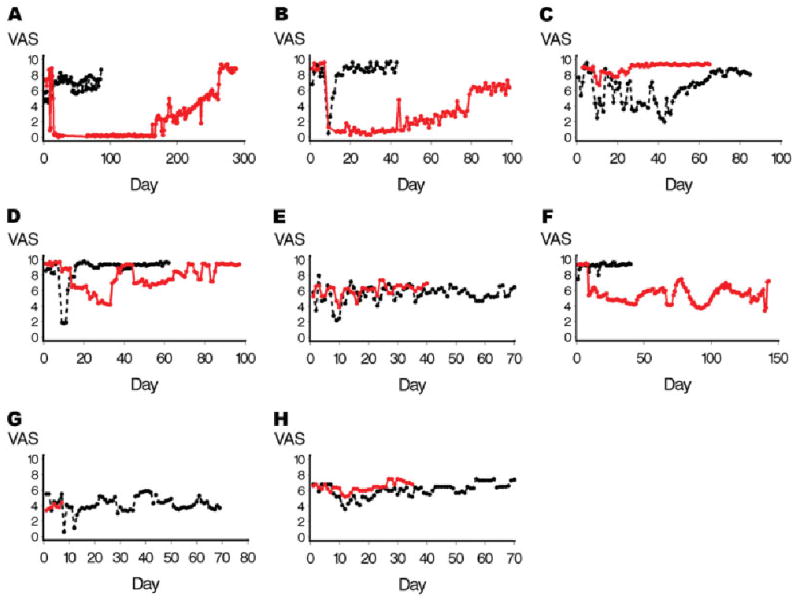
Change in visual analog pain score (VAS) over time. Botulinum toxin type A (BTA) reduces VAS sores of pain intensity over protracted time periods in responders. Assignment to the BTA-enhanced lumbar sympathetic block was associated with a mean decrease in VAS of 1.6 points (95% confidence interval, 1.2–2.0; p < 0.0001). Patients appear to be BTA responders or nonresponders. Despite refractory long-standing pain, Patients 1, 2, and 7 experienced long-term significant reduction in pain. Note time scales for each patient and each injection differ because each patient recorded pain until they perceived they had returned to baseline. All injections occurred on day 7 after a 1-week “run-in” period. Black lines represent bupivacaine; red lines represent bupivacaine plus BTA. Patients 1 (A), 2 (B), 4 (C), 5 (D), 6 (E), 7 (F), 8 (G), and 9 (H).
Adverse Events
One patient experienced significant nausea and emesis that started 5 hours after her BTA injection and lasted 2 days; it resolved spontaneously.
Discussion
This study presents preliminary evidence that BTA-supplemented sympathetic blocks may represent a significant novel therapeutic modality for the treatment of sympathetically maintained pain in patients with highly refractory CRPS. Within the context of the otherwise limited options for patients with refractory CRPS, these findings are striking. The small number of patients in this study should be noted. A larger study is now warranted to further test the safety and efficacy of this procedure.
Botulinum toxin is generally understood to be primarily, if not exclusively, a potent inhibitor of release of acetylcholine from cholinergic nerves. This inhibition is long lasting but not permanent, and it does not result in cytotoxicity or neural loss.10 Inhibition of sympathetic nerve transmission at cholinergic sympathetic ganglia in the lumbar sympathetic chain may be the mechanism of analgesia.
Manjunath and colleagues15 recently tested radiofrequency versus phenol-induced destruction of the lumbar sympathetic chain for patients with CRPS and SMP. They found no between-group differences and did not show superiority over standard LSB. Like other investigators attempting neurodestructive procedures, they note the occurrence of new neuralgias.4 In contrast, our results show that BTA is superior to the standard local anesthetic sympathetic block, and that such results can be attained without a neurodestructive procedure and its known complications.
The use of botulinum toxin in myofascial pain syndromes and headache conditions are well described.16,17 Furthermore, Argoff and others18 have anticipated its use both for neuropathic pain in general and for CRPS specifically, but trials have been lacking. In 2008, Ranoux and colleagues19 reported the first well-controlled trial of the use of intradermal botulinum toxin for pain and allodynia associated with peripheral nerve injury. Their results suggest efficacy and relief of a duration similar to those reported in this study. This study extends this work to specifically suggest efficacy in CRPS and efficacy when used to supplement traditionally approached sympathetic blockade of the lower extremity.
Although CRPS is more common among women, the predominance of women in the study population should be noted as a limitation to extrapolating the effect seen here to the afflicted population in general. The small number of patients completing the study and the failure to randomize by blocks allowed for a correlation of the order of injection with the use of botulinum toxin. Therefore, based on this study, an order effect cannot be excluded as a possible contributor to the magnitude of the effect seen with the botulinum toxin. Future work would also benefit from evaluating psychosocial variables known to affect pain perception such as mood and anxiety, both as baseline factors that might influence efficacy and as secondary outcome end points that speak to the multidimensional scope of clinical efficacy.
Conclusion
BTA enhances the analgesia from sympathetic block by profoundly extending analgesia among a subset of patients. Additional studies testing the efficacy of BTA-enhanced sympathetic blocks for the treatment of CRPS are warranted.
Footnotes
Potential conflict of interest: The authors report filing a patent for the use of botulinum toxins in sympathetic block.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Raja SN, Grabow TS. Complex regional pain syndrome I (reflex sympathetic dystrophy) Anesthesiology. 2002;96:1254–1260. doi: 10.1097/00000542-200205000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted County, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 3.de Mos M, de Bruijn AG, Huygen FJ, et al. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Mailis A, Furlan A. Sympathectomy for neuropathic pain. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD002918. CD002918. [DOI] [PubMed] [Google Scholar]
- 5.Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- 6.Birder LA, Perl ER. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J Physiol (Lond) 1999;515:533–542. doi: 10.1111/j.1469-7793.1999.533ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi B, Rowbotham MC. Effect of adrenergic receptor activation on post-herpetic neuralgia pain and sensory disturbances. Pain. 1997;69:55–63. doi: 10.1016/s0304-3959(96)03245-9. [DOI] [PubMed] [Google Scholar]
- 8.Xie J, Ho Lee Y, Wang C, et al. Differential expression of alpha1-adrenoceptor subtype mRNAs in the dorsal root ganglion after spinal nerve ligation. Brain Res Mol Brain Res. 2001;93:164–172. doi: 10.1016/s0169-328x(01)00201-7. [DOI] [PubMed] [Google Scholar]
- 9.Ali Z, Raja SN, Wesselmann U, et al. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain. 2000;88:161–168. doi: 10.1016/S0304-3959(00)00327-4. [DOI] [PubMed] [Google Scholar]
- 10.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 11.Oesch F, Thoenen H. Increased activity of the peripheral sympathetic nervous system: induction of choline acetyltransferase in the preganglionic cholinergic neurone. Nature. 1973;242:536–537. doi: 10.1038/242536a0. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Seo K, Yum KW, et al. Effects of botulinum toxin type A on the superior cervical ganglia in rabbits. Auton Neurosci. 2002;102:8–12. doi: 10.1016/s1566-0702(02)00093-0. [DOI] [PubMed] [Google Scholar]
- 13.Merskey H, Boqduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2nd. Seattle, WA: IASP Press; 1994. [Google Scholar]
- 14.Datta S, Pai U. Paradiscal extraforaminal technique for lumbar sympathetic block: report of a proposed new technique utilizing a cadaver study. Pain Physician. 2004;7:53–57. [PubMed] [Google Scholar]
- 15.Manjunath PS, Jayalakshmi TS, Dureja GP, Prevost AT. Management of lower limb complex regional pain syndrome type 1: an evaluation of percutaneous radiofrequency thermal lumbar sympathectomy versus phenol lumbar sympathetic neurolysis—a pilot study. Anesth Analg. 2008;106:647–649. doi: 10.1213/01.ane.0000298285.39480.28. table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Reilich P, Fheodoroff K, Kern U, et al. Consensus statement: botulinum toxin in myofascial [corrected] pain. J Neurol. 2004;251(suppl 1):I36–I38. doi: 10.1007/s00415-004-1109-5. [DOI] [PubMed] [Google Scholar]
- 17.Raj PP. Botulinum toxin therapy in pain management. Anesthesiol Clin North Am. 2003;21:715–731. doi: 10.1016/s0889-8537(03)00082-8. [DOI] [PubMed] [Google Scholar]
- 18.Argoff CE. A focused review on the use of botulinum toxins for neuropathic pain. Clin J Pain. 2002;18:S177–S181. doi: 10.1097/00002508-200211001-00010. [DOI] [PubMed] [Google Scholar]
- 19.Ranoux D, Attal N, Morain F, Bouhassira D. Botulinum toxin type a induces direct analgesic effects in chronic neuropathic pain. Ann Neurol. 2008;64:274–283. doi: 10.1002/ana.21427. [DOI] [PubMed] [Google Scholar]


