Abstract
Neutralizing antibodies are thought to be required at mucosal surfaces to prevent human papillomavirus (HPV) transmission. However, the potential for cell-mediated immunity in mediating protection against HPV infection has not been well explored. We generated recombinant Listeria monocytogenes (Lm) constructs that secrete listeriolysin O (LLO) fused with overlapping N-terminal (LLO-L11–258) or C-terminal (LLO-L1238–474) fragments of HPV type 16 major capsid protein L1 (HPV-16-L1). Oral immunization of mice with either construct induced IFN-γ-producing CD8+ and CD4+ T cells in the spleen and in the Peyer's patches with the C-terminal construct. Oral immunization with both constructs resulted in diminished viral titers in the cervix and uterus of mice after intravaginal challenge with vaccinia virus expressing HPV-16-L1.
Introduction
Oncogenic human papillomaviruses (HPVs) infect the genital tract and are associated with human anogenital cancers, particularly cervical cancer (1–3). HPV is the primary causative agent in more than 98% of cases, with HPV type 16 being linked with 50% of cervical cancers and high-grade cervical intraepithelial neoplasias (4). The discovery that expression of the major capsid protein L1 of papillomaviruses in different expression systems (e.g., insect cells or yeast) leads to self-assembly of virus-like particles (VLPs) was a breakthrough in the development of prophylactic vaccines against HPV infection (5). Several studies demonstrated that systemic immunization of animals with VLPs induces the generation of neutralizing antibodies and L1-specific cytotoxic T lymphocytes (CTLs), resulting in protection against challenge with the corresponding papillomavirus type (6–8). In humans, VLP-based HPV vaccines have been shown to be highly effective in protecting against HPV types covered by the vaccine (9–11). However, VLP-based HPV vaccines are not able to eliminate viral infections in already infected women in whom neutralizing antibodies, even if produced at mucosal surfaces, cannot eliminate already infected cells. In addition, high production costs are a major drawback of these vaccines, which can affect their distribution in developing countries, where over 80% of cervical cancer cases occur. An economically advantageous alternative to VLP-based vaccines might be the use of L1 pentamers (capsomeres), which can be purified from Escherichia coli and have been shown to induce a protective systemic immune response after parenteral administration (12–14). Furthermore, mucosal immunization has been investigated as an alternative immunization route that elicits an efficient immune response for several antigens (15–17). Indeed, intranasal as well as oral administration of VLPs induces neutralizing antibodies and L1-specific CTLs (18–21). An additional advantage of mucosal immunization is the induction of secretory IgA antibodies at the site of infection. It is not known how efficient the parenteral administration of L1 particles is in provoking the generation of secretory antibodies in the genital tract (22–24).
Listeria monocytogenes (Lm) is an intracellular pathogen that has direct access to the cytoplasm of antigen-presenting cells (APCs) due to the hemolytic activity of listeriolysin O (LLO) (25). LLO, a 529-aa protein, is secreted by Lm, allowing the bacterium to escape the vacuole and enter the cytosol. The hemolytic domain of LLO resides in the C-terminus of the protein. Proteins secreted by Lm during this intracellular phase of its life cycle are effectively targeted by the cellular immune system (26). We have taken advantage of Lm to target proteins to the immune system by engineering the bacterium to secrete fragments of the major capsid L1 protein of HPV16 by fusing it to LLO. We have previously shown the efficacy of Lm as a vaccine vector for combating viral infection at mucosal surfaces, with recombinant Lm expressing either the influenza nucleoprotein (27), SIV antigens (28–30), or HIV Gag (31). We have also shown that the treatment of mice bearing tumors expressing NP (32,33) or HPV16-E7 (34,35) with Lm-LLO-NP or Lm-LLO-E7, respectively, resulted in the eradication of tumors expressing these antigens. A further advantage of Listeria is that it can be cheaply produced in broth free of animal products and can be delivered by the “needle-free” oral route, which makes it an attractive vaccine for use in developing countries.
Because the L1 protein is too large to be expressed in a secretable form by L. monocytogenes, we have constructed two recombinant L. monocytogenes strains that express and secrete overlapping N-terminal (Lm-PL2-LLO-L11–258) and C-terminal (Lm-PL2-LLO-L1238–474) fragments of HPV16-L1 protein. The expression system used in these constructs was similar in that they both integrate a single copy of the fusion gene into the Lm chromosome, but differed in the fragment of L1 expressed. Oral immunization with both constructs induced systemic cell-mediated immune responses, whereas a mucosal cell-mediated immune response to L1 was induced only by the Lm-PL2-LLO-L1238–474 construct. Induction of cell-mediated immune responses to L1 in vaccinated mice resulted in evidence of protection after vaginal challenge with a recombinant vaccinia virus engineered to express the HPV16 L1 protein.
Materials and Methods
Construction of Lm-PL2-LLO-L11–258 and Lm-PL2-LLO-L1238–474 strains
We have often experienced difficulty in attempting the expression and secretion by Listeria of proteins greater than about 50 kD (28,36), thus we were not surprised that our first attempt in constructing a Listeria vaccine that expressed full-length L1 was unsuccessful. We thus used a previously successful strategy of expressing the protein as two overlapping peptide fragments, residues 1–258 and 238–474 (28). The L1 N-terminus fragment encoded by 3–774 bp was amplified by PCR from pGEX (kindly provided by Dr. Richard Roden, Johns Hopkins University) using the primers 5′-CTCGAGTCTCTTTGGCTGCCTAGTGAG G–3′ (XhoI site is in bold) and 5′-ACTAGTTTACTTGTCATCGTCGTCCTTGTAGTCTCTAACAAACATTTGTTCCC-3′ (SpeI site is in bold, and the flag sequence is underlined). The L1 C-terminus fragment encoded by 714–1422 bp was truncated by insertion of a stop codon at position 1422 bp (37), and amplified by PCR from pGEX using the primers 5′-CTCGAGTCAGAACCATATGGCGACAGC-3′ (XhoI site is in bold) and 5′-ACTAGTTTACTTGTCATCGTCGTCCTTGTAGTCCAATCCTGCTTGTAGTAAAAATTTGC-3′ (SpeI site is in bold, and the flag sequence is underlined). The amplified fragments were cloned into pCR2.1 (Invitrogen, San Diego, CA). These fragments were excised from pCR2.1 and ligated into an expression plasmid derived from the integration plasmid pPL2 (38).
Initially, we created a cassette containing the transcriptional terminator rrnBT1, the listerial actA promoter (PactA), and a gene encoding the first 420 residues of the listeriolysin O virulence factor (hly) fused to the gene encoding the HPV16 E7 protein to be inserted into the pPL2 plasmid. Briefly, a fragment of the origin of replication p15 including the AlwN I site was amplified from pTV3 plasmid (39) using the forward primer 5′-ACTGGCAG CAGCCACTGG-3′ (the AlwN I site is underlined), and the reverse primer 5′-TTGCGGCCGC GCTAGA AATATTTTATCTG ATT-3′ (the Not I site is underlined). The PCR product was cloned into the pCR2.1 plasmid. The PactA was amplified using the forward primer 5′-GGTAACC GCGGCCGC TGATTAACAATGTTAGAGAA-3′ (the Not I site is underlined), and the reverse primer 5′-TTATACTCCC TCCTCGTG-3′. A fragment of the hly gene including its HpaI site was amplified by PCR from pTV3 (39) using the forward primer 5′-GTATCAC GAGGAGGGAGTATAAAT GGAAAAAATAATGCTAGTTTT-3′ (the PactA sequence is in italics), and the reverse primer 5′-GTTAACGTTTGATTTAGTGGC-3′ (the Hpa I site is underlined). The PactA and hly PCR products were joined in a second PCR reaction and cloned into the pCR2.1 plasmid. The PactA-hly insert was subsequently excised from the pCR2.1 by double digestion with NotI and SpeI and ligated into the pCR2.1-oriP15 plasmid, which was previously linearized with Not I and Xba I. The resultant insert contained the p15 fragment, and the PactA and the hly gene fragment was finally digested as a cassette from pCR2.1 by double digestion with AlwN I and Hpa I and cloned in frame with the hly gene in the pTV3 plasmid (39) linearized with the same enzymes. The transcription terminator rrnBT1 was excised from the pL1V1 plasmid by double digestion with Eco0109I and EcoRI and the overhangs were filled by incubation with the Klenow fragment DNA polymerase for blunt ligation. The rrnBT1 terminator was cloned into the pTV3-PactA plasmid upstream the PactA in the Not I site, which was blunted by incubation with the Klenow polymerase. The cassette created in the pTV3 comprised the rrnBT1 terminator, PactA, and the hly gene fused to the HPV16 gene for the E7 protein. This rrnBT1-PactA-hly-e7 cassette was excised by double digestion with Not I and Spe I and then ligated to the pPL2 plasmid linearized using the same enzymes. The e7 gene was excised by double digestion with the XhoI and SpeI enzymes and replaced by the gene encoding either L11–258 or L1238–474.
The pPL2-LLO-L11–258 and pPL2-LLO-L238–474 were used to transform SM10 E. coli by electroporation and the resultant strains were mated with the L. monocytogenes strain 10403S for conjugation. A single colony of SM10-pPL2-LLO-L11–258 and SM10-pPL2-LLO-L1238–474 (donor strain) were individually grown in 5-mL LB medium with 34 μg/mL chloramphenicol. The 10403S (recipient strain) was grown in BHI with streptomycin 50 μg/mL. All cultures were incubated overnight at 37°C on a shaker. The overnight culture was used to inoculate new media and the bacteria were grown to mid-log phase. The donor and recipient were then mixed at a 5:3 ratio, pelleted down, and then washed twice with 30 mL BHI. The bacteria were then resuspended in 200 μL and applied to the center of a BHI agar plate. The plate was incubated for 4 h at 30°C, and the semi-dry central area of the plate was washed with 900 μL BHI. Aliquots of 50 and 100 μL were plated onto BHI agar plates with 6.8 μg/mL chloramphenicol and 50 μg/mL streptomycin and incubated at 30°C. After overnight incubation the plates were transferred to 37°C and incubated overnight. Transformed colonies with the plasmid integrated into the chromosome were selected by growth on BHI with 6.8 μg/mL chloramphenicol and 100 μg/mL streptomycin. The resultant L. monocytogenes strains expressed either the N-terminus (Lm-PL2-LLO-L11–258) or C-terminus (Lm-PL2-LLO-L1238–474) fragments of the HPV16 L1 protein. A previously described strain, Lm-NP (27), which is a similarly attenuated strain of Listeria that expresses the influenza nucleoprotein gene NP fused to LLO, was used as a control vaccine for the vaccinia challenge experiments.
Western blotting
Lm constructs were grown in LB media (Difco Labs, Detroit, MI) to stationary phase and supernatants were collected for evaluation of secreted proteins. The secreted proteins were precipitated using 10% TCA and resuspended in SDS loading sample buffer supplemented with 0.1 N NaOH. Identical amounts of each TCA-precipitated supernatant were loaded and separated on 4–12% Tris-glycine SDS-PAGE gels (Novex, San Diego, CA). The gel was transferred to polyvinylidene difluoride membrane (Nen Life Sciences, Boston, MA). Blots were blocked with 4% nonfat milk in PBS with 0.1% Tween 20 and probed with either an anti-HPV-mAb (HPV-16 Ab-3; NeoMarkers, Fremont, CA), or a polyclonal rabbit serum raised to the first 30 residues of the LLO protein (36). Subsequently the blot was incubated with HRP-conjugated anti-mouse secondary Ab (Amersham Pharmacia Biotech, Little Chalfont, U.K.) and HRP-conjugated anti-rabbit secondary Ab. Blots were developed with Amersham ECL detection reagents and exposed to Hyperfilm (Amersham Pharmacia Biotech).
Bacterial growth
Bacteria were grown in brain heart infusion medium supplemented with 6.8 μg/mL chloramphenicol. The bacteria were frozen in aliquots and stored at −80°C.
Generation of recombinant vaccinia virus Vac-L1
Similar to a prior recombinant vaccinia virus expressing L1 (40), a recombinant vaccinia virus was generated by transfection with pSC65-based plasmid construct (41). pSC65 is a modified shuttle vector that drives foreign gene expression with the synthetic vaccinia virus early-late promoter. However, unlike the previously described virus (40), we made a silent mutation within a cryptic termination signal that appears in the HPV L1 coding sequence to prevent expression of a truncated form of the protein at early times of infection. To accomplish this, we designed the following oligonucleotides and performed SOEing PCR. The oligonucleotides synthesized for this modification and amplification were as follows: 5′-CGCGTCGAC ATGTCTCTTTGGCTGCCTAGTGAGGCCACTG-3′, where the underlined sequence represents an introduced Sal I site; 5′-CCTTCGTAAATAAAAGAATAAGCTGTCG CC-3′ and 5′-GGCGACAGCTTATTCTTTTATTTACGAAGG-3′, where the bold nucleotide represents the base change to alter the termination signal (this would result in a codon change from TTT to TTC, which both represent a phenylalanine), and 5′-GGACCCGGG TTA CAATCCTGCTTGTAGTAAAAATTTGCG- 3′, where the underlined sequence represents an introduced Sma I site. After amplification, the resultant fragment was digested with Sal I and Sma I and then ligated into pSC-65. Successful introduction of this modification was confirmed by sequencing. The recombinant virus was isolated by standard procedure. Briefly, pSC65-L1 was used to transfect CV1 cells that had been infected with wild-type vaccinia virus strain WR (Western Reserve). Cell lysates obtained with this infection-transfection step contained vaccinia virus recombinant that was plaque purified three times on a thymidine kinase–deficient cell line in the presence of bromodeoxyuridine along with staining with X-Gal. Expression of L1 product by plaque-purified vaccinia virus was verified by Western blotting using BSC-1 cells.
Mice
Female C57BL/6 mice were purchased from Charles River Breeding Laboratories (Wilmington, MA) or The Jackson Laboratory (Bar Harbor, ME). All mice were maintained in a pathogen-free environment. The mice used in this study were 6–12 wk old.
Immunization of mice
C57BL/6 mice were orally immunized and boosted 3 wk later, as previously described (31). Briefly, the oral immunization was performed by intragastric inoculation using feeding needles to deliver 109 CFU of bacteria in 200 μL PBS. The animals were not fasted before immunization. Control mice received the same treatment using Lm-LLO-NP (27). Seven days after the boost the mice were sacrificed and spleens and Peyer's patches were harvested for evaluation of the immune responses.
Bacterial translocation studies
Spleen and Peyer's patches (PPs) were removed on days 2, 3, and 5 after oral immunization with 109 Lm constructs. Four mice were used for each time point for each group. Each tissue was homogenized in 1 mL PBS, and bacterial growth was determined by plating serial 10-fold dilutions on BHI agar supplemented with the appropriate concentration of chloramphenicol. The detection limit was set to 102 CFU per organ. The colonies were counted after 24–48 h of incubation at 37°C. Bacterial levels in each tissue were calculated as CFU per tissue homogenate for each mouse at each time point and plotted individually. Statistical analyses were performed using a non-parametric Mann-Whitney test.
Preparation of tissue for T-cell analysis
A single-cell suspension was prepared from the spleen and PP by homogenization of the tissues in RPMI medium and filtration of the homogenate through a nylon mesh. Cells were pelleted gently and washed three times with RPMI supplemented with 10% FCS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 100 μM non-essential amino acids, and 1 mM sodium pyruvate.
Oligonucleotide primers
Primers were synthesized by Operon Technologies (Alameda, CA) and were resuspended in Tris-EDTA and stored at −20°C.
Synthetic peptide and VLP
Synthetic L1 (165–173) peptide was synthesized by EZBiolab (Westfield, IN) and purified by HPLC to 97.2% purity. A stock solution was prepared in PBS at a concentration of 2 mg/mL. VLP was a kind gift from Dr. Richard Roden (Department of Pathology, Johns Hopkins University), and Dr. Robert Rose (Department of Microbiology, University of Rochester).
Flow cytometric analysis
Because the immunodominant peptide for H-2b mice is known (14) and is an epitope in the N-terminal construct, C57BL/6 mice were immunized orally with 1 × 109 CFU of Lm-PL2-LLO-L11–258 and boosted 3 wk later. Splenocytes harvested 7 d after the boost were stimulated in vitro with the L1165–173 peptide (AGVRNERCI) (2 μM), PMA (25 ng/mL), and ionomycin (1 μM), or left unstimulated for 5 h at 37°C. L1165–173 has been identified as the immunodominant epitope for L1 recognition by CTL in the H-2b mouse (14). Three-color flow cytometry for CD8β (53-6.7, PE conjugated; BD Pharmingen, San Diego, CA), CD62L (MEL-14, APC conjugated; BD Pharmingen), and IFN-γ (PE conjugated XMG 1.2; BD Pharmingen) was performed using a FACS Calibur flow cytometer and analyzed with CellQuest software (Becton Dickinson, Mountain View, CA).
ELISPOT assay
Multiscreen ninety-six-well plates (Millipore, Bedford, MA) were coated with 15 μg/mL rat anti-mouse IFN-γ antibody (clone AN18; Mabtech, Mariemont, OH) in 100 μL of PBS and incubated overnight at 4°C. The wells were washed and blocked with culture medium containing 10% fetal bovine serum. Serial dilutions of 4 × 105, 2 × 105, and 1 × 105/well of pooled splenocytes and Peyer's patch lymphocytes from each vaccinated mouse group were applied to the wells in triplicate. The cells were incubated at 37°C for 24 h with IL-2 (5 U/mL) and stimulated with VLP (10 μg/mL), concanavalin A (2.5 μg/mL; Sigma) or left unstimulated. The plates were then washed and followed by incubation with 1 μg/mL biotinylated IFN-γ antibody (clone R4-6A2; Mabtech) in 100 μL PBS at 4°C overnight. After washing, 1:100 streptavidin-horseradish peroxidase in 100 μL PBS were added and incubated for 1 h at room temperature. The spots were developed after washing, the addition of 100 μL of substrate, and incubation at room temperature for 15 min. Color development was stopped by washing extensively in tap water. The spots were counted on an ELISPOT reader. The results were normalized by subtraction of background spots in the corresponding unstimulated wells from the VLP-stimulated wells, and the results were expressed as the mean number and standard deviation of IFN-γ-secreting cells per 4 × 105 splenocytes or PP lymphocytes.
Depletion experiment
Splenocytes were harvested by physical disaggregation, passed through a 100-micron sieve, and incubated with rat antibodies designed to deplete all cells except CD4 or CD8 T cells (per the instructions for use of the Dynal negative isolation kits). Subsequently, washed Dynal beads (coated with anti-rat Ig) were added to the cells in 14-mL tubes, and the tubes were placed on a magnet until the labeled cells adhered to the magnet side of the tube. Supernatant containing either purified CD4 or CD8 T cells was removed from the tubes and the cell concentration was counted on a hemocytometer. The purity of the CD4 or CD8 T cells was verified as ≥90% by flow cytometry analysis using a combination of conjugated CD3, CD4, and CD8 antibodies.
Recombinant vaccinia virus Vac-L1 challenge
Mice were immunized as described above. Nine days after the boost, the mice were subcutaneously injected with 2 mg progesterone (Depo-Provera; Pharmacia & Upjohn, Kalamazoo, MI) to thin the vaginal mucosa (42). Five days later the mice were anesthetized via IP injection of ketamine (100 mg/kg body weight; Wyeth, Madison, NJ) and xylazine (10 mg/kg; Butler Co.) in 100 μL 0.9% NaCl, and infected intravaginally with a total dose of 4 × 107 PFU of recombinant Vac-L1 (given as two 10-uL instillations of 2 × 107 PFU over a 1-h period). Six days after the challenge, the mice were sacrificed and the uterus and cervix were dissected from each mouse. The tissues were then homogenized in nylon mesh and viral titers determined on BSC-1 cell monolayers by serial dilutions. The resulting plaques were stained with 0.1% crystal violet. The number of PFU of virus in each tissue was plotted for individual mice. Statistical analyses of the average viral titer within each group of mice were performed using a non-parametric Mann-Whitney test.
Results
Construction of chromosomal rL. monocytogenes that express and secrete HPV-16-L1 protein
We have designed and constructed two Lm strains that express and secrete LLO as a fusion protein with overlapping fragments of HPV-16 L1. In Lm-PL2-LLO-L11–258 and Lm-PL2-LLO-L1238–474 constructs, a site-specific single copy phage integration vector (38) was used to express and secrete a fusion protein consisting of a non-functional LLO joined with either the L11–258 or L1238–474 fragment. Typically, large proteins are not well tolerated by prokaryotic organisms and we have frequently resorted to expressing proteins greater than 50 kD as overlapping polypeptide fragments (28,36). Since the immune response generated by Listeria is predominantly cell-mediated, any impact this vaccine design may have on the proper folding of the foreign protein, and thus its ability to generate neutralizing antibodies, is not of consequence.
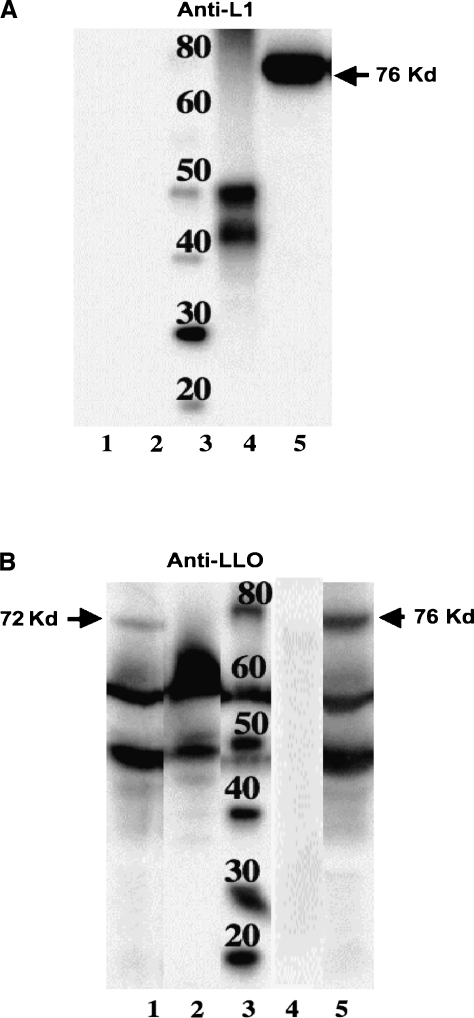
To evaluate the expression of the recombinant fusion proteins by these constructs, we used Western blot analysis of the protein secreted by these constructs. As shown in Fig. 1A, Lm-PL2-LLO-L11–258 secreted a 76-kDa LLO-L11–258 recombinant protein that was detected when probed with an antibody that is thought to recognize a linear internal epitope of L1 (NeoMarkers data sheet, Fremont, CA). Lm-PL2-LLO-L1238–474 did not show a secreted L1 product when probed with anti-L1 antibody in this blot, as presumably the epitope recognized is not within this fragment. However, when an anti-LLO antibody was used, a 72-kDa protein corresponding to the secreted LLO-L1238–474 fusion protein was detected in Lm-PL2-LLO-L1238–474 culture supernatant (Fig. 1B). As expected, the anti-LLO antibody also detected the LLO-L11–258 secreted by the Lm-PL2-LLO-L11–258, and indicates that there is poorer expression of the C-terminal fusion protein (Fig. 1B). In addition to the fusion proteins (72 and 76 kDa) the anti-LLO antibody (Fig. 1B) also detects endogenous full-length LLO (58 kDa) and a degradation product of LLO (48 kDa), which we commonly detect in expression systems of this type.
FIG. 1.
LLO-L11–258 and LLO-L1238–474 secretion were analyzed by Western blots. Lm-PL2-LLO-L1238–474 (lane 1), wild-type Listeria monocytogenes 10403s (lane 2), molecular weight marker (lane 3), HPV-16 VLP (lane 4), and Lm-PL2-LLO-L11–258 (lane 5) were grown on a shaker overnight at 37°C in Lauria-Bertoni broth. Equivalent numbers of bacteria, as determined by OD600-nm absorbance, were pelleted and 18 mL of each supernatant was TCA precipitated. The blots were probed with anti-HPV-16-L1 monoclonal antibody (Neomarker) (A) and anti-LLO polyclonal antibody (B), followed by HRP-conjugated anti-mouse or anti-rabbit secondary antibody (Amersham). The blots were developed using ECL detection reagents (Amersham). Note that in Fig. 1B some lanes have been removed from the original Western blots, but all the lanes shown came from a single Western blot.
Gut translocation of Lm recombinant constructs
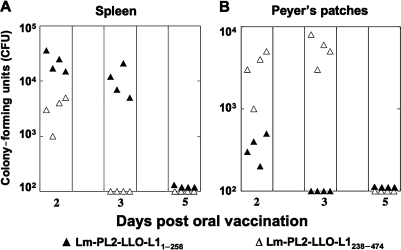
Mucosal immune responses induced by oral immunization could be dependent on antigen presentation at the mucosal induction sites by infected cells. Induction of CD8+ T cells strongly depends on the presence of Listeria in the induction sites for successful T-cell-mediated immunity (43). Therefore, we investigated the kinetics of bacterial translocation into the spleen and PP after primary oral immunization. When bacteria are given orally, they are challenged by the acidic environment of the stomach, the commensal flora of the intestinal tract, and the mucus and epithelial layers as physical barriers. To induce immunity, the bacteria must overcome these considerable challenges to translocate to immune-inductive sites. As shown in Fig. 2A and B, oral immunization resulted in transient colonization of the spleen and PP. The colonization profile of the recombinant Lm constructs differed among the tissues evaluated. Higher levels of Lm-PL2-LLO-L11–258 were detectable in the spleen until day 3 post-inoculation (Fig. 2A), whereas Lm-PL2-LLO-L1238–474 had somewhat lower levels that were detectable for only 2 d. In contrast, in the PP, Lm-PL2-LLO-L1238–474 had high levels in the Peyer's patches that were detectable until day 3 post-inoculation, while Lm-PL2-LLO-L11–258 was rapidly cleared (Fig. 2B). The number of bacteria in the spleen and PP of Lm-PL2-LLO-L11–258 compared to Lm-PL2-LLO-L1238–474 on days 2 and 3 was statistically significant (p < 0.05). All mice in both groups immunized with the two constructs successfully cleared the bacteria 5 d after immunization in both tissues, and levels of bacteria were below the lowest detection limit (Fig. 2A and B).
FIG. 2.
Time course of translocation to the spleen (A) and Peyer's patches (B) after a single oral immunization of C57BL/6 mice with 109 CFU of the recombinant L. monocytogenes strains Lm-PL2-LLO-L11–258 (solid triangles) and Lm-PL2-LLO-L1238–474 (open triangles).. Data are shown as colony-forming units (CFU) per organ homogenate after immunization as described in materials and methods. Individual data are shown for each of four mice per group per time point.
Induction of cell-mediated immunity after oral immunization with Lm-L1 constructs
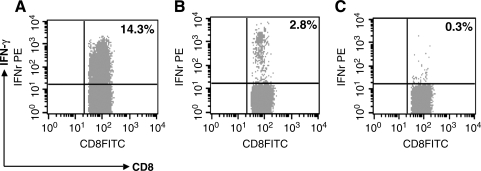
To determine whether oral immunization with Lm-PL2-LLO-L11–258, which expresses the N-terminus of HPV16 L1 protein, could generate CD8+ T cells specific to L1, we measured the immune response to an MHC class I-restricted epitope previously identified in the C57BL/6 mouse that comprises the residues 165–173 of the L1 protein (14). IFN-γ production in spleen and PP from immunized mice after in-vitro stimulation with the L1 peptide was measured by intracellular cytokine staining. Fig. 3 shows the induction of activated IFN-γ-producing CD8+ T cells in the spleens of mice immunized with Lm-PL2-LLO-L11–258, corresponding to approximately 3% of the total CD8+ T cells in these mice (Fig. 3B). In the PP, however, L1-specific CD8+ IFN-γ-producing T cells were not detected by intracellular cytokine staining in mice immunized with Lm-PL2-LLO-L11–258 (data not shown). Mice immunized with the L1 C-terminus-expressing construct (Lm-PL2-LLO-L1238–474) were not tested in this assay, as no CTL epitope has been mapped out in the C-terminus fragment that can be used for in-vitro restimulation.
FIG. 3.
The induction of L1-specific CD8+ T cells in the spleen after oral immunization and boost with 109 CFU Lm-PL2-LLO-L11–258. Four C57BL/6 mice were immunized and boosted 3 wk after the primary immunization. Spleens were removed on day 7 after the boost, and splenocytes were analyzed for the presence of activated IFN-γ-producing CD8+ T cells. Splenocytes were restimulated ex vivo for 5 h with (A) PMA and ionomycin, (B) L1165–173-specific peptide, or (C) left without stimulation. The plots show CD8+ CD62Llow cells gated using CellQuest software. These data are representative of three different experiments.
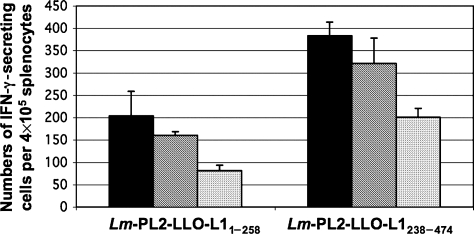
To further test the induction of T-cell responses and determine whether oral immunization could generate functional CD8+ and CD4+ T cells in the spleen and PP from mice immunized and boosted orally by both Lm strains, we measured antigen-specific IFN-γ production by ELISpot assay. Splenocytes and PP lymphocytes from immunized mice were restimulated in vitro with HPV16 VLPs. After in-vitro stimulation with VLPs for 24 h, L1-specific IFN-γ-secreting cells were detected in the spleens of mice immunized with either Lm-PL2-LLO-L11–258 or Lm-PL2-LLO-L1238–474 (Fig. 4). The individual contributions of L1-specific CD8+ and CD4+ T cells were evaluated after depletion of each subset of these T lymphocytes. Upon immunization, both CD8+ and CD4+ T-cell responses were induced in the spleen, and L1-specfic CD4+ T cells outnumbered CD8+ T cells (Fig. 4). Interestingly, L1-specific IFN-γ-producing cells (122 ± 40 per 4 × 105 cells) were observed in the PP from mice immunized with the Lm-PL2-LLO-L1238–474 (data not shown). In contrast, T-cell responses were undetectable in the PP after oral immunization with Lm-PL2-LLO-L11–258 (data not shown). These findings are consistent with our data on gut translocation for each construct, since only the Lm-PL2-LLO-L1238–474 but not the Lm-PL2-LLO-L11–258 construct could be detected in the PP 3 d after primary immunization. Depletion of CD4+ and CD8+ T cells was not performed on PP lymphocytes, as we recovered insufficient number of cells to perform these experiments.
FIG. 4.
The induction of L1-specific CD8+ T cells in the spleen after oral immunization with 109 colony forming units (CFU) of Lm-PL2-LLO-L11–258 and Lm-PL2-LLO-L1238–274. C57BL/6 mice were immunized and boosted 3 wk after the primary immunization. Spleens were removed on day 7 after the boost, and analyzed for the presence of IFN-γ-secreting CD8+ and CD4+ T cells. Splenocyte suspensions were pooled from four mice from each group and were restimulated ex vivo for 24 h with VLP or left unstimulated. Unstimulated background spots were subtracted from stimulated samples to standardize the results. Whole splenocyte responses are shown in black. CD4+ T-cell enriched splenocytes (i.e., depleted of CD8+ T cells) are shown as white bars, and CD8+ T-cell enriched splenocytes (i.e., depleted of CD4+ cells) are shown as grey bars. Data are presented as mean and standard deviation of three independent experiments.
Protection against mucosal virus challenge
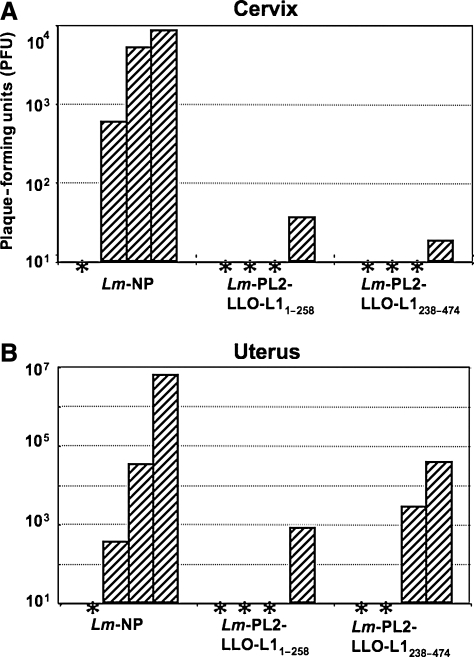
We determined that cell-mediated immunity can be induced by oral immunization with either Lm-PL2-LLO-L11–258 or Lm-PL2-LLO-L1238–474 vaccines. To evaluate whether these responses are protective, we challenged immunized mice in the vaginal mucosa with a recombinant vaccinia virus that expresses the HPV16-L1 protein (Vac-L1). Mice were orally immunized twice with either Lm-PL2-LLO-L11–258 or Lm-PL2-LLO-L1238–474 vaccines and then progesterone-treated animals were challenged intravaginally with Vac-L1. Six days after the challenge the mice were sacrificed, the cervix and uterus harvested, and viral titers determined. Because of the degree of variance of viral titers between individual mice, statistical significance was not achieved (p < 0.09) between the average viral load for immunized versus the control groups of mice. However, in mice immunized with a control Lm vaccine expressing the influenza nucleoprotein as an irrelevant antigen (Lm-NP), high titers of Vac-L1 were observed in the cervix of three out of four mice (Fig. 5A). In contrast, three out of four mice immunized with either construct had viral titers below the level of detection, and a low amount of virus was detected in the cervix in one mouse in each group (Fig. 5A). Similarly, viral titers were detected in the uterus of three out of four mice immunized with Lm-NP and challenged with Vac-L1 (Fig. 5B). Three out of four mice immunized with Lm-PL2-LLO-L11–258 showed no viral titers in the uterus. On the other hand, in mice immunized with Lm-PL2-LLO-L1238–474, two mice were below the level of detection and the other two mice had moderate levels of virus detected (Fig. 5B).
FIG. 5.
Oral immunization with recombinant L. monocytogenes constructs expressing and secreting overlapping HPV16-L1 fragments results in diminished viral titers in the cervix and uterus. Groups of four C57BL/6 female mice were orally immunized and boosted 3 wk later with Lm-PL2-LLO-L11–258, Lm-PL2-LLO-L1238–474, or a control Listeria expressing an irrelevant protein (Lm-NP). The mice were treated with progesterone and then challenged intravaginally with a recombinant vaccinia virus expressing HPV16-L1. The mice were sacrificed 6 d after challenge and the cervices and uteri were harvested. Shown are titers of vaccinia virus in the cervix (A) and uterus (B) from individual mice. The asterisks represent levels below the level of detection (<5 plaques/organ).
Discussion
The natural route of transmission of HPV and many other pathogens is through mucosal surfaces, thus vaccines applied through mucosal surfaces offer the opportunity to induce both secretory antibody and CTL mucosal immune responses and protection against these pathogens (15,44–46). Although mucosal immunity can be induced by parenterally administered vaccines, a more robust response is usually elicited by vaccines that are administered through the oral, nasal, vaginal, or rectal route. The oral vaccination route also offers certain advantages over other routes, including ease of administration and reduced cost of production compared with other vaccines formulated for injection.
This study addressed whether immune responses and protection specific to the HPV16 L1 protein could be generated by a recombinant Lm as a mucosal vaccine. For this purpose, we generated two recombinant Lm strains, Lm-PL2-LLO-L11–258 and Lm-PL2-LLO-L1238–474, which respectively express fragments of the N- and C-terminal region of the HPV16 L1 protein as a fusion protein to a non-hemolytic LLO. In previous studies we have shown that fusing a target antigen to a non-functional LLO increases the immunogenicity of the antigen and the efficacy of the recombinant Lm as a vaccine vector (34,36).
We first determined the effectiveness of Lm-PL2-LLO-L11–258 and Lm-PL2-LLO-L1238–474 administered orally to translocate to the gut and persist in the mucosal and systemic immune response inductive sites. Both Lm-PL2-LLO-L11–258 and Lm-PL2-LLO-L1238–474 constructs translocated and could be found in the spleen and Peyer's patches on day 2 after immunization (Fig. 2). Interestingly, only Lm-PL2-LLO-L11–258 persisted in the spleen until day 3, whereas Lm-PL2-LLO-L1238–474 only transiently infected the spleen but persisted in Peyer's Patches until day 3 (Fig. 2). Given that colonization of Listeria by the oral route first results in its appearance in Peyer's patches followed by spread to the mesenteric lymph nodes and then the spleen (31), this suggests that Lm-PL2-LLO-L1238–474 is less able to spread than Lm-PL2-LLO-L11–258, due to lower virulence. The mechanism of spread is not well known but probably involves the colonization of macrophages that then carry the bacteria through the lymphatics to distant organs. Although both recombinant strains are isogenic, except for the HPV-16 L1 fragment they express, differences in virulence and the ability to persist in macrophages can vary substantially among isogenic strains of bacteria associated with foreign antigen expression (47).
We further found that oral immunization with both constructs induced splenic IFN-γ-producing CD8+ T cells (Figs. 3 and 4). Both vaccines induced substantial numbers of these cells, although an immunodominant epitope for HPV-16 L1 has been identified only in the 1–258 fragment of the molecule (14). However, we have previously shown that delivery of a foreign antigen by Listeria and also fusion to LLO can enhance antigen processing and reveal sub-dominant epitopes that do not emerge using other vaccine approaches (36). Importantly, in this study we also show the induction of splenic L1-specific CD4+ T cells after oral immunization with our Lm recombinant constructs (Fig. 4). The IFN-γ produced by CD4+ T cells may be a necessary supplement for protection against antigenic challenge; however, they are also likely to be playing a role in the induction of CTL. Interestingly, IFN-γ-producing cells in the PP were induced only after oral immunization with Lm-PL2-LLO-L1238–474. Our finding that only Lm-PL2-LLO- L1238–474 persisted in the PP until day 3 after oral immunization (Fig. 2B) is consistent with the notion that persistent antigenic exposure by recombinant Lm is a prerequisite for induction of antigen-specific T cells (43).
The efficacy of vaccines depends on their generation of a potent pool of Ag-specific T cells ready to expand rapidly to express killing function and protect against re-exposure to the antigen. The induced L1-specific CD8+ and CD4+ T cells after immunization should provide protection against HPV challenge and constitute an effective line of defense against antigenic challenge. As indicated above, the L1-specific CD8+ and CD4+ T cells induced after oral immunization with the two recombinant Lm constructs showed evidence of protection against Vac-L1 challenge in mice immunized with Lm-PL2-LLO-L11–258 and Lm-PL2-LLO-L1238–474 (Fig. 5). Previous studies have shown that parenteral immunization with VLP or capsomeres induces potent systemic cell-mediated immune responses resulting in protection or regression of established C3 tumors, which express HPV16 L1, in mice (14). Intranasal and oral vaccination with different recombinant strains of Salmonella have shown induction of neutralizing antibodies in the serum and genital tract, as well as protection from experimental tumor challenge with C3 cells (48,49). Mucosal intranasal immunization with VLP has also been shown to induce serum and genital antibody responses (50). In humans, VLP vaccination induces neutralizing antibody responses that results in protection against HPV infection (9,11). In fact, studies in animal models and humans have clearly shown the importance of neutralizing antibody responses in protection from or prevention of HPV transmission. In this study we were not able to detect antibody titers against L1 in the serum or vaginal secretions (data not shown). This is in agreement with previous observations showing that Lm in not a potent inducer of humoral immune responses since it is an intracellular pathogen (32). Thus the protection conferred by the Lm vaccines in this study is clearly cell mediated. This is consistent with studies performed in the cotton-tailed rabbit papillomavirus model of papilloma infections, where cell-mediated immune responses against L1 have been shown to be effective at limiting papilloma formation in both a prophylactic (51) and therapeutic (52) setting.
Although VLP vaccination has shown clear success in preventing HPV infection, vaccination with VLPs is ineffective against established HPV infections (53). In this case mucosal cell-mediated immune responses to HPV antigens may be more effective for viral clearance, instead of neutralizing antibodies. However, targeting L1 by cellular immunity is limited to active HPV infections and low-grade squamous intraepithelial lesions, because L1 is generally lost in high-grade squamous intraepithelial lesions and invasive cancers (54). For these advanced lesions, vaccines targeting the oncoproteins E6 and E7 from HPV have a clear advantage, as these genes are required in the malignant transformation process and are retained in cervical carcinomas (55,56).
Conclusion
In conclusion, this study describes new oral Lm-based HPV-L1-expressing vaccines, which induce potent antigen-specific CD8+ and CD4+ T-cell responses and show evidence of protection against a heterologous challenge. Clinical trials have shown the efficiency of parenteral VLP vaccines in prevention of cervical infection with certain HPV types and subsequent cervical cancer. This work is an initial step toward examining the contribution of cellular immune responses to L1 generated by mucosally administered Lm constructs expressing L1.
Acknowledgments
This work was supported by CA69632 (Y.P.) and AI066333 (S.N.I.). W.M. was partially supported by training grant T32CA09140.
Disclosure Statement
Yvonne Paterson wishes to disclose that she has a financial interest in Advaxis, Inc., a vaccine and therapeutic company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of Listeria monocytogenes or listerial products as vaccines.
References
- 1.Ho GY. Burk RD. Klein S, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–1371. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 2.Nobbenhuis MA. Walboomers JM. Helmerhorst TJ, et al. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20–25. doi: 10.1016/S0140-6736(98)12490-X. [DOI] [PubMed] [Google Scholar]
- 3.Wallin KL. Wiklund F. Angstrom T, et al. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N Engl J Med. 1999;341:1633–1638. doi: 10.1056/NEJM199911253412201. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX. Lorincz A. Muñoz N. Meijer CJ. Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J. Sun XY. Davies H. Crawford LV. Park D. Frazer IH. Definition of linear antigenic regions of the HPV16 L1 capsid protein using synthetic virion-like particles. Virology. 1992;189:592–529. doi: 10.1016/0042-6822(92)90582-a. [DOI] [PubMed] [Google Scholar]
- 6.Gissmann L. Osen W. Muller M. Jochmus I. Therapeutic vaccines for human papillomaviruses. Intervirology. 2001;44:167–175. doi: 10.1159/000050044. [DOI] [PubMed] [Google Scholar]
- 7.Schiller JT. Lowy DR. Papillomavirus-like particle vaccines. J Natl Cancer Inst Monogr. 2001;28:50–54. doi: 10.1093/oxfordjournals.jncimonographs.a024258. [DOI] [PubMed] [Google Scholar]
- 8.Schiller JT. Davies P. Delivering on the promise: HPV vaccines and cervical cancer. Nat Rev Microbiol. 2004;2:343–347. doi: 10.1038/nrmicro867. [DOI] [PubMed] [Google Scholar]
- 9.Harper DM. Franco EL. Wheeler C et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 10.Lowy DR. Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116:1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villa LL. Costa RL. Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose RC. White WI. Li M. Suzich JA. Lane C. Garcea RL. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J Virol. 1998;72:6151–6154. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan H. Estes PA. Chen Y. Newsome J. Olcese VA. Garcea RL. Schlegel R. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J Virol. 2001;759:7848–7853. doi: 10.1128/JVI.75.17.7848-7853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohlschlager P. Osen W. Dell K, et al. Human papillomavirus type 16 L1 capsomeres induce L1-specific cytotoxic T lymphocytes and tumor regression in C57BL/6 mice. J Virol. 2003;77:4635–4645. doi: 10.1128/JVI.77.8.4635-4645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmgren J. Czerkinsky C. Mucosal immunity, vaccines. Nat Med. 2005;11(4 Suppl):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 16.Neutra MR. Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 17.Aziz MA. Midha S. Waheed SM. Bhatnagar R. Oral vaccines: new needs, new possibilities. Bioessays. 2007;29:591–604. doi: 10.1002/bies.20580. [DOI] [PubMed] [Google Scholar]
- 18.Balmelli C. Roden R. Potts A. Schiller J. De Grandi P. Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998;72:8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupuy C. Buzoni-Gatel D. Touze A. Bout D. Coursaget P. Nasal immunization of mice with human papillomavirus type 16 (HPV-16) virus-like particles or with the HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymph nodes. J Virol. 1999;73:9063–9071. doi: 10.1128/jvi.73.11.9063-9071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose RC. Lane C. Wilson S. Suzich JA. Rybicki E. Williamson AL. Oral vaccination of mice with human papillomavirus virus-like particles induces systemic virus-neutralizing antibodies. Vaccine. 1999;179:2129–2135. doi: 10.1016/s0264-410x(98)00484-8. [DOI] [PubMed] [Google Scholar]
- 21.Gerber S. Lane C. Brown DM, et al. Human papillomavirus virus-like particles are efficient oral immunogens when coadministered with Escherichia coli heat-labile enterotoxin mutant R192G or CpG DNA. J Virol. 2001;75:4752–4760. doi: 10.1128/JVI.75.10.4752-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mestecky J. Kutteh WH. Jackson S. Mucosal immunity in the female genital tract: relevance to vaccination efforts against the human immunodeficiency virus. AIDS Res Hum Retroviruses. 1994;10(Suppl 2):S11–S20. [PubMed] [Google Scholar]
- 23.Kozlowski PA. Cu-Uvin S. Neutra MR. Flanigan TP. Mucosal vaccination strategies for women. J Infect Dis. 1999;179(Suppl 3):S493–S498. doi: 10.1086/314810. [DOI] [PubMed] [Google Scholar]
- 24.Nardelli-Haefliger D. Roden R. Balmelli C. Potts A. Schiller J. De Grandi P. Mucosal but not parenteral immunization with purified human papillomavirus type 16 virus-like particles induces neutralizing titers of antibodies throughout the estrous cycle of mice. J Virol. 1999;73:9609–9613. doi: 10.1128/jvi.73.11.9609-9613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gedde MM. Higgins DE. Tilney LG. Portnoy DA. Role of listeriolysin in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pamer EG. Sijts AJ. Villanueva MS. Busch DH. Vijh S. MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses. Immunol Rev. 1997;158:129–136. doi: 10.1111/j.1600-065x.1997.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 27.Ikonomidis G. Portnoy DA. Gerhard W. Paterson Y. Influenza-specific immunity induced by recombinant Listeria monocytogenes vaccines. Vaccine. 1997;15:433–440. doi: 10.1016/s0264-410x(96)00188-0. [DOI] [PubMed] [Google Scholar]
- 28.Boyer JD. Robinson TM. Maciag PC, et al. DNA prime Listeria boost induces a cellular immune response to SIV antigens in the rhesus macaque model that is capable of limited suppression of SIV239 viral replication. Virology. 2005;333:88–101. doi: 10.1016/j.virol.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Boyer JD. Maciag PC. Parkinson R. Wu L. Lewis MG. Weiner DB. Paterson Y. Rhesus macaques with high levels of vaccine induced IFN-gamma producing cells better control viral set-point following challenge with SIV239. Vaccine. 2006;24:4498–4502. doi: 10.1016/j.vaccine.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Neeson P. Boyer J. Kumar S, et al. A DNA prime-oral Listeria boost vaccine in rhesus macaques induces a SIV-specific CD8 T cell mucosal response with high levels of α4β7 integrin and effector memory phenotype. Virology. 2006;354:299–315. doi: 10.1016/j.virol.2006.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters C. Peng X. Douven D. Pan ZK. Paterson Y. The induction of HIV-Gag specific CD8 + T cells in the spleen and GALT by parenteral or mucosal immunization using recombinant Listeria monocytogenes-HIV-Gag. J Immunol. 2003;170:5176–5187. doi: 10.4049/jimmunol.170.10.5176. [DOI] [PubMed] [Google Scholar]
- 32.Pan ZK. Ikonomidis G. Lazenby A. Pardoll D. Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 33.Pan ZK. Weiskirch LM. Paterson Y. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res. 1999;59:5264–5269. [PubMed] [Google Scholar]
- 34.Gunn GR. Zubair A. Peters C. Pan ZK. Wu TC. Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 35.Souders NC. Sewell DA. Pan ZK, et al. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8 + T cells capable of killing a solid tumor in a transgenic mouse model of cancer. Cancer Immunity. 2007;7:2. [PMC free article] [PubMed] [Google Scholar]
- 36.Singh R. Dominiecki ME. Jaffee EM. Paterson Y. Fusion to listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663–3673. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- 37.Touze A. Mahe D. El Mehdaoui S, et al. The nine C-terminal amino acids of the major capsid protein of the human papillomavirus type 16 are essential to DNA binding and gene transfer capacity. FEMS Microbiol Lett. 2000;189:121–127. doi: 10.1111/j.1574-6968.2000.tb09217.x. [DOI] [PubMed] [Google Scholar]
- 38.Laure P. Chow MY. Loessner MJ. Portnoy DA. Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verch T. Pan ZK. Paterson Y. Listeria monocytogenes-based antibiotic resistance gene-free antigen delivery system applicable to other bacterial vectors and DNA vaccines. Infect Immun. 2004;72:6418–6425. doi: 10.1128/IAI.72.11.6418-6425.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marais D. Passmore JA. Maclean J. Rose R. Williamson AL. A recombinant human papillomavirus (HPV) type 16 L1-vaccinia virus murine challenge model demonstrates cell-mediated immunity against HPV virus-like particles. J Gen Virol. 1999;80:2471–2475. doi: 10.1099/0022-1317-80-9-2471. [DOI] [PubMed] [Google Scholar]
- 41.Chakrabarti S. Sisler JR. Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 42.Jiang JQ. Patrick A. Moss RB. Rosenthal KL. CD8 + T-cell-mediated cross-clade protection in the genital tract following intranasal immunization with inactivated human immunodeficiency virus antigen plus CpG oligodeoxynucleotides. J Virol. 2005;79:393–400. doi: 10.1128/JVI.79.1.393-400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.North RJ. Berche PA. Newborg MF. Immunologic consequences of antibiotic-induced abridgement of bacterial infection: effect on generation and loss of protective T cells and level of immunologic memory. J Immunol. 1981;127:342–346. [PubMed] [Google Scholar]
- 44.Guerrero RA. Ball JM. Krater SS. Pacheco SE. Clements JD. Estes MK. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J Virol. 2001;75:9713–9722. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi W. Liu J. Huang Y. Qiao L. Papillomavirus pseudovirus: a novel vaccine to induce mucosal and systemic cytotoxic T-lymphocyte responses. J Virol. 2001;75:10139–10148. doi: 10.1128/JVI.75.21.10139-10148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niikura M. Takamura S. Kim G, et al. Chimeric recombinant hepatitis E virus-like particles as an oral vaccine vehicle presenting foreign epitopes. Virology. 2002;293:273–280. doi: 10.1006/viro.2001.1240. [DOI] [PubMed] [Google Scholar]
- 47.Galen JE. Levine MM. Can a “flawless” live vector vaccine strain be engineered? Trends Microbiol. 2001;9:372–376. doi: 10.1016/s0966-842x(01)02096-0. [DOI] [PubMed] [Google Scholar]
- 48.Revaz V. Benyacoub J. Kast WM. Schiller JT. De Grandi P. Nardelli-Haefliger D. Mucosal vaccination with a recombinant Salmonella typhimurium expressing human papillomavirus type 16 (HPV16) L1 virus-like particles (VLPs) or HPV16 VLPs purified from insect cells inhibits the growth of HPV16-expressing tumor cells in mice. Virology. 2001;279:354–360. doi: 10.1006/viro.2000.0717. [DOI] [PubMed] [Google Scholar]
- 49.Fraillery D. Baud D. Pang SY, et al. Salmonella enterica serovar typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin Vaccine Immunol. 2007;14:1285–1295. doi: 10.1128/CVI.00164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasagawa T. Tani M. Basha W, et al. A human papillomavirus type 16 vaccine by oral delivery of L1 protein. Virus Res. 2005;110:81–90. doi: 10.1016/j.virusres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Hu J. Cladel NM. Budgeon LR. Reed CA. Pickel MD. Christensen ND. Protective cell-mediated immunity by DNA vaccination against papillomavirus L1 capsid protein in the cottontail rabbit papillomavirus model. Viral Immunol. 2006;19:492–507. doi: 10.1089/vim.2006.19.492. [DOI] [PubMed] [Google Scholar]
- 52.Govan VA. Rybicki EP. Williamson AL. Therapeutic immunisation of rabbits with cottontail rabbit papillomavirus (CRPV) virus-like particles (VLP) induces regression of established papillomas. Virol J. 2008;5:45. doi: 10.1186/1743-422X-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hildesheim A. Herrero R. Wacholder S, et al. the Costa Rican HPV Vaccine Trial Group. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 54.Stoler MH. Rhodes CR. Whitbeck A. Wolinsky SM. Chow LT. Broker TR. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992;23:117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- 55.Choo KB. Pan CC. Han SH. Integration of human papillomavirus type 16 into cellular DNA of cervical carcinoma: preferential deletion of the E2 gene and invariable retention of the long control region and the E6/E7 open reading frames. Virology. 1987;161:259–261. doi: 10.1016/0042-6822(87)90195-4. [DOI] [PubMed] [Google Scholar]
- 56.Vousden KH. Doniger J. DiPaolo JA. Lowy DR. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncogene Res. 1988;3:167–175. [PubMed] [Google Scholar]