Abstract
As a result of their pluripotency and potential for unlimited self-renewal, human embryonic stem cells (hESCs) hold tremendous promise in regenerative medicine. An essential prerequisite for the widespread application of hESCs is the establishment of effective and efficient protocols for large-scale cell culture, storage, and distribution. At laboratory scales hESCs are cultured adherent to tissue culture plates; these culture techniques are labor-intensive and do not scale to high cell numbers. In an effort to facilitate larger scale hESC cultivation, we investigated the feasibility of culturing hESCs adherent to microcarriers. We modified the surface of Cytodex 3 microcarriers with either Matrigel or mouse embryonic fibroblasts (MEFs). hESC colonies were effectively expanded in a pluripotent, undifferentiated state on both Matrigel-coated microcarriers and microcarriers seeded with a MEF monolayer. While the hESC expansion rate on MEF-microcarriers was less than that on MEF-plates, the doubling time of hESCs on Matrigel-microcarriers was indistinguishable from that of hESCs expanded on Matrigel-coated tissue culture plates. Standard hESC cryopreservation methodologies are plagued by poor viability and high differentiation rates upon thawing. Here, we demonstrate that cryopreservation of hESCs adherent to microcarriers in cryovials provides a higher recovery of undifferentiated cells than cryopreservation of cells in suspension. Together, these results suggest that microcarrier-based stabilization and culture may facilitate hESC expansion and storage for research and therapeutic applications.
Introduction
Human embryonic stem cells (hESCs) were derived from the inner cell mass of blastocysts by Thomson et al. in 1998 (1). These pluripotent cells can be propagated indefinitely in vitro, yet still retain the capacity to differentiate into a wide variety of somatic tissues representing progenies of the three embryonic germ layers. In fact, hESCs have been shown to differentiate into cell types from each of the primary embryonic lineages in vitro, including neural cells, myocytes, hematopoietic cells, and insulin-secreting cells (2–11). Therefore, hESCs possess significant promise to advance cell-based regenerative medicine (12–15), and also provide a powerful in vitro model for the studies of early human developmental events (16–19).
A prerequisite for the transition of hESC research advances to the clinic is the establishment of effective and efficient cell culture and cryopreservation protocols for large-scale cell expansion, storage, and distribution. In the laboratory, hESCs are typically batch cultured on tissue culture plates, and require medium replacement on a daily basis and passaging every 4 to 7 days. This procedure is labor-intensive, space-consuming, and the culture is subject to high risk of contamination (20). Cell expansion surface area and rapid depletion of culture medium also limit the scalability of current culture methods (21). Accordingly, efforts have been implemented recently to improve ESC scalability and robustness. For example, intermittent perfusion feeding in tissue culture plates can increase murine ESCs (mESCs) yield by over 200% and hESC yield by 70% as compared to conventional static culture (21,22). However, these perfusion systems still operate at a low cell density compared to suspension culture as a result of limiting culture surface area. Suspension culture in bioreactors can provide high cell volume density, and also benefits from a homogenous, easy-to-monitor microenvironment which, in the case of ESC culture, can be regulated to favor either self-renewal or directed differentiation. Suspension bioreactors have been used to expand human hematopoietic stem cells (23,24) and neural stem cells (25). Nevertheless, suspension culture of embryonic stem cells, including mESCs and hESCs, has been hindered by the excessive colony agglomeration, perhaps resulting from high levels of E-cadherin expression in undifferentiated ESCs (26,27). To reduce agglomeration, Dang et al. (27) encapsulated undifferentiated ESCs in size-specified agarose capsules, and before the cells outgrew the capsule the E-cadherin expression diminished with the progress of differentiation. While this method was effective for producing embryoid bodies (EBs) in suspension, its application to culture of undifferentiated cells is limited.
In 2005, Fok et al. (20) reported a method to propagate mESCs in stirred-suspension culture as cell aggregates. Stir-related shear stress reduced colony agglomeration and regulated the size of aggregates. Cormier et al. (28,29) reported a similar mESC culture system. By varying inoculation densities and stirring speeds, the culture doubling time was shortened. hESCs possess a lower clonal recovery and appear to be more anchorage-dependent than mESCs. One possible method to assist suspension culture of hESCs involves use of microcarriers as substrates. Microcarriers are spherical particles, composed of various materials including cellulose, glass, plastic, and polyester, with a typical diameter of 100–250 μm. Because of their high surface area:volume ratio, microcarriers are commonly used to scale culture of anchorage-dependent cells, including human hepatocytes (30), human retinal pigment epithelial cells (31) and co-cultures of neurons and astrocytes (32). Glass and Cytodex 3 microcarriers have been used for mESC suspension culture and resulted in shorter doubling times than those of mESCs cultured as substrate-free aggregates in suspension (20).
Inefficient hESC cryopreservation also limits cell expansion via an extended lag in establishing a dense, viable culture following thawing. Even though hESCs are considered to be “immortal”, aging cultures can acquire chromosomal abnormalities and can lose differentiation potential (33,34). Therefore, techniques to preserve stocks of early passage cells are indispensable. Moreover, efficient cryopreservation will facilitate banking hESC lines with different major histocompatibility complex genotypes and genetically-modified clones (35). Also, cryopreserved hESCs can be conveniently transferred between research centers, enabling scientific collaboration and widespread research and clinical uses of the cells.
Ji et al. reported an hESCs cryopreservation method in which cells were frozen as adherent, intact colonies on their culture plate, instead of freely-suspended colonies (36). This technique substantially improved post-thaw survivability and reduced spontaneous differentiation when compared to cryopreservation of colonies in suspension. However, large scale storage of hESC colonies attached to plates does not make efficient use of freezer space. In addition, tissue culture plates are unable to be sealed or fastened as cryovials, increasing the risk of sample cross-contamination during storage in liquid nitrogen.
Here we report a scalable method of culturing and cryopreserving hESCs on microcarriers. We modified the surface of Cytodex 3 beads with Matrigel or irradiated mouse embryonic fibroblasts (MEFs) to enhance hESC seeding and self-renewal. The growth rate of hESCs on microcarriers was comparable to that of hESCs on conventional Matrigel-coated culture plates and hESCs cultured on microcarriers maintained self-renewal and pluripotency. The recovery of hESCs cryopreserved adherent to microcarriers was also significantly improved compared to that of hESCs frozen as freely-suspended colonies. Our results suggest that microcarriers may facilitate an integrated distribution, storage, and culture scale-up system.
Materials and Methods
Cell lines
Human embryonic stem cell lines H1 and H9 (1), passages 25 to 40, were used in this study. hESCs were cultured as undifferentiated colonies attached to 6-well tissue culture plates, incubated inside a humidified 5% CO2 incubator at 37°C, as described previously (36). The 6-well plates were either pre-seeded with irradiated mouse embryonic fibroblasts (MEFs) as a feeder layer, or pre-coated with Matrigel®(37). hESCs on MEFs were fed with UM/F+ medium (20% knockout serum replacement, 0.5% 200mM L-glutamine solution, 1% 10 mM MEM nonessential amino acids solution, 78.5% DMEM/F12 medium, supplemented with 0.1 mM β-mercaptoethanol and 4 ng/mL bFGF). While on Matrigel, hESCs were cultured in CM/F+ medium (UM/F+ medium without bFGF, pre-conditioned on MEFs, then supplemented with 4ng/mL bFGF before fed to hESCs). To passage hESCs, collagenase or dispase was used to partially detach cell colonies on MEF-plate or Matrigel-plate, followed by scraping with the tip of a glass pipette. Matrigel was purchased from BD Biosciences and β-mercaptoethanol was from Sigma. Dispase, collagenase and all the other medium components were obtained from Invitrogen.
hESC cultivation on microcarriers
In order to evaluate a suitable microcarrier for hESC culture, we screened a variety of commercially-available materials. Cultisphere-S was purchased from Persell and a Microcarrier Beads Starter Kit was purchased from Solo Hill, containing the following microcarriers: F102-1521, C102-1521, G102-1521, P102-1521, PP102-1521, PF102-1521, H11-921. Cytodex 1 and 3, Cytopore1 and 2 microcarriers were purchased from GE Healthcare as dry powders. Dry microcarrier material was pretreated for cell culture according to the manufacturer’s recommended procedures. Briefly described for Cytodex 3, 1 g of microcarrier powder was hydrated and swollen overnight in 80 mL Ca2+ and Mg2+ free PBS (Invitrogen). The next day the PBS was decanted and the microcarrier beads were twice washed and sedimented in 50 mL fresh PBS prior to sterilization by autoclaving.
Initial trials with available microcarrier materials were performed in CM/F+ with pre-coating using a mixture of laminin and fibronectin. Microcarriers were pre-incubated for 1 hr at 37°C in CM/F+ with laminin and fibronectin (each 8.5 μg per ml; BD Biosciences) in a 15 ml tube. An equivalent of 10 cm2 surface area for each carrier was used for each material and microcarriers were maintained in an untreated polystyrene 12-well plate to examine initial cell attachment. To seed the cells onto microcarriers, one well of culture (2–3×106 cells) was treated with dispase and added directly to the microcarriers in CM/F+. The plate was gently agitated on an orbital shaker inside the incubator. Media changes were accomplished by carefully aspirating the supernatants from the settled microcarriers. After seeding, culture medium was changed every other day for 5 days and microcarriers were visually examined for attachment.
For all subsequent studies with Cytodex 3, the sterilized microcarriers were pre-seeded with MEFs or pre-coated with Matrigel before used for hESC culture. The PBS was decanted, and then MEFs, suspended in MEF culture medium (10% FBS, 90% DMEM, both ingredients from Invitrogen), were added onto the microcarriers at a surface concentration of 3–5×104 cells/cm2. MEF culture medium was added to a final volume of 2.5 mL medium for every 1×106 MEFs. Then the MEF-seeded microcarriers were transferred into a 100 mL Wheaton Magna-Flex spinner flask (Fisher Scientific) which was placed on a Wheaton Micro-stir stirrer (Fisher Scientific) inside the CO2 incubator. The mixture was stirred at 70 rpm for 5 min and let rest for 55 min; this stirring cycle repeated overnight to allow MEFs to attach onto the microcarriers (MEF-microcarriers). The next day, the MEF medium was decanted, and MEF-microcarriers were washed with DMEM/F12 three times and then seeded with hESCs. To prepare Matrigel-coated microcarriers (Matrigel-microcarriers), 2 mg Matrigel was dissolved in ice-cold DMEM/F12 and added to 65 mg (dry weight) microcarriers. DMEM/F12 was added to bring the final volume to 60 mL. Then the mixture was transferred into the spinner flask and stirred continuously overnight at 70 rpm. Matrigel-microcarriers were washed with DMEM/F12 before seeding with hESCs.
hESC colonies cultured on 6-well plates were washed with Ca2+ and Mg2+-free PBS. Then 1 mL trypsin (Invitrogen), pre-warmed to 37°C, was added to each well and the plate was incubated at 37°C for 5–10 min. The plates were monitored visually until the cell suspension consisted of small clumps (1–20 cells). At this time, 1 mL hESC culture medium was added to each well to neutralize the trypsin. The cells were collected and washed in hESC medium, and resuspended in CM/F+ at a concentration of 0.6–1×106/mL. These cells were added to MEF-microcarriers or Matrigel-microcarriers at the surface concentration of 3–7×104 cells/cm2. The mixture was transferred into Corning Ultra Low Attachment 6-well plates (Fisher Scientific) at 2 mL/well, then placed on a rocker inside the incubator and gently rocked overnight. The medium was replaced with 3 mL/well fresh CM/F+ medium the following day. The cells were cultured on the rocker and medium was changed every day until the microcarriers were confluent (>90% coverage, estimated visually).
Long-term cultivation of hESCs on microcarriers and karyotyping
hESCs were seeded onto MEF-microcarriers and Matrigel-microcarriers, and cultured in CM/F+ as described above. When the cultures reached confluence, the cells were harvested. hESCs adherent on microcarriers were washed once in DMEM/F12, and treated with trypsin for 5–10 min at 37°C before the trypsin was neutralized with hESC culture medium. Then the cells were further dissociated from the beads and individualized by pipetting. The mixture was filtered through a 40 or 70 μm mesh (cell strainer) to remove the beads. The cells were then pelleted, resuspended in fresh CM/F+, and seeded onto newly-made MEF-microcarriers and Matrigel-microcarriers. hESCs (H9, passage 26) were continuously cultured on MEF-microcarriers for 10 passages, and on Matrigel-microcarriers for 11 passages. On the fourth day of the fifth passage, both cultures were sampled for karyotype analyses, which were performed at the cytogenetics lab of WiCell Research Institute (adison, WI).
hESC cryopreservation
Freezing in suspension
hESC colonies were pretreated with collagenase or dispase for 3–5 min at 37°C and then scraped off culture plates with the tip of a glass pipette. The cells were collected, pelleted at 1000 rpm, and washed in culture medium. They were then resuspended in CM/F+ and the total cell number was determined by counting on a hemocytometer. CM/F+ was added to generate a final cell concentration of 2×106 cells/mL. Freezing medium (20% DMSO, 60% FBS, 20% CM/F+) was added dropwise to the cells, so that the final cell concentration was 1×106 cells/mL and the final composition of the medium was 10% DMSO, 30% FBS, and 60% CM/F+. The solution was aliquoted into cryovials, 1 mL/tube, then placed inside an Nalgene Cryo 1°C Freezing container (Fisher Scientific) and frozen in an −80 °C freezer. The frozen hESCs were transferred into liquid nitrogen for long-term storage the next day.
Freezing on microcarriers
hESC-microcarriers were collected, washed, and resuspended in fresh CM/F+. The hESC microcarriers were treated similar to cryopreserved hESC colonies, except the cells were not removed from the microcarriers prior to freezing. hESC-microcarriers were suspended in 10% DMSO, 30% FBS, and 60% CM/F+ at ~1×106 cells/mL on ~10 cm2 microcarriers. The cells were divided into cryovials, frozen inside the Nalgene Cryo 1°C Freezing container in the −80 °C freezer, and transferred into liquid nitrogen within the next 24 hr.
Thawing
The procedure for thawing hESCs frozen in suspension or on microcarriers followed the same protocol. Cryovials were quickly thawed in a 37°C water bath. Fresh hESC culture medium was added dropwise into the vials to dilute the cryoprotectants. The cells were then pelleted and washed in culture medium. Finally, they were resuspended in UM/F+ and plated onto a MEF feeder layer on a culture plate. Cells frozen on microcarriers were not detached from the microcarriers prior to replating.
Differentiation
After hESC-microcarrier cultures became confluent, CM/F+ was replaced with UM/F− (UM/F+ medium without bFGF) to facilitate hESC differentiation via embryoid body (EB) formation on the microcarriers. The EBs were cultured in ultra-low adhesion plates with gentle agitation to prevent aggregation. After suspension culture in UM/F− for about 1 week, the microcarriers were allowed to settle, collected, and transferred into a gelatin-coated conventional tissue culture plate, and the EBs were allowed to attach to the plate. Cells were maintained in UM/F− for one additional week, with daily medium changes, before analysis by immunocytochemistry.
Immunocytochemistry
Cells were washed in PBS and fixed in 4% paraformaldehyde for 15 min at room temperature (RT). Cells were then permeabilized by incubating in PBS-T (PBS+0.4% Triton X-100) for 30 min and blocked in 5% milk in PBS-T for 1 h at RT. Primary antibodies were added onto the cells as a 1:50 or 1:100 dilution in PBS-T and samples were gently rocked overnight at 4°C. The next day, the samples were washed with PBS-T and secondary antibodies were added as a 1:500 or 1:1000 dilution in PBS-T. After gentle rocking for 1 hr at RT, the samples were washed with PBS-T before imaging with epifluorescence or confocal microscopy. The primary antibodies used here included anti-Oct3/4 (Santa Cruz Biotechnology) for detecting undifferentiated hESCs, anti-a-fetoprotein (Biodesign International) for endodermal cells, anti-brachyury (Santa Cruz Biotechnology) for mesodermal cells, and anti-nestin (Santa Cruz Biotechnology) for ectodermal cells. The secondary antibodies were Alexa fluor 488 or 594 conjugates (Molecular Probes).
The presence of the Matrigel layer on the surface of microcarriers after coating was confirmed by anti-laminin staining of Matrigel-microcarriers. The immuno-staining procedure was similar to that described above. The dilution of primary anti-laminin antibody (Sigma) was 1:300.
Flow cytometry
hESCs were detached from 6-well plates or microcarriers with 5–10 min trypsin (supplemented with 2% chicken serum) treatment, and filtered through a 40 micron mesh to eliminate cell aggregates and/or microcarriers. Then the cells were washed with PBS and fixed in 1% paraformaldehyde for 10 min at 37 °C. After chilling on ice for 1 min, the cells were washed in FACS buffer (Ca2+ and Mg2+ free PBS, 2% FBS). To permeabilize the cells, they were resuspended in ice-cold 90% methanol and incubated on ice for 30 min. The cells were then washed and resuspended in FACS+T buffer (FACS buffer + 0.1% Triton X-100) at the concentration of 1×106 cells/mL. The cell solution was divided into FACS tubes, 100 μL/tube, and 1μL of the primary antibody anti-Oct3/4 was added into each tube. The samples were incubated overnight at 4°C. The next day the cells were washed and the secondary antibody was added as a 1:1000 dilution in FACS-T buffer. The samples were then incubated at RT in the dark for 30 min and washed. Cell fluorescence was assessed with a FACScan flow cytometer (Beckton Dickinson) to quantify the Oct4 expression.
The viability of hESCs growing on microcarriers was quantified via a Calcein-AM assay. Cells were detached and filtered as described above. They were then washed and resuspended in FACS buffer. Calcein-AM (Invitrogen) was added into the samples as a 1:1000 dilution, and incubated 20 min at RT. The cells were then washed in FACS buffer and analyzed with flow cytometry.
Results
hESCs cultured on MEF-microcarriers and Matrigel-microcarriers remain undifferentiated
Culturing hESCs in their self-renewing, undifferentiated state requires an appropriate extracellular matrix (ECM). As reported to date, hESCs can be cultured on feeder cells (mainly MEFs and human fibroblasts), or on Matrigel, laminin, fibronectin, collagen IV, and vitronectin substrates (37–41). In a preliminary screen of 12 different commercially-available microcarriers we determined Cytodex 3 (GE Healthcare) to be suitable for further studies based on qualitative visual evaluation of attachment and survival after 5 days (Table 1).
Table 1.
Qualitative visual assessment of microcarrier materials during preliminary screen
| Name | Manufacturer | Material | Visual assessment | |
|---|---|---|---|---|
| Attachment | Viability | |||
| Cytodex 1 | GE Healthcare | cross-linked dextran with N, N-diethylaminoethyl groups | fair | poor |
| Cytodex 3 | GE Healthcare | crosslinked dextran, denatured collagen on surface | fair | fair |
| Cytopore 1 | GE Healthcare | Macroporous cross-linked dextran with N, N-diethylaminoethyl groups, charge density of 1.1 meq/g | poor* | poor |
| Cytopore 2 | GE Healthcare | Macroporous cross-linked dextran with N, N-diethylaminoethyl groups, charge density of 1.8 meq/g | poor* | poor |
| CultiSphere-S | PerCell | Crosslinked pharmaceutical grade porcine gelatin | fair | poor |
| H11-921 | Solo Hill | Crosslinked polystyrene, modified with cationic trimethyl-ammonium | poor | none |
| F102 | Solo Hill | Crosslinked polystyrene, modified with cationic gelatin | poor | none |
| C102 | Solo Hill | Crosslinked polystyrene, modified with gelatin | poor | none |
| G102 | Solo Hill | Crosslinked polystyrene, modified with high silica glass | poor | none |
| P102 | Solo Hill | Crosslinked polystyrene | poor | none |
| PP102 | Solo Hill | Crosslinked polystyrene, cationic | poor | none |
| PF102 | Solo Hill | Crosslinked polystyrene, modified with recombinant fibronectin | poor | none |
Summary of a preliminary screen of 12 different available microcarriers. Microcarriers were pre-incubated in CM/F+ with laminin and fibronectin. An equivalent of 10 cm2 surface area for each microcarrier class was used. The cells were cultured in CM/F+ in an untreated polystyrene 12-well plate to examine initial attachment. Culture medium was changed every other day for 5 days. Performance was judged by qualitative visual evaluation of attachment and survival after 5 days.
Because of the macroporous structure of Cytopore 1 and Cytopore 2, the visual accessibility of cells attachment inside the microcarrier beads was poor. However, since no outgrowth of cells from inside of the beads was observed, the attachment inside was presumed to be low.
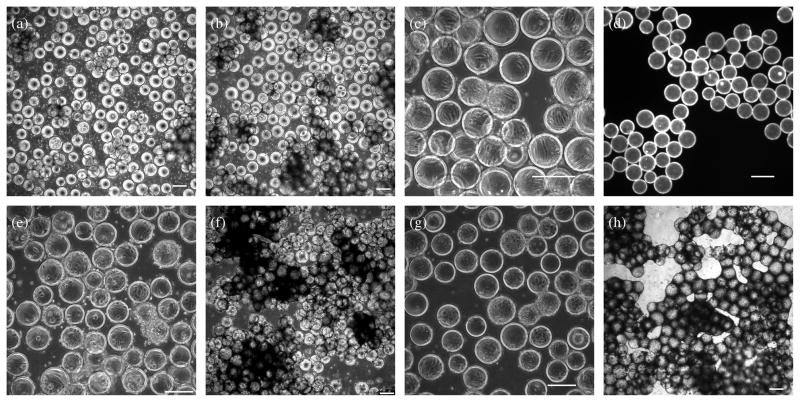
Cytodex 3 microcarriers consist of a thin layer of denatured collagen covalently coupled to a matrix of cross-linked dextran. These microcarriers have been used as a substrate for mass cultivation of various types of mammalian cells, including human retinal pigment epithelial cells, human hepatocytes, and murine neurons and astrocytes (30–32). However, our initial attempts to culture hESCs directly on Cytodex 3 microcarriers were impeded by low attachment (Figure 1a, 1b). Moreover, collagen is much less effective than MEFs or Matrigel in long-term maintenance of undifferentiated hESCs (37). Therefore, we modified the surface of Cytodex 3 microcarriers by formation of a MEF monolayer on the surface, or incubation in Matrigel. Unlike hESCs, MEFs easily attached to the collagen outer layer of the Cytodex 3 microcarriers (Figure 1c). MEFs on the microcarrier surface exhibited the same spread morphology as on a conventional tissue culture plate. To verify the effectiveness of Matrigel coating, we labeled the laminin on the surface of the microcarrier beads with immunocytochemistry (Figure 1d).
Figure 1. hESCs cultured on microcarriers.
hESCs were seeded onto microcarriers at ~6.7×104 cells/cm2 in Corning Ultra Low Attachment 6-well plates and rocked gently overnight inside an incubator. (a), (b) Phase contrast images of hESCs growing on bare Cytodex 3 microcarriers, one day (a) and four days (b) after seeding. (c) Phase contrast image of MEFs on Cytodex 3 microcarriers. MEFs were seeded onto the microcarriers at ~3.7×104 cells/cm2 in a spinner flask overnight with intermittent stirring (cycle: 70 rpm for 5 min followed by 55 min without stirring). (d) Fluorescence image of anti-laminin staining of Matrigel-coated microcarriers. 65 mg (dry weight) microcarriers were coated with 2 mg Matrigel in 60 mL DMEM/F12 in spinner flask and stirred overnight at 70 rpm. (e), (f) Phase contrast images of hESCs growing on MEF-microcarriers, one day (e) and four days (f) after seeding. (g), (h) Phase contrast images of hESCs cultured on Matrigel-microcarriers, one day (g) and four days (h) after seeding. Scale bars: 200 μm.
Surface modification of Cytodex 3 microcarriers with MEFs and Matrigel improved the seeding of hESCs onto microcarriers (Figure 1e, 1g). As described in Materials and Methods, hESCs were seeded onto microcarriers in low-attachment plates with a hydrophilic and neutrally-charged surface, and were gently rocked, which diminished the cell loss caused by attachment to the plate surface and enhanced the interactions between cells and microcarriers. Using this protocol with a seeding density of 3–7×104 cells/cm2, over 80% of the microcarriers were covered with hESCs by the time the hESC-microcarrier culture reached confluence 4–6 days after seeding (Figure 1f, 1h). As shown in Figure 1, hESC-microcarriers agglomerated in culture. The sizes of hESC-microcarrier aggregates did not increase indefinitely, however; once the culture reached confluence, the cells of the outer layer started to dissociate and numerous dead cells were observed floating in the culture.
In this study, hESCs were serially cultured on microcarriers for up to 11 passages. To harvest or passage, hESCs were detached from microcarriers by trypsin, and microcarriers were removed using cell strainers. Compared to hESC colonies cultured on Matrigel-microcarriers, hESC colonies growing on MEF-microcarriers were more compact. This morphology difference is consistent with differences in shapes of hESC colonies cultured on tissue culture plates.
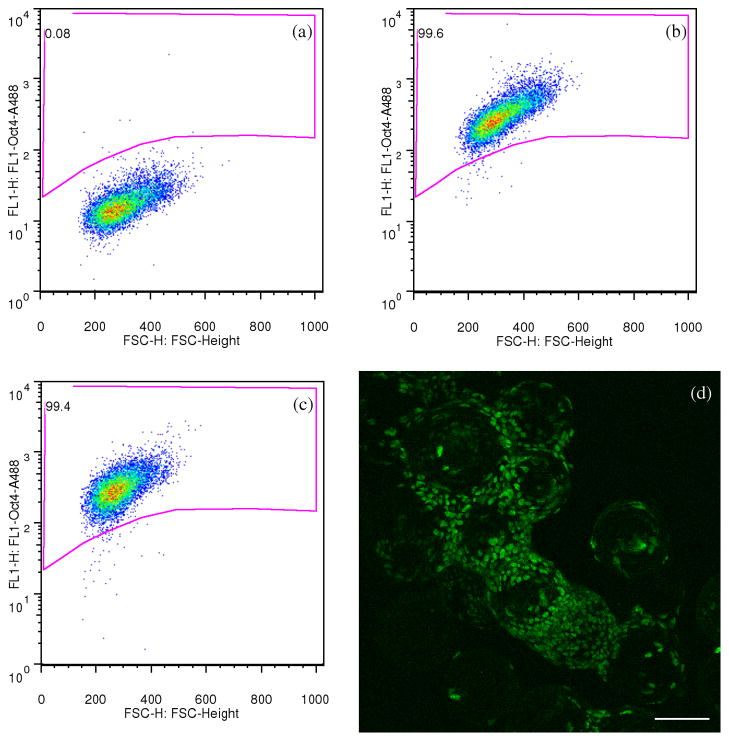
To verify the undifferentiated status of hESCs growing on microcarriers, the expression of transcription factor Oct4 was assessed via flow cytometry (Figure 2a–2c) and immunocytochemistry (Figure 2d). After 10 passages on Matrigel-microcarriers, hESCs were harvested as single cells using trypsin and the percentage of Oct4 positive cells was measured. hESCs cultured on a Matrigel-plate were used as a positive control. Of the hESCs cultured on Matrigel-microcarriers, 98.7 ± 0.6% were Oct4 positive, comparable to that of the hESCs cultured on MEF-plate (99.5 ± 0.1%). At each passage up to passage 10, >98% of the cells in each culture expressed Oct4 (not shown). Confocal fluorescence micrographs further confirmed that hESCs cultured on Matrigel-microcarriers were Oct4 positive. Additionally, we transferred hESC-microcarriers to a conventional tissue culture plate, let the hESCs attach and spread onto the plate, then stained the colonies with an anti-Oct4 antibody. These cells also possessed positive Oct4 immunoreactivity (data not shown).
Figure 2. hESCs cultured on Matrigel-microcarriers remained Oct4 positive.
(a)–(c) Flow cytometry density dot plots of fluorescence showing Oct4 expression of hESCs cultured on Matrigel-plates (b) and Matrigel microcarriers (c). hESCs from the same culture were simultaneously seeded on Matrigel-microcarriers and Matrigel-plates, cultured for 10 passages, harvested as single cells using trypsin on the 6th day of the 10th passage, then immuno-stained for Oct4 and analyzed with flow cytometry. The gated region was determined with the reference to the plot of hESC sample treated with only secondary antibodies (a). The percentages of events located inside the gated region (Oct4 positive) are indicated on the plots. (d) Confocal microscopy fluorescence image showing undifferentiated hESCs cultured on Matrigel-microcarriers with positive Oct4 immunoreactivity. Scale bars: 100 μm.
hESCs cultured on microcarriers are pluripotent
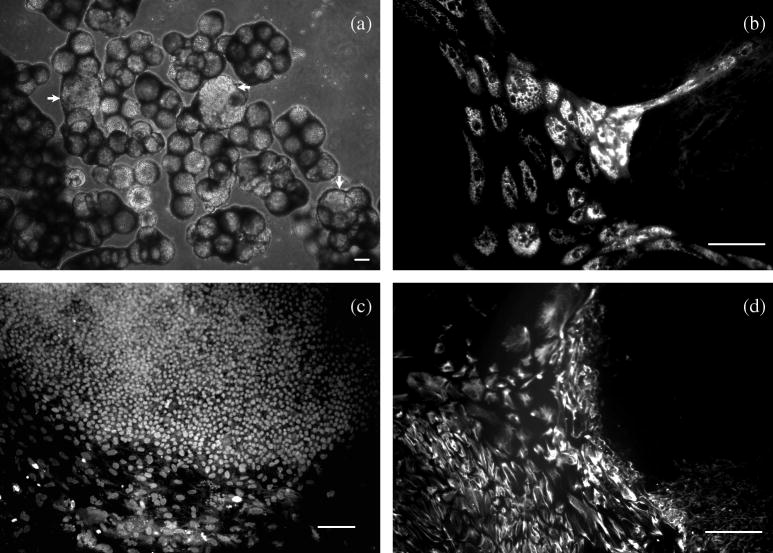
The ability of hESCs cultured on microcarriers to differentiate into progeny cells in each of the three embryonic germ layers was assessed in vitro as an indication of their pluripotency. In typical hESC differentiation protocols, embryoid bodies (EBs) are formed via suspension culture of hESCs colonies in bFGF-free medium (19,42,43). Instead of detaching the cells from microcarriers to form EBs, we directly transferred hESC-microcarriers from CM/F+ into UM/F− to facilitate hESC differentiation. As shown in Figure 3a, some of the hESC colonies differentiated on microcarriers formed cyst-like structures after 4–7 days in suspension. After 7 days in suspension, the microcarriers were transferred to a gelatin-coated plate and cells cultured for another week. Immunocytochemistry was used to detect the expression of the markers for progeny cells in each of the three germ layers, i.e. α-fetoprotein (endoderm), Brachyury (mesoderm), and nestin (ectoderm) (Figure 3), indicating pluripotency. Oct4 expression was not observed in microcarrier cultured EBs, while nestin-expressing cells exhibited a neural morphology and many also stained positive for βIII tubulin (data not shown).
Figure 3. hESCs cultured on microcarriers differentiated into progeny of all three germ layers.
hESCs were cultured on Matrigel-microcarriers in CM/F+ until confluent, transferred to UM/F − and cultured in suspension for 1 week, then transferred to a gelatin-coated culture plate and cultured for another week before fixation. (a) phase contrast image of differentiating hESCs on microcarriers suspending in UM/F −. The arrows indicate cyst-like structures formed during differentiation. (b–d) fluorescence image of differentiated hESCs stained for a-fetoprotein (endoderm, b), Brachyury (mesoderm, c), and nestin (ectoderm, d). Scale bars: 100 μm.
Expansion rates of microcarrier-cultured hESCs
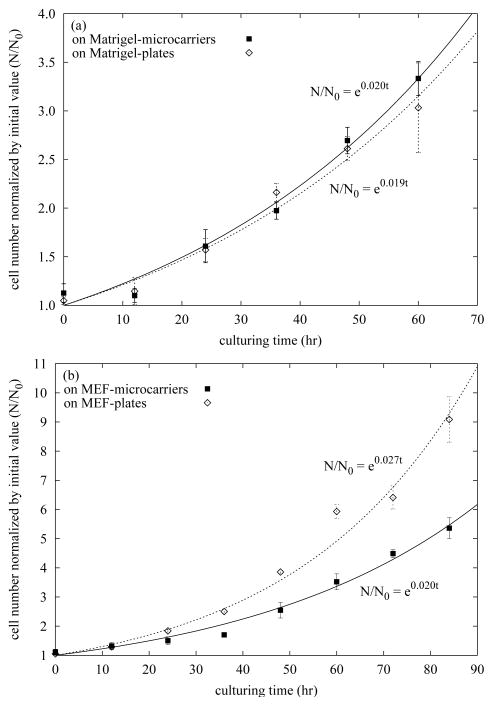
Maintaining a high expansion rate is an important consideration in scaling cell culture. We measured the doubling time of hESCs growing on microcarriers and compared it with that of hESCs cultured on tissue culture plates. The cells, harvested from the same mother culture, were seeded onto microcarriers as small clumps of trypsinized cells and onto culture plates as dispase-treated colonies. After seeding, both cultures were incubated overnight to permit cell attachment to the culture substrate. The initial cell count (0 hr) was performed 20 hr after seeding. Subsequent counts were conducted every 12 hr. Samples harvested from each culture were trypsinized into single cells for counting. Trypan blue was used to exclude cells lacking an intact plasma membrane. Greater than 90% of the cells excluded Trypan blue at each time point.
The growth curves of cells cultured on Matrigel-microcarriers and Matrigel-plates for 60 hr are shown in Figure 4a, and those of cells cultured on MEF-microcarriers and MEF-plates for 84 hr are shown in Figure 4b. Growth curves were fit to an exponential growth model N = N0ekt; thus the cell doubling time is t2 = ln 2/k. Fitting the data in Figure 4a, k = 0.020hr−1 for Matrigel-microcarrier culture and k = 0.019hr−1 for Matrigel-plate culture, which are equivalent to t2 = 35hr for Matrigel-microcarrier culture and t2 = 36hr for Matrigel-plate culture (P>0.1, 2-tailed Student’s t test for kmicrocarrier = kplate). Similarly, from the data in Figure 4b, k = 0.020hr−1 for MEF-microcarrier culture and k = 0.027hr −1 for MEF-plate culture, which are equivalent to t2 = 35hr and t2 =26hr, respectively (P<0.05, 2-tailed Student’s t test for kmicrocarrier=kplate). The doubling time values of microcarrier cultures and Matrigel-plate culture obtained here are similar to those reported by Xu et al.: 31–33 hr for hESCs on Matrigel plates (37). These data suggest that hESCs cultured on Matrigel-microcarriers grow as fast as hESCs on Matrigel-plates. Nevertheless the doubling time of hESCs cultured on MEF-plates was about 9 hr less than that of hESCs on MEF-microcarriers.
Figure 4. Growth curves of hESCs cultured on microcarriers and 6-well tissue culture plates in their exponential growth stages.
Cell number normalized to the initial value (N/N0) was quantified as a function of time during exponential growth. (a) Matrigel-microcarriers vs. Matrigel-plates. hESCs (cell line: H1, passage: 30) were seeded onto microcarriers as small clumps of trypsinized cells at 3.0×104 cells/cm2; they were also seeded onto 6-well tissue culture plates as dispase-treated colonies at the density of 2.8×105 cells/well. The first cell count (0 hr) was performed 20 hr after seeding. (b) MEF-microcarriers vs. MEF-plates. hESCs (cell line: H9, passage: 36) were seeded onto MEF-microcarriers at 3.3×104 cells/cm2; they were seeded onto plates at the density of 3.0×105 cells/well. The first counting (0 hr) was performed 17 hr after seeding. Cells were trypsinized into single cells and counted. Trypan blue was used to exclude cells lacking an intact plasma membrane. Error bars indicate SEM.
The viability of hESCs growing on MEF-microcarriers was quantified via Calcein-AM reduction using flow cytometry, and was compared with that of hESCs cultured on a MEF-plate. Propidium iodide (PI) was also used to identify dead cells. Of hESCs cultured on microcarriers, 91.5% were PI-negative and 89.0% were both PI-negative and Calcein-positive (live), compared to 94.1% PI-negative and 80.6% PI-negative and Calcein-positive hESCs cultured on a MEF-plate. Together, these results suggest that rates of microcarrier and plate-based cell expansion and viability are similar.
hESCs can be passaged on microcarriers
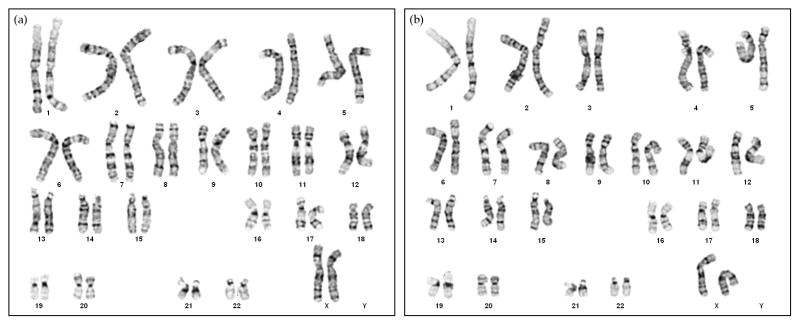
Chromosomal abnormalities, such as trisomy of chromosomes 17q and 12, are commonly detected in long-term culture of hESCs (34). To determine whether hESCs cultured on microcarriers contained any chromosomal abnormalities, we performed karyotype analyses of these cells after several passages. hESCs from the same mother culture on MEF-plates were subcultured on MEF-microcarriers and Matrigel-microcarriers separately for 5 consecutive passages before they were harvested for G-band chromosome analysis. The karyotypes of hESCs in both MEF-microcarrier and Matrigel-microcarrier cultures were found to be normal female at the standard level of resolution (Figure 5). The cultures of hESCs on MEF-microcarriers and Matrigel-microcarriers were maintained for over 2 months for 10 passages and 11 passages, respectively. Both H1 and H9 lines were able to be expanded in an undifferentiated state through ten passages.
Figure 5. Karyotypes of hESCs cultured on (a) MEF-microcarriers and (b) Matrigel-microcarriers.
hESCs (H9, passage 26) cultured on MEF-plates were seeded onto microcarriers, and consecutively cultured on microcarriers for five passages. On the 4th day of the 5th passage on microcarriers, the cells were analyzed for G-band karyotype.
hESC recovery improves when cryopreserved on microcarriers
In a previous study by Ji et al., adherent hESCs cryopreserved directly on a culture plate exhibited higher recovery and less spontaneous differentiation than hESCs frozen as free colonies in suspension (36). However, large scale storage of hESCs in tissue culture plates is not feasible because cells on plates cannot easily be stored at high density. Since hESCs can be successfully cultured on microcarriers, we assessed survival of microcarrier-immobilized colonies following cryopreservation to determine whether the same survival cues provided by plate immobilization improve viability of microcarrier-cultured cells.
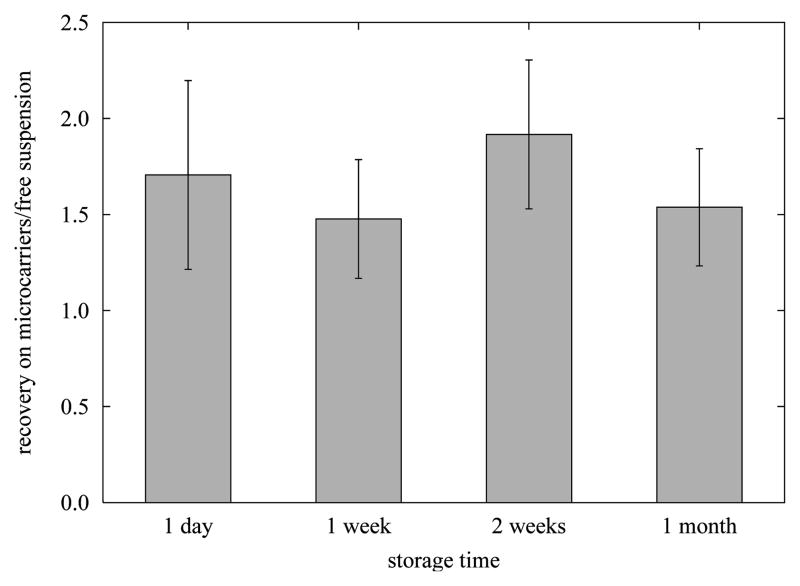
The relative recoveries of hESCs cryopreserved on microcarriers to those of hESCs cryopreserved as freely-suspended colonies (conventional hESC cryopreservation method) are shown in Figure 6. In this experiment, H1 hESCs were cultured on MEF-microcarriers to a density of ~1.1×105 cells/cm2, then frozen on the microcarriers. For each batch of hESCs cultured and cryopreserved on microcarriers, hESCs from the same mother culture were cultured on a 6-well MEF plate to a density of 1.5–2×106/well and then frozen as freely-suspended colonies after removal from the plate. Approximately 1×106 hESCs in a 1 mL sample volume were frozen in each cryovial with a cooling rate of ~ −1 °C/min in a −80 °C freezer. The hESCs were then stored in liquid nitrogen for one day, one week, two weeks or one month. After thawing, the hESCs were plated onto fresh MEF-plates and cultured for one week before the cell number was counted. This cell number, divided by the number of cells in each sample at the time of freezing, represents the recovery. Although the results indicate that cryopreservation of hESCs adherent to microcarriers was superior to freezing hESCs as freely-suspended colonies, the recoveries varied significantly from one batch of cells to another, ranging from 20–200% for the freely-suspended colonies. Therefore we normalized the recoveries of hESCs frozen on microcarriers to the recoveries of hESCs frozen as free colonies in the same experiment. The consequential ratios of all the three cell batches were then averaged and the results are displayed in Figure 6. The recovery of adherent hESCs frozen on microcarriers was 1.5–1.9 times of the recovery of hESCs frozen as free colonies (P<0.05). The improvement in recovery did not change significantly over 1 month in storage (P>0.05). hESCs adherent on Matrigel-microcarriers were also cryopreserved, and the recovery was similar to the recovery of hESCs frozen as freely-suspended colonies (data not shown). Compared to hESCs on MEF-microcarriers, hESCs adherent to Matrigel-microcarriers were more prone to detach from the beads after thawing.
Figure 6. Recovery of hESCs cryopreserved on MEF-microcarriers (RM), compared to that of hESCs cryopreserved as freely-suspended colonies (RF).
hESCs (H1, passage 32–37) were frozen in a medium composed of 10% DMSO, 30% FBS and 60% CM/F+, with a cooling rate of ~ −1 °C/min, and stored in LN2 for 1 day, 1 week, 2 weeks or 1 month as indicated. After rapid thawing in a 37 °C water bath, cells were cultured on fresh MEF-plate for 1 week before recovered cells were trypsinized from the plate and the cell number was counted using Trypan blue to exclude cells lacking an intact plasma membrane. Recovery is defined as the number of cells that exclude Trypan blue divided by the number of cells in each sample at the time of freezing. The recoveries of hESCs frozen on microcarriers were normalized by recoveries of hESCs frozen as free colonies (RM/RF). The value represented by each bar is the average RM/RF ratio of three samples of cryopreserved hESCs in each of three independent experiments. Error bars represent SEM.
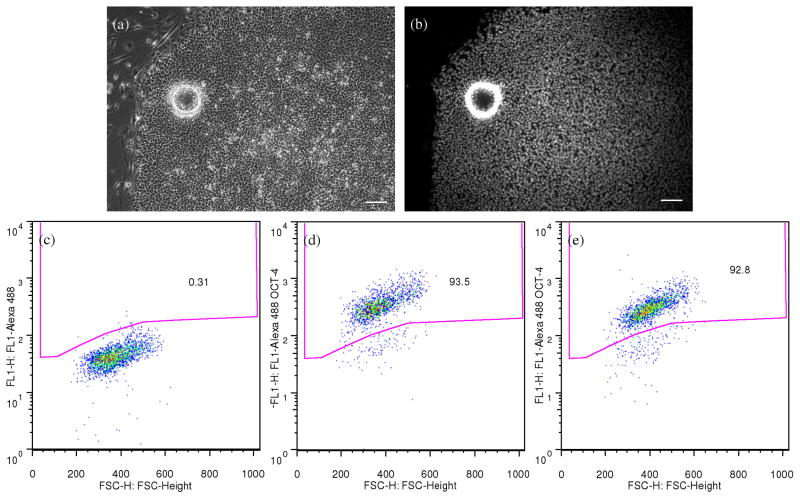
hESCs remained undifferentiated after cryopreservation on microcarriers, as determined by anti-Oct4 immunocytochemistry and flow cytometry (Figure 7). After thawing, hESC-microcarriers were plated onto a MEF-plate, cultured for one week and analyzed for differentiation state. Figure 7a and 7b show a thawed undifferentiated hESC colony recovered from a microcarrier. Flow cytometry for Oct4 expression indicated that 91.1 ± 1.0% of the cells cryopreserved on MEF-microcarriers and recovered on MEF-plate for 7 days were Oct4-positive, while 93.8 ± 0.7% of cells recovered from the conventional hESC cryopreservation were Oct4-positive (Figure 7c and 7d).
Figure 7. hESCs remained undifferentiated after cryopreservation on microcarriers.
After thawing, hESCs frozen on microcarriers were plated onto an MEF-plate, and cultured on the plate for one week before fixation, immuno-staining and flow cytometry analysis. Phase contrast (a) and Oct4 immunofluorescence (b) images of an undifferentiated hESC colony recovered from cryopreservation on MEF-microcarriers. The ring shown in both images is the outline of a microcarrier bead. Scale bars: 100 μm. (c)–(e) Density dot plots of fluorescence from flow cytometry showing the Oct4 expression of hESCs recovered from cryopreservation with (d) or without (e) microcarriers. The gated region was determined with the reference to the plot of hESC sample treated with only secondary antibodies (c). The percentages of events located inside the gated region (Oct4 positive) are indicated on the plots.
Discussion
Current hESC expansion methods rely on laborious feeding and propagation techniques. Microcarriers permit culture of adherent cells in suspension and typically afford higher cell density and scalability than cell growth on culture plates. In this study, we report a method to culture hESCs on microcarriers. The surface of Cytodex 3 microcarriers was modified with Matrigel or MEFs to enhance hESC attachment and inhibit hESC differentiation. Our experiments have shown that hESCs cultured on Matrigel-microcarriers have similar growth rates to hESCs grown on Matrigel-coated tissue culture plates. hESCs cultured on MEF-plates, however, possessed a higher growth rate than hESCs cultured on MEF-microcarriers. Due to the differences in microcarrier and plate surfaces and MEF seeding protocols, the surface concentration of MEFs on microcarriers varied from that on plates, which may also contribute to the disparity in the growth rates of hESCs on MEF-microcarriers and on MEF-plates. The mother cultures used in this growth-curve experiment were the hESCs of passage 36 (Figure 4b) grown on MEF-plates, i.e., the cells had been cultured on MEF-plates for long-term before they were transferred onto new culture surfaces and grown in new conditions. During their 36 passages, hESCs may have been selected to adapt to MEF-plate culture conditions, which may also contribute to the faster growth rate on MEF-plates than on MEF-microcarriers as shown in Figure 4b. In fact, we have qualitatively observed that, to a certain extent (passage < 50), hESCs cultured in constant conditions grow faster as passage number increases.
Since hESCs grow in multiple layers on microcarriers and multiple microcarriers can agglomerate into larger cell masses, inefficient transport of nutrients, growth factors and metabolic wastes may eventually exert detrimental effects on hESCs and cause inconsistency in cell growth and differentiation. However, after 4–6 days, the cells of the outer layer start to dissociate and the aggregate size reaches an apparent steady-state confluence. We have transferred the confluent hESC-microcarriers onto a plate. Similar as shown in Figure 7, the cells spread on plate surface and exhibited consistent hESC morphology and strong Oct4 expression throughout the entire colony. This implies that the diffusion in the hESC-microcarrier suspension culture was sufficient to support the growth of undifferentiated hESCs throughout the multiple layers of cells wrapping around the microcarrier by the time of culture confluence. Passaging from microcarriers to plates or expansion to additional microcarriers requires efficient detachment of viable cells. At harvest, trypsin was used to detach hESCs from microcarriers. We have also tried dextranase to digest Cytodex 3; however this enzyme caused severe hESC toxicity before the breakdown of the beads.
The success of culturing undifferentiated and pluripotent hESCs on microcarriers opens the path of large-scale cultivation of hESCs. Further improvements are likely to improve the performance of microcarrier hESC culture methods. For example, a perfusion system which continuously feeds the cells may increase the culture density (22). However, some challenges have to be addressed before the hESC-microcarrier culture technique is fully developed and applied into high-yield production of hESCs. hESCs are “highly-cooperative”; they cannot be efficiently passaged as individual cells (44). The clonal efficiency of individualized H1 and H9 hESCs is less than 7% under the conventional culture conditions (45). In plate culture protocols, hESCs are treated with collagenase or dispase and passaged as relatively large colony fragments which are too large to be seeded onto microcarriers. Instead, hESCs are seeded onto microcarriers as a mixture of very small cell clumps and single cells. Therefore the seeding efficiency of hESCs onto microcarriers is currently very low, which would not only compromise the hESC production efficiency, but also exert selective pressure on the cultures. The seeding efficiency was very sensitive to cell density. Confluent cultures were not obtained using seeding densities below 104 cells/cm2 (data not shown). At cell densities greater than 105 cells/cm2, confluent microcarriers were readily achieved, but seeding efficiency was low since the microcarrier surface was saturated. Seeding densities from 3–7 × 104 cells/cm2 represented an optimum for expansion. Furthermore, seeding efficiency was sensitive to the size of clumps used to seed the microcarriers. Single cell suspensions did not attach and grow. Likewise, large clusters of cells did not efficiently adhere to the microcarrier surface. We found that a mixture of 1–20 cells/cluster was optimal for seeding. A possible way to improve the seeding efficiency is to lower the oxygen level of the culture from 21% to 2%, since it was reported that physiologic oxygen concentration (2%) increased H1 and H9 hESC clonal efficiency to 25–31% (45). Alternatively, inhibition of Rho-associated kinase (ROCK) may likewise increase clonal survival and microcarrier seeding efficiency (46). Like mESCs cultured in suspension, hESC-microcarriers also agglomerate, which diminishes accessible surface area, reducing the ratio of hESC yield to the mass of microcarriers used. Agglomeration of mESC aggregates was significantly reduced by appropriate levels of agitation (20,28). Hence it is expected that appropriately-designed agitation for the hESC-microcarrier culture could also reduce agglomeration while avoiding deleterious mechanical damage to the cells.
The ECM and culture media used in this study contain poorly-defined components of animal origin. hESCs cultured under these conditions were reported to express nonhuman sialic acid Neu5Gc which would evoke an immune response if these cells were transplanted (47). Another enhancement to the hESC-microcarrier culture would be xeno-free and defined components for ECM and medium as desired for future clinical applications in regenerative medicine (38). However preliminary studies with mTeSR1 medium in our lab (data not shown) showed low attachment and survival of hESCs on microcarriers after trypsinization, possibly due to their known limited cloning efficiency in this defined medium.
With the efforts to advance hESCs cultivation techniques, significant effort has been placed on development of effective, robust hESC cryopreservation techniques. The reported recoveries after conventional slow freezing in a variety of labs vary from 0% to 48% (48–54). However, each study uses different metrics to evaluate effectiveness of their protocol, complicating comparison of results. Typically colony-based measurements of recovery provide higher apparent viabilities than cell-based measurements, as a colony may attach and grow even if not all cells in that colony survived the freeze-thaw process. We stored our frozen hESC samples in LN2 for up to one month, and cultured the hESCs for 7 days after thawing before assessing viability. Considering the sizes of the colonies vary greatly, we chose cell-based recovery to evaluate the effectiveness of the cryopreservation of hESCs adherent on microcarriers, which was compared to that of the conventional hESC slow freezing method. As shown in Figure 6, hESC cryopreservation on microcarriers resulted in 1.7 times the recovery of hESCs cryopreserved in free suspension, suggesting that adherence of hESCs to microcarriers aids cell survival during the freeze-thaw process.
ECM has been shown to help certain cells survive freezing and thawing. For example, rat hepatocytes cryopreserved in collagen displayed a higher viability than cells cryopreserved in suspension (55). hESCs embedded in Matrigel exhibit an approximately ten-fold improvement in recovery as compared to the ~1.5-fold improvement found in this study (36). Heng et al showed that post-thaw apoptosis was the predominant mechanism of death when hESCs were cryopreserved adherent on plate (56). They argued that Matrigel entrapment further improved hESC colony recovery by preserving gap-junctions and other intercellular adhesion contacts during freezing and thawing. Although the mechanism is far from fully understood, this study supports the notion that maintaining cell adhesion improves hESC recovery following cryopreservation. Furthermore, storing frozen colonies on microcarriers in cryovials is more scalable than storing culture plates.
This study reveals a promising approach to improve hESC culture which may allow efficient scale-up of hESC storage, distribution and culture systems. Although culturing and stabilizing hESCs on microcarriers still requires further optimization and translation to defined xeno-free culture conditions, our approach serves as a proof-of-principle for future larger-scale investigations.
Acknowledgments
This work was supported by the National Stem Cell Bank, NIH contract 06-W226. The authors would like to thank Christian Metallo and Somen Saha for initial work in culturing hESCs on microcarriers. We also thank Seth Taapken, Casey Stankewicz and Karen Montgomery at the cytogenetics lab of WiCell Research Institute for the karyotyping study, and also the other staff at WiCell for their assistance in culturing hESCs. We thank Kathy Schell, Erik Puffer and their colleagues at the UW Comprehensive Cancer Center Flow Cytometry Facility for their technical support in the flow cytometry experiments. We also thank Clay Glennon at the UW Primate Research Center for his assistance in acquiring the confocal images.
References and Notes
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein RS, Benvenisty N. Induced neuronal differentiation of human embryonic stem cells. Brain research. 2001;913:201–205. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 5.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nature biotechnology. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 6.Slukvin II, Vodyanik MA, Thomson JA, Gumenyuk ME, Choi KD. Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. J Immunol. 2006;176:2924–2932. doi: 10.4049/jimmunol.176.5.2924. [DOI] [PubMed] [Google Scholar]
- 7.Metallo CM, Azarin SM, Ji L, de Pablo JJ, Palecek SP. Engineering tissue from human embryonic stem cells. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem cells (Dayton, Ohio) 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem cells (Dayton, Ohio) 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 10.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 11.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 12.Hentze H, Graichen R, Colman A. Cell therapy and the safety of embryonic stem cell-derived grafts. Trends Biotechnol. 2007;25:24–32. doi: 10.1016/j.tibtech.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Ho HY, Li M. Potential application of embryonic stem cells in Parkinson’s disease: drug screening and cell therapy. Regenerative medicine. 2006;1:175–182. doi: 10.2217/17460751.1.2.175. [DOI] [PubMed] [Google Scholar]
- 14.Lyon A, Harding S. The potential of cardiac stem cell therapy for heart failure. Current opinion in pharmacology. 2007;7:164–170. doi: 10.1016/j.coph.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Gerecht-Nir S, Itskovitz-Eldor J. Cell therapy using human embryonic stem cells. Transplant immunology. 2004;12:203–209. doi: 10.1016/j.trim.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Golos TG, Pollastrini LM, Gerami-Naini B. Human embryonic stem cells as a model for trophoblast differentiation. Seminars in reproductive medicine. 2006;24:314–321. doi: 10.1055/s-2006-952154. [DOI] [PubMed] [Google Scholar]
- 17.Clark AT, Reijo Pera RA. Modeling human germ cell development with embryonic stem cells. Regenerative medicine. 2006;1:85–93. doi: 10.2217/17460751.1.1.85. [DOI] [PubMed] [Google Scholar]
- 18.Menendez P, Bueno C, Wang L. Human embryonic stem cells: A journey beyond cell replacement therapies. Cytotherapy. 2006;8:530–541. doi: 10.1080/14653240601026654. [DOI] [PubMed] [Google Scholar]
- 19.Vallier L, Pedersen RA. Human embryonic stem cells: an in vitro model to study mechanisms controlling pluripotency in early mammalian development. Stem cell reviews. 2005;1:119–130. doi: 10.1385/SCR:1:2:119. [DOI] [PubMed] [Google Scholar]
- 20.Fok EY, Zandstra PW. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333–1342. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 21.Oh SK, Fong WJ, Teo Y, Tan HL, Padmanabhan J, Chin AC, Choo AB. High density cultures of embryonic stem cells. Biotechnology and bioengineering. 2005;91:523–533. doi: 10.1002/bit.20650. [DOI] [PubMed] [Google Scholar]
- 22.Fong WJ, Tan HL, Choo A, Oh SK. Perfusion cultures of human embryonic stem cells. Bioprocess and biosystems engineering. 2005;27:381–387. doi: 10.1007/s00449-005-0421-5. [DOI] [PubMed] [Google Scholar]
- 23.Cabrita GJ, Ferreira BS, da Silva CL, Goncalves R, Almeida-Porada G, Cabral JM. Hematopoietic stem cells: from the bone to the bioreactor. Trends in biotechnology. 2003;21:233–240. doi: 10.1016/S0167-7799(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay A, Madhusudhan T, Kumar R. Hematopoietic stem cells: clinical requirements and developments in ex-vivo culture. Advances in biochemical engineering/biotechnology. 2004;86:215–253. doi: 10.1007/b12444. [DOI] [PubMed] [Google Scholar]
- 25.Kallos MS, Sen A, Behie LA. Large-scale expansion of mammalian neural stem cells: a review. Medical & biological engineering & computing. 2003;41:271–282. doi: 10.1007/BF02348431. [DOI] [PubMed] [Google Scholar]
- 26.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442–453. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 27.Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem cells (Dayton, Ohio) 2004;22:275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 28.Cormier JT, zur Nieden NI, Rancourt DE, Kallos MS. Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 2006;12:3233–3245. doi: 10.1089/ten.2006.12.3233. [DOI] [PubMed] [Google Scholar]
- 29.zur Nieden NI, Cormier JT, Rancourt DE, Kallos MS. Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors. J Biotechnol. 2007;129:421–432. doi: 10.1016/j.jbiotec.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Tang NH, Chen YL, Wang XQ, Li XJ, Yin FZ, Wang XZ. Construction of IL-2 gene-modified human hepatocyte and its cultivation with microcarrier. World J Gastroenterol. 2003;9:79–83. doi: 10.3748/wjg.v9.i1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuriyama S, Nakano T, Yoshimura N, Ohuchi T, Moritera T, Honda Y. Mass cultivation of human retinal pigment epithelial cells with microcarrier. Ophthalmologica. Journal international d’ophtalmologie International journal of ophthalmology. 1992;205:89–95. doi: 10.1159/000310319. [DOI] [PubMed] [Google Scholar]
- 32.Westergaard N, Sonnewald U, Petersen SB, Schousboe A. Characterization of microcarrier cultures of neurons and astrocytes from cerebral cortex and cerebellum. Neurochemical research. 1991;16:919–923. doi: 10.1007/BF00965542. [DOI] [PubMed] [Google Scholar]
- 33.Buzzard JJ, Gough NM, Crook JM, Colman A. Karyotype of human ES cells during extended culture. Nat Biotechnol. 2004;22:381–382. doi: 10.1038/nbt0404-381. author reply 382. [DOI] [PubMed] [Google Scholar]
- 34.Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, Meisner L, Zwaka TP, Thomson JA, Andrews PW. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 35.Gearhart J. New potential for human embryonic stem cells. Science. 1998;282:1061–1062. doi: 10.1126/science.282.5391.1061. [DOI] [PubMed] [Google Scholar]
- 36.Ji L, de Pablo JJ, Palecek SP. Cryopreservation of adherent human embryonic stem cells. Biotechnol Bioeng. 2004;88:299–312. doi: 10.1002/bit.20243. [DOI] [PubMed] [Google Scholar]
- 37.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, Thomson JA. Derivation of human embryonic stem cells in defined conditions. Nature biotechnology. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23:699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- 40.Rosler ES, Fisk GJ, Ares X, Irving J, Miura T, Rao MS, Carpenter MK. Long-term culture of human embryonic stem cells in feeder-free conditions. Dev Dyn. 2004;229:259–274. doi: 10.1002/dvdy.10430. [DOI] [PubMed] [Google Scholar]
- 41.Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Developmental biology. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 42.Tian X, Kaufman DS. Hematopoietic development of human embryonic stem cells in culture. Methods Mol Biol. 2005;290:149–162. doi: 10.1385/1-59259-838-2:149. [DOI] [PubMed] [Google Scholar]
- 43.Weitzer G. Embryonic stem cell-derived embryoid bodies: an in vitro model of eutherian pregastrulation development and early gastrulation. Handbook of experimental pharmacology. 2006:21–51. [PubMed] [Google Scholar]
- 44.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 45.Forsyth NR, Musio A, Vezzoni P, Simpson AH, Noble BS, McWhir J. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning and stem cells. 2006;8:16–23. doi: 10.1089/clo.2006.8.16. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 47.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 48.Ha SY, Jee BC, Suh CS, Kim HS, Oh SK, Kim SH, Moon SY. Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Human reproduction (Oxford, England) 2005;20:1779–1785. doi: 10.1093/humrep/deh854. [DOI] [PubMed] [Google Scholar]
- 49.Zhou CQ, Mai QY, Li T, Zhuang GL. Cryopreservation of human embryonic stem cells by vitrification. Chinese medical journal. 2004;117:1050–1055. [PubMed] [Google Scholar]
- 50.Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- 51.Fujioka T, Yasuchika K, Nakamura Y, Nakatsuji N, Suemori H. A simple and efficient cryopreservation method for primate embryonic stem cells. Int J Dev Biol. 2004;48:1149–1154. doi: 10.1387/ijdb.041852tf. [DOI] [PubMed] [Google Scholar]
- 52.Heng BC, Clement MV, Cao T. Caspase Inhibitor Z-VAD-FMK Enhances the Freeze-Thaw Survival Rate of Human Embryonic Stem Cells. Biosci Rep. 2007 doi: 10.1007/s10540-007-9051-2. [DOI] [PubMed] [Google Scholar]
- 53.Richards M, Fong CY, Tan S, Chan WK, Bongso A. An efficient and safe xeno-free cryopreservation method for the storage of human embryonic stem cells. Stem Cells. 2004;22:779–789. doi: 10.1634/stemcells.22-5-779. [DOI] [PubMed] [Google Scholar]
- 54.Wu CF, Tsung HC, Zhang WJ, Wang Y, Lu JH, Tang ZY, Kuang YP, Jin W, Cui L, Liu W, Cao YL. Improved cryopreservation of human embryonic stem cells with trehalose. Reproductive biomedicine online. 2005;11:733–739. doi: 10.1016/s1472-6483(10)61692-6. [DOI] [PubMed] [Google Scholar]
- 55.Borel Rinkes IH, Toner M, Ezzell RM, Tompkins RG, Yarmush ML. Effects of dimethyl sulfoxide on cultured rat hepatocytes in sandwich configuration. Cryobiology. 1992;29:443–453. doi: 10.1016/0011-2240(92)90047-6. [DOI] [PubMed] [Google Scholar]
- 56.Heng BC, Ye CP, Liu H, Toh WS, Rufaihah AJ, Yang Z, Bay BH, Ge Z, Ouyang HW, Lee EH, Cao T. Loss of viability during freeze-thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. Journal of biomedical science. 2006;13:433–445. doi: 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]