Abstract
The basolateral amygdala is critical for generation of anxiety. Additionally, exposure to both stress and glucocorticoids induce anxiety. Demonstrated ability of the amygdala to change in response to stress and glucocorticoids could thus be important therapeutic target for anxiety management. Several studies have reported a relationship between anxiety and dendritic arborization of the amygdaloid neurons. In this study we employed a gene therapeutic approach to reduce anxiety and dendritic arborization of the amygdala neurons. Specifically we over-expressed SK2 potassium channel in the basolateral amygdala using a herpes simplex viral system. Our choice of therapeutic cargo was guided by the indications that activation of the amygdala might underlie anxiety and that SK2 could reduce neuronal activation by exerting inhibitory influence on action potentials. We report that SK2 over expression reduced anxiety and stress-induced corticosterone secretion at a systemic level. SK2 overexpression also reduced dendritic arborization of the amygdala neurons. Hence, SK2 is a potential gene therapy candidate molecule which can be used against stress-related neuropsychiatric disorders like anxiety.
Keywords: gene-therapy, brain, stress hormone, hypertrophy, herpes simplex virus, rats
INTRODUCTION
Fear is an adaptive behavioral response to danger, enabling organisms to evade stimuli that are threatening. Anxiety, on the other hand, is mal-adaptive expression of fear response. It is usually expressed as generalized and heightened response to stimuli that do not pose or predict danger (1-4). The amygdala is an important mediator of fear and anxiety (5-8). Among the heterogeneous nuclei of the amygdala, basolateral amygdala (BLA) is especially important, because it serves as an interface between sensory and cognitive realm (5, 9).
It is interesting from a clinical perspective that the triad of amygdala, anxiety and stress is reciprocally inter-linked (1). Stress or stress hormones enhance anxiety (10-12). Stress and stress hormones also change structure and function of the BLA, resulting in hypertrophy and increased excitability (11-15). BLA, in turn, can enhance secretion of stress hormone, thus potentiating stress response (16-20). Additionally, a stronger BLA can enhance anxiety (11, 12, 14). These positive feedbacks might explain the incidence of high stress hormone levels in anxiety disorders (1, 21, 22).
Diminution of these positive feedbacks is of immense clinical significance. We postulate that influencing the BLA plasticity is central to any such approach. Dendritic arborization of principal BLA neurons is tightly linked to anxiety (11, 13). Dendritic hypertrophy could also affect secretion of stress hormones because of positive feedbacks emanating from BLA on hypothalamic nuclei signaling stress hormone release (23). Thus, a therapeutic approach targeting dendritic structure of BLA neurons could prove useful in reducing anxiety and stress hormone secretion.
Gene-therapy is a powerful mean to manipulate the molecular milieu in a targeted population of cells. Within the nervous system, this approach has been primarily used to rescue neurons in response to necrotic neurological insults (24-26). As one strategy relevant to the current study, over-expression of the SK2 potassium channel via a herpes simplex viral vector protects hippocampal neurons from seizure-induced excitotoxicity (27). Over-expression of SK2 channels is known to reduce excitatory drive by augmenting the afterhyperpolarization phase of action potential (28). Thus, in theory, such a reduction in excitatory drive and amygdala activation could affect dendritic architecture and could blunt anxiety and stress hormone secretion. This is the goal of the present study.
MATERIALS AND METHODS
Subjects
Adult male Wistar rats (10 weeks, Charles River Laboratories) were used for both morphological and behavioral analysis. Animals were maintained in a 14:10 hours light-dark cycle (lights on at 7 am) and provided with food and water ad libitum. All procedures were carried out according to NIH guidelines for animal care and were approved by the Stanford University institutional animal care committee (APLAAC).
Gene therapy
We employed over-expression of a calcium-activated potassium channel, SK2, through a herpes simplex viral system (HSV). SK2 is responsible for the slow component of afterhyperpolarization of an action potential. Overexpression of SK2 should thus prolong afterhyperpolarization and reduce frequency of action potentials. Generation and in vivo over-expression of the SK2 potassium channel using HSV has been previously described in detail (27). Experimental vector (SK2) consisted of SK2 cDNA under the control of a HSV α4 promoter and β-gal reporter gene under a α22 promoter. Control vector (β-Gal) consisted of β-Gal reporter gene alone under a α4 promoter. Both amplicons were packaged into replicationdeficient modified HSV vectors and purified with a yield of 1-15 × 106 viral vectors/ml and 106 - 107 helper virus particles/ml. Vector-induced expression of SK2 in rat brain was originally reported for this vector by Lee et al in 2003 (27). Similarly, in this report we show the overexpression of SK2 in BLA (Figure 1).
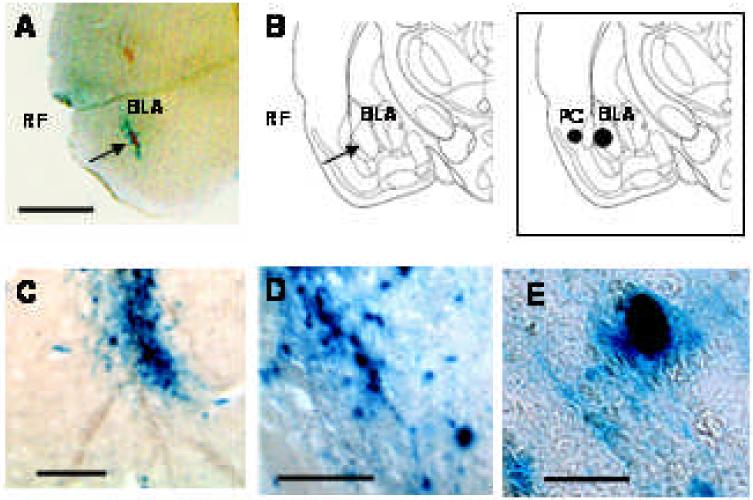
Figure 1.
SK2 viral vector resulted in over expression of reporter gene β-gal, stained with X-gal (blue), in the BLA, 48 hours post-infusion. (A) β-gal staining in BLA (next to RF, an identifiable anatomical landmark); scale = 2.5 mm (B) Diagram depicting coronal section of rat brain from comparable level as shown in A (C) β-gal stained cells of BLA at higher magnification; scale = 250 μm. (D) scale = 125 μm (E) Single cell stained with β-gal in BLA; scale = 20 μm. Inset: Coronal section depicting BLA and PC in rat brain.
Treatments and Experimental groups
Control (β-Gal) or experimental (SK2) vectors were infused (2 μl over a 10-minute period with a titer of ~1 × 106) in the basolateral amygdala (BLA; anterior/posterior, -2.3 mm from bregma; medial/lateral, ± 5.1 mm from midline; dorsal/ventral, 7 mm from dura). For behavioral and endocrine studies, a given rat received bilateral infusion of the same vector (β-Gal or SK2). For morphological analysis, β-Gal vector was infused in the left hemisphere and SK2 vector was infused in the right hemisphere, unilaterally.
The viral vector expresses cargo for about 3-4 days post-infusion. Most of the parameters (behavior, morphology and endocrine) in this manuscript were measured at a time point 12 days post-infusion when the cargo ceased to express (time-lines for each experiment given in Inset of Figures 2-7).
Figure 2.
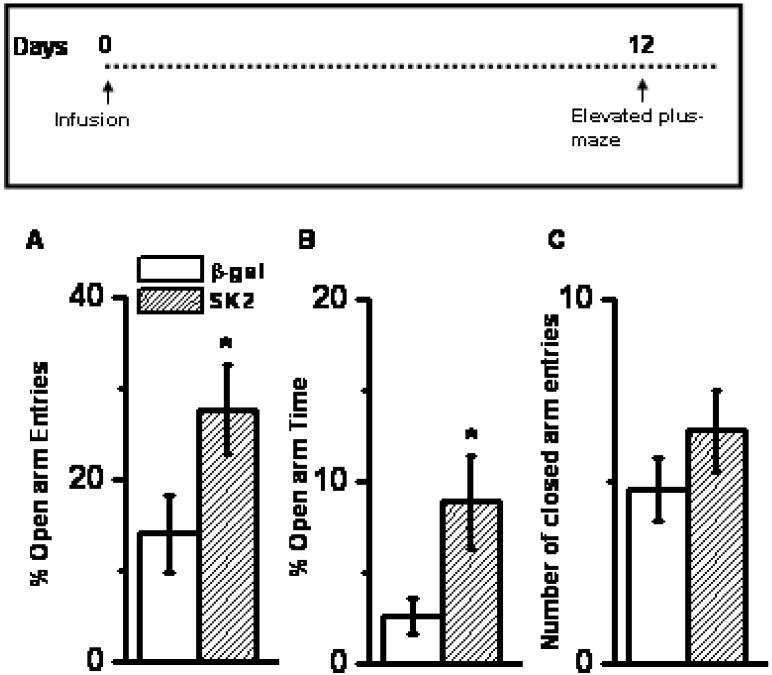
Effect of SK2 over-expression on anxiety and locomotion on EPM. SK2 infusion enhanced percentage open-arm entries (A) and percentage open-arm time (B). This enhancement was not accompanied by differences in entries in enclosed arm (C) Mean ± SEM, n = 9 animals each group; * p < 0.001, Student's t-test. Inset: Time profile of experiment and endpoint.
Figure 7.
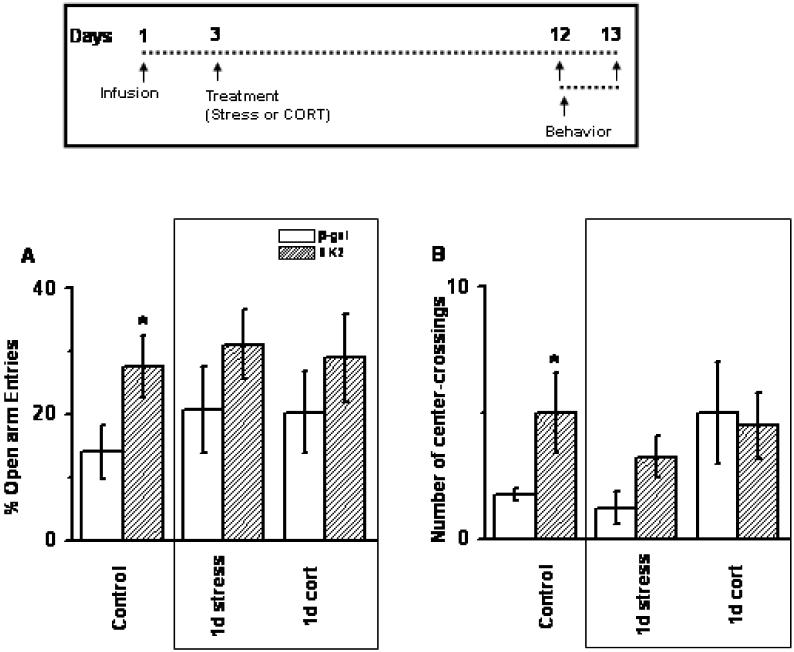
SK2 did not change anxiety in stress or CORT-treated animals in either open-arm entries (A) or number of center crossings (B); n = 8-10 animals per group. Control data in Fig 7 has been shown earlier in Fig 2 and 3. Inset: Time profile for infusion and behavior.
Animals were allowed to recover for 72 hours and then randomly divided in three experimental groups. First group was left undisturbed and served as control. Second group was subjected to a single session of two hours of immobilization stress (10 am to noon). Third group was treated with a single injection of corticosterone (CORT, principal glucocorticoid hormone in rats; 10 mg dissolved in 1 ml peanut oil per kg body weight; subcutaneously). These treatments are known to produce sustained elevations of circulating CORT concentrations in the range seen during major stressors (11, 29, 30).
Separate sets of animals were employed for behavioral, endocrine and morphological measurements, in order to avoid cross-over effects. First set of animals underwent behavioral analysis. Second set of animals were tested for morphological parameters. A third set of animals was tested for secretion of basal and stress-induced CORT. Some animals had their blood drawn in basal conditions without stress and were later also employed in behavioral testing.
Behavioral analysis: Starting twelve days after infusion of vectors, animals were tested for anxiety and locomotor activity in three different tasks, as detailed below. A single observer (blinded for the experimental groups) rated performance in all trials. Individual trials lasted for 5 min each. An entry into arm or center of arena was deemed to have occurred when all four paws and base of tail were inside the arm or center. Details of the number of animals employed per experiment are specifically noted in results and figure legends.
Elevated Plus-Maze. The elevated plus-maze consisted of two opposite open arms (60 × 15 cm) and two enclosed arms (60 × 15 cm, surrounded by a 15-cm-high black wall) elevated 75 cm from the ground. The open arms were more brightly illuminated (60 lux) compared to the center (16 lux) and the closed arms (<10 lux). At the beginning of each trial, animals were placed at the center of the maze, facing an enclosed arm. The maze was cleaned with 70% (v/v) ethanol solution after each trial. The number of entries and the time spent in open arms were measured in addition to the number of entries in enclosed arms. Open-arm exploration was measured by normalizing a. open arm entries to total entries (open arm + close arm) and b. open arm time to total time (time spent in open arm + time spent in close arm). In this paradigm, anxiety is measured as a function of decreased open arm exploration (31).
Circular Open Arena: Animals were allowed to explore a circular open arena (radius = 75 cm). An illumination gradient was established using incandescent lamp (95 lux at the center and 65 lux at the periphery). Anxiety was measured as the time spent at the center (defined as at least 18.75 cm away from wall). Locomotion was measured as the total distance traveled in the arena.
Rectangular Open Field: Animals were allowed to explore a rectangular open field. This open field (60 cm × 60 cm × 45 cm; length, width and height) was smaller than circular open arena described above. The arena was lighted with an illumination of 222 lux at the centre and 180 lux at the periphery. Anxiety was measured as the number of crossings into the center arena (20 cm away from wall).
A subset of animals was tested sequentially for performance in the elevated plus-maze and in the circular open arena. A second subset was used to measure performance in rectangular open field. All behavioral tests were conducted in same room.
To address specificity of BLA-function through gene-therapeutic intervention, we carried out additional infusion in a nearby brain region, the piriform cortex (PC: anterior/posterior, -2.3 mm from bregma; medial/lateral, ± 6.1 mm from midline; dorsal/ventral, 5.2 mm from dura) and tested the animals (n = 6) for anxiety on EPM.
Plasma corticosterone concentration
Concentration of circulating plasma corticosterone was quantified three days (basal and stress-induced) and twelve days (basal) after the infusion. Same animals were not always used for blood collection at all three points. A smaller subset of animals was employed for collecting stress-induced samples. A total of 4-6 animals were used for different time points of CORT collection.
Stress induced samples were collected by immobilizing animals for thirty minutes and collecting blood immediately thereafter. For blood collection, animals were gently held inside a dark towel and up to 100 μl blood was collected in heparinised tubes through a tail nick. This method is known to induce minimal stress during repeated blood collection (32). The tubes were kept on ice and were subsequently centrifuged to collect the plasma (5415C, Eppendorf; 10000 rpm for 10 minutes). Corticosterone titers in plasma (diluted 11 times) were assessed using a competitive enzyme immunoassay kit (Assay Design, Minneapolis, MN). This assay typically results in a sensitivity value of 27 pg/ml (concentration of CORT two standard deviation away from zero on standard curve). This assay method has low cross-reactivity to testosterone (< 0.15%).
Morphological studies and analysis
Morphological analysis was conducted 12 days after the infusion. Animals were decapitated under deep flurothane anesthesia. Freshly dissected brain tissues containing the amygdala were processed for staining individual neurons using rapid Golgi method (15,16). Golgi-stained BLA tissue was sectioned (120 μm thick), mounted with cover slip and used for morphological analysis. Camera Lucida tracings (500 X) were obtained (Nikon, USA) from selected neurons and were then scanned (8-bit grayscale TIFF images with 600 dpi resolution, Canon MultiPass MP360) along with a calibrated scale for subsequent computerized image-analysis. Custom-designed macros embedded in `Scion Image' software (http://www.scioncorp.com/) were used for morphometric analysis of digitized images. Using the center of the soma as the reference point, dendritic length and branch points were measured as a function of radial distance from the soma by adding up all values in each successive concentric segment (Sholl's analysis). An average of 40-50 neurons from 4-5 animals per group was used for morphological analysis.
Statistical analysis
Values are reported as mean ± SEM, and percentage changes are calculated with respect to corresponding control values. The differences between naïve animals infused with SK2 and β-gal were analyzed using Student's t-test. Effects of infusion on secretion of endogenous CORT were assessed using non-parametric Mann Whitney U-test, because this dataset was not normally distributed. Two way analysis of variance was conducted to measure effects of treatment (control, stress or CORT treatment) and infusion (β-Gal or SK2) on behavioral and morphological endpoints. Orthogonal planned comparisons were used to compare effects of infusion (β-Gal versus SK2, Student's t-test). No mean was compared more than once during the planned comparison.
RESULTS
SK2 overexpression in basolateral amygdala (BLA) reduced anxiety
Rats over-expressing SK2 in the BLA exhibited more open arm exploration in an elevated plus-maze compared to rats over-expressing β gal. Such enhanced open arm exploration was evident in terms of both percentage open-arm entries (Figure 2A, 97% increase; p < 0.001, n = 9) and percentage time spent in open arms (Figure 2B, 242% increase; p < 0.001, n = 9). Moreover, the increase in open arm exploration was not due to an increase in locomotor activity, as demonstrated by comparable number of entries in the enclosed arms (Figure 2C; p > 0.05, n = 9). SK2-induced facilitation of open arm exploration was specific to BLA; not evident when SK2 was infused in a neighboring brain region (piriform cortex; % open arm entries = 18.1 ± 3.5 %).
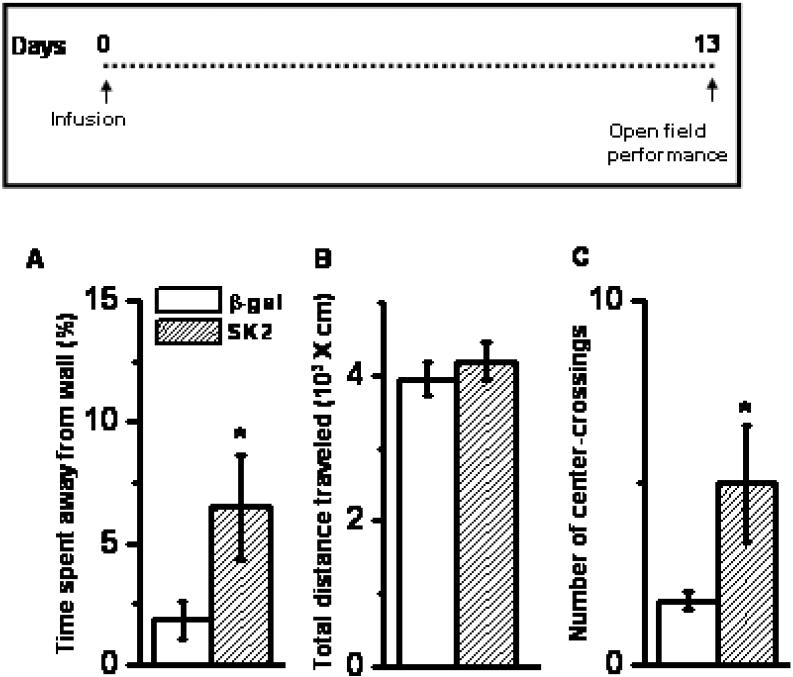
We further investigated the effects of SK2 on anxiety by employing a circular open arena. SK2-treated rats spent more time away from the wall (Figure 3A, center defined as at least 18.75 cm away from wall; 253% increase; p < 0.05, n = 12). This was not due to a generic increase in locomotion, because SK2 and control vector rats did not differ in the total distance traveled (Figure 3B, p > 0.4, n = 12). A different set of animals was also tested in a smaller rectangular open field. SK2-treated animals made more number of crossings into the center of the arena (Figure 3C; p < 0.05, n = 5-6). In summary, animals treated with SK2 exhibited enhanced exploration of anxiogenic compartments in elevated plus-maze and open fields, without affecting locomotion itself. Thus we conclude that SK2 infusion in BLA reduced anxiety.
Figure 3.
Effect of SK2 over-expression on anxiety and locomotion as measured in open field. SK2 over-expression enhanced time spent away from wall in a big open arena (A) without affecting the total distance traveled (B) Mean ± SEM. n = 12 animals per group. SK2 over-expression also increased the number of crossings made at the center of a smaller open field (C) Mean ± SEM. n = 5-6 animals per group; * p < 0.001, Student's t-test. Inset: Time profile for infusion and behavior.
SK2 overexpression in basolateral amygdala reduced stress-induced CORT levels
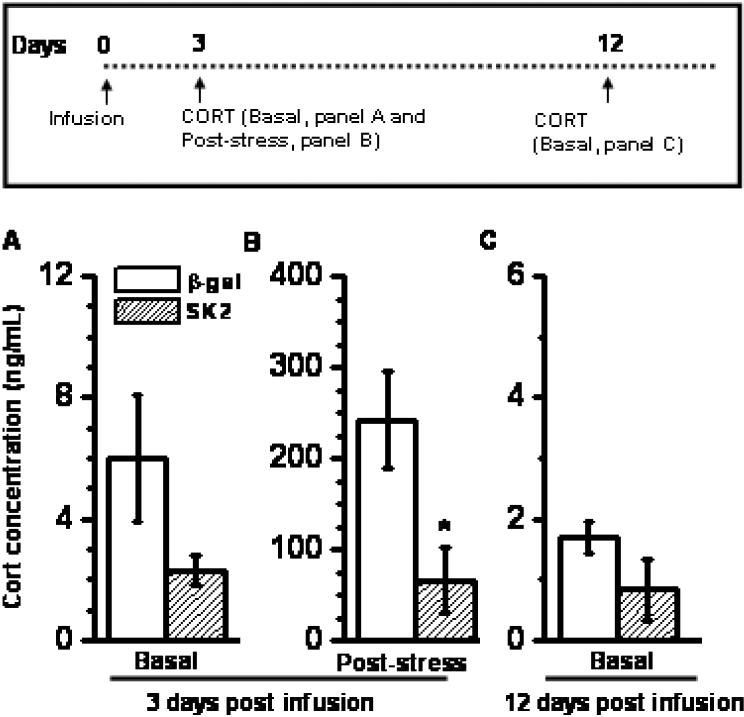
SK2 overexpression did not affect baseline plasma CORT levels 3 days after infusion of vector (Figure 4A; p > 0.13). Immobilization stress (30 minutes) increased circulating CORT. Magnitude of stress-induced plasma CORT was significantly reduced in animals overexpressing SK2 (Figure 4B, 74% reduction; p < 0.005, MW test). No differences in baseline CORT were found at 12 days post-treatment (Figure 4C; p > 0.3), a time-point when infused vectors had ceased to express cargo. A total of 4-6 animals per group were used for CORT measurements.
Figure 4.
SK2 reduced secretion of CORT when measured after a 30-minute session of stress (B) SK2 did not affect basal CORT at 3 days (A) or 12 days post infusion (C) Mean ± SEM. n = 4-6 animals, * p < 0.01, Student's t-test. Inset: Time profile for infusion and CORT measurement. Note: The ordinate scale in panel A, B and C are different.
SK2 overexpression in BLA reduced dendritic length of neurons
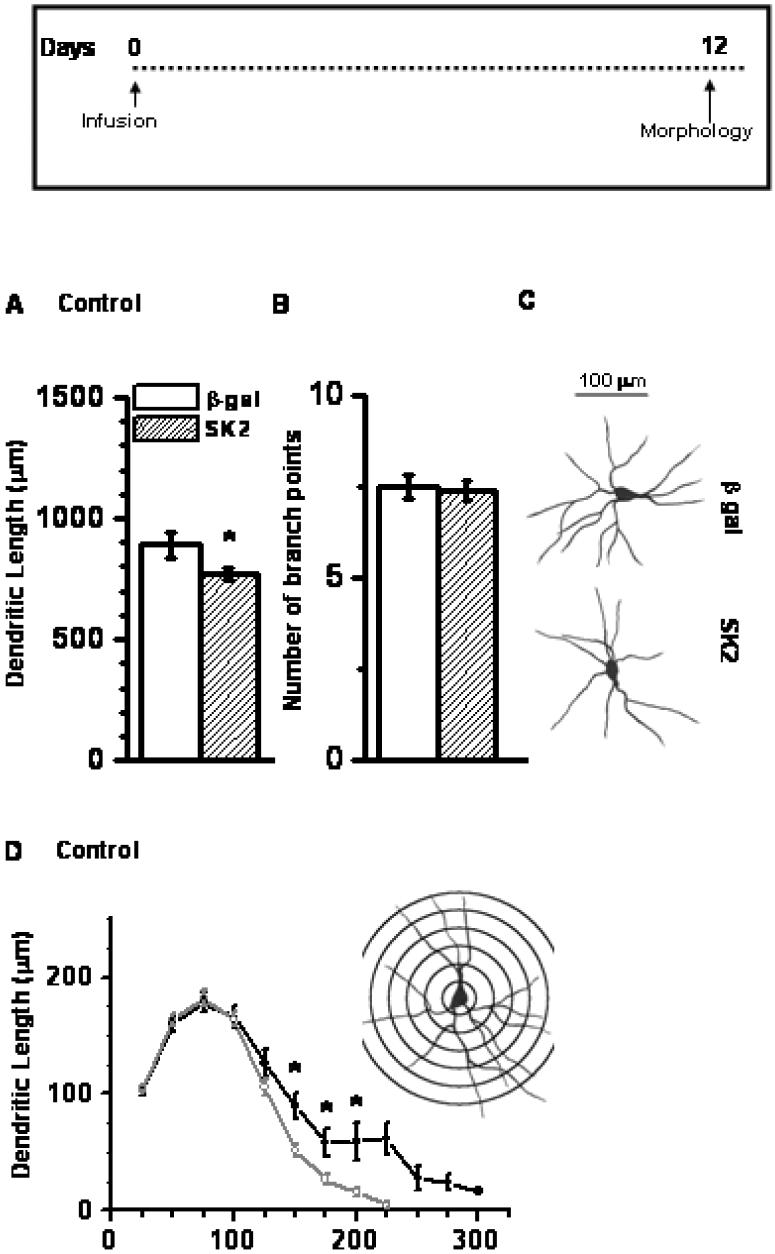
We studied the effects of SK2 on dendritic arborization of BLA principal neurons by infusing SK2 in one hemispheric BLA and β-Gal in the contra lateral BLA. Morphological measures were performed 12 days after infusion on 40-50 neurons from 4-5 animals. Neurons from SK2-infused BLA showed reduced dendritic length (Figure 5A, 14% reduction; p < 0.05), with no differences in the number of branch points (Figure 5B; p > 0.4). Figure 5C depicts representative camera lucida drawings obtained from neurons treated with either SK2 (lower) or β-Gal (upper). A detailed segmental analysis revealed that SK2-over-expression reduced dendritic length along distal segments of BLA neuron, between 160 μm to 200 μm away from the cell soma (Figure 5D).
Figure 5.
SK2 over-expression decreased dendritic arborization of neurons in the BLA. The decrease in dendritic arborization was reflected in the total dendritic length (A) but not in the number of branch points (B). (C) Camera Lucida drawings of representative neuron from β-gal-expressing (upper) or SK2-over-expressing (lower) animals. Scale bar = 100 μm. (D) Segmental analysis of dendritic length in SK2-infused animals showing reduction in segments 150-200 μm away from cell body. * p < 0.05; Student's t-test; n= 40-50 neurons from 4-5 animals per group. Inset (D): Sholl's analysis of a typical pyramidal neuron of BLA. Inset: Time profile for morphological measurement post infusion.
SK2 reduced dendritic length in stressed and CORT-treated animals
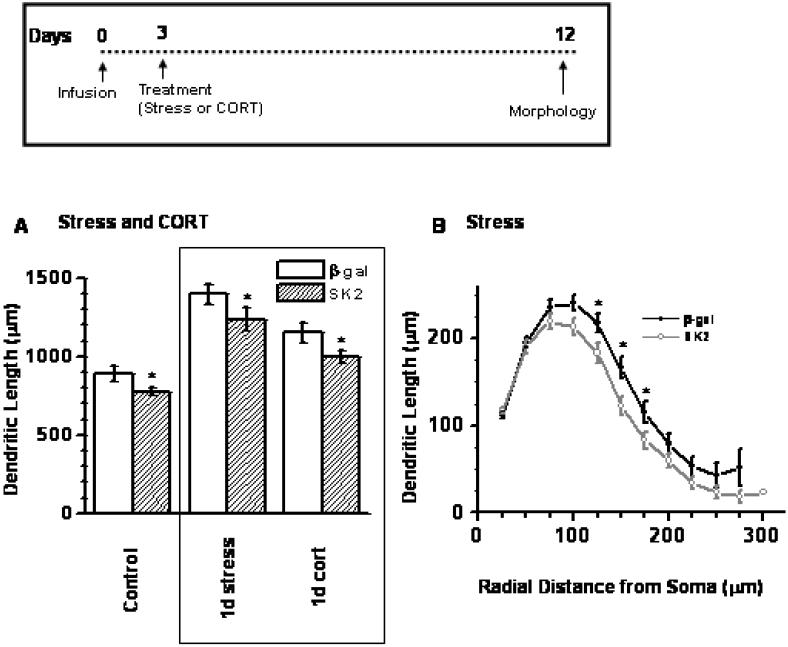
Previous studies have reported that stress and stress hormones cause BLA dendritic expansion (11, 13). In that context, we studied the effects of SK2 infusion on dendritic arborization in animals treated with either acute stress or acute stress hormone injection (40-50 neurons from 5-6 animals). Morphological parameters were collected 10 days after the stress or stress hormone treatment (12 days post-infusion). A two-way ANOVA revealed a significant main effect of SK2 infusion (Figure 6A; p = 0.001; F1,277 = 15.2, SK2 < β-Gal) and treatment (p < 0.001; F2,277 = 37.5, stress > CORT > naive) on dendritic length. Interaction between treatment and infusion was not significant (F2,277 = 0.51; p > 0.5). Planned comparisons revealed that over-expression of SK2 significantly reduced the effects of stress and CORT on dendritic length (p < 0.05). Such dendritic retraction is similar to the effects of SK2 in naïve unstressed animals (Figure 5). However, segmental analysis showed that SK2 over expression in stressed animals reduced dendritic length more proximally (110-180 μm away from cell body; Figure 6B), in contrast to distal retraction observed in naïve animals (Figure 5D).
Figure 6.
Stress and CORT-treatment enhanced dendritic length, and SK2 over-expression decreased dendritic length in Control, Stress and CORT-treated animals, compared to animals of each group expressing β-gal (A). * p < 0.05; planned comparison using Student's t-test; n = 40-50 neurons from 5-6 animals per group. Control data in Fig 6A is same as in Fig 5A. (B) Segmental analysis showed a reduction in segments 100-150 μm away from cell body in stress-treated animals. Inset: Time profile for morphological measurement post infusion.
SK2 did not affect anxiety in stressed and CORT-treated animals
As described earlier (Figure 2 and 3), SK2 infusion in naïve animals reduced anxiety. We tested if SK2 also reduced anxiety in animals treated with stress or stress hormone (8-10 animals per group). Two-way ANOVA revealed significant main effect of SK2 infusion on percentage open arm entries made in the elevated plus-maze (F1,47 = 5.5, SK2 > β-Gal; p < 0.05). Main effect of treatment and interaction failed to reach statistical significance (F2,277 < 0.5, p > 0.5).
Although SK2-treated animals showed a trend of increase in open arm exploration in stress and CORT-treated animals, planned comparison between the groups failed to reach statistical significance (Figure 7A; p > 0.2). A similar trend was observed in open-field exploration in case of stressed animals (Figure 6B; p > 0.08), while CORT-treated animals were not different than controls (Figure 7B; p > 0.84).
DISCUSSION
Anxiety disorders are among the most debilitating psychiatric diseases, characterized by a maladaptive fear response. The amygdala is critical for both fear and anxiety. Thus modulation of amygdaloid function will be relevant to management of anxiety disorders. Previously, gene therapeutic approaches have been used to manipulate the amygdala and amygdala-dependent behaviors. For example, virally-mediated over expression of enkephlin in the amygdala reduces pain responsiveness and augments the ability of benzodiazepines to reduce anxiety (33, 34). Virally-mediated blockage of synaptic incorporation of AMPA receptors also blocks associative learning (35). In this study we demonstrate that over expression of a potassium channel, SK2, can alter amygdala-dependent behavior, structure and function. Moreover, these effects are present long after the viral vector has ceased to express its cargo. To our knowledge, the present report is the first gene-therapeutic approach to target stress hormone secretion and long-term anxiety.
Pathological anxiety is thought to reflect a maladaptive state characterized by exaggerated fear mismatched with actual environmental stimuli. There has been growing interest in the role of the amygdala in anxiety, because the amygdala is known to be central for fear responses (5-9). Manipulations that enhance anxiety also enhance dendritic arborization, number of spines, gross volume and synaptic transmission in the amygdala (11, 12, 14, 15). NMDA receptor antagonists in the amygdala blunt anxiety (36), while reduction in inhibitory tone by GABA receptor blockade facilitates anxiety (37). These observations in rats are supported by parallel studies in primates and humans. For example, lesion studies in rhesus monkeys suggest that bilateral destruction of the central amygdala reduces fear and anxiety-related behaviors (38). In humans, amygdala activation is a reliable predictor of anxiety (39-43). Activation of the amygdala is positively correlated with severity of social anxiety symptoms (44). Moreover, amygdala volume is increased in generalized anxiety disorders (45, 46). These observations suggest that the amygdala is an important target for therapeutic interventions against anxiety.
In this study, we focused on virally-mediated over-expression of SK2 in the BLA. As noted, abnormal activation of BLA is thought to be relevant to anxiety disorders (11, 12, 14, 36). We have previously observed that SK2 over-expression can protect hippocampal neurons from excitotoxicity, both in vitro and in vivo (27). Here we report that SK2 over-expression reduces anxiety, causes dendritic retraction in BLA and reduces stress-induced CORT secretion peripherally. These effects are not related to an acute decrease in activity of BLA neurons. This is because the HSV amplicon vectors only express their cargo for 3-5 days and in this study behavioral and anatomical effects were observed long after the vector had ceased to express SK2. Hence a long-term plastic change rather than acute suppression of neuronal activity is more likely to be involved in bringing about the effects. As is typical with HSV vectors, only a small percentage of neurons were infected. This was nonetheless sufficient to drive long-term changes. This is in agreement with previous reports where infection of only a small percentage of neurons can potentially alter function (35, 47). Finally, although HSV is primarily a neurotropic virus, it still does infect some glia. Thus, a role of glia in indirectly modulating neuronal and functional change can not be ruled out.
Various studies have investigated the relationship between dendritic changes in the BLA and behavioral changes. Of particular interest to this report, stress and stress hormones cause dendritic expansion of BLA neurons and such dendritic expansion is thought to be important for ensuing enhancement in anxiety (11, 14). This is in agreement with reports that link dendritic changes in hippocampus to behavioral changes in memory (48). Changes in dendritic arborization can directly affect passive electrotonic property of neurons (49, 50). Coupled with passive changes, there is possibility that active ion channels could also get purged during dendritic retraction. Because of previous reports linking dendritic expansion in BLA to anxiety and because SK2-induced dendritic retraction could affect electrical property of neurons, the effects of SK2 on anxiety might have arisen from SK2-induced dendritic retraction.
Similar to its effects on naïve animals, SK2 over-expression also reduced dendritic length in animals exposed to either acute stress or acute treatment of CORT. Both of these treatments cause dendritic hypertrophy (11, 14). While we did see a trend of reduced anxiety in SK2-treated animals exposed to stress or CORT, stress and CORT themselves did not have much effect on anxiety. One possibility is that anxiety in our animals was already very high, perhaps due to the stress of the bilateral stereotaxic surgery. Further enhancement of anxiety by stress or CORT was not possible within the dynamic range of behavioral tests we employed.
As noted, the mechanisms underlying dendritic expansion and retraction in the BLA are poorly understood. One possibility is that the activity of neurons regulate level of cytosolic Ca+2 and that in turn influences cytoarchitectural reorganization. For example, activation of Ca+2 permeable NMDA channels in Xenopus tectal neurons promotes dendritic outgrowth (51). In rat motorneurons, in vivo delivery of DNA coding for GluR1, a subunit of glutamatergic AMPA channel, results in enhanced branching of the neurons (52). It is possible that by virtue of reduced neuronal activity, SK2 reduces the level of cytosolic Ca+2 and thus influences cytoarchitecture promoting dendritic retraction.
We also observed that over-expression of SK2 attenuated secretion of CORT in response to stress. Our results show that stress-induced levels of CORT in β-gal animals is comparable to stress-induced levels reported in animals left untreated with any vector (53-56). In contrast, SK2 infused animals have lower levels of CORT compared to widely known levels of stress-induced CORT. Interestingly, hypersecretion of CORT is known to occur in human anxiety as well as in rodent models of anxiety (1, 21, 22). In rats, treatment with anxiety-reducing drugs (such as chlordiazepoxide or diazepam) reduces stress-induced CORT secretion (57). Similarly, an anxiolytic acetylcholine receptor antagonist, mecamylamine, reduces stress-induced CORT secretion in rats (58). Our findings are in agreement with these reports, suggesting that anxiolysis is related to decrease in circulating CORT. A possible mechanistic explanation for this relationship is the fact that amygdala activity can stimulate CORT secretion, and thus mediate the activating effects of many stressors on the adrenocortical axis (19, 23).
In summary, we show that transient over-expression of SK2 in the BLA can protect against anxiety. It can also reduce stress-induced peripheral CORT-secretion and dendritic arbors of the BLA neurons, both of which has been shown to be directly related to anxiety. Hence, this approach is of heuristic value in understanding the neurobiology of anxiety, and may also pave the way for eventual therapeutic interventions.
ACKNOWLEDGEMENTS
This study is funded by RO1 AGO20633 (NIH).
ABBREVIATIONS
- β gal
β galactosidase
- BLA
Basolateral Amygdala
- CORT
Corticosterone
- EPM
Elevated Plus Maze
- GABA
Gamma amino butyric acid
- HSV
Herpes Simplex Virus
- PC
Piriform Cortex
- RF
Rhinal Fissure.
REFERENCES
- 1.Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 1):S2–5. [PubMed] [Google Scholar]
- 2.Shearer SL. Recent advances in the understanding and treatment of anxiety disorders. Prim Care. 2007;34(3):475–504. v–vi. doi: 10.1016/j.pop.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.WHO . The ICD-10 Classification of Mental and Behavioral Disorders-Diagnostic Criteria for Research. World Health Organization; Geneva: 1993. [Google Scholar]
- 4.APA . Diagnostic and statistical manual of mental disorders. fourth edition (DSM-IV) Washington DC: 1994. [Google Scholar]
- 5.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4-5):727–38. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- 6.Quirk GJ. Fear research: implications for anxiety disorders. Bol Asoc Med P R. 1998;90(1-3):27–9. [PubMed] [Google Scholar]
- 7.Miller LA, Taber KH, Gabbard GO, Hurley RA. Neural underpinnings of fear and its modulation: implications for anxiety disorders. J Neuropsychiatry Clin Neurosci. 2005;17(1):1–6. doi: 10.1176/jnp.17.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med. 2006;73(7):941–9. [PubMed] [Google Scholar]
- 9.Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17(5):208–14. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 10.Mitra R, Vyas A, Chatterjee G, Chattarji S. Chronic-stress induced modulation of different states of anxiety-like behavior in female rats. Neurosci Lett. 2005;383(3):278–83. doi: 10.1016/j.neulet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 11.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;I105(14):5573–8. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24(14):3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102(26):9371–6. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res Dev Brain Res. 2004;148(2):159–67. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Feldman S, Conforti N, Itzik A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res. 1994;658(1-2):21–6. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- 17.Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull. 1998;45(4):389–93. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 18.Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003;963(1-2):203–13. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- 19.Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10(3-4):371–94. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 20.Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–9. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- 21.Greaves-Lord K, Ferdinand RF, Oldehinkel AJ, Sondeijker FE, Ormel J, Verhulst FC. Higher cortisol awakening response in young adolescents with persistent anxiety problems. Acta Psychiatr Scand. 2007;116(2):137–44. doi: 10.1111/j.1600-0447.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- 22.Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, et al. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33(6):773–81. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin J, Roozendaal B, Dumas T, Gupta A, Ajilore O, Hsieh J, et al. Sparing of neuronal function postseizure with gene therapy. Proc Natl Acad Sci U S A. 2000;97(23):12804–9. doi: 10.1073/pnas.210350097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimazaki K, Urabe M, Monahan J, Ozawa K, Kawai N. Adeno-associated virus vector-mediated bcl-2 gene transfer into post-ischemic gerbil brain in vivo: prospects for gene therapy of ischemia-induced neuronal death. Gene Ther. 2000;7(14):1244–9. doi: 10.1038/sj.gt.3301211. [DOI] [PubMed] [Google Scholar]
- 26.Roy M, Hom JJ, Sapolsky RM. HSV-mediated delivery of virally derived anti-apoptotic genes protects the rat hippocampus from damage following excitotoxicity, but not metabolic disruption. Gene Ther. 2002;9(3):214–9. doi: 10.1038/sj.gt.3301642. [DOI] [PubMed] [Google Scholar]
- 27.Lee AL, Dumas TC, Tarapore PE, Webster BR, Ho DY, Kaufer D, et al. Potassium channel gene therapy can prevent neuron death resulting from necrotic and apoptotic insults. J Neurochem. 2003;86(5):1079–88. doi: 10.1046/j.1471-4159.2003.01880.x. [DOI] [PubMed] [Google Scholar]
- 28.Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984;354:319–31. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson D, Sapolsky R. Overexpression of mineralocorticoid and transdominant glucocorticoid receptor blocks the impairing effects of glucocorticoids on memory. Hippocampus. 2008 doi: 10.1002/hipo.20467. [DOI] [PubMed] [Google Scholar]
- 30.Stein-Behrens B, Mattson MP, Chang I, Yeh M, Sapolsky R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J Neurosci. 1994;14(9):5373–80. doi: 10.1523/JNEUROSCI.14-09-05373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mechiel Korte S, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463(1-3):163–75. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- 32.Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab Anim. 2000;34(4):372–8. doi: 10.1258/002367700780387714. [DOI] [PubMed] [Google Scholar]
- 33.Kang W, Wilson SP, Wilson MA. Overexpression of proenkephalin in the amygdala potentiates the anxiolytic effects of benzodiazepines. Neuropsychopharmacology. 2000;22(1):77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 34.Kang W, Wilson MA, Bender MA, Glorioso JC, Wilson SP. Herpes virus-mediated preproenkephalin gene transfer to the amygdala is antinociceptive. Brain Res. 1998;792(1):133–5. doi: 10.1016/s0006-8993(98)00194-2. [DOI] [PubMed] [Google Scholar]
- 35.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308(5718):83–8. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 36.Adamec RE, Burton P, Shallow T, Budgell J. Unilateral block of NMDA receptors in the amygdala prevents predator stress-induced lasting increases in anxiety-like behavior and unconditioned startle--effective hemisphere depends on the behavior. Physiol Behav. 1999;65(4-5):739–51. doi: 10.1016/s0031-9384(98)00225-x. [DOI] [PubMed] [Google Scholar]
- 37.Sanders SK, Morzorati SL, Shekhar A. Priming of experimental anxiety by repeated subthreshold GABA blockade in the rat amygdala. Brain Res. 1995;699(2):250–9. doi: 10.1016/0006-8993(95)00915-d. [DOI] [PubMed] [Google Scholar]
- 38.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24(24):5506–15. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 40.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24(46):10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55(9):897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann N Y Acad Sci. 2003;985:370–88. doi: 10.1111/j.1749-6632.2003.tb07095.x. [DOI] [PubMed] [Google Scholar]
- 43.Amaral DG, Corbett BA. The amygdala, autism and anxiety. Novartis Found Symp. 2003;251:177–87. discussion 187-97, 281-97. [PubMed] [Google Scholar]
- 44.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59(5):424–9. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 46.De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48(1):51–7. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 47.Dumas TC, Sapolsky RM. Gene therapy against neurological insults: sparing neurons versus sparing function. Trends Neurosci. 2001;24(12):695–700. doi: 10.1016/s0166-2236(00)01956-1. [DOI] [PubMed] [Google Scholar]
- 48.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–77. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 49.Rall W. Cable properties of neurons with comples dendritic trees. In: Segev I, Rinzel J, Shepherd GM, editors. The theoretical foundation of dendritic function. MIT Press; Cambridge, MA: 1995. pp. 25–104. [Google Scholar]
- 50.Rall W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- 51.Rajan I, Witte S, Cline HT. NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J Neurobiol. 1999;38(3):357–68. doi: 10.1002/(sici)1097-4695(19990215)38:3<357::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 52.Inglis FM, Crockett R, Korada S, Abraham WC, Hollmann M, Kalb RG. The AMPA receptor subunit GluR1 regulates dendritic architecture of motor neurons. J Neurosci. 2002;22(18):8042–51. doi: 10.1523/JNEUROSCI.22-18-08042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roman E, Gustafsson L, Berg M, Nylander I. Behavioral profiles and stress-induced corticosteroid secretion in male Wistar rats subjected to short and prolonged periods of maternal separation. Horm Behav. 2006;50(5):736–47. doi: 10.1016/j.yhbeh.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Spencer RL, Miller AH, Moday H, Stein M, McEwen BS. Diurnal differences in basal and acute stress levels of type I and type II adrenal steroid receptor activation in neural and immune tissues. Endocrinology. 1993;133(5):1941–50. doi: 10.1210/endo.133.5.8404640. [DOI] [PubMed] [Google Scholar]
- 55.Karssen AM, Meijer OC, Berry A, Sanjuan Pinol R, de Kloet ER. Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinology. 2005;146(12):5587–95. doi: 10.1210/en.2005-0501. [DOI] [PubMed] [Google Scholar]
- 56.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 57.Mikkelsen JD, Soderman A, Kiss A, Mirza N. Effects of benzodiazepines receptor agonists on the hypothalamic-pituitary-adrenocortical axis. Eur J Pharmacol. 2005;519(3):223–30. doi: 10.1016/j.ejphar.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 58.Newman MB, Nazian SJ, Sanberg PR, Diamond DM, Shytle RD. Corticosterone-attenuating and anxiolytic properties of mecamylamine in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(3):609–20. doi: 10.1016/s0278-5846(00)00178-0. [DOI] [PubMed] [Google Scholar]