Abstract
Family history is a strong predictor of colorectal cancer risk; however, a diagnosis of colorectal cancer among first-degree relatives has not been systematically investigated as a function of the colorectal cancer molecular subtypes related to tumor microsatellite instability (MSI) status. We investigated whether the observable familial colorectal cancer risks differed according to tumor MSI subtypes, stratified as MSI-High (>30% instability), MSI-Low (<30% instability), and MSS (no instability). Data from 3,143 population-based colorectal cancer cases from five institutions were assessed for family history according to the Amsterdam criteria and the Bethesda guidelines, age at diagnosis, sex, tumor location, and MSI status. The distribution of patient characteristics by MSI status was compared using polytomous logistic regression. Overall, 2.8% colorectal cancer cases met the Amsterdam criteria and 37% met the Bethesda guidelines. There were 14% MSI-High, 13% MSI-Low, and 73% MSS colorectal cancers. MSI-High (P < 0.0001) and MSI-Low tumors (P = 0.01) were more proximally located than MSS tumors. MSI-High tumors were more common among females (P < 0.001). The highest proportion of MSI-High tumors occurred in cases <40 years of age whereas the age-dependent distribution of MSI-Low tumors was unchanged. MSI-High tumors showed a statistically significant association with increasing numbers of first-degree relatives with colorectal cancer (P = 0.002); this association disappeared, however, when MSI-High cases meeting Amsterdam criteria were removed from the analysis. MSI-Low tumors did not show a similar association with family history of colorectal cancer. Familial risk associated with MSI-High tumors is primarily driven by the Amsterdam-criteria patients. MSI-Low tumors may represent a distinct subtype of colorectal cancer with respect to certain epidemiologic variables studied here.
Introduction
In 2006, in the United States, there were 148,610 new cases of colorectal cancer, and mortality from colorectal cancer ranked second only to lung cancer (1). At a population level, one challenge has been to determine how best to stratify individuals by levels of risk, so as to provide optimal screening. Age is the strongest risk factor for colorectal cancer. Family history is also a strong predictor of colorectal cancer risk, and large population-based studies from multiple countries have repeatedly documented increased risk of colorectal cancer in the relatives of those with colorectal cancer (2–19). The risk is greater if the relative is young or if a person has more than one affected relative. These epidemiologic studies, however, did not examine familial risk as a function of the recently defined molecular subtypes of colorectal cancer related to tumor microsatellite instability (MSI).
Colorectal cancers can be divided by molecular phenotyping into those with normal DNA mismatch repair (MMR) function (around 85% of all colorectal cancers) and those with DNA MMR deficiency. The laboratory feature observed in those with DNA MMR deficiency is a distinct form of genetic instability known as microsatellite instability (MSI), which is characterized by small deletions or insertions within short tandem repeats (“microsatellites”) in tumor DNA not seen in corresponding DNA from normal tissue. Microsatellite stable (MSS) tumors have no evidence of instability at any marker. Tumors are classified as having a high level of MSI (MSI-High or MSI-H) if >30% of tested microsatellites show instability (20, 21). Tumors with 1% to 29% of microsatellites showing instability are said to have a low level of MSI (MSI-Low or MSI-L). Depending on the number and type of microsatellite loci showing instability, and the threshold criteria used to score MSI status, the reported frequency of MSI-L tumors has ranged from 3% to 35% of all colorectal cancers (22–25). MSI-L tumors do not seem to arise due to a defective DNA MMR function like MSI-H tumors; thus, in clinical practice, MSI-L tumors have been commonly grouped together with MSS tumors. Recent studies suggest, however, that compared with MSS tumors, MSI-L colorectal cancers are associated with less desirable histopathologic features and may confer a poorer prognosis, raising the possibility that MSI-L tumors represent a distinct subgroup (26–28).
About 15% to 20% of all colorectal cancers are MSI-H tumors. Few (about 2–3%) of these are due to inherited syndrome hereditary nonpolyposis colorectal cancer (HNPCC), also known as Lynch syndrome, which is characterized by germline defects of DNA MMR genes (29, 30). Besides colorectal cancer, Lynch syndrome patients are also at an increased risk of developing extracolonic cancers, including but not limited to the endometrium, small bowel, ureter and renal pelvis. Extracolonic cancers in Lynch syndrome patients also manifest MSI-H status. Among extracolonic cancers, endometrial cancer is the second most common cancer occurring in these patients. This clustering of cancers often makes the diagnosis of Lynch syndrome difficult, and several clinical diagnostic criteria have been developed to address this issue. Traditional and more stringent Amsterdam type I criteria are based on a family history of colorectal cancer associated with a dominant mode of inheritance combined with an early age of onset (31). These criteria were subsequently revised and updated, termed Amsterdam type II criteria, to include extracolonic cancers observed in Lynch syndrome families (32). Other widely used criteria include the Bethesda Guidelines, which are based on clinical and histopathologic characteristics of tumors. The Bethesda Guidelines were primarily developed to identify individuals with MSI tumors, who should be further considered for evaluation of germline MMR gene mutation status (33–36).
In population-based studies (as opposed to studies conducted in high-risk clinics) the MSI-H tumor phenotype is predominantly caused by somatic (not germline) biallelic inactivation of the MMR gene MLH1 via promoter hypermethylation. DNA methylation is associated with the aging process, and sporadic MSI-H tumors with MLH1 deficiency are more commonly seen in older patients, in women, and in proximal colon tumors (37, 38). Recently, reports have noted that the process of DNA methylation may be influenced by genetic factors such as germline, and such MSI-H patients may have variable family histories of colorectal cancer and/or other extracolonic malignancies (39, 40). However, these seem to be rather exceptional families and likely do not account for most of the MLH1 methylation in MSI-H tumors associated with aging. Thus, the heritability of colorectal cancer of the MSI-H type that is not due to Lynch syndrome is poorly defined.
By stratifying colorectal cancers by DNA MMR–dependent tumor MSI status in this study, we sought to assess family history in the context of this information. Furthermore, we attempted to stratify the MSI-H group by excluding putative Lynch syndrome families, in order to observe the residual familial risk in the MSI-H group that likely does not have Lynch syndrome. Lastly, this large population-based study provided an opportunity to compare and contrast the controversial MSI-L group with the MSS and MSI-H groups with regard to family history and basic demographic categories.
Materials and Methods
Patients
The study subjects were patients diagnosed with primary colorectal cancer, from ages 18 to 75 y, ascertained from population-based registries through participating centers in the Colon Cancer Family Registries (C-CFRs), a National Cancer Institute–supported consortium established in 1997 to create a multinational comprehensive collaborative infrastructure for interdisciplinary studies in the genetic and molecular epidemiology of colorectal cancer.15 The participating registries and collaborating institutions use standardized instruments and protocols to collect family history information, epidemiologic and clinical data, and related biological specimens, with quality control measures throughout the collection, processing, and storing of data and samples. All CFR sites have institutional review board approval of protocols. Since 1997, eligible patients were identified and relevant data collected by Cancer Care Ontario, Toronto, Canada; The Fred Hutchinson Cancer Research Center, Seattle, Washington; the Mayo Clinic, Rochester, Minnesota; the University of Southern California Consortium, Los Angeles, California; the University of Queensland, Brisbane and the University of Melbourne, Melbourne, Australia; and the University of Hawaii, Honolulu, Hawaii.
The sampling and recruitment methods varied across CFR sites. Some centers recruited all colorectal cancer cases within a stipulated age range, whereas others sampled cases for recruitment according to age and/or family history, as previously described (41).
Family History and Tumor Phenotyping
After consenting to participate in research, cases (probands) provided a family history, and enrollment of additional relatives began. Verification of reported cancer diagnoses included use of multiple family member interviews, review of medical records, death certificates, pathology reports, and tumor tissues. The pedigrees of probands were reviewed to identify those meeting the Amsterdam I and Amsterdam II criteria and the Bethesda Guidelines. Amsterdam I criteria require three relatives with colorectal cancer (the “triad”), one of whom is a first-degree relative of the other two; colorectal cancer involving at least two generations; one or more colorectal cancer cases diagnosed at <50 y of age at onset; and exclusion of the diagnosis of familial adenomatous polyposis (31). Amsterdam II criteria are similar to Amsterdam I except that they specify three relatives with a “HNPCC-associated” tumor (defined, for these purposes, as colorectal, endometrial, small-bowel, ureter, or renal-pelvis tumors; ref. 32). A colorectal cancer proband was classified as meeting the Amsterdam I or II criteria if the proband was any part of the Amsterdam triad, or the proband was not part of the triad but there was a continuous lineage through first-degree relatives affected with Amsterdam I- or II-related cancers from the proband to the triad.
The Bethesda Guidelines refer to an individual with colorectal cancer. Each affected individual can be scored for five specific criteria (34–36), namely: (a) a proband with colorectal cancer diagnosed <50 y of age; (b) a proband with colorectal cancer and the presence of synchronous or metachronous colorectal or other HNPCC-associated tumors, regardless of age; (c) a proband with colorectal cancer with MSI-H histology diagnosed in a patient <60 y of age; (d) a proband with colorectal cancer plus a colorectal cancer or HNPCC-associated tumor diagnosed at <50 y of age in at least one first-degree relative; and (e) a proband with colorectal cancer plus a colorectal cancer or HNPCC-associated tumor diagnosed at any age in two first- or second-degree relatives. As we did not have a systematic review of colorectal tumor pathology across all CFR sites, we did not apply criteria 3 in scoring our families for the Bethesda Guidelines. In keeping with the presumed intent of this criterion, we made a rule that the two relatives used to fulfill criteria 5 had to be related by blood to one another, even though this was not stated explicitly in the publication of the Revised Bethesda Guidelines (35). The “HNPCC-associated” tumors in the published guidelines included colorectal, endometrial, stomach, ovarian, pancreas, ureter and renal pelvis, biliary tract, and brain (usually glioblastoma as seen in Turcot syndrome) tumors; sebaceous gland adenomas and keratoacanthomas in Muir-Torre syndrome; and carcinoma of the small bowel, regardless of age. We excluded those reported only as “kidney” as most of these were likely not to be renal pelvis. We included “brain,” not otherwise specified, as specific brain tumor histology was not mandated in the guidelines.
Colon tumors were stratified as “proximal” when located proximal to hepatic flexure, and “distal” when localized to rectosigmoid junction, rectum, rectum overlap with other sites and anus unspecified; those with missing location information were considered to be “other” category.
MSI Testing
Colorectal tumor blocks were collected from all eligible patients from hospitals throughout the United States, Canada, and Australia. DNA was extracted from paraffin-embedded matched normal and tumor tissue specimens, and tested for MSI using 10 microsatellite loci (four mononucleotide markers: BAT25, BAT26, BAT40, BAT34C4; five dinucleotide markers: D5S346, D17S250, ACTC, D18S55, D10S197; and penta-mono-tetra compound marker MYCL as described previously; ref. 42). Tumors were classified as MSI-H if >30% markers showed instability, MSI-L if ≤30% and >0% showed instability, and MSS if no marker exhibited instability. A minimum of four interpretable markers (maximum up to 10) was required to be included in this study.
Statistical Analysis
This was a case-case study using the largest subgroup, the MSS group, as the reference against which the MSI-H and the MSI-L groups were compared. To account for the sampling of subjects at some CFR centers, we used proband weighting so that the recruited sample was a statistical representation of the entire population of colorectal cancer cases at each site. Subjects included in this study were from all CFR sites except Hawaii, where the ascertainment design did not permit this proband weighting.
The association between patient characteristics and MSI status was assessed using polytomous logistic regression, with MSI category as the outcome variable; odds of MSI-H or MSI-L versus MSS were computed. All analyses use sampling weights to account for the probability that a proband was selected for recruitment into the Colon CFR. There was negligible effect on our estimates from adjustment for study center (Ontario, USC Consortium, Australia, Mayo Clinic, Seattle), sex, and age at diagnosis (<40, 40–49, 50–59, 60–69, ≥70 y). As a result we report frequencies from the unadjusted data only. All analyses were conducted using SAS version 9.1 (SAS Institute Inc.) and Stata 9 (Stata Corporation).
Results
Characteristics of Study Population
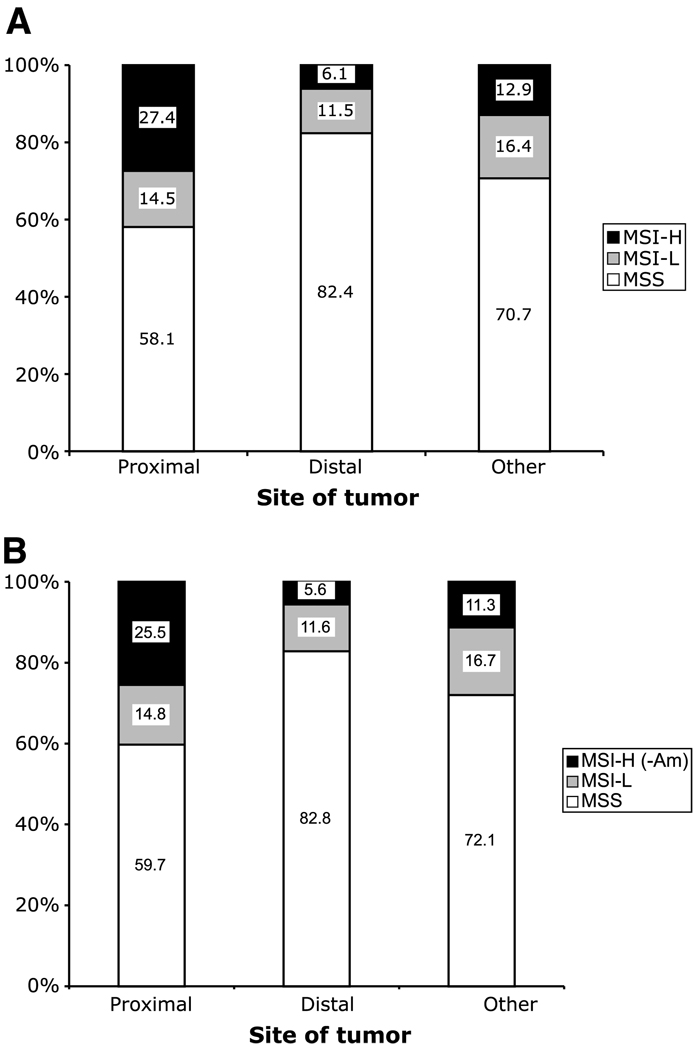
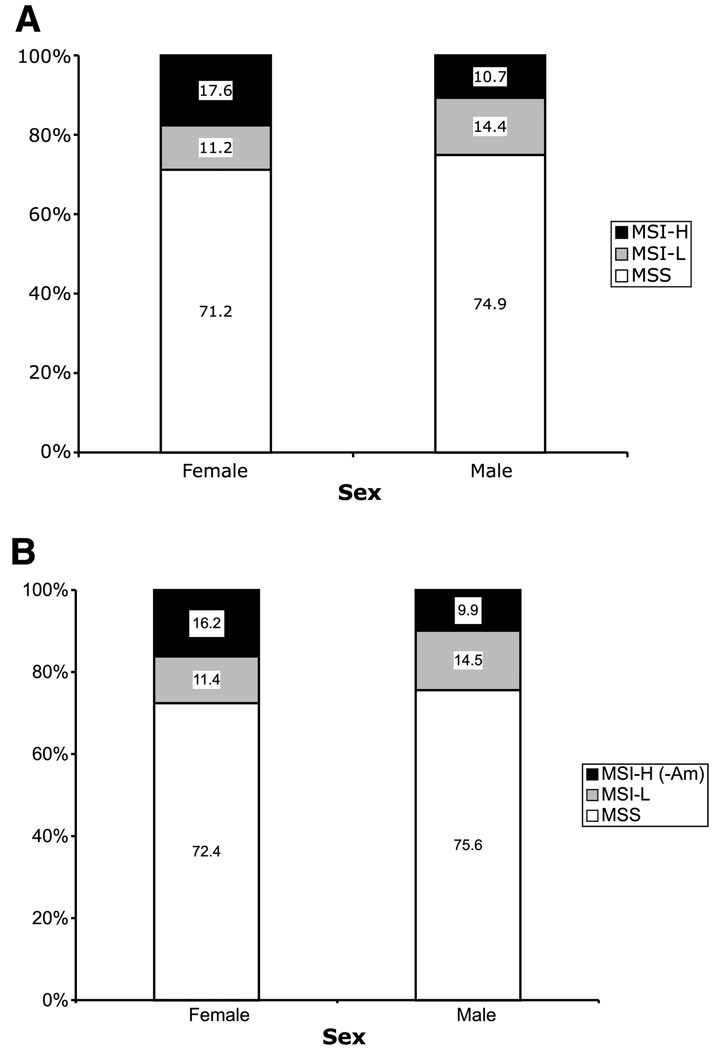
A total of 3,143 cases were included in the analysis, representing (after weighting) an estimated 6,796 colorectal cancer cases in the underlying populations. All cited statistics and comparisons use the proband weights to estimate the number of cases represented by these data. The clinico-pathologic features of the tumors for all colorectal cancer probands, and for those that did not meet the Amsterdam criteria, are shown in Table 1 and Fig. 1 to Fig. 4. Overall, 56% probands were men and 44% were women. The ethnicity of study participants was Caucasian (81%), African American/Caribbean (3%), South-East Asian (2%), Latin/Hispanic origin (2%), or unknown (12%). Among all cases, 14% of colorectal cancers were MSI-H, 13% MSI-L, and 73% MSS. MSI-H tumors accounted for more proximal than distal tumors, whereas MSI-L tumors were about equally represented in the two regions of the bowel (Fig. 1). MSI-H tumors were more common among females than among males, even after the removal of the probands meeting Amsterdam criteria, but this pattern was not observed for MSS or MSI-L tumors (Fig. 2).
Table 1.
Clinicopathologic features of colorectal cancer probands stratified by MSI status
| MSS (73%) | MSI-L (13%) | MSI-H (14%) | OR (95% CI) | OR (95% CI) | OR vs. OR | |
|---|---|---|---|---|---|---|
| Wgt N* (%)† | Wgt N*(%)† | Wgt N*(%)† | MSI-L vs MSS | MSI-H vs MSS | P‡ | |
| Age at diagnosis (y) | ||||||
| <40 | 153 (3.1) | 22 (2.5) | 55.0 (5.7) | 1 | 1 | |
| 40–49 | 674 (13.7) | 125 (13.9) | 115.1 (12.0) | 1.28 (0.63–2.63) | 0.48 (0.27–0.84) | 0.01 |
| 50–59 | 1,480 (30.0) | 252 (27.8) | 148.8 (15.5) | 1.17 (0.59–2.34) | 0.28 (0.16–0.49) | 0.0004 |
| 60–69 | 1,709 (34.6) | 271 (30.0) | 376.8 (39.2) | 1.1 (0.54–2.21) | 0.61 (0.35–1.07) | 0.15 |
| +70 | 921 (18.7) | 234 (25.9) | 265.6 (27.6) | 1.75 (0.8–3.85) | 0.8 (0.45–1.42) | 0.08 |
| Heterogeneity P | 0.49 | <0.0001 | ||||
| Sex | ||||||
| Female | 2,116 (42.9) | 341 (37.7) | 533.5 (55.5) | 1 | 1 | |
| Male | 2,814 (57.1) | 564 (62.3) | 427.7 (44.5) | 1.24 (0.88–1.76) | 0.6 (0.44–0.82) | 0.001 |
| Heterogeneity P | 0.21 | 0.0014 | ||||
| Site of tumor | ||||||
| Proximal | 1,234 (25.0) | 305 (33.7) | 622.4 (64.8) | 1 | 1 | |
| Distal | 3,281 (66.5) | 487 (53.8) | 253.8 (26.4) | 0.6 (0.42–0.87) | 0.15 (0.11–0.22) | <0.0001 |
| Other | 421 (8.5) | 113 (12.5) | 85.0 (8.8) | 1.08 (0.52–2.23) | 0.4 (0.23–0.69) | 0.02 |
| Heterogeneity P | 0.01 | <0.0001 | ||||
| Number of first-degree relatives with colorectal cancer | ||||||
| 0 | 4,024 (81.5) | 756 (83.6) | 728.2 (75.8) | 1 | 1 | |
| 1 | 774 (15.7) | 134 (14.8) | 165.7 (17.2) | 0.92 (0.63–1.33) | 1.18 (0.86–1.62) | 0.26 |
| 2 | 117 (2.4) | 13 (1.4) | 51.1 (5.3) | 0.57 (0.28–1.16) | 2.42 (1.41–4.14) | 0.0002 |
| ≥3 | 22 (0.5) | 2 (0.2) | 16.3 (1.7) | 0.47 (0.08–2.66) | 4.01 (1.19–13.51) | 0.007 |
| Heterogeneity P | 0.38 | 0.002 | ||||
| Number of first-degree relatives with endometrial cancer | ||||||
| 0 | 4,793 (97.1) | 891 (98.5) | 912.7 (95.0) | 1 | 1 | |
| ≥1 | 144 (2.9) | 14 (1.5) | 48.5 (5.0) | 0.52 (0.18–1.49) | 1.77 (1.07–2.93) | 0.02 |
| Heterogeneity P | 0.22 | 0.02 | ||||
| Number of first-degree relatives with any other cancer than colorectal cancer or endometrial cancer | ||||||
| 0 | 2,239 (45.4) | 410 (45.4) | 394.9 (41.1) | 1 | 1 | |
| 1 | 1,672 (33.9) | 303 (33.5) | 354.3 (36.9) | 0.99 (0.69–1.42) | 1.2 (0.85–1.7) | 0.4 |
| 2 | 754 (15.3) | 106 (11.7) | 149.7 (15.6) | 0.76 (0.39–1.5) | 1.13 (0.73–1.74) | 0.31 |
| ≥3 | 272 (5.5) | 86 (9.5) | 62.3 (6.5) | 1.73 (0.88–3.39) | 1.3 (0.7–2.4) | 0.48 |
| Heterogeneity P | 0.30 | 0.69 | ||||
| Had first-degree relatives with both colorectal cancer and endometrial cancer | ||||||
| No | 4,896 (99.2) | 903 (99.9) | 933.6 (97.1) | 1 | 1 | |
| Yes | 41 (0.8) | 1 (0.1) | 27.6 (2.9) | 0.13 (0.02–1.02) | 3.54 (1.72–7.25) | 0.0015 |
| Heterogeneity P | 0.05 | 0.0006 | ||||
| Amsterdam I | ||||||
| No | 4,860 (98.4) | 894 (98.8) | 904.9 (94.1) | 1 | 1 | |
| Yes | 78 (1.6) | 11 (1.2) | 56.3 (5.9) | 0.76 (0.34–1.69) | 3.9 (2.21–6.87) | <0.0001 |
| Heterogeneity P | 0.49 | <0.001 | ||||
| Amsterdam II | ||||||
| No | 4,828 (97.8) | 893 (98.7) | 889.9 (92.6) | 1 | 1 | |
| Yes | 109 (2.2) | 12 (1.3) | 71.3 (7.4) | 0.59 (0.28–1.25) | 3.55 (2.13–5.92) | <0.0001 |
| Heterogeneity P | 0.16 | <0.0001 | ||||
| Bethesda | ||||||
| No | 3,150 (75.1) | 626 (69.2) | 524.5 (54.6) | 1 | 1 | |
| Yes | 1,787 (71.4) | 279 (30.8) | 436.7 (45.4) | 0.78 (0.57–1.09) | 1.47 (1.09–1.98) | 0.002 |
| Heterogeneity P | 0.14 | 0.01 | ||||
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
Per text, these are estimated numbers of probands corrected for proband weighting based on ascertainment criteria.
Proportion of covariates within each tumor MSI category (MSS/MSI-L/MSI-H) is calculated based on Wgt N.
Comparison between OR of MSI-L vs. MSS and OR of MSI-H vs. MSS.
Figure 1.
Distribution of colorectal cancer (CRC) MSI subtype by tumor location, including all probands (A) and after exclusion of MSI-H probands meeting the Amsterdam criteria I and II (B). P < 0.001.
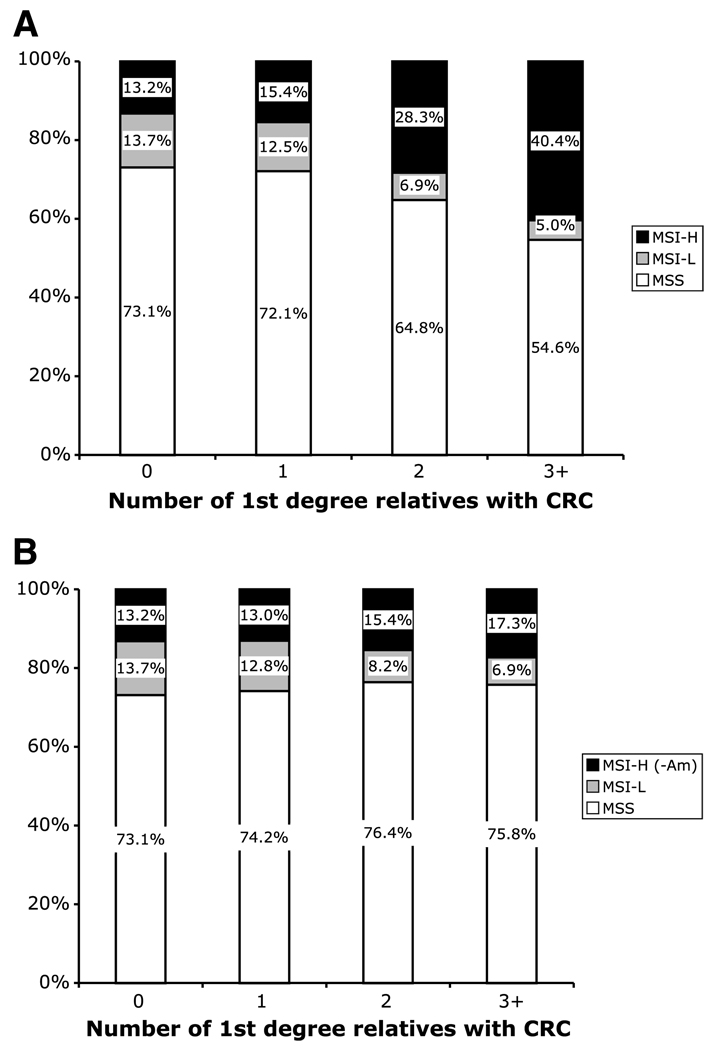
Figure 4.
Distribution of MSI subtypes to familial aggregation of CRC, showing distribution of probands by the number of first-degree relatives diagnosed with CRC, including all probands (A; P < 0.01) and after exclusion of MSI-H probands meeting the Amsterdam criteria I and II (B; P = 0.713).
Figure 2.
Histogram showing the distribution of different CRC MSI subtypes by sex, including all probands (A; P < 0.001) and after exclusion of MSI-H probands meeting the Amsterdam criteria I and II (B; P < 0.01).
Assessment of MSI status versus Age at Diagnosis
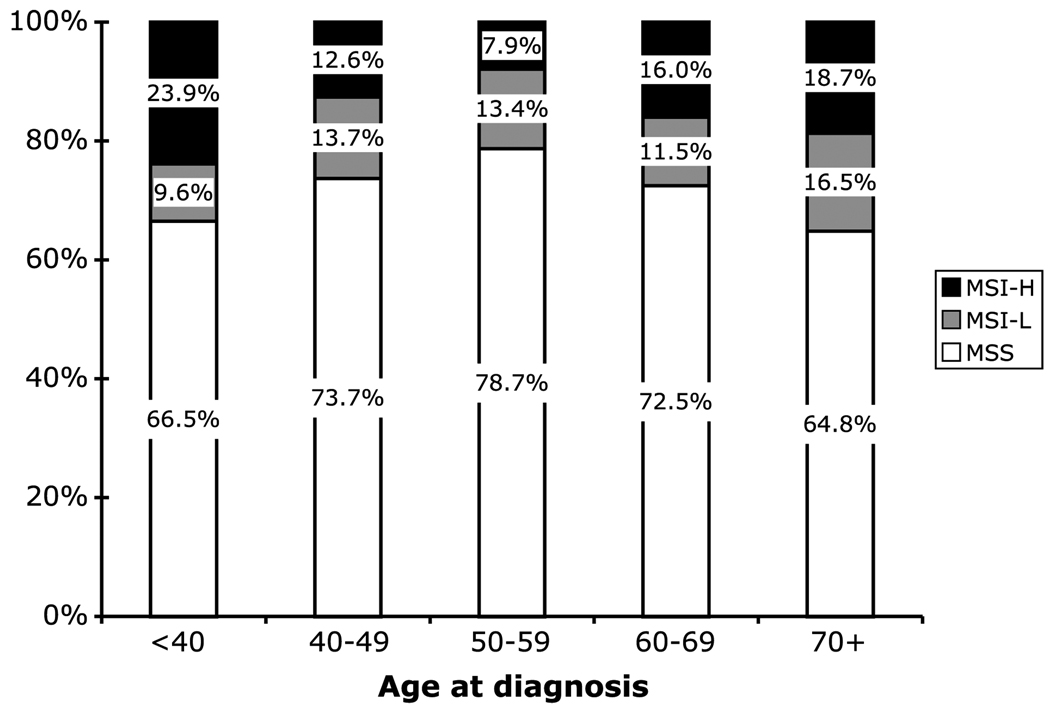
MSI-H tumors accounted for significantly different proportions of colorectal cancers across the different age groups (P < 0.001). Among cases under age 40, 24% had MSI-H cancer; whereas in 50- to 59-year-olds, only 8% were MSI-H (Fig. 3). In contrast, the proportion of tumors that were MSI-L was relatively constant across age groups, varying between 10% and 17%. Both younger and older age at diagnosis increased the risk of a MSI-H cancer versus MSS (P < 0.0001), but a similar variation was not observed in MSI-L versus MSS (P = 0.49; Table 1).
Figure 3.
Distribution of MSS, MSI-L, and MSI-H tumor subtypes by age at diagnosis of CRC probands. P < 0.001.
Assessment of MSI Status and Family History Characteristics
The proportion of cases that were MSI-H varied directly with family history (P = 0.002; Table 1). As shown in Fig. 4, among probands who reported no first-degree relatives with a diagnosis of colorectal cancer, 13% had MSI-H tumors. This rose to 28% among those with two affected first-degree relatives and 40% among those with three or more. No similar trend was seen for the MSI-L probands. There were even suggestions that the proportions that were MSI-L decreased with family history (Table 1 and Fig. 4).
The association of extracolonic cancers in relatives with the tumor MSI status of patients was also examined (Table 1). Among colorectal cancer cases having one or more first-degree relatives diagnosed with endometrial cancer, 23.4% were diagnosed with MSI-H tumors versus 13.8% among those without such a family history (P = 0.02). Family history of endometrial cancer increased the risk of a MSI-H versus MSS cancer by 77% (95% confidence interval, 7–193%). This was significantly different (P = 0.02) from the pattern for MSI-L tumors, which were nonsignificantly less common among cases with a family history of endometrial cancer (P = 0.22). We also looked at associations with any noncolon/endometrial cancer, but neither the MSI-H nor the MSI-L subgroup of colorectal cancer seemed to show overrepresentation relative to the MSS group. Of the 70 cases with first-degree relatives diagnosed with double-primary cancers of the colorectum and endometrium, 41% were MSI-H.
Assessment of MSI Status and Amsterdam Criteria and Bethesda Guidelines
There were an estimated 145 probands (2%) that met the Amsterdam I criteria; of these, 39% had MSI-H, 7.6% MSI-L, and 53.4% MSS tumors (computed from weighted Ns in Table 1). Among tumors of the 192 (2.8%) Amsterdam-II-criteria probands (which included those meeting Amsterdam I criteria), 37% were MSI-H, 6% MSI-L, and 57% MSS. At the same time, an Amsterdam family history was associated with a significantly higher risk of a MSI-H cancer in comparison with an MSS tumor (P < 0.001) although it conferred a nonsignificantly reduced risk of a MSI-L tumor (P for difference between MSI-L and MSI-H odds ratios < 0.0001). There were similar patterns for an Amsterdam II family history.
Among an estimated 2,503 probands that met one or more of the revised Bethesda Guidelines (37% of the total), 18% had MSI-H, 11% MSI-L, and 71% MSS tumors. The Bethesda criteria conferred an increased risk of a MSI-H cancer versus MSS and a lower risk of a MSI-L tumor versus MSS (P for difference between the MSI-L and MSI-H odds ratios = 0.002). However, the differences were less marked than in the Amsterdam analysis (Table 1). Among probands that did not meet the Bethesda criteria, 11% were MSI-H, 14% MSI-L, and 75% MSS.
Assessment of MSI-H Status and Nonsyndromic Familial Colorectal Cancer Risk
To investigate the residual familial colorectal cancer risk associated with MSI-H tumor phenotype among probands who do not meet the Amsterdam criteria (and so are unlikely to have Lynch syndrome in a population-based registry), the family history analysis was repeated after excluding 71 MSI-H probands who met the Amsterdam criteria I or II (1% of total; putative Lynch syndrome; ref. 43). As shown in Fig. 4, the trend of increasing risk of MSI-H tumors versus MSS with increasing number of first-degree relatives diagnosed with colorectal cancer, completely disappeared when analysis was done after exclusion of Amsterdam-positive probands with MSI-H tumors (Supplemental Table S1). Similarly, the association with increasing number of first-degree relatives diagnosed with endometrial cancer or with a double-primary diagnosis of colon and endometrial cancer also disappeared after the exclusion of Amsterdam criteria MSI-H probands.
Analysis of Tumor MSI Status by Age, Sex, and Anatomic Location
Table 2 shows the distribution of MSI subtypes stratified by age at diagnosis, sex, and tumor location. MSI-H tumors have a strong predilection for proximal location in both men and women, but the age-related increase in MSI-H tumors is more striking in women. Across all ages, those ages 50 to 59 years have the lowest proportion of MSI-H tumors, in both men and women. The increase in contribution of the MSI-H tumors in the older population was balanced by a decrease in the proportion of MSS tumors in the same age groups. The proportion of MSI-L tumors remained relatively unchanged across the same age groups (Table 1 and Table 2).
Table 2.
Tumor MSI status versus age, sex, and anatomic location
| Age at diagnosis (y) | MSS | MSI-L | MSI-H | P‡ | |||||
|---|---|---|---|---|---|---|---|---|---|
| Wgt N* | Row %† | Wgt N* | Row %† | Wgt N* | Row %† | ||||
| Males | All colorectal cancers | <40 | 59.2 | 55.3 | 14.2 | 14.8 | 28.6 | 29.9 | <0.001 |
| 40–49 | 306.5 | 69.9 | 58.3 | 14.4 | 62.3 | 15.7 | |||
| 50–59 | 848.9 | 79.8 | 146.6 | 13.9 | 65.0 | 6.3 | |||
| 60–69 | 1035.0 | 74.4 | 190.9 | 13.0 | 183.8 | 12.6 | |||
| ≥70 | 564.8 | 71.6 | 153.7 | 18.1 | 88.0 | 10.4 | |||
| Distal | <40 | 38.4 | 63.2 | 10.2 | 19.4 | 10.2 | 17.4 | 0.12 | |
| 40–49 | 210.4 | 75.2 | 41.1 | 16.2 | 26.8 | 8.6 | |||
| 50–59 | 599.3 | 83.1 | 98.1 | 13.9 | 22.7 | 3.0 | |||
| 60–69 | 662.6 | 83.5 | 121.3 | 14.2 | 23.0 | 2.2 | |||
| ≥70 | 392.8 | 81.7 | 73.3 | 13.4 | 30.0 | 4.9 | |||
| Proximal | <40 | 14.4 | 36.9 | 4.0 | 11.7 | 17.4 | 51.5 | 0.09 | |
| 40–49 | 65.9 | 58.6 | 15.6 | 16.4 | 25.9 | 25.1 | |||
| 50–59 | 157.6 | 68.3 | 38.2 | 15.9 | 35.3 | 15.8 | |||
| 60–69 | 264.8 | 59.3 | 39.0 | 8.3 | 150.1 | 32.4 | |||
| ≥70 | 146.2 | 64.2 | 41.0 | 15.7 | 48.0 | 20.0 | |||
| Females | All colorectal cancers | <40 | 94.0 | 73.3 | 8.0 | 6.1 | 26.4 | 20.7 | <0.001 |
| 40–49 | 367.2 | 75.7 | 67.1 | 13.6 | 52.8 | 10.8 | |||
| 50–59 | 630.7 | 77.2 | 105.1 | 12.9 | 83.9 | 10.0 | |||
| 60–69 | 670.3 | 71.2 | 80.6 | 8.4 | 192.9 | 20.4 | |||
| ≥70 | 354.0 | 58.1 | 80.1 | 12.8 | 177.5 | 29.0 | |||
| Distal | <40 | 74.6 | 84.5 | 6.0 | 7.1 | 10.6 | 8.4 | 0.02 | |
| 40–49 | 258.5 | 87.2 | 29.7 | 10.1 | 10.6 | 2.7 | |||
| 50–59 | 421.0 | 85.9 | 55.1 | 11.3 | 16.0 | 2.8 | |||
| 60–69 | 409.2 | 84.3 | 29.3 | 5.8 | 55.7 | 9.9 | |||
| ≥70 | 212.1 | 78.3 | 22.9 | 8.3 | 48.3 | 13.4 | |||
| Proximal | <40 | 18.4 | 51.0 | 2.0 | 4.2 | 14.8 | 44.8 | 0.12 | |
| 40–49 | 83.4 | 62.6 | 21.8 | 15.1 | 30.7 | 22.3 | |||
| 50–59 | 156.5 | 60.9 | 47.6 | 16.9 | 60.8 | 22.2 | |||
| 60–69 | 230.1 | 59.6 | 41.2 | 9.6 | 125.3 | 30.8 | |||
| ≥70 | 93.1 | 38.1 | 54.6 | 19.9 | 114.2 | 42.0 | |||
Per text, these are estimated numbers of probands corrected for proband weighting based on ascertainment criteria.
Percentage adjusting for center from polytomous logistic regression.
Heterogeneity test across the explanatory variable in polytomous logsitic after adjustment.
Discussion
In this large population-based study, we systematically investigated the association of tumor MSI status with basic demographics and family history variables. Two novel and clinically relevant observations emerged. The first observation illustrates the major contribution of Lynch syndrome to colorectal cancer cases presenting with a family history of colorectal cancer; after subtracting MSI-H Amsterdam families, the proportions of MSI-H, MSI-L, and MSS cancers did not vary significantly with the numbers of affected family members diagnosed with colorectal cancer. Prior studies have not examined risks in relatives after subtracting out the syndromic groups. Second, we observed that MSI-L cancers represent a distinct tumor subtype because patients with MSI-L cancers exhibit certain epidemiologic characteristics different from both MSI-H and MSS tumors. Specifically, the distribution of MSI-L colorectal cancers by tumor location and sex differs from both MSI-H and MSS cases. The relationships with family history and age are also quite distinct for patients with MSI-L and MSI-H cancers.
Assessment of clinicopathologic features of colorectal cancer cases stratified by tumor MSI status showed that MSI-H tumors were more proximally located, and were more common among female cases than males. We observed that MSI-H tumors, but not MSI-L tumors, were significantly associated with colorectal cancer cases meeting the clinical diagnostic Amsterdam criteria and Bethesda Guidelines, when compared with MSS tumors. Furthermore, only MSI-H tumors showed a significant association with a diagnosis of colorectal cancer or endometrial cancer, or both, among first-degree relatives of the colorectal cancer cases (Table 1). Taken together, MSI-H tumors show a preferential association with familial colorectal cancer.
We observed that the proportion of MSI-H tumors varied by age in a U-shaped pattern; the younger cases undoubtedly reflect germline involvement of the DNA MMR genes, whereas the late-onset MSI-H cases arise nearly always through methylation of the promoter of MLH1 (29). As a surrogate for having germline testing and tumor methylation testing on all registry patients, the combination of MSI-H tumors plus Amsterdam pedigree criteria was used to define a subset that is highly likely to possess DNA germline MMR mutations. This definition has been shown to be quite specific for the presence of DNA MMR germline mutations although the converse is not true. It is widely acknowledged that some Lynch syndrome cases will be missed by use of the conventional Amsterdam criteria (30), that is, they lack high sensitivity due to several possible reasons, which include incomplete penetrance, variable expressivity, small families, nonpaternity, adoption, and loss of contact with family members.
As expected, in determining the association of tumor MSI status with clinical and family history characteristics, we observed a robust association between the MSI-H status of colorectal cancer probands and the Amsterdam Criteria and the Bethesda Guidelines. MSI-H tumor status was significantly associated with familial cancer predisposition, with the proportion of MSI-H cases increasing as the number of first-degree relatives diagnosed with such cancers increased. Of interest, however, when these putative Lynch syndrome probands were excluded from the analysis, there was no similar association between MSI-H group and family history. This observation may suggest that there is not another common highly penetrant single-gene hereditary disorder contained in the MSS, MSI-L, and MSI-H groups, once the Lynch syndrome families have been removed, or alternatively, the single-gene syndromes are equally represented in these three remaining groups.
Among Bethesda criteria –negative families, about 11% colorectal cancers were MSI-H. These are likely to be sporadic cases. Possible factors contributing to MSI-H status in these patients are epigenetic silencing mechanisms, including MLH1 promoter hypermethylation, and/or other mechanisms of somatic MMR inactivation, including mutations or allelic deletions.
This case-case study was not designed to assign or to be capable of assigning absolute risks for colorectal cancer among relatives, but rather to compare the proportions of MSS, MSI-L, and MSI-H across groups of patients defined by family history. Seeing the excess familial risk peel away from the MSI-H group with subtraction of the putative Lynch syndrome cases may raise the question of whether all familial risk in colorectal cancer studies is driven by the syndromic causes, of which Lynch syndrome is by far the most common. The design of this study does not permit analysis of this possibility because it could only assess differences in risk across MSI subgroups. Pinsky modeled familial clustering of colorectal cancer populations, incorporating what was then known about the inheritance, prevalence, and penetrance of HNPCC, and found the calculated risks closely matched the risks reported in the literature, raising the possibility that all the familial risk in colorectal cancer was driven by HNPCC (44). Given the relative rarity of syndromic causes among all colorectal cancers, (e.g., 2.2% of all colorectal cancer have Lynch syndrome; ref. 30), the increase in familial risks reported in the large population-based studies is expected to persist but a case-control study that incorporates tumor phenotyping will be required to address this point.
A second line of information that emerged from this study was characterization of MSI-L probands and their families. The very existence of a MSI-L subgroup has been controversial. Might not all cancers have some MSI if one searched through enough markers? Or perhaps these were MSI-H tumors that had not yet evolved to a high level of MSI? In previous studies, MSI-L cases have generally been categorized with the MSS cases; clinically, however, it has been less clear if this lumping was justified, as family history characteristics had not been carefully evaluated in a larger series as we have done in this study. It was therefore interesting to note that the clinicopathologic characteristics of this group did not follow the pattern of either the MSS group or the MSI-H group. MSI-L tumors have a significant predilection to proximal location compared with MSS tumors, but gender distribution and family history association are similar to the MSS tumors (Supplemental Table S2). We looked carefully to determine if there was any association with endometrial cancer or other cancers in general category with MSI-L tumors but no association was detected.
Our study has several strengths. Results from this large, multicentered study have general applicability across different populations. We used a rigorous definition of family history to examine its association with colorectal cancers stratified by MSI-H, MSI-L, and MSS subtypes. The weaknesses of this study are the case-case design and a lack of germline MMR mutation data which would have helped to increase the specificity of our designation of Lynch Syndrome. Also, tumor location information is missing for a small number of colorectal cancers in our study.
In summary, this study made two observations. First, nearly all of the observed excess familial risk of colorectal cancer in MSI-H tumors is driven by the Lynch syndrome. When those families are subtracted from the analysis, even using as blunt an instrument as MSI-plus-Amsterdam Criteria, the familial aggregation is indistinguishable from all other colorectal cancer subtypes. This suggests there will not be a common, highly penetrant, single-gene predisposition syndrome that accounts for the late-onset MSI-H cases that have MLH1 promoter methylation. A case-control study design will be required to assess the actual colorectal cancer risks among relatives of non–Lynch Syndrome cases. Second, we observed that MSI-L tumors may be biologically different from MSS or MSI-H tumors. MSI-L tumors are more proximal than MSS tumors, but the gender distribution of the MSI-L group of colorectal cancers was quite similar to MSS tumors and they do not seem to be MSI-H tumors that have not yet accumulated enough microsatellite alterations to be detected. The contribution of MSI-L tumors does not vary across different age groups, unlike both MSI-H and MSS tumors. Further analyses are encouraged to further define characteristics such as response to therapy and prognosis among these different tumor subtypes.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute (RFA CA-95-011) and through cooperative agreements with members of the colon family registry and principal investigators from Australian Colorectal Cancer Family Registry (UO1 CA097735), USC Familial Colorectal Neoplasia Collaborative Group (UO1 CA074799), Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (UO1 CA074800), Ontario Registry for Studies of Familial Colorectal Cancer (UO1 CA074783), Seattle Colorectal Cancer Family Registry (UO1 CA074794), University of Hawaii Colorectal Cancer Family Registry (UO1 CA074806), and University of California, Irvine Informatics Center (UO1 CA078296).
Footnotes
Note: Supplementary materials for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Bonelli L, Martines H, Conio M, Bruzzi P, Aste H A case-control study. Family history of colorectal cancer as a risk factor for benign and malignant tumours of the large bowel. Int J Cancer. 1988;41:513–517. doi: 10.1002/ijc.2910410407. [DOI] [PubMed] [Google Scholar]
- 3.Cannon-Albright LA, Thomas A, Goldgar DE, et al. Familiality of cancer in Utah. Cancer Res. 1994;54:2378–2385. [PubMed] [Google Scholar]
- 4.Duncan JL, Kyle J. Family incidence of carcinoma of the colon and rectum in north-east Scotland. Gut. 1982;23:169–171. doi: 10.1136/gut.23.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331:1669–1674. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 6.Grossman S, Milos ML. Colonoscopic screening of persons with suspected risk factors for colon cancer. I. Family history. Gastroenterology. 1988;94:395–400. doi: 10.1016/0016-5085(88)90427-1. [DOI] [PubMed] [Google Scholar]
- 7.Guillem JG, Neugut AI, Forde KA, Waye JD, Treat MR. Colonic neoplasms in asymptomatic first-degree relatives of colon cancer patients. Am J Gastroenterol. 1988;83:271–273. [PubMed] [Google Scholar]
- 8.Hall NR, Finan PJ, Ward B, Turner G, Bishop DT. Genetic susceptibility to colorectal cancer in patients under 45 years of age. Br J Surg. 1994;81:1485–1489. doi: 10.1002/bjs.1800811029. [DOI] [PubMed] [Google Scholar]
- 9.Hemminki K, Chen B. Familial risk for colorectal cancers are mainly due to heritable causes. Cancer Epidemiol Biomarkers Prev. 2004;13:1253–1256. [PubMed] [Google Scholar]
- 10.Kune GA, Kune S, Watson LF. The role of heredity in the etiology of large bowel cancer: data from the Melbourne Colorectal Cancer Study. World J Surg. 1989;13:124–129. doi: 10.1007/BF01671173. discussion 129 – 31. [DOI] [PubMed] [Google Scholar]
- 11.Lovett E. Family studies in cancer of the colon and rectum. Br J Surg. 1976;63:13–18. doi: 10.1002/bjs.1800630103. [DOI] [PubMed] [Google Scholar]
- 12.Negri E, Braga C, La Vecchia C, et al. Family history of cancer and risk of colorectal cancer in Italy. Br J Cancer. 1998;77:174–179. doi: 10.1038/bjc.1998.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponz de Leon M, Antonioli A, Ascari A, Zanghieri G, Sacchetti C. Incidence and familial occurrence of colorectal cancer and polyps in a health-care district of northern Italy. Cancer. 1987;60:2848–2859. doi: 10.1002/1097-0142(19871201)60:11<2848::aid-cncr2820601141>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Rozen P, Fireman Z, Figer A, Legum C, Ron E, Lynch HT. Family history of colorectal cancer as a marker of potential malignancy within a screening program. Cancer. 1987;60:248–254. doi: 10.1002/1097-0142(19870715)60:2<248::aid-cncr2820600223>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Slattery ML, Kerber RA. Family history of cancer and colon cancer risk: the Utah Population Database. J Natl Cancer Inst. 1994;86:1618–1626. doi: 10.1093/jnci/86.21.1618. [DOI] [PubMed] [Google Scholar]
- 16.Sondergaard JO, Bulow S, Lynge E. Cancer incidence among parents of patients with colorectal cancer. Int J Cancer. 1991;47:202–206. doi: 10.1002/ijc.2910470207. [DOI] [PubMed] [Google Scholar]
- 17.St John DJ, McDermott FT, Hopper JL, Debney EA, Johnson WR, Hughes ES. Cancer risk in relatives of patients with common colorectal cancer. Ann Intern Med. 1993;118:785–790. doi: 10.7326/0003-4819-118-10-199305150-00005. [DOI] [PubMed] [Google Scholar]
- 18.Stefansson T, Moller PH, Sigurdsson F, Steingrimsson E, Eldon BJ. Familial risk of colon and rectal cancer in Iceland: evidence for different etiologic factors? Int J Cancer. 2006;119:304–308. doi: 10.1002/ijc.21835. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson BM, Finan PJ, Gascoyne J, Garbett F, Murday VA, Bishop DT. Frequency of familial colorectal cancer. Br J Surg. 1991;78:1162–1166. doi: 10.1002/bjs.1800781005. [DOI] [PubMed] [Google Scholar]
- 20.Bocker T, Diermann J, Friedl W, et al. Microsatellite instability analysis: a multicenter study for reliability and quality control. Cancer Res. 1997;57:4739–4743. [PubMed] [Google Scholar]
- 21.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 22.Halford S, Sasieni P, Rowan A, et al. Low-level microsatellite instability occurs in most colorectal cancers and is a nonrandomly distributed quantitative trait. Cancer Res. 2002;62:53–57. [PubMed] [Google Scholar]
- 23.Jass JR, Young J, Leggett BA. Biological significance of microsatellite instability-low (MSI-L) status in colorectal tumors. Am J Pathol. 2001;158:779–781. doi: 10.1016/s0002-9440(10)64020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laiho P, Launonen V, Lahermo P, et al. Low-level microsatellite instability in most colorectal carcinomas. Cancer Res. 2002;62:1166–1170. [PubMed] [Google Scholar]
- 25.Tomlinson I, Halford S, Aaltonen L, Hawkins N, Ward R. Does MSI-low exist? J Pathol. 2002;197:6–13. doi: 10.1002/path.1071. [DOI] [PubMed] [Google Scholar]
- 26.Kets CM, Hoogerbrugge N, Bodmer D, et al. Unfavorable pathological characteristics in familial colorectal cancer with low-level microsatellite instability. Mod Pathol. 2006;19:1624–1630. doi: 10.1038/modpathol.3800701. [DOI] [PubMed] [Google Scholar]
- 27.Kohonen-Corish MR, Daniel JJ, Chan C, et al. Low microsatellite instability is associated with poor prognosis in stage C colon cancer. J Clin Oncol. 2005;23:2318–2324. doi: 10.1200/JCO.2005.00.109. [DOI] [PubMed] [Google Scholar]
- 28.Wright CM, Dent OF, Newland RC, et al. Low level microsatellite instability may be associated with reduced cancer specific survival in sporadic stage C colorectal carcinoma. Gut. 2005;54:103–108. doi: 10.1136/gut.2003.034579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham JM, Christensen ER, Tester DJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998;58:3455–3460. [PubMed] [Google Scholar]
- 30.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 31.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 32.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 34.Umar A. Lynch syndrome (HNPCC) and microsatellite instability. Dis Markers. 2004;20:179–180. doi: 10.1155/2004/486032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umar A, Risinger JI, Hawk ET, Barrett JC. Testing guidelines for hereditary non-polyposis colorectal cancer. Nat Rev Cancer. 2004;4:153–158. doi: 10.1038/nrc1278. [DOI] [PubMed] [Google Scholar]
- 37.Jass JR, Do KA, Simms LA, et al. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakar S, Burgart LJ, Thibodeau SN, et al. Frequency of loss of hMLH1 expression in colorectal carcinoma increases with advancing age. Cancer. 2003;97:1421–1427. doi: 10.1002/cncr.11206. [DOI] [PubMed] [Google Scholar]
- 39.Hitchins M, Williams R, Cheong K, et al. MLH1 germline epimutations as a factor in hereditary nonpolyposis colorectal cancer. Gastroenterology. 2005;129:1392–1399. doi: 10.1053/j.gastro.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Hitchins MP, Wong JJ, Suthers G, et al. Inheritance of a cancer-associated MLH1 germ-line epimutation. N Engl J Med. 2007;356:697–705. doi: 10.1056/NEJMoa064522. [DOI] [PubMed] [Google Scholar]
- 41.Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–2343. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 42.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 43.Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA. 2005;293:1979–1985. doi: 10.1001/jama.293.16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinsky PF. Does hereditary nonpolyposis colorectal cancer explain the observed excess risk of colorectal cancer associated with family history? Epidemiology. 2000;11:297–303. doi: 10.1097/00001648-200005000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.