Abstract
Objective
To determine the effect of upper limb effort on maximal lower limb muscle activation in individuals with incomplete spinal cord injury.
Methods
Fifteen individuals with incomplete spinal cord injury performed recumbent stepping using different combinations of upper and lower limb efforts.
Results
There was no significant difference in active lower limb electromyography amplitudes regardless of whether the upper limbs were resting or exerting maximal effort. Upper limb effort increased passive lower limb muscle activation and likewise, lower limb effort increased passive upper limb muscle activation.
Conclusions
Upper limb effort did not increase lower limb muscle activation during active lower limb effort in individuals with incomplete spinal cord injury during recumbent stepping. This suggests that individuals with incomplete spinal cord injury cannot recruit additional lower limb motor units using maximal volitional effort of their upper limbs.
Significance
Understanding how upper limb effort and movement influences lower limb muscle activation patterns in incomplete spinal cord injury patients has implications for prescribing therapies for lower limb rehabilitation.
Keywords: neural coupling, rehabilitation, interlimb coupling, incomplete spinal cord injury, electromyography, locomotion
INTRODUCTION
Upper limb muscle activation can increase muscle activity in the passive lower limbs during a rhythmic motor task (Ferris DP, et al., 2006). In previous studies, we examined neurologically intact subjects performing recumbent stepping on an exercise device that coupled motion of the upper and lower limbs. We found that increasing upper limb muscle activation through greater resistance (Huang HJ and Ferris DP, 2004) or higher movement frequency (Kao PC and Ferris DP, 2005) resulted in greater lower limb muscle electromyography amplitudes in passively moving legs. The most likely explanation for the observed lower limb muscle recruitment with active upper limb exertion is an excitatory connection between upper limb motor neurons and lower limb motor neurons involving the neural networks controlling locomotion (Ferris DP, et al., 2006).
Clinically, it has been suggested that active upper limb movement during gait training can be beneficial for rehabilitation (Behrman and Harkema 2000). When subjects with incomplete spinal cord injury freely swing their arms, their lower limb muscle activity looks more symmetric and has greater rhythmic bursts (Visintin M and Barbeau H, 1994). Kawashima and colleagues recently showed that individuals with incomplete spinal cord injury using an upright exercise device had improved muscle activation in passively moved lower limbs with passive arm swing compared to a stationary arm condition (Kawashima N, et al., 2008). These studies support the idea that reciprocal upper limb movement can enhance lower limb muscle activation patterns and promote activity-dependent neural plasticity during gait rehabilitation (Ferris DP, et al., 2006). An unanswered question is whether active upper limb exertion provides a means to increase lower limb muscle recruitment over what could be achieved without active upper limb exertion. Therapeutic interventions after incomplete spinal cord injury often focus on increasing volitional muscle activation through strength training and exercise. Both muscle hypertrophy and enhanced neural drive contribute to the increased muscle strength that accompanies resistance training. If active upper limb exertion allows individuals with incomplete spinal cord injury to increase maximal recruitment of lower limb muscles during resistance training, then it could be helpful to include simultaneous upper and lower limb maximal exercise in their rehabilitation.
The purpose of this study was to determine the effect of upper limb effort on maximal lower limb muscle activation in individuals with incomplete spinal cord injury. Previous work (Huang HJ and Ferris DP, 2004; Kao PC and Ferris DP, 2005; Kawashima N, et al., 2008) has focused on passive lower limb muscle activation rather than active lower limb muscle activation, but active lower limb effort is more characteristic of exercise during rehabilitation. Gaining a more thorough understanding of how upper limb effort influences lower limb muscle activation during active voluntary effort is important for incorporating combined upper and lower limb exercise into neurological rehabilitation practices (Ferris DP, et al., 2006).
METHODS
Subjects
Fifteen individuals with incomplete spinal cord injury participated in this study after providing written informed consent. There were six subjects with a cervical injury, five with a thoracic injury, and four with a lumbar injury (Table 1). All subjects were at least 12 months post-injury and free of any conditions that would limit their ability to exercise safely. Subjects had to be able to perform the recumbent stepping task with just their upper limbs to participate in the study. All subjects were screened and approved for participation by a physician from the Physical Medicine and Rehabilitation Department at the University of Michigan. The University of Michigan Medical School Institutional Review Board approved the protocol and consent form in accord with the Declaration of Helsinki.
Table 1.
Subject informaion. Data for each subject showing age, injury level, and walking ability.
| Subject | Age | Gender | ASIA Level * | Injury Level |
Post Injury (yrs) |
Injury Etiology | Est. WISCI † |
|---|---|---|---|---|---|---|---|
| A | 51 | F | D | C3 | 7 | Epidural Abcess | 13 |
| B | 62 | M | D | C3 | 5 | Cervical Stenosis | 19 |
| C | 64 | M | D | C4 | 4 | Trauma | 15 |
| D | 40 | M | D | C6 | 5 | Trauma | 9 |
| E | 42 | M | D | C6 | 28 | Trauma | 18 |
| F | 61 | M | D | C7 | 42 | Trauma | 13 |
| G | 41 | F | C | T5 | 28 | Transverse Myelitis | 15 |
| H | 78 | M | E | T7 | 8 | Sarcoma | 20 |
| I | 47 | F | D | T9 | 8 | Transverse Myelitis | 13 |
| J | 57 | F | C | T11 | 4 | Trauma | 18 |
| K | 53 | F | C | T11 | 6 | Dermoid Cyst | 12 |
| L | 30 | M | C | L1 | 3 | Trauma | 9 |
| M | 46 | M | C | L3 | 4 | Surgical Complications | 18 |
| N | 41 | F | C | L3 | 20 | Trauma | 9 |
| O | 23 | M | D | L3 | 3 | Trauma | 20 |
ASIA = American Spinal Injury Association Impairment Scale. A = complete. E = normal.
Est. WISCI = Estimated Walking Index of Spinal Cord Injury based on observation and subject self-reported walking ability.
0 = unable to walk with assistance. 20 = unassisted walking.
Computer-controlled Recumbent Stepper
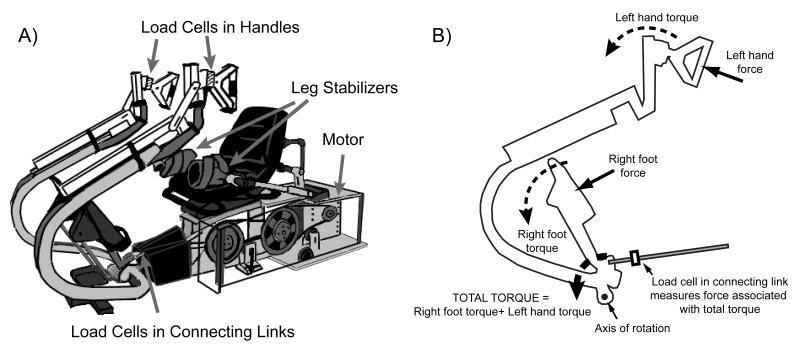
We have taken a commercially available recumbent stepper (TRS 4000, NuStep Inc., Ann Arbor, MI) and modified it to have computer-controlled real-time resistance (Fig. 1) (Huang HJ and Ferris DP, In Press). We also instrumented the recumbent stepper with load cells to measure handle and pedal forces. For this study, the stepper followed a prescribed sine-wave position profile with a stepping frequency of 75 beats per minute (equivalent to the stepping frequency of walking at ~1.25 m/s). If subjects were unable to step at the desired frequency, then the stepper drove the stepping motion. If subjects were strong enough to drive the stepping motion faster than the desired stepping frequency, the motor generated a torque to oppose the subject’s effort. This allowed the stepper to have a fixed position profile and to maintain the desired stepping frequency.
Figure 1.
A) Recumbent stepping machine with real-time computer-controlled resistance and force and position sensors (modified TRS 4000, NuStep Inc, Ann Arbor, MI). The handles and seat are adjustable. Velcro gloves, foot straps, and a torso belt help minimize unwanted movement. Leg stabilizers also help prevent excessive medial-lateral movement. B) Schematic of the forces and torques for one handle-pedal unit on the recumbent stepper.
Experimental Set Up
We adjusted the stepper to make the range of the stepping motion as comfortable as possible for each subject. The seat position was set so that the knees were near full extension but could not lock out. For some more hyper-reflexive subjects, we had to set the seat so that their legs were more flexed for safety reasons. If needed, we used leg stabilizers to prevent the subject’s legs from abducting and potentially colliding with the moving handles. We aligned each foot to be centered within the pedal. As the pedal was only 5.5 inches wide, it prevented subjects from rotating their feet medially or laterally. We used a torso strap to minimize torso movement during stepping. We also used Velcro gloves to attach the hands to the handles and used foot straps to attach the feet to the pedals during passive conditions. This allowed subjects to be as passive as possible because they did not have to actively hold the handles or keep their feet on the pedals throughout the stepping motion.
Protocol
Subjects performed recumbent stepping using different combinations of upper (U) and lower (L) limb effort. For active effort, we instructed subjects to use maximal effort. For passive effort, we instructed subjects to relax as much as possible. We tested three active lower limb conditions: a) Resting Upper & Active Lower [RU-AL], b) Passive Upper & Active Lower [PU-AL], c) Active Upper & Active Lower [AU-AL]. For the resting upper limb condition, we had subjects cross their arms and rest them on his/her lap. These active lower limb conditions examined whether different upper limb states altered active lower limb muscle electromyography amplitudes. We also tested two passive lower limb conditions, d) Passive Upper & Passive Lower [PU-PL], and e) Active Upper & Passive Lower [AU-PL], to determine how upper limb effort influences passive lower limb muscle activation in individuals with incomplete spinal cord injury.
We collected two sets of data, with each set consisting of five trials for each of the five conditions. Conditions were randomized for each subject. Before each trial, we verbally described the combination of arm and leg effort to the subject. Subjects were instructed to relax and use the first fifteen seconds to get used to the stepping frequency as the stepper slowly ramped up to full range of motion. Then on a verbal cue, we instructed subjects to perform the stepping condition with maximal effort for approximately fifteen seconds. This yielded six to eight strides of data. Throughout the trial, we gave the subject verbal cues and encouragement. Subjects were also given an opportunity to practice the condition prior to testing at their discretion. The average length of rest between trials was one minute.
Data Acquisition
We collected data signals using two computer systems at a sampling rate of 1000 Hz. One computer was used to collect electromyography, load cell, and joint angle data signals. The other computer ran the real-time software program and sampled data signals related to the recumbent stepper hardware. We used a common data signal sampled in both systems to synchronize the data offline.
Electromyography (EMG)
We measured surface electromyography (Delsys, Boston, MA) from sixteen muscles, four muscles on each limb. On each lower limb, we measured muscle activity from the vastus medialis (VM), medial hamstrings (MH), tibialis anterior (TA), and soleus (SO). On each upper limb, we measured muscle activity from the anterior deltoid (AD), posterior deltoid (PD), biceps brachii (BB), and lateral head of the triceps brachii (TB). We shaved each electrode site and cleaned them with rubbing alcohol. We then placed the electrode sensor over the muscle belly along the long axis, secured the electrode with tape, and wrapped excess loose electrode wires to the limbs with elastic foam wrap. We processed the EMG data with a second order high-pass Butterworth filter with zero lag (cutoff frequency of 20 Hz) to attenuate low frequency components such as mechanical artifact. We then full wave rectified the EMG data signals.
Joint Angles
We measured bilateral joint angles of the ankles, knees, hips, and elbows using twin-axis electrogoniometers placed along the sagittal plane (Biometrics Ltd, Ladysmith, VA). Electrogoniometers were zeroed with the limbs in the anatomically neutral position. Joint angle data were processed with a second order low-pass Butterworth filter with zero lag (cutoff frequency of 6 Hz). Because of equipment malfunctions, we were not able to obtain data from all eight goniometers during all conditions for every subject.
Kinetics
We calculated the forces each hand and foot contributed to the stepping motion via single axis load cells (Fig. 1). Because the handle and contralateral pedal were part of a single rigid body, the torques generated by a force from the hand and a force from the contralateral foot summed and yielded a net torque for the handle-pedal unit (Fig. 1B). We measured directly the force exerted by each hand through a load cell mounted in the handle. We also measured the force associated with the net torque for each handle-pedal unit through a load cell mounted in a connecting link between the handle-pedal unit and a cam. Using the measured forces and moment arm relationships, we calculated the torques associated with each handle and handle-pedal unit. We subtracted the handle torque from the handle-pedal unit torque to determine the pedal torque of that contralateral hand-foot pair. We then divided the pedal torques by the pedal moment arm to find the pedal forces. We filtered measured force data using a second order low-pass Butterworth filter with zero lag (cutoff frequency of 6 Hz).
Data Analysis
For all subjects, we analyzed the data from the second set. The subjects with incomplete spinal cord injury were more consistent during the second set.
Calculation of Mean Profiles
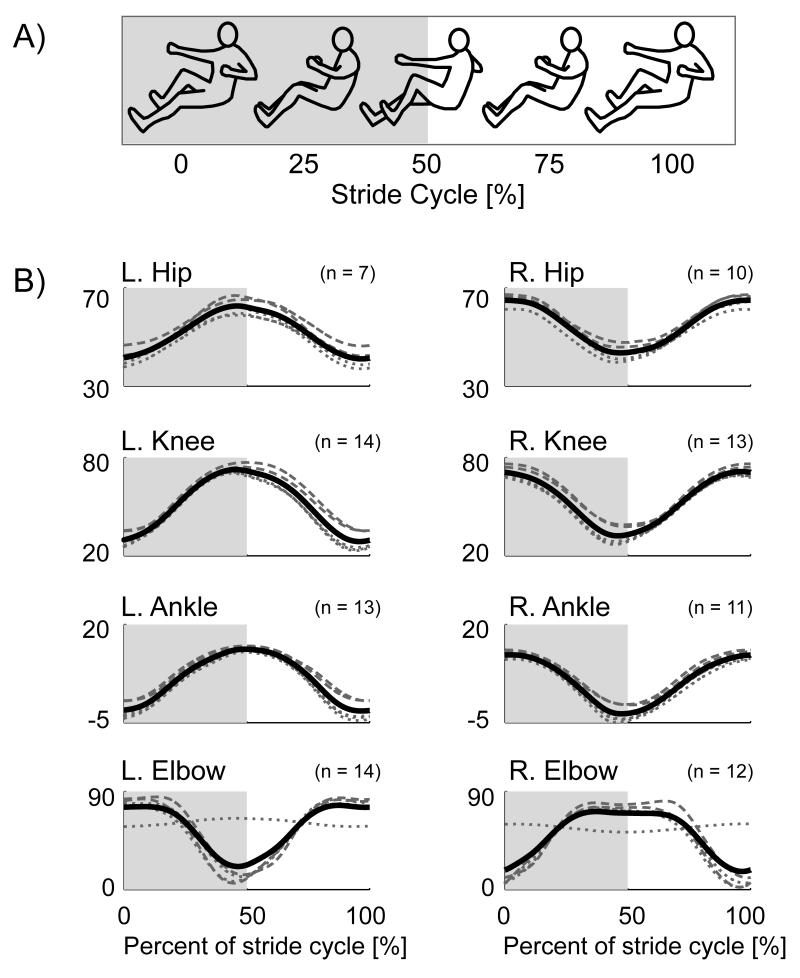
To compare EMG patterns between conditions, we calculated group normalized EMG mean profiles over a stride cycle for each condition. The beginning and end of each stride corresponded with the left lower limb and right upper limb at full extension as indicated from the position data (Fig. 2A). We first calculated an intra-subject EMG mean profile for a stride cycle per condition. We then normalized the intra-subject EMG mean profiles to the maximum value among all conditions. We then calculated a group normalized EMG mean profile for each condition by averaging all of the intra-subject normalized EMG mean profiles for that condition. We used the same general procedure, but without normalization, for the joint angle and force mean profiles.
Figure 2.
A) Schematic of recumbent stepping motion. At 0% of the stride cycle, the left lower limb and right upper limb are at full extension. From 0% to 50% of the stride cycle, the left lower limb and right upper limb are flexing while the right lower limb and left upper limb are extending. At 50% of the stride cycle, the right lower limb and left upper limb are at full extension. From 50% to 100% of the stride cycle, the right lower limb and left upper limb are flexing while the left lower limb and right upper limb are extending. B) Group mean joint angle profiles for the bilateral hip, knee, ankle, and elbow during one stepping cycle. Black solid line: average for all conditions. Dotted grey lines: active lower limb conditions, Resting Upper & Active Lower, Passive Upper & Active Lower, and Active Upper & Active Lower. Dashed grey lines: passive lower limb conditions, Passive Upper & Passive Lower and Active Upper & Passive Lower.
Calculation of EMG Amplitudes
To compare EMG amplitudes across conditions, we calculated a group averaged normalized root-mean-square (RMS) EMG for each muscle and condition. For each muscle, we only calculated RMS EMG during the half of the stride when the muscle was concentrically contracting. For example, for the right vastus medialis, we calculated the RMS EMG during the first half of the stride cycle when the knee was extending (Fig. 2 grey blocks). We calculated each muscle’s RMS EMG for the concentric half of the cycle for each subject-condition data set. We calculated an intra-subject average RMS EMG for each muscle per condition. We then normalized the intra-subject RMS EMG amplitudes for each muscle (left and right vastus medialis, medial hamstrings, soleus, tibialis anterior, anterior deltoid, posterior deltoid, biceps brachii, and triceps brachii ) to the maximum intra-subject average RMS EMG amplitude across all conditions. We excluded any subject’s data that did not have at least a 10% difference between Passive Upper & Passive Lower and Passive Upper & Active Lower conditions for lower limb RMS EMG and between Passive Upper & Passive Lower and Active Upper & Passive Lower conditions for upper limb RMS EMG. We then averaged across subjects to calculate the group averaged normalized RMS EMG amplitude for each muscle per condition.
Statistical Analysis
We used a repeated measures analysis of variance (rmANOVA) to determine if there were significant differences in lower limb muscle activation among active lower limb conditions. We also ran another rmANOVA to determine if there were significant differences in lower (or upper) limb muscle activation among passive lower (or upper) limb conditions. If the rmANOVA showed a significant difference among conditions, we used a Tukey’s honestly significant difference (THSD) post hoc test to determine which conditions were significantly different (P < 0.05).
RESULTS
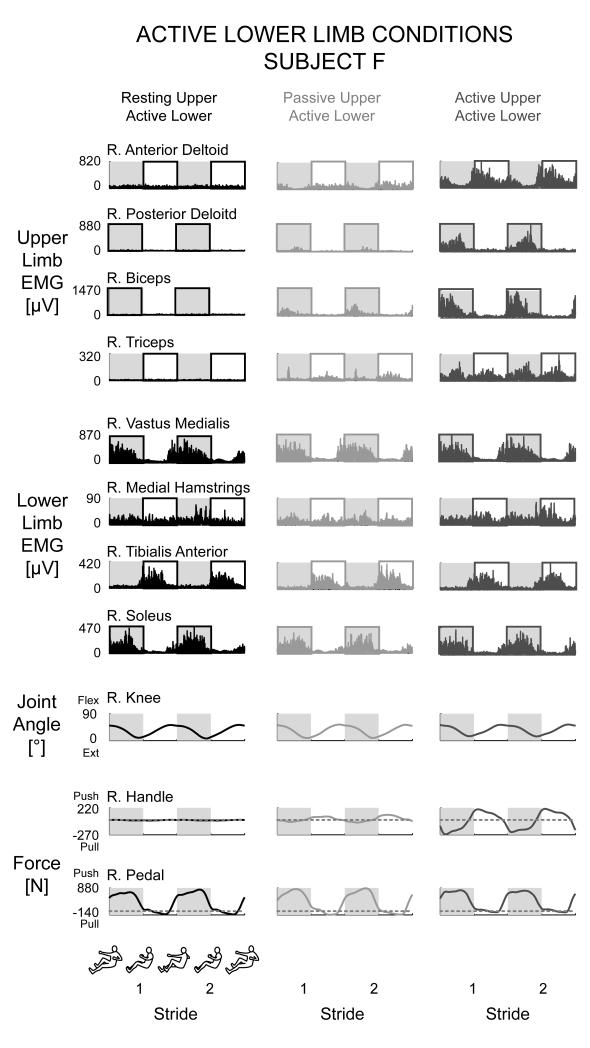
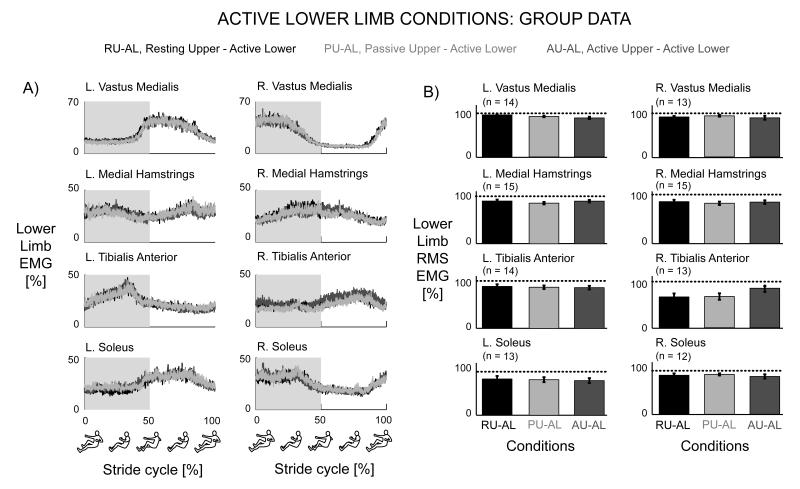
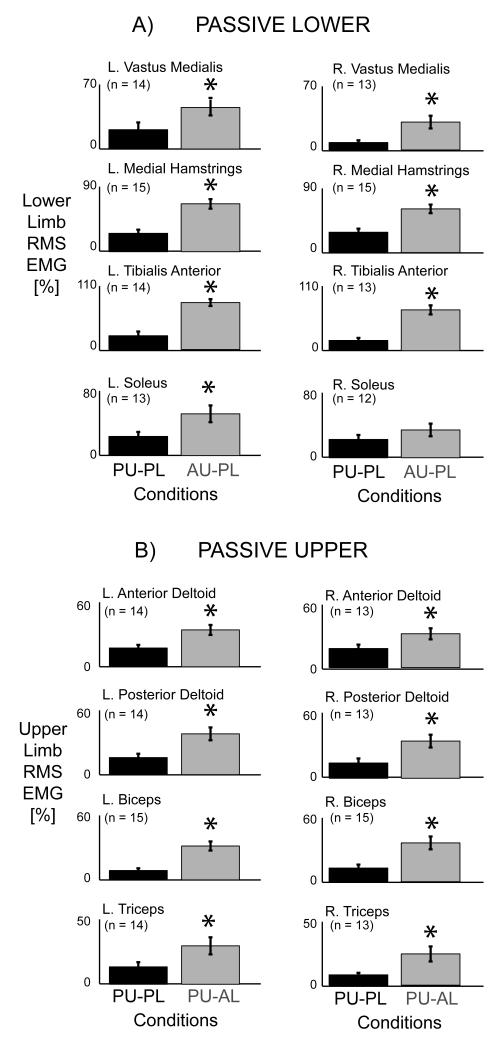
Adding upper limb effort did not enhance lower limb muscle activation during active lower limb effort in subjects with incomplete spinal cord injuries. The group mean joint angle profiles were consistent among the different conditions for the bilateral hip, knee, ankle, and elbow joints (Fig. 2B). In a representative single subject, the muscle activation patterns for the active lower limbs for the Resting Upper & Active Lower, Passive Upper & Active Lower, and Active Upper & Active Lower conditions were similar in amplitude and had a rhythmic burst like pattern, particularly for the vastus medialis, tibialis anterior, and soleus muscles (Fig. 3). In this particular subject, the medial hamstrings muscle activity was not rhythmic, but the muscle activation shape and amplitude were similar among the three conditions. The upper limb muscle patterns corresponded to the different upper limb states of resting, passive, and active. There was minimal upper limb EMG during the resting upper limb condition, small amplitudes of EMG during the passive upper limb condition, and greater burst-like EMG during the active upper limb condition. The active knee joint angle and pedal forces were similar across the three active lower limb conditions. The handle forces indicated that the subject correctly followed directions for each condition. Only the Active Upper & Active Lower handle forces had a distinct pushing and pulling handle force while the Resting Upper & Active Lower and Passive Upper & Active Lower conditions had minimal handle forces (Fig. 3). Looking at the group averaged EMG data for all the subjects (Fig. 4), the data showed the same effects as the representative single subject data. There were no observable differences in the left and right lower limb muscle activation patterns among any of the active lower conditions despite varying levels of upper limb effort. The group EMG mean profiles for the active left and right lower limb muscles all overlapped one another (Fig. 4A). The group averaged RMS EMG amplitudes for the lower limb muscles also indicated no significant differences among active lower limb conditions (Fig. 4B, rmANOVA P > 0.05). The left and right vastus medialis, left tibialis anterior, and left and right soleus muscles had slight reductions in group averaged RMS EMG of 3.4%, 4.6%, 0.82%, 2.3%, and 4.7%, respectively, during Active Upper & Active Lower compared to Passive Upper & Active Lower. The left medial hamstrings, right medial hamstrings, and right tibialis anterior had slight increases in group averaged RMS EMG of 4.2%, 2.4%, and 17%, respectively during Active Upper & Active Lower compared to Passive Upper & Active Lower. Statistical powers for the active lower limb conditions for the left and right vastus medialis, medial hamstring, tibialis anterior, and soleus muscles were 0.541, 0.130, 0.165, 0.081, 0.068, 0.380, 0.064, and 0.098, respectively. In comparison, statistical powers for the passive lower limb conditions were 0.514, 0.829, 0.997, 0.980, 1.000, 0.999, 0.623, and 0.218 for the left and right vastus medialis, medial hamstring, tibialis anterior, and soleus muscles, respectively. Active upper limb effort resulted in greater passive lower limb muscle activation. Single subject data and group mean EMG profiles showed greater burst-like muscle activation in the passive lower limbs when coupled with active upper limb effort. Group RMS EMG data indicated that for the passive lower limbs, Active Upper & Passive Lower RMS EMG amplitudes were significantly greater than Passive Upper & Passive Lower RMS EMG amplitudes for the bilateral vastus medialis, bilateral medial hamstring, bilateral tibialis anterior, and left soleus muscles (Fig. 5A*, THSD P < 0.05). Similarly, active lower limb effort resulted in greater passive upper limb muscle activation. Single subject data and group mean EMG profiles showed greater burst-like muscle activation in the passive upper limbs when coupled with active lower limb effort. The group RMS EMG amplitudes of the passive upper limb muscles were significantly greater during the Passive Upper & Active Lower condition compared to the Passive Upper & Passive Lower for the bilateral anterior deltoid, posterior deltoid, biceps brachii, and triceps brachii (Fig. 5B*, THSD P < 0.05).
Figure 3.
Right limb data from a single representative subject for the active lower limb conditions, Resting Upper & Active Lower (black), Passive Upper & Active Lower (light grey), Active Upper & Active Lower (dark grey). There was no observable difference in active lower limb muscle activation patterns regardless of the activity in the upper limbs. There was minimal upper limb muscle activation during the resting and passive conditions and increased burst-like activity during the active condition. Boxed halves indicate concentric half of the cycle. The knee joint angle profiles and active pedal forces were similar between the conditions. The Resting Upper & Active Lower and Passive Upper & Active Lower handle forces were minimal while the Active Upper & Active Lower handle forces had a large pushing and pulling force. Dashed lines in the force data is zero force.
Figure 4.
Left and right limb group data for the active lower limb conditions, Resting Upper & Active Lower (black), Passive Upper & Active Lower (light grey), Active Upper & Active Lower (dark grey). A) Group normalized EMG mean profiles showed no observable difference in active lower limb muscle activation patterns regardless of the activity in the upper limbs. B) Group normalized RMS EMG amplitudes with standard error bars for the active lower limb conditions. There were no significant differences among the three conditions (rmANOVA P > 0.05). Error bars are standard error of the means.
Figure 5.
A) Normalized RMS EMG group data for the left and right lower limb for the passive lower limb conditions, Passive Upper & Passive Lower (PU-PL, black) and Active Upper & Passive Lower (AU-PL, grey). The RMS EMG for the bilateral vastus medialis, bilateral medial hamstrings, bilateral tibialis anterior, and left soleus muscles during the Active Upper & Passive Lower condition were significantly greater compared to the Passive Upper & Passive Lower condition (* THSD P < 0.05). B) Normalized RMS EMG group data for the left and right upper limb for the passive upper limb conditions, Passive Upper & Passive Lower (PU-PL, black) and Passive Upper & Active Lower (PU-AL, grey). The RMS EMG amplitudes for the bilateral anterior deltoid, bilateral posterior deltoid, bilateral biceps brachii, and bilateral triceps brachii muscles during the Passive Upper & Active Lower condition were significantly greater compared to the Passive Upper & Passive Lower condition (* THSD P < 0.05). Error bars are standard error of the means.
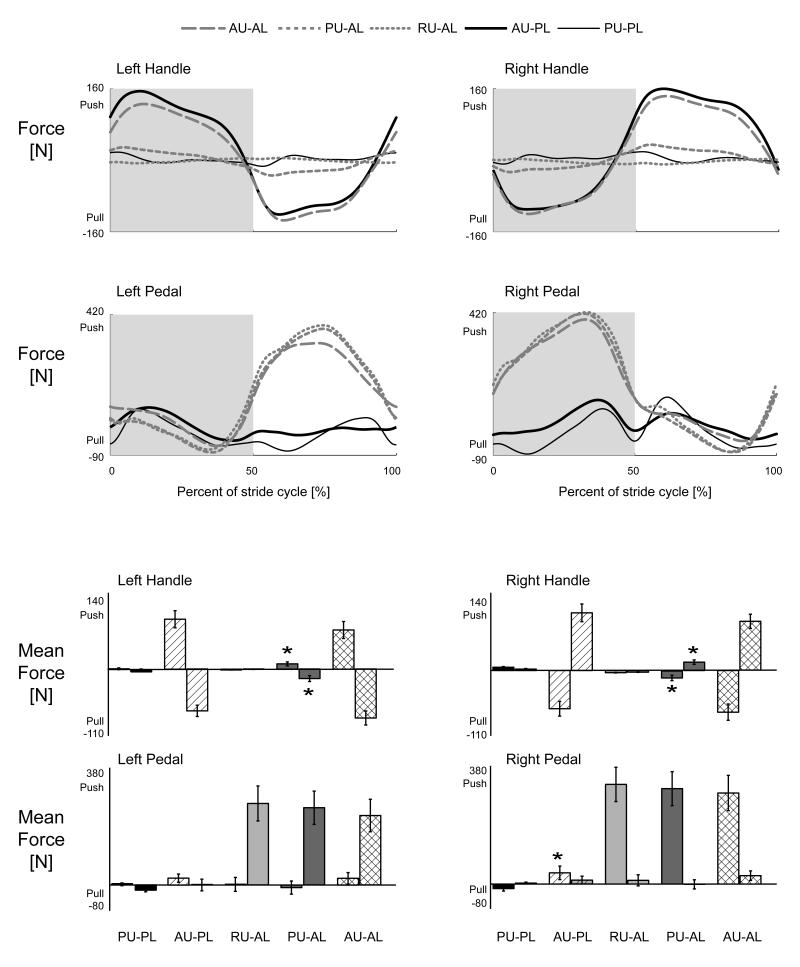
Group averaged force data indicated that subjects performed conditions as instructed. Group mean force profiles for the active lower limb conditions had similar shapes and amplitudes for the pedal forces while the handle forces reflected the different levels of upper limb effort, resting, passive, and active (Fig. 6A). There were also no significant differences among the mean handle and pedal forces for the active lower limb conditions (Fig. 6B, rmANOVA P > 0.05). Passive lower limb pedal forces were small compared to active lower limb conditions. The mean force of the right pedal during the first half stepping cycle for the Active Upper & Passive Lower condition was significantly greater than the Passive Upper & Passive Lower condition (Fig. 6B*, THSD P < 0.05).
Figure 6.
A) Group mean force profiles for the left and right handles and pedals. The force profiles match the levels of effort required for each of the conditions. Handle forces only had substantial pushing and pulling phases for the active upper limb effort conditions. Pedal forces only had substantial pushing phases for the active lower limb effort conditions. B) Mean forces for each half of the stepping cycle for the left and right handles and pedals. For each condition, the bar on the left is the mean force for the first half of the stepping cycle and the bar on the right is for the second half of the stepping cycle. *Significantly different from the Passive Upper & Passive Lower condition during the same half of the stepping cycle. PU-PL, Passive Upper & Passive Lower. AU-PL, Active Upper & Passive Lower. RU-AL, Resting Upper & Active Lower. PU-AL, Passive Upper & Active Lower. AU-AL, Active Upper & Passive Lower. Error bars are standard error of the means.
DISCUSSION
Our main finding was that upper limb effort did not increase muscle activation during active lower limb effort in individuals with incomplete spinal cord injury during recumbent stepping. During active lower limb conditions, subjects generated similar lower limb EMG amplitudes regardless of whether the upper limbs were resting, passive, or exerting maximal effort. The mean forces mirrored the RMS EMG data for the knee and ankle musculature, suggesting that hip musculature EMG would not have shown substantially different findings from the ankle and knee EMG. If hip muscles had a different muscle activation pattern compared to the ankle and knee muscles then the force data would have shown different changes in pedal forces by condition (Fig. 6). The finding that active lower limb muscle activation was indifferent to upper limb passive or active effort was contrary to our hypothesis. This result suggests that individuals with incomplete spinal cord injury are not able to recruit additional lower limb motor units during maximal volitional effort by actively using their upper limbs. This result is also similar to our results on neurologically intact individuals who showed no significant change in maximal lower limb muscle activation despite the effort level of the upper limbs (Huang HJ and Ferris DP, In Press). We expected that we might find different results for individuals with incomplete spinal cord injury compared to neurologically intact individuals because the spinal cord injury patients had a lower capacity to maximally recruit their lower limb motor neurons.
We did find that upper limb effort increased muscle activation in the passive lower limbs and lower limb effort increased muscle activation in the passive upper limbs. These results on individuals with incomplete spinal cord injury were similar to our previous results on neurologically intact individuals (Huang HJ and Ferris DP, 2004; Huang HJ and Ferris DP, In Press; Kao PC and Ferris DP, 2005). These results differ somewhat from Kawashima and colleagues recent work (Kawashima N, et al., 2008). They found no significant differences in lower limb EMG amplitudes between passive and active arm swing conditions for stepping movements in a standing frame glider. In contrast, we found a significant increase in passive lower limb muscle activation amplitude when coupled with active upper limbs compared to passive upper limbs (Fig. 5). The differences between the two studies may be a result of differences between the two tasks, reciprocal leg swing versus recumbent stepping. In the standing frame glider, there was no knee flexion during the rhythmic motion unlike in recumbent stepping. Another difference in movement kinematics between the two devices was that the standing frame glider had hip motion more similar to the hip excursions seen during locomotion. As hip afferents have been found to play an important role in the neural control of walking in humans (Dietz V, et al., 2002), this could be an important difference between the two movement tasks.
The increase in muscle activation of passive limbs when coupled with maximal effort in the other limb pair is likely a result of the convergence of multiple neural drives. Spinal interneurons could relay increased locomotor output in the networks of the upper limb pair to the networks of the lower limb pair and vice versa (Dietz V, 2002; Zehr EP and Duysens J, 2004). Furthermore, even though reflexes in one limb are often suppressed with movements of the other limbs (Collins DF, et al., 1993; Frigon A, et al., 2004; Knikou M, 2007), reflex facilitation from other sensory feedback pathways such as cutaneous stimulation can prevail (Zehr EP, et al., 2004; Zehr EP, et al., 2007a). Decreased inhibition to the passive limbs from supraspinal centers or spinal interneurons could also allow the emergence of a rhythmic motor pattern. Additionally, an excitatory locomotor drive from the mesencephalic locomotor region in the brain could lead to increased recruitment of passive limb motor neurons (Shik ML, et al., 1966). Descending supraspinal drive from regions other than the mesencephalic locomotor region could also produce unintended muscle activation, possibly through general motor neuron excitation (Cernacek J, 1961; Dimitrijevic MR, et al., 1992; Shinohara M, et al., 2003; Zijdewind I, et al., 2006). Clearly, there are multiple neural drives which could contributors to our results. Future studies using other neural techniques (transcranial magnetic stimulation, electrophysiological reflex testing, etc.) may provide greater insight about specific mechanisms.
Our findings suggest that the maximal recruitment of lower limb motor neurons in individuals with incomplete spinal cord injury has a neural limit despite the convergence of multiple neural drives. The lack of a change in muscle activation when subjects simultaneously use their upper and lower limbs at maximal effort compared to only using their lower limbs at maximal effort suggests that individuals with incomplete spinal cord injury did not gain significant additional recruitment from using their upper limbs with maximal lower limb stepping. Despite the convergence of neural drives from spinal neural networks and supraspinal centers, muscle activation associated with maximal effort was not enhanced. It is also possible that suppressive effects such as a bilateral deficit masked facilitatory effects. A bilateral deficit occurs when the combined output force or output muscle activation during a simultaneous multi-limb exertion is less than the sum of the individual limb’s forces or muscle activation amplitudes. The exact mechanisms responsible for the bilateral deficit are not known, but it is thought to be neurally mediated (Howard JD and Enoka RM, 1991). There are several proposed neural mechanisms for bilateral deficit including spinally based neural inhibition (Khodiguian N, et al., 2003), interhemispheric inhibition (Oda S, 1997), and decreased input to the primary motor cortex (Post M, et al., 2007). Bilateral deficits are often observed in tasks using homologous muscles during isometric and isokinetic contractions in the upper limbs (Oda S and Moritani T, 1994; Ohtsuki T, 1983) and in the lower limbs (Khodiguian N, et al., 2003; Simon AM and Ferris DP, 2008; Vandervoort AA, et al., 1984). Because our task combined maximal effort of not just two limbs, but all four limbs, it is possible that a bilateral deficit or a quadrupedal deficit was present but unobserved due to enhancement from interlimb neural coupling. This will also require additional techniques to determine.
Grouping subjects by injury level produced similar results and led to the same conclusions, indicating that the results of this study were robust. When we grouped subjects into cervical, thoracic, or lumbar groups, we observed increased passive limb muscle activation with maximal effort for the other limb pair in all three groups. We also performed statistical analysis on the grouped data which revealed nearly identical results compared to all of the subjects grouped together. The cervical group (n = 6) showed a significant increase in passive lower limb muscle activation for the bilateral vastus medialis, medial hamstrings, and tibialis anterior muscles with maximal upper limb effort (THSD P < 0.05). Likewise, the thoracic group (n= 5) had significant increases in the left vastus medialis, bilateral medial hamstrings, and bilateral tibialis anterior muscles (THSD P < 0.05). The lumbar group (n = 4) only reached significance for the bilateral medial hamstrings and the right tibialis anterior (THSD P < 0.05). Because we found similar trends and results regardless of how subjects were grouped, we feel confident that analyzing all subjects together was appropriate.
Normalization procedure used and cycle portion analyzed also did not affect the results. For this study, we used a non-zero variance normalization procedure for the RMS EMG data. We also analyzed the data using a zero-variance normalization condition similar to our normalization procedure in our study of neurologically intact subjects (Huang HJ and Ferris DP, In Press). For that procedure, we normalized lower (or upper) limb RMS EMG amplitudes to the Passive Upper & Active Lower (or Active Upper & Passive Lower) condition such that the Passive Upper & Active Lower (or Active Upper & Passive Lower) condition had zero variance. Regardless of normalization, passive lower (or upper) limb muscle activation significantly increased with maximal upper (or lower) limb effort. Lastly, we also analyzed RMS EMG with respect to the eccentric half of the stride cycle and the full cycle. The results had the same trends and led to the same conclusions regardless of the portion of the cycle used in the RMS EMG calculation.
A limitation of this study was that we had to rely on the subjects’ confirmation of their effort for each stepping condition. During the Passive Upper & Passive Lower condition, the motor moved with the subject, promoting subject passivity because the subject did not need to do any work. During maximal effort conditions however, the device was still moving but would increase its resistance to maintain a constant stepping frequency if the subject’s maximal effort was strong enough to drive the stepping motion faster than the specified stepping frequency (75 BPM). This means that the harder subjects worked, the more resistance they encountered. The only motivation for the subject to exert maximal effort was to comply with the instructions given. We provided verbal encouragement, but did not provide any other forms of feedback. Providing feedback such as a display of the subject’s force production would alter the task and involve more voluntary and supraspinal processes. Another limitation was that we did not examine any submaximal levels of recumbent stepping. Based on our previous work, we chose to have subjects use maximum effort to produce the greatest change in passive limb motor neuron recruitment (Huang HJ and Ferris DP, 2004; Kao PC and Ferris DP, 2005). Submaximal levels of effort, however, correspond better to daily tasks and customary therapeutic exercise.
Based on the results in this study, studies on neurologically intact individuals (Huang HJ and Ferris DP, 2004; Huang HJ and Ferris DP, In Press; Kao PC and Ferris DP, 2005) and clinical observations (Behrman AL and Harkema SJ, 2000; Visintin M and Barbeau H, 1994), it is likely that upper limb effort would result in an increase in submaximal effort lower limb muscle activation. Therefore, an experiment examining submaximal active upper and lower limb exercise combined with other neural techniques such as transcranial magnetic stimulation might provide valuable insight. Zehr and colleagues used transcranial magnetic stimulation to show that rhythmic arm movement decreased cortiospinal excitability of a forearm muscle compared to tonic voluntary contraction (Carroll TJ, et al., 2006). In a similar experiment, they demonstrated that rhythmic leg cycling increased corticospinal excitability of a forearm muscle compared to a static position (Zehr EP, et al., 2007b). Likewise, we could determine if adding upper limb effort to lower limb stepping decreases supraspinal descending neural drive compared to just lower limb stepping. This would support the idea that upper limb effort aids lower limb muscle recruitment along a convergent pathway from descending supraspinal drives (Ferris DP, et al., 2006).
We found that upper limb effort did not increase lower limb muscle activation when subjects with incomplete spinal cord injury were already using their lower limbs maximally during rhythmic exercise. Upper limb effort did increase lower limb muscle activation when the subject’s lower limbs were passive. Likewise, lower limb effort increased upper limb muscle activation when the subject’s upper limbs were passive. Combined with our previous work, individuals with incomplete spinal cord injury behaved similarly to neurologically intact individuals performing a similar stepping protocol and maximal effort (Huang HJ and Ferris DP, In Press). These findings suggest that despite presumed interlimb neural connections (Dietz V, 2002; Zehr EP and Duysens J, 2004), any excitatory influence from the interlimb neural connections does not add on to the maximal motor recruitment. There is a neural limit on muscle activation in actively moving lower limbs during rhythmic whole body exercise. Even though these results do not indicate that maximal upper limb effort increases muscle activation in active lower limbs, they do not rule out the possibility that at submaximal levels, upper limb effort may improve lower limb muscle activation. Understanding how upper limb effort and movement influences lower limb muscle activation patterns has implications for designing exercise therapies for lower limb rehabilitation. If adding upper limb effort increases lower limb muscle activation and improves muscle activation patterns at submaximal levels, then incorporating upper limb effort in lower limb rehabilitation may help patients regain lower limb functionality more quickly (Ferris DP, et al., 2006).
ACKNOWLEDGEMENTS
We would like to thank the subjects who participated in this study and the University of Michigan Physical Medicine and Rehabilitation staff for screening subjects with spinal cord injury. We would also like to thank NuStep, Inc. for their support of our research and members of the University of Michigan Human Neuromechanics Laboratory for help with data collections. This research was supported in part by Award Number F31NS056504 from the National Institute of Neurological Disorders And Stroke and Award Number 2293-01 from the Paralyzed Veterans of America Spinal Cord Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80:688–700. [PubMed] [Google Scholar]
- Carroll TJ, Baldwin ER, Collins DF, Zehr EP. Corticospinal excitability is lower during rhythmic arm movement than during tonic contraction. J Neurophysiol. 2006;95:914–921. doi: 10.1152/jn.00684.2005. [DOI] [PubMed] [Google Scholar]
- Cernacek J. Contralateral motor irradiation--cerebral dominance. Its changes in hemiparesis. Arch Neurol. 1961;4:165–172. doi: 10.1001/archneur.1961.00450080047005. [DOI] [PubMed] [Google Scholar]
- Collins DF, McIlroy WE, Brooke JD. Contralateral inhibition of soleus H reflexes with different velocities of passive movement of the opposite leg. Brain Res. 1993;603:96–101. doi: 10.1016/0006-8993(93)91303-a. [DOI] [PubMed] [Google Scholar]
- Dietz V. Do human bipeds use quadrupedal coordination? Trends Neurosci. 2002;25:462–467. doi: 10.1016/s0166-2236(02)02229-4. [DOI] [PubMed] [Google Scholar]
- Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, McKay WB, Sarjanovic I, Sherwood AM, Svirtlit L, Vrbovà G. Co-activation of ipsi- and contralateral muscle groups during contraction of ankle dorsiflexors. Journal of the Neurological Sciences. 1992;109:49–55. doi: 10.1016/0022-510x(92)90092-y. [DOI] [PubMed] [Google Scholar]
- Ferris DP, Huang HJ, Kao PC. Moving the arms to activate the legs. Exerc Sport Sci Rev. 2006;34:113–120. doi: 10.1249/00003677-200607000-00005. [DOI] [PubMed] [Google Scholar]
- Frigon A, Collins DF, Zehr EP. Effect of rhythmic arm movement on reflexes in the legs: modulation of soleus H-reflexes and somatosensory conditioning. J Neurophysiol. 2004;91:1516–1523. doi: 10.1152/jn.00695.2003. [DOI] [PubMed] [Google Scholar]
- Howard JD, Enoka RM. Maximum bilateral contractions are modified by neurally mediated interlimb effects. J Appl Physiol. 1991;70:306–316. doi: 10.1152/jappl.1991.70.1.306. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Ferris DP. Neural coupling between upper and lower limbs during recumbent stepping. J Appl Physiol. 2004;97:1299–1308. doi: 10.1152/japplphysiol.01350.2003. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Ferris DP. Upper and Lower Limb Muscle Activation Is Bidirectionally and Ipsilaterally Coupled. Medicine & Science in Sports & Exercise. doi: 10.1249/MSS.0b013e31819f75a7. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao PC, Ferris DP. The effect of movement frequency on interlimb coupling during recumbent stepping. Motor Control. 2005;9:144–163. doi: 10.1123/mcj.9.2.144. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Nozaki D, Abe MO, Nakazawa K. Shaping appropriate locomotive motor output through interlimb neural pathway within spinal cord in humans. J Neurophysiol. 2008;99:2946–2955. doi: 10.1152/jn.00020.2008. [DOI] [PubMed] [Google Scholar]
- Khodiguian N, Cornwell A, Lares E, DiCaprio PA, Hawkins SA. Expression of the bilateral deficit during reflexively evoked contractions. J Appl Physiol. 2003;94:171–178. doi: 10.1152/japplphysiol.00703.2002. [DOI] [PubMed] [Google Scholar]
- Knikou M. Neural coupling between the upper and lower limbs in humans. Neurosci Lett. 2007;416:138–143. doi: 10.1016/j.neulet.2007.01.072. [DOI] [PubMed] [Google Scholar]
- Oda S, Moritani T. Maximal isometric force and neural activity during bilateral and unilateral elbow flexion in humans. Eur J Appl Physiol Occup Physiol. 1994;69:240–243. doi: 10.1007/BF01094795. [DOI] [PubMed] [Google Scholar]
- Oda S. Motor control for bilateral muscular contractions in humans. Jpn J Physiol. 1997;47:487–498. doi: 10.2170/jjphysiol.47.487. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T. Decrease in human voluntary isometric arm strength induced by simultaneous bilateral exertion. Behav Brain Res. 1983;7:165–178. doi: 10.1016/0166-4328(83)90190-0. [DOI] [PubMed] [Google Scholar]
- Post M, van Duinen H, Steens A, Renken R, Kuipers B, Maurits N, et al. Reduced cortical activity during maximal bilateral contractions of the index finger. Neuroimage. 2007;35:16–27. doi: 10.1016/j.neuroimage.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Shinohara M, Keenan KG, Enoka RM. Contralateral activity in a homologous hand muscle during voluntary contractions is greater in old adults. J Appl Physiol. 2003;94:966–974. doi: 10.1152/japplphysiol.00836.2002. [DOI] [PubMed] [Google Scholar]
- Simon AM, Ferris DP. Lower limb force production and bilateral force asymmetries are based on sense of effort. Exp Brain Res. 2008;187:129–138. doi: 10.1007/s00221-008-1288-x. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, Sale DG, Moroz J. Comparison of motor unit activation during unilateral and bilateral leg extension. J Appl Physiol. 1984;56:46–51. doi: 10.1152/jappl.1984.56.1.46. [DOI] [PubMed] [Google Scholar]
- Visintin M, Barbeau H. The effects of parallel bars, body weight support and speed on the modulation of the locomotor pattern of spastic paretic gait. A preliminary communication. Paraplegia. 1994;32:540–553. doi: 10.1038/sc.1994.86. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist. 2004;10:347–361. doi: 10.1177/1073858404264680. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Frigon A, Hoogenboom N, Collins DF. Facilitation of soleus H-reflex amplitude evoked by cutaneous nerve stimulation at the wrist is not suppressed by rhythmic arm movement. Exp Brain Res. 2004;159:382–388. doi: 10.1007/s00221-004-2092-x. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Klimstra M, Dragert K, Barzi Y, Bowden MG, Javan B, et al. Enhancement of arm and leg locomotor coupling with augmented cutaneous feedback from the hand. J Neurophysiol. 2007a;98:1810–1814. doi: 10.1152/jn.00562.2007. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Klimstra M, Johnson EA, Carroll TJ. Rhythmic leg cycling modulates forearm muscle H-reflex amplitude and corticospinal tract excitability. Neurosci Lett. 2007b;419:10–14. doi: 10.1016/j.neulet.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res. 2006;175:526–535. doi: 10.1007/s00221-006-0570-z. [DOI] [PubMed] [Google Scholar]