Abstract
In this study, we investigated the functional elements of the flaB promoter of Borrelia burgdorferi. Promoter function was examined in a high-passage variant of strain JD1 using a set of 5′ deletions and mutations within the flaB promoter. Expression from the modified flaB promoters was assayed using the gene for green fluorescent protein (gfp) as a reporter. Although the -35 element of the promoter stimulated promoter activity, its disruption did not negate expression. Sequence upstream of the -35 had no effect on expression. The -35/-10 spacer region composed of a T-rich sequence was critical for optimal promoter function. Surprisingly, a Cytosine at the -13 site was found to be more favorable for transcription compared to a Guanosine at the same site. Based on these results and other characteristics, we propose that the B. burgdorferi flaB promoter is an example of an extended -10 promoter. Further, the T-rich spacer is a key element of the flaB promoter that contributes to the abundance of the flagellar core protein in Borrelia species.
Keywords: Borrelia burgdorferi, flaB, promoter, transcription
Introduction
Lyme disease is caused by the spirochete Borrelia burgdorferi and transmitted by ticks. It is primarily a chronic inflammatory disease and often involves multiple organs (Steere et al., 1977). Motility plays an important role in the ability of this pathogen to journey to and invade distal sites. As in other bacteria, motility in B. burgdorferi is enabled by the presence of flagella in these organisms. However, the flagella in B. burgdorferi are periplasmic in location with several flagella attached to each end. The periplasmic flagella also influence the shape of the cell (reviewed in Li et al., 2000, Charon & Goldstein, 2002).
The motility genes are uniquely regulated in B. burgdorferi (Li et al., 2000, Charon & Goldstein, 2002). In contrast to other bacteria, these genes are initiated by the housekeeping sigma factor, sigma 70 (σ70 or RpoD) and not σ28 (Ge et al., 1997a, 1997b, Sal et al., 2008). The major component of the flagellar filament is a 41-kDa protein, FlaB. Inactivation of the flaB gene results not only in nonmotile but also rod shaped spirochetes in contrast to the helical shape of the wild-type bacterium (Sadziene et al., 1991, Motaleb et al., 2000, Sartakova et al., 2001). Multiple studies have established that the flaB gene is constitutively expressed in B. burgdorferi.
Initial studies noted that the flaB promoter did not resemble a prototypical RpoD promoter but rather most closely to the bacteriophage SP01 sigma gp33-34 promoter sequence of Bacillus subtilis (Noppa et al., 1995). A subsequent study recognized and designated a σ70 promoter upstream of the flaB gene (Ge et al., 1997b). Nevertheless, the early confusion regarding the sigma specificity of the flaB promoter underscores the unusual nature of this promoter. The atypical features include an uncharacteristic C at the -13 site, a base at this site normally associated with RpoS promoters (Lee & Gralla, 2001) and a poor resemblance to the consensus -10 and the -35 elements. Despite these apparent deficiencies, the expression of flaB is robust in B. burgdorferi. Therefore, other features must exist within the flaB promoter that adequately compensate for the lack of a strong resemblance to the consensus σ70 promoter. We undertook a systematic study to tease out these hitherto unrecognized elements of the flaB promoter that strengthen its activity. We determined that the flaB promoter of B. burgdorferi carries an extended -10 element and further that the T-rich -35/-10 spacer is very important for its optimal activity. Within this extended -10 core of the flaB promoter, a C at the -13 site is more favorable to transcription in comparison to a G.
Materials & methods
Bacterial strains
Low-passage, infectious B. burgdorferi B315A4NP1 (cp9-bbe02::kanr) (Kawabata et al., 2004) as well as a high-passage (passage 20) variant of strain JD1 (Ramamoorthy et al., 2005) were used in the current study. The E. coli strain Top 10 (Invitrogen) was generally used in the generation of constructs and for the preparation of plasmids for the transformation of B. burgdorferi.
Generation of pBSV2G-flaBp-gfp fusion constructs
All constructs were generated by amplifying the flaBp-gfp sequence by PCR using the appropriate primers (Table 1) and then cloning them into the E. coli-B. burgdorferi shuttle vector pBSV2G using different combinations of restriction enzymes. The template for amplification was pQE30-flaBp-gfp or pBSV2-flaBp-gfp (Ramamoorthy et al., 2005). The flaBp2T→C-gfp fusion was first cloned in the vector pREP4 (Qiagen, USA) before transfer to pBSV2G. Transformants in E. coli TOP10 strain were selected by plating on Luria broth (LB)-Agarose supplemented with 10 μg gentamicin mL-1. Plasmids from the positive clones were prepared using Tip100 columns (Qiagen) under sterile conditions and the flaB promoter sequences in these plasmids were confirmed prior to their utilization in this study.
Table 1. Primers used in this study.
| Primer | Sequencea | Gene(s)/Plasmid | Use |
|---|---|---|---|
| T116 | TAATTATTTTAATGCTATTGCTATTTGCGTTTCTTTTTTTTTAA | flaBp | pBSV2G-flaBp2-gfp |
| T123 | TTTTTTTTAATTTTTGTGCTATTCTTTTTAACAGGCAAAAGGATT | flaBp | pBSV2G-flaBp3-gfp |
| T230 | TTAATGCTATTGCTATTTGCGTTTCTTTTTTTTTAATTTTTGTGCCATTCTTTTTAAC | flaBp | pBSV2G-flaBp2T→C-gfp |
| T240 | gcatgcaccggtaccTAATTATTTTTAATGCTATTGCTATTTGCG | flaBp | Multiple constructs |
| T316 | tcccccgggATGCTATTGCTATTTGCGTTTCTTTTTGTTGGATTGTTGTGCTATTCT | flaBp | pBSV2G-flaBp2sm-gfp |
| T317 | tcccccgggATGCTATTGCTATTTGCGTTTCTTTTTTTTTAATTTTTGTGGTATTCT | flaBp | pBSV2G-flaBp2C→G-gfp |
| T319 | gatcagataggtaccCTTCTTTTTTTTTAATTTTTGTGC | flaBp | pBSV2G-flaBp35-gfp |
| B93 | cgatagcatatcgatGTTCTTTACGATGCCATTGGGATAT | pQE30 | pBSV2G-flaBp2C→G-gfp |
| B172 | tagcatgagcatgcCTGCAGGCGGCCGCACTAGTG | gfp 3 ′ | Multiple constructs |
| B197 | ATGCGATCCTCTCATAGTTAATTTCTCCTC | gfp | DNA sequencing |
| B234 | CGACAGGTAATGGTTGTCTG | gfp | pBSV2G-flaBp2C→G-gfp |
gene sequences are in capitals and unrelated sequences are in small letters. Functional restriction enzyme sites added to the primers are underlined and mutations are underlined and bolded.
Transformation of B. burgdorferi
The preparation of electrocompetent cells, electroporation conditions and selection of transformants were all done as described (Samuels, 1995). Transformants were selected on solid agar supplemented with 40 μg gentamicin mL-1 (for B315A4NP1) or 20 μg gentamicin mL-1 (for JD1-P20). Ten to 14 days after plating, colonies were picked and inoculated into 4 ml of complete BSK II medium containing 20 μg gentamicin mL-1. When the density of the culture reached >5 × 107 cells mL-1, freezer stocks were prepared and 14 ml of fresh medium was inoculated at a density of 1 × 106 cells mL-1 for the preparation of whole cell lysates (WCL) for protein analysis. Cultures were harvested at stationary phase (~1 × 108 mL-1). At least two colonies were included for each construct.
Western blotting
Whole cell lysates were prepared and normalized as described (Ramamoorthy et al., 1995). Samples (usually 10 μl) were electrophoresed through a 12.5% SDS-PAGE gel and the gel was either stained with Coomassie Blue R250 dye or the proteins transferred to nitrocellulose for Western blotting. The expression of GFP was detected using a polyclonal rabbit anti-GFP antibody and quantified by densitometry as described (Gautam et al., 2008).
Results
Confirmation of the flaB promoter -10 element by targeted mutation of the -12T
Two different transcriptional start sites have been identified for the B. burgdorferi flaB promoter (Gassmann et al., 1991, Noppa et al., 1995, Ge et al., 1997b). These initial studies mapped the transcription sites to adjacent bases, and more importantly, also designated two overlapping, but distinct, hexamers as the -10 element. Therefore, it was essential to identify the authentic -10 hexamer as a first step in the functional characterization of this promoter. We resorted to a mutational approach to resolving this difference taking advantage of the observation that the -12 T, the first base of the hexamer sequence, is highly conserved in Escherichia coli and very important for promoter recognition (Lisser and Margalit, 1993). In B. burgdorferi, this base has also been shown to be crucial for optimal gene expression from the ospC promoter (Eggers et al., 2004). Thus it seemed likely that the sequence TATTCT (Fig. 1A) represents the flaB promoter -10 element. We changed the -12T to a C in order to confirm this assignment. These initial experiments were carried out in the low-passage infectious strain, B315A4NP1 (Kawabata et al., 2004). Furthermore, a shorter version of the flaB promoter, flaBp2, was utilized as this version exhibited a level of expression similar to that measured for the previously used (Ramamoorthy et al., 1995) longer flaB promoter (data not shown). Two random transformants were characterized. In both clones, the loss of the -12T resulted in a marked decrease in expression to levels undetectable by Western blotting (Fig. 2). This result provides mutational evidence in support of the previous designation of the -10 element (Gassmann et al., 1991, Ge et al., 1997b).
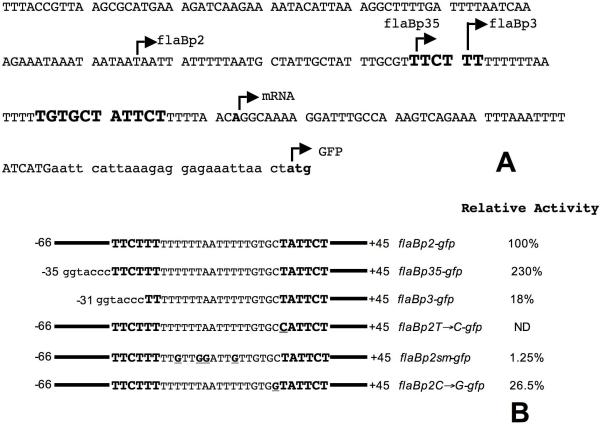
Figure 1. A. Sequence of the flaB promoter and the junction region of the flaBp-gfp fusion.
The locations of the deletions and the transcriptional and translational start sites are all shown by bent arrow above the sequence. The sequence of the fusion junction is shown in small letters. B. The boundaries and sequences of the various promoter fragments used in the study. The -35 and the -10 elements are shown in bold and bigger font size. Mutations introduced in the flaB promoter are in bold and underlined. ND, not detected.
Figure 2. Substitution of the -12 T with a C results in loss of promoter function.
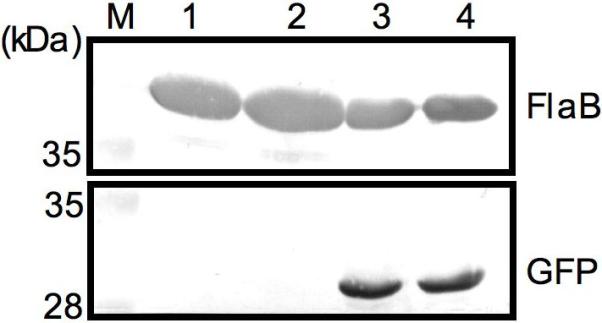
The effect of the mutation in comparison to the wild-type promoter was assessed in strain B315A4NP1. Two transformants were tested for each construct but the amount of the whole cell lysate used from the mutant construct (lanes 1 and 2) was four times the amount of the lysate from the wild-type construct (lanes 3 and 4). The expression of GFP and FlaB was examined by Western blotting using monospecific antibodies.
Delineating the functional regions of the flaB promoter by progressive deletion of the upstream sequence
To delineate the sequences essential for optimal expression from the flaB promoter, a set of flaBp-gfp fusion constructs were generated that contained progressive 5′ deletions of the flaBp upstream sequence (Fig. 1B). The expression of GFP from these constructs was assayed in a high-passage (passage 20) variant of JD1 (JD1-P20) (Ramamoorthy et al., 2005). The disruption of the -35 element resulted in a significant reduction in expression but did not eliminate promoter activity (Fig. 3, Panel B, lanes 5 and 6, and Fig. 1B). On the contrary, the retention of just the -35 without any additional upstream sequence boosted expression by 2.3 fold over the activity level obtained with the longer flaBp2-gfp fusion construct (Panel B, lanes 3 and 4, and Fig. 1B). These results establish the importance of the -35 element for flaB promoter activity.
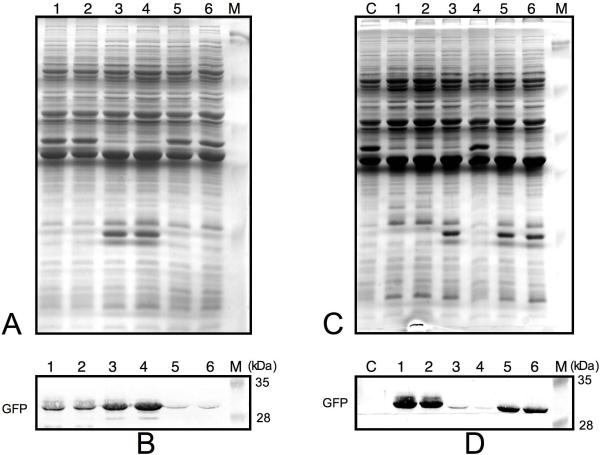
Figure 3. Effect of deletions and mutations of the flaB promoter on gene expression.
Panels A and C. To ensure that equivalent amounts of total protein from each sample were assayed, the same volume of lysate from each sample was electrophoresed through an SDS-polyacrylamide and stained with Coomassie Blue R250 dye. Panels B and D. Western blots showing the relative expression of GFP from the different promoter-gfp constructs. The expression of GFP was detected using a rabbit anti-GFP antibody. Two transformants carrying the following constructs, pBSV2G-flaBp2-gfp (lanes 1 and 2), pBSV2G-flaBp35-gfp (lanes 3 and 4) and pBSV2G-flaBp3-gfp (lanes 5 and 6), were tested (Panel B). Similarly, GFP expression was assayed from transformants carrying the following mutations within the flaB promoter: pBSV2G-flaBp2-gfp (lanes 1 and 2) and pBSV2G-flaBp2sm-gfp (lanes 3 and 4), and pBSV2G-flaBp2C→G-gfp (lanes 5 and 6). Lane C is a promoterless negative control (pBSV2G-gfp) (Panel D).
The AT-rich -35/-10 spacer sequence is essential for optimal gene expression
The -35/-10 spacer sequence of the B. burgdorferi flaB promoter is remarkably AT-rich (14/17 bases or 82%). Therefore, it was of interest to ascertain if this sequence played any role in promoter function. We substituted 4 nucleotides in this sequence with Gs (Fig. 1B, flaBp2sm-gfp) to not only reduce the overall AT-richness from 82% to 59%, but also to eliminate even short stretches of AT sequences. The expression of GFP from these constructs was also assayed in the JD1-P20 variant. The loss of the AT-rich sequence from the -35/-10 spacer resulted in a dramatic 80-fold reduction in expression (Fig. 3, Panel D, lanes 3 and 4, and Fig. 1B).
The -13C facilitates expression of flaB
Among B. burgdorferi genes not known to require the alternative sigma factor RpoS for expression and whose promoters have been experimentally confirmed, the flaB promoter is unique in possessing a C at the -13 site (our analysis of the literature). A C at the -13 site is usually associated with RpoS-dependent promoters in E. coli (Lee & Gralla, 2001). Moreover, the promoters for at least four B. burgdorferi RpoS-dependent genes (Caimano et al., 2007) including ospC (Padula et al., 1993), dbpAB (Hagman et al., 1998), bmpC (Dobrikova et al., 2001) and bba66 (Clifton et al., 2006) also harbor a C at the equivalent site. The C at the -13 site is not essential for activity but does enhance expression in the context of the ospC promoter (Eggers et al., 2004, Yang et al, 2005). However, it is not known if a similar C in the context of a σ70 promoter discriminates against σ70 transcription in B. burgdorferi. Therefore, to address the importance of the uncharacteristic -13C in the context of the RpoS-independent flaB promoter, we altered this base to a G. Contrary to expectation, the substitution of the C resulted in a significant decrease in expression suggesting that the presence of the C at the -13 site was highly favorable, rather than inhibitory, to gene expression (Fig. 3, Panel D, lanes 5 and 6, and Fig. 1B).
DISCUSSION
In this study, we dissected the flaB promoter of B. burgdorferi to identify individual bases and regions that are involved in promoter function. The identity of the flaB promoter initially designated by Charon and colleagues (Ge et al., 1997b) was further confirmed experimentally in this study. Interestingly, the -12T→C mutation in case of the flaBp was more sensitive unlike the T→A change in case of ospCp (Eggers et al., 2004). Although the EσS enzyme appears capable of utilizing, albeit inefficiently, an A at the -12 site, by contrast, the EσD enzyme was incapable of recognizing the -12C in the context of flaBp. This is not surprising as a C at -12 in an E. coli promoter serves to discriminate against EσD and favor EσS (Lacour & Landini, 2004).
Deletion analysis established the importance of the -35 element in flaBp despite the fact that it shows a poor identity (2 out of 6) to the consensus TTGACA (Lisser and Margalit, 1993). By comparison a total loss of this element, such as occurs in the case of flaBp3, resulted in a significant reduction in activity. This is not surprising given that the flaBp35-gfp fusion, but not the flaBp3-gfp fusion, preserves an optimal 17 bp -35/-10 spacer region as well the 2 Ts, the most-conserved bases of the -35 hexamer (Lisser & Margalit, 1993). The flaBp sequence upstream of the -35 has very little role in promoter function. Although the deletion of this sequence resulted in a modest increase in expression, an increase in expression was also noted in E. coli (data not shown). In light of this, it seems unlikely that the flaBp sequence upstream of the -35 harbors any cryptic regulatory or promoter function element. These conclusions are further supported by a previous study that demonstrated that a minimal flaB promoter lacking the sequence upstream of the -35 element was equally proficient as the longer version of the flaB promoter (Xu et al., 2008).
The disruption of the AT-rich -35/-10 spacer sequence resulted in a drastic 80-fold reduction in expression. The substitution of the wild-type spacer with a mutated spacer led to a drop in the AT content of the 17 bp spacer from 82% to 59% and in the interruption of T-stretches. Therefore, we believe it is the loss of one or more elements and/or features within the space region rather than the inadvertent introduction of an inhibitory sequence that is responsible for the diminished expression. This explanation is consistent with recent observations in E. coli associating optimal gene expression with an AT-rich spacer sequence (Liu et al., 2004). Although the latter study suggested that a specific sequence was involved in boosting expression, our study cannot distinguish between several different possibilities for the enhancing element within the flaBp -35/-10 spacer region including a specific sequence, overall AT content and one or more T-stretches. In light of the conservation among Borrelia species of just the T stretches in the spacer (Fig. 4), it is highly likely that one or both of the T-stretches is the enhancing element within the spacer region. Interestingly, a T-rich region, albeit upstream of the -35 element, is also involved in enhancing expression from the ospAB promoter of B. burgdorferi (Sohaskey et al., 1999).
Figure 4. The conservation of the extended -10 flaB promoter configuration and other features among Borrelia species.
The -35 and the extended -10 elements are shown in bold and the conserved intervening spacer sequences are shown in small case letters.
Closer inspection of the flaB promoter reveals the existence of a sequence, TGTGCTATTCT, which is very similar to the consensus extended -10 element, TRTGNTATAAT (Mitchel et al., 2003). Therefore, we believe the flaB promoter is an example of an extended -10 element. This notion is further supported by the following observations. Extended -10 promoters exhibit a poor resemblance to the consensus -35 element and, in fact, do not need the -35 for transcription (Kumar et al., 1993). The moderate expression of gfp from the flaBp3-gfp fusion despite the disruption of the native -35 is consistent with this criterion for an extended -10 promoter. Further, such promoters have also been reported to include short stretches of Ts in the spacer region (Mitchel et al., 2003), features that are also conserved in the flaB promoter. However, it is more than likely, and as demonstrated in this study, that the T-stretches influence the strength of the promoter rather than constitute an integral part of the extended -10 promoter. Finally, the fact that the -13 C was not inhibitory also is consistent with the observation that this site shows no base preference in an extended -10 promoter configuration. To our knowledge, we believe our study is the first to demonstrate an effect of a mutation at the -13 site on gene expression from an extended -10 promoter. It remains to be determined if C is the optimal base for this site.
The flaB promoters of other agents of Lyme disease including B. afzelii, B. garinii, B. spielmanii and B. valaisiana are nearly identical to that of B. burgdorferi (Fig. 4). Moreover, the extended -10 promoter configuration and the T-rich spacer are also conserved in the flaBp of several other Borrelia species including B. anserina, B. crocidurae, B. duttonii, B. hermsii, B. turicatae and B. recurrentis (Fig. 4) with some minor differences. These orthologs carry an A instead of a C at the -13 site, exhibit a better match (3 out of 6 bases) of the -35 hexamer to the consensus, and with the exception of B. anserina, carry a longer -35/-10 spacer. It is not known if these changes affect promoter strength or if these are compensatory changes with no net effect on promoter activity. It is apparent from the conservation of the functional elements that the flaB promoter in Borrelia has evolved to deliver constitutive and abundant expression of the flagellar core protein.
A survey of 26 experimentally confirmed promoters in B. burgdorferi revealed the presence of an extended -10 promoter configuration in three other genes including bba74 (Mulay 2007), ospE (Eggers et al., 2006) and bbk2.10 (Hefty et al., 2001). The frequency (15%) of occurrence of extended -10 promoters in B. burgdorferi, albeit in a small sample size, is nevertheless similar to that reported for E. coli (20%) (Burr et al., 2000). Therefore, it is likely that additional genes with extended -10 promoters exist in the B. burgdorferi genome. Our study extends the understanding of promoter architecture in B. burgdorferi and also identifies a key feature of the flaB promoter underlying the abundance of FlaB, an integral component of the flagellar structure in this organism.
Acknowledgements
This work was funded by grants AI 49293 from the National Institute of Allergy and Infectious Diseases and RR00164 from the National Institutes of Health.
References
- Burr T, Mitchell J, Kolb A, Minchin S, Busby S. DNA sequence elements located immediately upstream of the -10 hexamer in Escherichia coli promoters: a systematic study. Nucleic Acis Res. 2000;28:1864–1870. doi: 10.1093/nar/28.9.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano M, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilberty MA, Schwarz I, Radolf JA. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon NW, Goldstein SF. Genetics of motility and chemotaxis of a fascinating group of bacteria: The spirochetes. Ann Rev Genet. 2002;36:47–73. doi: 10.1146/annurev.genet.36.041602.134359. [DOI] [PubMed] [Google Scholar]
- Clifton DR, Nolder CL, Hughes JL, Nowalk AJ, Carroll JA. Regulation of bba66 encoding an immunogenic and infection-associated lipoprotein in Borrelia burgdorferi. Mol Microbiol. 2006;61:243–258. doi: 10.1111/j.1365-2958.2006.05224.x. [DOI] [PubMed] [Google Scholar]
- Dobrikova EY, Bugrysheva J, Cabello FC. Two independent transcriptional units control the complex and simultaneous expression of the bmp paralogous chromosomal gene family in Borrelia burgdorferi. Mol Microbiol. 2001;39:370–378. doi: 10.1046/j.1365-2958.2001.02220.x. [DOI] [PubMed] [Google Scholar]
- Eggers CH, Caimano MJ, Radolf JD. Analysis of promoter elements involved in the transcriptional initiation of RpoS-dependent Borrelia burgdorferi genes. J Bacteriol. 2004;186:7390–7402. doi: 10.1128/JB.186.21.7390-7402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers CH, Caimano MJ, Radolf JD. Sigma factor selectivity in Borrelia burgdorferi: RpoS recognition of the ospE/ospF/elp promoters is dependent on the sequence of the -10 region. Mol Microbiol. 2006;59:1859–1875. doi: 10.1111/j.1365-2958.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Gassmann GS, Jacobs E, Deutzmann R, Gobel U. Analysis of the Borrelia burgdorferi GeHo fla gene and antigenic characterization of its gene product. J Bacteriol. 1991;173:1452–1459. doi: 10.1128/jb.173.4.1452-1459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A, Hathaway M, McClain N, Ramesh G, Ramamoorthy R. Analysis of the determinants of bba64 (P35) gene expression in Borrelia burgdorferi using a gfp reporter. Microbiology. 2008;154:275–285. doi: 10.1099/mic.0.2007/011676-0. [DOI] [PubMed] [Google Scholar]
- Ge Y, Old LG, Saint-Girons I, Charon NW. The flgK motility operon of Borrelia burgdorferi is initiated by a σ70-like promoter. Microbiol. 1997a;143:1681–1690. doi: 10.1099/00221287-143-5-1681. [DOI] [PubMed] [Google Scholar]
- Ge Y, Old LG, Saint-Girons I, Charon NW. Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus σ70 promoter. J Bacteriol. 1997b;179:2289–2299. doi: 10.1128/jb.179.7.2289-2299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman KE, Lahdenne P, Popova TG, Porcella SF, Akins DR, Radolf JD, Norgard MV. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme Borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefty PS, Jolliff SE, Caimano MJ, Wikel SJ, Radolf JD, Akins DR. Regulation of OspE-related, OspF-related, and Elp lopoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect Immun. 2001;69:3618–3627. doi: 10.1128/IAI.69.6.3618-3627.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata H, Norris S, Watanabe H. BBE02 disruption mutants of Borrelia burgdorferi have a highly transformable, infectious phenotype. Infect Immun. 2004;72:7147–7154. doi: 10.1128/IAI.72.12.7147-7154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Malloch RA, Fujita N, Smillie DA, Ishihama A, Hayward RS. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- Lacour S, Landini P. σS-Dependent Gene Expression at the Onset of Stationary Phase in Escherichia coli: Function of σS -Dependent Genes and Identification of Their Promoter Sequences. J Bacteriol. 2004;186:7186–7195. doi: 10.1128/JB.186.21.7186-7195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Gralla JD. Sigma 38 (rpoS) RNA Polymerase Promoter Engagement via -10 Region Nucleotides. J Biol Chem. 2001;276:30064–30071. doi: 10.1074/jbc.M102886200. [DOI] [PubMed] [Google Scholar]
- Lescot M, Audic S, Robert C, Nguyen TT, Blanc G, Cutler SJ, Wincker P, Couloux A, Claverie J-M, Raoult D, Drancourt M. The Genome of Borrelia recurrentis, the Agent of Deadly Louse-Borne Relapsing Fever, Is a Degraded Subset of Tick-Borne Borrelia duttonii. PLoS Genet. 2008;4:1–11. doi: 10.1371/journal.pgen.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisser S, Margalit H. Compilation of E. coli mRNA promoter sequences. Nuc Acids Res. 1993;21:1507–1516. doi: 10.1093/nar/21.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Motaleb MA, Sal M, Goldstein SF, Charon NW. Spirochete periplasmic flagella and motility. J Mol Microbiol Biotechnol. 2000;2:345–354. [PubMed] [Google Scholar]
- Liu M, Tolstorukov M, Zhurkin V, Garges S, Sankar Adhya S. A mutant spacer sequence between -35 and -10 elements makes the Plac promoter hyperactive and cAMP receptor protein-independent. Proc Natl Acad Sci USA. 2004;101:6911–6916. doi: 10.1073/pnas.0401929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE, Zheng D, Busby SJW, Minchin SD. Identification and analysis of `extended -10′ promoters in Escherichia coli. Nuc Acids Res. 2003;31:4689–4695. doi: 10.1093/nar/gkg694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Corum L, Bono JL, Elias AF, Rosa P, Samuels DS, Charon NW. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc Natl Acad Sci USA. 2000;97:10899–10904. doi: 10.1073/pnas.200221797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulay V. PhD Thesis. New York Medical College; Valhalla: 2007. Functional characterization of Borrelia burgdorferi BBA74, a periplasmic protein expressed in feeding ticks. [Google Scholar]
- Noppa L, Burman N, Sadziene A, Barbour AG, Bergström S. Expression of the flagellin gene in Borrelia is controlled by an alternative sigma factor. Microbiology. 1995;141:85–93. doi: 10.1099/00221287-141-1-85. [DOI] [PubMed] [Google Scholar]
- Padula SJ, Sampieri A, Dias F, Szczepanski A, Ryan RW. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect Immun. 1993;61:5097–5105. doi: 10.1128/iai.61.12.5097-5105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken RN. Polymerase chain reacion primers and probes derived from flagellin gene sequences for specific detection of the agents of Lyme disease and North American relapsing fever. J Clin Microbiol. 1992;30:99–114. doi: 10.1128/jcm.30.1.99-114.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy R, Povinelli L, Philipp MT. Molecular characterization, genomic arrangement, and expression of bmpD, a new member of the bmp class of genes encoding membrane proteins of Borrelia burgdorferi. Infect Immun. 1995;64:1259–1264. doi: 10.1128/iai.64.4.1259-1264.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy R, McClain NA, Gautam A, Scholl-Meeker D. Expression of the bmpB gene of Borrelia burgdorferi is modulated by two distinct transcription termination events. J Bacteriol. 2005;187:2592–2600. doi: 10.1128/JB.187.8.2592-2600.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Thomas DD, Bundoc V, Holt SC, Barbour AG. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J Clin Invest. 1991;88:82–92. doi: 10.1172/JCI115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sal MS, Li C, Motalab MA, Shibata S, Aizawa S, Charon NW. Borrelia burgdorferi uniquely regulates its motility genes and has an intricate flagellar hook-basal body structure. J Bacteriol. 2008;190:1912–1921. doi: 10.1128/JB.01421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol. 1995;47:253–259. doi: 10.1385/0-89603-310-4:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartakova ML, Dobrikova EL, Motaleb MA, Godfrey HB, Charon NW, Cabello FC. Complementation of a nonmotile flaB mutant of Borrelia burgdorferi by chromosomal integration of a plasmid containing a wild-type flaB allele. J Bacteriol. 2001;183:6558–6564. doi: 10.1128/JB.183.22.6558-6564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohaskey CD, Zückert WR, Barbour A. The extended promoters for two outer membrane lipoprotein genes of Borrelia spp. uniquely include a T-rich region. Mol Microbiol. 1999;33:41–51. doi: 10.1046/j.1365-2958.1999.01443.x. [DOI] [PubMed] [Google Scholar]
- Steere AC, Malawista SE, Hardin JA, Ruddy S, Askenase W, Andiman WA. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med. 1977;86:685–698. doi: 10.7326/0003-4819-86-6-685. [DOI] [PubMed] [Google Scholar]
- Xu Q, McShan K, Liang FT. Verification and dissection of the ospC operator by using flaB promoter as a reporter in Borrelia burgdorferi. Microbial Path. 2008;45:70–78. doi: 10.1016/j.micpath.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Lybecker MC, Pal U, Alani SM, Blevins J, Revel AT, Samuels DS, Norgard MV. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J Bacteriol. 2005;187:4822–4829. doi: 10.1128/JB.187.14.4822-4829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]