Abstract
Objectives:
In order for therapies for Alzheimer's disease (AD) to have the greatest impact, it will likely be necessary to treat individuals in the “preclinical” (presymptomatic) stage. Fluid and neuroimaging measures are being explored as possible biomarkers of AD pathology that could aid in identifying individuals in this stage in order to target them for clinical trials and to direct and monitor therapy. The objective of this study was to determine if cerebrospinal fluid biomarkers for AD suggest the presence of brain damage in the preclinical stage of AD.
Methods:
We investigated the relationship between structural neuroimaging measures (whole brain volume) and levels of cerebrospinal fluid (CSF) amyloid-β (Aβ)40, Aβ42, tau, and phosphorylated tau181 (ptau181), and plasma Aβ40 and Aβ42 in well-characterized research subjects with very mild and mild dementia of the Alzheimer type (DAT; n=29) and age-matched, cognitively normal controls (n=69).
Results:
Levels of CSF tau and ptau181, but not Aβ42, correlated inversely with whole brain volume in very mild/mild DAT, whereas levels of CSF Aβ42, but not tau or ptau181, was positively correlated with whole brain volume in non-demented controls.
Interpretation:
Reduction in CSF Aβ42, likely reflecting Aβ aggregation in the brain, is associated with brain atrophy in the preclinical phase of AD. This suggests that there is toxicity associated with Aβ aggregation prior to the onset of clinically detectable disease. Increases in CSF tau (and ptau181) are later events that correlate with further structural damage and occur with clinical onset and progression.
Keywords: Alzheimer's disease, amyloid-β, biomarker, brain atrophy, cerebrospinal fluid, MRI, preclinical AD, tau
Introduction
Alzheimer's disease (AD) will become a public health crisis within 2-3 decades if left untreated. Although there are currently no proven therapies that delay the onset or prevent the progression of AD, several promising candidates are being developed. The testing and ultimate implementation of emerging therapies will require identification of affected and “at risk” individuals in order to target them for clinical trials and to direct and monitor therapy. AD pathology is estimated to begin a decade or longer prior to the appearance of any cognitive symptoms and signs of dementia (a stage termed “preclinical AD”), with impairments becoming clinically detectable only after substantial neuronal cell death has taken place.1-5 Thus, fluid and neuroimaging measures are being explored as possible biomarkers for early stage and preclinical AD diagnosis since it is in these initial stages that disease-modifying therapies are likely to have the greatest chance of preserving normal brain function.
Whole brain atrophy, the presumed consequence of years of axonal and neuronal degeneration, is a fundamental but not specific feature of AD and has been quantified antemortem by magnetic resonance imaging (MRI) in both cross-sectional and longitudinal studies. 6, 7 The best studied fluid analytes in AD have been cerebrospinal fluid (CSF) levels of Aβ42, the primary constituent of amyloid plaques, and tau, the primary component of neurofibrillary tangles. Levels of CSF Aβ42 are typically reduced in dementia of the Alzheimer type (DAT),8-11 reflecting its aggregation and deposition as amyloid in the brain,12 whereas levels of CSF tau and phosphorylated tau (ptau) species are elevated in DAT8, 13, 14 and are hypothesized to reflect the presence of neurofibrillary tangles and/or neurodegeneration,15, 16 although not all studies support this conclusion.17 The extent to which these fluid measures relate to brain volumetric measures remains unclear, especially in non-demented elderly individuals and those with early stage DAT. To this end, we investigated the relationship between CSF (and plasma) analytes and brain structural measures as determined by quantitative MRI in well-characterized cohorts of non-demented elders and those with very mild or mild DAT. Elucidation of these relationships may not only provide information regarding the possible utility of these measures as biomarkers of preclinical and early stage AD but may also shed light on the natural progression of neuropathological changes in AD and whether or not protein misfolding in the brain results in neurodegeneration prior to clinically detectable disease.
Subjects and Methods
Subjects
Subjects (n=98) were community-dwelling volunteers enrolled in longitudinal studies of healthy aging and dementia through the Washington University Alzheimer Disease Research Center (ADRC). Subjects were 60-91 years of age and in good general health, having no other neurological, psychiatric or systemic medical illness that could contribute importantly to dementia, nor medical contraindication to lumbar puncture (LP) or magnetic resonance imaging (MRI). Cognitive status was determined annually in accordance with standard protocols and criteria.18, 19 A Clinical Dementia Rating (CDR) 20 of 0 (n=69) indicated no dementia whereas CDR 0.5 (n=21) and CDR 1 (n=8) indicated very mild and mild AD dementia, respectively, consistent with NINCDS/ADRDA criteria.21 The rate of postmortem confirmation of AD clinical diagnosis in our Center is 92% 22, including the very mild CDR 0.5 stage. Studies were approved by the Human Studies Committee at Washington University, and written informed consent was obtained from all subjects. All subjects in the study underwent LP for the collection of CSF, venipuncture for the collection of plasma (n=84 subjects), and MRI for assessment of brain volumetric measures. LP/blood collection and MRI were performed within 2 years of each other.
CSF and Plasma Collection, Processing, and Biomarker Measurement
CSF (20-30 mL) was collected in polypropylene tubes at 8:00 am after overnight fasting as previously described.12 CSF samples were free from any blood contamination. Samples were gently inverted to avoid gradient effects, briefly centrifuged at low speed to pellet any cellular elements, and aliquoted (500 μL) into polypropylene tubes prior to freezing at −84° C. Fasted blood (10-15 mL) was obtained at the time of LP and collected into polypropylene tubes containing ethylenediaminetatraacetic acid. Plasma was prepared by standard centrifugation methods prior to aliquoting and freezing at −84° C. CSF samples were analyzed for total tau, phosphorylated tau181 (ptau181), and Aβ42 by commercial enzyme-linked immunosorbant assay (ELISA) (Innotest, Innogenetics, Ghent, Belgium). CSF Aβ40 and plasma Aβ40 and Aβ42 were assayed by ELISA, as described.23 For all biomarker measures, samples were continuously kept on ice, and assays were performed on sample aliquots after a single thaw following initial freezing.
Magnetic Resonance Imaging Acquisition and Image Processing
MR imaging was performed using a Siemens 1.5 Tesla Vision scanner (Erlangen, Germany). Cushions and a thermoplastic mask were used to reduce head movement. A scout image (TR = 15 ms, TE = 6 ms, flip angle = 30°, 2.34 × 1.17 × 8 mm resolution) was acquired to center the field of view, and then multiple (three or four) T1-weighted sagittal MP-RAGE24 scans (TR = 9.7 ms, TE = 4 ms, flip angle = 10°, TI = 20 ms, TD = 200 ms, 1 × 1 × 1.25 mm resolution) were acquired in each subject. Images were processed for motion correction, atlas transformation and inhomogeneity correction as previously described.25-27 Processing resulted in registered structural data resampled to 1 mm3 voxels in Talairach atlas space.28 Whole brain volume was obtained using commercially available FSL software,29, 30 with segmentation classifying each voxel of the MR image as CSF, gray or white matter. Normalized whole brain volumes (nWBV) were computed as the proportion of all voxels occupied by gray and white matter (equivalent to 100-%CSF) voxels, yielding a unit that represents the proportion of estimated total intracranial volume. Hippocampal (hippocampal formation and dentate gyrus) volume measures were obtained using Freesurfer software. 31 This automated technique registers an individual's MR image to a probabilistic brain atlas acquired from a predetermined training set (in this case healthy adults and AD patients), and has been reported to generate volumes with a high correspondence to manually generated volumes. 31 Hippocampal volumes were obtained from all but 12 of the 98 subjects (omissions due to Freesurfer processing errors). Hippocampal volumes (combined left and right) were adjusted for body size by correcting for intracranial volume (ICV) (representing all voxels within the cranium) using a covariance approach: Adjusted volume in cubic millimeters = raw volume − b × (ICV − mean ICV), where b is the slope of the regression of an ROI volume on ICV. Adjusted hippocampal volumes (HC) are expressed as cubic millimeters.
In Vivo Amyloid Imaging with PET PIB
Thirty-seven of the 98 participants who had CSF and MRI measures underwent in vivo amyloid imaging via positron emission tomography (PET) with Pittsburgh Compound B (PIB) ([N-methyl-[11C]]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole)32 within two years of LP, as described.12 Three-dimensional regions of interest were created for each participant based on their individual MRI scans. A mean cortical PIB value was obtained by averaging the prefrontal cortex, precuneus, lateral temporal cortex, and gyrus rectus regions, as described previously.12 In qualitative analyses, presence of cortical amyloid (PIB-positive) was defined by a mean cortical PIB binding potential ≥0.16 based on visual inspection of more than 200 scans performed at Washington University. Signal from PIB binding potentials above this value are easily visualized and appear qualitatively to be above background levels. Individuals with mean cortical values <0.16 were considered to be PIB-negative.
Statistical Analyses
Unpaired Student's t-tests or Chi square analyses were used to determine whether demographic and biomarker variables differed between the two clinical groups (CDR 0 vs CDR >0). The relationship between imaging measures and CSF biomarker measures was examined using bivariate (Pearson's r) correlations and partial correlations controlling for age (SPSS v.15) without correction for multiple comparisons. Since the Type I error rate rises with increasing numbers of statistical tests, some statistically significant differences reported may be due to chance. However, the likelihood for such an error is highest for p-values closest to 0.05 and decreases with smaller p-values. Mahalanobis Distance and Cook's Distance methods 33 were used to define multivariate outliers, and partial correlations (controlling for age) were again performed on the new data set in which outliers were omitted. A p-value of 0.05 defined statistical significance for all analyses.
Results
Since there were only 8 CDR 1 subjects in the present cohort, for comparative purposes we combined the CDR 1 (mild DAT, n=8) group with the CDR 0.5 group (very mild DAT, n=21) to make a CDR>0 group (very mild/mild DAT, n=29). We then investigated whether the two clinical groups (non-demented, CDR 0 vs. very mild/mild DAT, CDR>0) differed according to the various demographic and biomarker variables. Comparisons between these groups are shown in Table 1. The groups differed significantly with regard to age (slightly older in the CDR>0 group), education (less in the CDR>0 group), and MMSE scores (lower in the CDR>0 group). Subjects with very mild/mild DAT also exhibited the typical AD fluid biomarker phenotype characterized by significantly lower mean levels of CSF Aβ42, higher CSF tau, ptau181 and the tau(s)/Aβ42 ratios, 8-11, 13, 14 with no change in mean levels of CSF Aβ40 or plasma Aβ40 or Aβ42. The very mild/mild DAT group also exhibited significantly reduced whole brain (nWBV) and hippocampal (HC) volumes, as has been reported previously.6, 27
Table 1.
Subject Demographic Characteristics
| Characteristic | CDR 0 | CDR > 0a | P value |
|---|---|---|---|
| N | 69 | 29 | |
| Age at LP, mean (SD), y | 71.41 (8.62) | 75.1 (5.0) | 0.0337* |
| Gender, F/M (% F) | 52/17 (75%) | 17/12 (59%) | 0.6092 |
| APOE genotype, % ε4+ | 41% | 59% | 0.4598 |
| Education, mean (SD), y | 15.7 (3.0) | 14.0 (3.1) | 0.0128* |
| MMSE score (range 0-30), mean (SD) | 29.32 (0.98) | 25.24 (4.15) | <0.0001* |
| nWBV, mean (SD), fractional ICV | 0.765 (0.038) | 0.728 (0.033) | <0.0001* |
| HC, mean (SD), mm3b | 7084 (793) | 6009 (1236) | <0.0001 |
| CSF Aβ40, mean (SD), pg/mL | 10524 (3837) | 10613 (3787) | 0.9164 |
| CSF Aβ42, mean (SD), pg/mL | 572 (208) | 472 (186) | 0.0275* |
| CSF tau, mean (SD), pg/mL | 334 (180) | 534 (304) | <0.0001* |
| CSF ptau181, mean (SD), pg/mL | 61 (27) | 82 (46) | 0.0059* |
| CSF tau/Aβ42, mean (SD) | 0.684 (0.559) | 1.363 (1.176) | <0.0002* |
| CSF ptau181/Aβ42, mean (SD) | 0.124 (0.087) | 0.211 (0.197) | 0.0030* |
| Plasma Aβ40, mean (SD), pg/mLc | 220 (113) | 194 (81) | 0.2942 |
| Plasma Aβ42, mean (SD), pg/mLd | 64 (120) | 40 (44) | 0.3137 |
CDR 0.5, n=21; CDR 1, n=8
CDR 0, n=58; CDR > 0, n=28
CDR 0, n=57; CDR > 0, n=27
CDR 0, n=57; CDR > 0, n=27
Abbreviations: APOE, apolipoprotein E; CDR, clinical dementia rating; HC, hippocampal complex (combined left and right hippocampal formation); ICV, intracranial volume; LP, lumbar puncture; N, number; nWBV, normalized whole brain volume; v, volume; y, years;
statistically significant
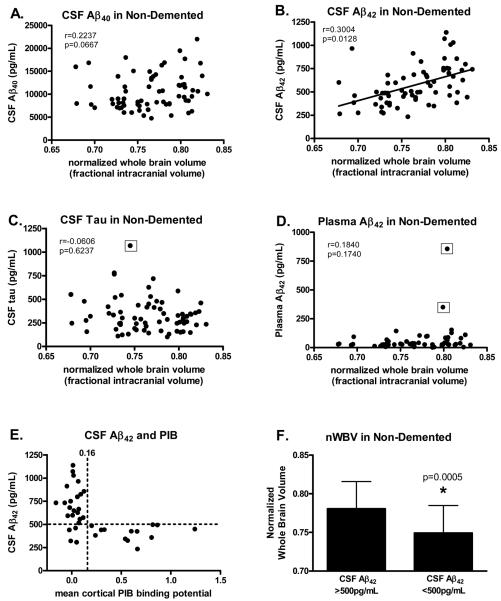
In order to better understand the relationship between the structural and fluid biomarker measures in cognitively normal elderly, we next compared the CSF and plasma measures with nWBV in non-demented individuals (Figure 1). As chronological age was associated with fluid and imaging measures, correlations were adjusted for age in all analyses. In this non-demented group, brain volume was not associated with levels of CSF Aβ40 (Fig. 1A), tau (Fig. 1C) or ptau181 (r=−0.0020, p=0.987), nor with plasma Aβ40 (r=−0.0390, p=0.776) or Aβ42 (Fig. 1D), even when the five outliers (CSF tau=1068 pg/mL, plasma Aβ42=351 and 856 pg/mL, and plasma Aβ40=518 and 599 pg/mL) were omitted from the analysis. However, nWBV was significantly correlated with CSF Aβ42 (Fig. 1B); non-demented individuals with low CSF Aβ42 had smaller brain volumes than those with higher CSF Aβ42. None of the fluid measures correlated with hippocampal volume in the non-demented cohort (Table 2).
Figure 1.
Scatterplots showing the relationship between the various fluid and imaging measures in non-demented (CDR 0) subjects (n=69). Normalized whole brain volume (nWBV) in non-demented subjects is not correlated with levels of (A) CSF Aβ40, (C) CSF tau (even when the outlier is omitted, r=−0.0280, p=0.8210), or (D) plasma Aβ42 (even when the two outliers are omitted, r=0.0150, p=0.9170), but is positively correlated with (B) CSF Aβ42. E) In the subset (n=37) of subjects in this study who underwent in vivo amyloid imaging with Pittsburgh Compound B (PIB), all subjects with cortical amyloid (PIB-positive, mean cortical PIB binding potential ≥0.16) had low levels of CSF Aβ42 (<500 pg/mL) whereas the majority (83%) of PIB-negative subjects (mean cortical PIB binding potential <0.16) had high CSF Aβ42 (>500 pg/mL). F) As a group, non-demented subjects with CSF Aβ42 <500 pg/mL had significantly smaller brain volumes than subjects with CSF Aβ42 ≥500 pg/mL. CSF tau and plasma Aβ42 values surrounded by the open squares in panels C and D, respectively, indicate statistical outliers as defined by the Mahalanobis Distance and Cook's Distance methods.33
Table 2.
Correlations Between HC Imaging and Fluid Measures
| CSF tau | CSF ptau181 | CSF Aβ40 | CSF Aβ42 | Plasma Aβ40 | Plasma Aβ42 | ||
|---|---|---|---|---|---|---|---|
| CDR 0 | Pearson r | −0.2180 | −0.1330 | 0.0330 | 0.0430 | 0.2260 | 0.1380 |
| p-value | 0.1030 | 0.3250 | 0.8100 | 0.7490 | 0.1150 | 0.3390 | |
| df | 55 | 55 | 55 | 55 | 48 | 48 | |
| CDR >0 | Pearson r | −0.3170 | −0.2470 | −0.0040 | −0.1540 | 0.0430 | 0.0610 |
| p-value | 0.1070 | 0.2140 | 0.9850 | 0.4420 | 0.8370 | 0.7710 | |
| df | 25 | 25 | 24 | 25 | 23 | 23 |
Correlations were controlled for age. Abbreviations: df, degrees of freedom; HC, hippocampal complex (combined left and right hippocampal formation).
We previously reported that low CSF Aβ42 was highly sensitive and specific for identifying individuals with cortical amyloid as assessed by PET PIB.12, 34 In particular, all individuals who were PIB-positive had a CSF Aβ42 <500 pg/mL (100% sensitivity) and the majority of individuals who were PIB-negative had a CSF Aβ42 >500 pg/mL (84% specificity). This same pattern was observed in the subset of individuals in the present cohort who had undergone PET PIB (n=37); all PIB-positive individuals had CSF Aβ42 values below the cut-off of 500 pg/mL (13/13, 100% sensitivity) and most PIB-negative individuals had CSF Aβ42 values ≥500 (20/24; 83% specificity) (Fig. 1E). Therefore, using the CSF Aβ42 level as a surrogate for the presence of brain amyloid, we next compared whole brain volumes in CDR 0 subjects in the present cohort whose CSF Aβ42 was below or above this 500 pg/mL cut-off. Non-demented individuals with CSF Aβ42 <500 pg/mL (likely indicative of brain amyloid) exhibited significantly smaller brain volumes (p=0.0005) on average than those with CSF Aβ42 ≥500 pg/mL (Fig. 1F). The mean hippocampal volume was also smaller in CDR 0 subjects with CSF Aβ42 <500 pg/mL (mean=6793.59, SD=786.46, n=26) compared to those with CSF Aβ42 values ≥500 pg/mL (mean=7338.48, SD=720.09, n=32) (p=0.008). These observations strongly suggest that alterations in Aβ42 metabolism linked with Aβ aggregation are associated with structural change (i.e., brain atrophy) prior to the ability to detect any cognitive impairment.
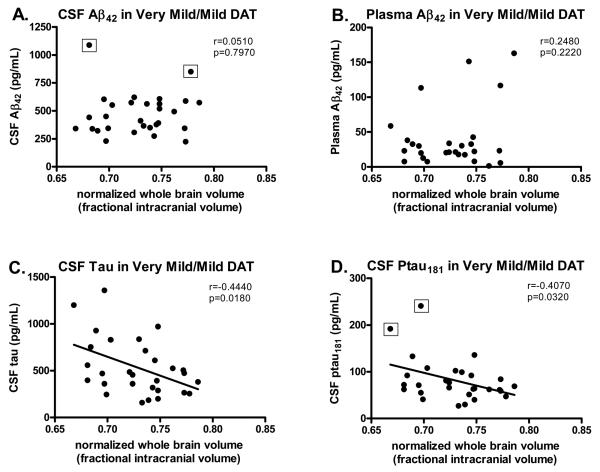
Different results were observed in individuals with very mild/mild DAT. In contrast to the non-demented group, nWBV in subjects with very mild/mild DAT was not associated with levels of CSF Aβ42 (Fig. 2A), nor with levels of CSF Aβ40 (r=0.0830, p=0.6820), or plasma Aβ40 (r=0.1240, p=0.5470) or Aβ42 (Fig. 2B). Instead, whole brain volume was inversely correlated with levels of CSF tau (Fig. 2C) and ptau181 (Fig. 2D). The same results were obtained even when the five outliers (CSF Aβ42= 849 and 1088 pg/mL, CSF ptau181=181 and 241 pg/mL, plasma Aβ40=563 pg/mL) were omitted from the analysis. These very mildly and mildly impaired individuals with high CSF tau and ptau181 had smaller brain volumes than those with lower CSF tau and ptau181. Similar results were obtained when we restricted our analyses to just CDR 0.5 cases (n=21); subjects with very mild DAT showed correlations between nWBV and CSF tau (r=−0.4810, p=0.0320) and ptau181 (r=−0.4340, p=0.0560, approaching statistical significance) as in the combined CDR 0.5/CDR 1 group. Interestingly, nWBV was now also correlated with CSF Aβ42 (r=0.4930, p=0.0270) in the CDR 0.5 group, similar to what we observed in the CDR 0 group. There were too few subjects in the CDR 1 group (n=8) to make meaningful statements about this group on its own. None of the fluid measures correlated with hippocampal volume in the very mildly/mildly demented cohort (Table 2).
Figure 2.
Scatterplots showing the relationship between the various fluid and imaging measures in subjects with very mild/mild dementia of the Alzheimer type (DAT; CDR >0) (n=29). Normalized whole brain volume (nWBV) in subjects with early stage DAT is not correlated with levels of Aβ42 in (A) CSF (even when the two outliers are omitted, r=0.1920, p=0.3510) or (B) plasma, but is inversely correlated with (C) CSF tau and (D) CSF ptau181 (approaching significance even when the outlier is omitted, r=−0.3780, p=0.0520). CSF Aβ42 and ptau181 values surrounded by the open squares in panels A and D, respectively, indicate statistical outliers as defined by the Mahalanobis Distance and Cook's Distance methods. 33
Discussion
As disease-modifying therapies for AD are being developed, there is great need to identify biomarkers that will serve as surrogates of underlying disease pathology. In the eventual clinical setting, such biomarkers might be used to improve the accuracy of clinical diagnosis and to track disease progression. As an immediate application, biomarkers may be useful in the design and evaluation of clinical trials; for example, to assess the effect of a therapy on its intended target in early phase studies, to optimize patient enrollment in prevention trials (to increase homogeneity, decrease sample size and shorten trial duration), and to track disease progression (by providing quantifiable, pathology-related endpoints). Results of the present study suggest that alterations in Aβ42 metabolism, likely reflecting Aβ aggregation in the brain, are associated with brain atrophy in the “preclinical” phase of AD (as well as the very early CDR 0.5 stage), whereas alterations in tau and ptau181 are later events in the disease that correlate with further structural damage and occur with clinical (i.e., dementia) progression. Thus, CSF Aβ42 may be considered a useful biomarker for the presence of amyloid plaques regardless of clinical status, whereas CSF tau measures may have more utility in tracking disease progression once clinical symptoms and signs have appeared.
The correlations we observed between levels of CSF tau (and ptau181), but not Aβ42, and whole brain volume in DAT are consistent with histopathologic findings of associations between atrophy measures and NFT burden or Braak NFT stage35-38 but not Aβ plaque load.37, 38 In addition, studies suggest that by the time individuals become cognitively impaired due to AD, cortical amyloid deposition is close to reaching its maximal extent.39, 40 A recent antemortem study reported a positive correlation between whole brain atrophy and cortical amyloid as detected by PET PIB in a small number of demented subjects (n=9);41 however, evaluation of the robustness of this finding awaits further study in larger subject groups. Previous results regarding the relationship between CSF measures and atrophy in DAT (or MCI) have been mixed,42-47 likely due to the small number of subjects evaluated and the differences in subject characteristics and methodologies between the studies.
To our knowledge, only one study has investigated the relationship between CSF and volumetric measures in non-demented controls, and no associations between any CSF marker and hippocampal atrophy were found in that very small cohort (n=9)42, consistent with our hippocampal analyses. It is possible that many of the CDR 0 subjects who have brain amyloid and low CSF Aβ42 still have many years before they convert to dementia . During this period, lasting possibly a decade or more, amyloid is depositing in several brain regions, including cortical regions that have recently been shown to exhibit thinning during this presymptomatic stage.48 The hippocampus proper is not a region with prominent early amyloid deposition so hippocampal volume may not be expected to strongly correlate with CSF Aβ42 in the presymptomatic stage, even though volume may be reduced in this stage. Reduction in hippocampal volume in the presymptomatic stage may relate to processes taking place in addition to amyloid deposition, especially tangle formation. Consistent with this hypothesis, we did observe a non-significant trend for an association between hippocampal volume and CSF tau (p=0.1030) in non-demented controls.
The present study is the first to characterize the relationship between CSF and volumetric measures in a large cohort (n=69) of well-characterized, cognitively normal controls. The positive correlation we observe between CSF Aβ42 and whole brain volume in this cohort may reflect the death of neurons in the preclinical stage of AD. However, cell death is not a fundamental feature of preclinical AD, 1, 49 and the lack of relationship between CSF Aβ40 and brain volume also argues against this hypothesis. Alternatively, because individuals with brain amyloid, as detected by PET PIB, have low levels of CSF Aβ42, 12, 34 the association between low CSF Aβ42 and small brain volume in this non-demented cohort may reflect underlying Aβ aggregation and deposition and consequent and/or concomitant axonal and synaptic degeneration leading to measurable atrophy in the preclinical phase. Reductions in CSF Aβ42 reflect the presence of amyloid plaques but perhaps also diffuse (non-fibrillar) plaques and/or concomitant Aβ oligomer formation, both of which could contribute to neurotoxicity (neuritic and/or cellular) and consequent volume loss, but would not be visualized with PET PIB. In support of this hypothesis, we have recently observed low CSF Aβ42 in the absence of cortical PIB binding in a subject later found to exhibit an abundance of diffuse, but not neuritic, plaques at autopsy two years after LP and PIB testing (N. Cairns, A. Fagan, M. Mintun, D. Holtzman, J. Morris, unpublished observations). Since the present data demonstrate that CSF Aβ42 and tau measures correlate with meaningful structural change in the preclinical and clinical stages of AD, respectively, and that the CSF tau/Aβ42 ratio predicts future cognitive decline in cognitively normal elders 34, 50 and individuals with MCI,51 we propose that CSF Aβ42, either alone or in combination with tau measures, may be especially useful for the selection of presymptomatic individuals with known preclinical AD pathology for enrollment in prevention trials of disease-modifying therapies. As robust changes in CSF tau (and ptau) and further volumetric loss appear to occur later in the disease process, these biomarkers may be useful for evaluating the effects of a given treatment on disease progression.
Acknowledgments
We gratefully acknowledge the contributions of Sushyla Sathyan, MS, Catherine C. Roe, PhD, our lumbar puncture physicians, and the Clinical, Psychometrics and Biostatistics Cores of the Washington University Alzheimer Disease Research Center.
Funding Sources: This study was supported by the NIH (P30-NS057105, D.M.H.), the National Institute on Aging (P01-AG03991, J.C.M.; P50-AG05681, J.C.M.; P01-AG026276, J.C.M.), National Institute of Neurological Disorder and Stroke (P30 NS048056, M.A.M.), a grant from the Dana Foundation (J.C.M.), and a generous gift to J.C.M. from Charles F. and Joanne Knight. This publication was made possible by grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
References
- 1.Price J, Ko A, Wade M, et al. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer's disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 2.Gomez-Isla T, Price JL, McKeel DW, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris J, Price J. Pathologic correlates of nondemented aging, mild cognitive impairment, and early stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 4.Hulette CM, Welsh-Bohmer KA, Murray MG, et al. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Markesbery W, Schmitt F, Kryscio R, et al. Neuropathologic substrate of Mild Cognitive Impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 6.Kantarci K, Jack CR., Jr Quantitative magnetic resonance techniques as surrogate markers of Alzheimer's disease. NeuroRx. 2004;1:196–205. doi: 10.1602/neurorx.1.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scahill RI, Fox NC. Longitudinal imaging in dementia. Br J Radiol. 2007;80(Spec No 2):S92–98. doi: 10.1259/bjr/78981552. [DOI] [PubMed] [Google Scholar]
- 8.Sunderland T, Linker G, Mirza N, et al. Decreased β-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer's disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 9.Galasko D, Chang L, Motter R, et al. High cerebrospinal fluid tau and low amyloid β42 levels in the clinical diagnosis of Alzheimer's disease and relation to apolipoprotein E genotype. Arch. Neurol. 1998;55:937–945. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- 10.Kanai M, Matsubara E, Isoe K, et al. Longitudinal study of cerebrospinal fluid levels of tau, Aβ1-40, and Aβ1-42(43) in Alzheimer's disease: A study in Japan. Ann. Neurol. 1998;44:17–26. doi: 10.1002/ana.410440108. [DOI] [PubMed] [Google Scholar]
- 11.Motter R, Vigo-Pelfrey C, Kholodenko D, et al. Reduction of β-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann. Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 12.Fagan A, Mintun M, Mach R, et al. Inverse relation between in vivo amyloid imaging load and CSF Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen N, Vanmechelen E, Van de Voorde A, et al. Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer's disease: A community based follow up study. J. Neurol. Neurosurg. Psych. 1998;64:298–305. doi: 10.1136/jnnp.64.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arai H, Terajima M, Miura M, et al. Tau in cerebrospinal fluid: A potential diagnostic marker in Alzheimer's disease. Ann. Neurol. 1995;38:649–652. doi: 10.1002/ana.410380414. [DOI] [PubMed] [Google Scholar]
- 15.Tapiola T, Overmyer M, Lehtovirta M, et al. The level of cerebrospinal fluid tau correlates with neurofibrillary tangles in Alzheimer's disease. Neuroreport. 1997;8:3961–3963. doi: 10.1097/00001756-199712220-00022. [DOI] [PubMed] [Google Scholar]
- 16.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 17.Engelborghs S, Sleegers K, Cras P, et al. No association of CSF biomarkers with APOEepsilon4, plaque and tangle burden in definite Alzheimer's disease. Brain. 2007;130:2320–2326. doi: 10.1093/brain/awm136. [DOI] [PubMed] [Google Scholar]
- 18.Morris J, McKeel D, Fulling K, et al. Validation of clinical diagnostic criteria for Alzheimer's disease. Ann Neurol. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- 19.Berg L, McKeel DW, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease - Relation of histologic markers to dementia severity, age, sex, and apoE genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. The Clinical Dementia Rating (CDR). Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Storandt M, Grant E, Miller J, Morris J. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 23.Cirrito J, May P, O'Dell M, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mugler JP, 3rd, Brookeman JR. Rapid three-dimensional T1-weighted MR imaging with the MP-RAGE sequence. J Magn Reson Imaging. 1991;1:561–567. doi: 10.1002/jmri.1880010509. [DOI] [PubMed] [Google Scholar]
- 25.Buckner R, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Head D, Snyder A, Girton L, et al. Frontal-hippocampal double dissociation between normal aging and Alzheimer's Disease. Cereb. Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- 27.Fotenos AF, Snyder AZ, Girton LE, et al. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- 28.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; Stuttgart, Germany: 1988. [Google Scholar]
- 29.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imag. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischl B, Salat D, Busa E, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 32.Klunk W, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J, Cohen P, West S, Aiken L. Applied Multiple Regression/Correlations Analysis for the Behavioral Sciences. Third Edition Lawrence Erlbaum Associates; Mahwah, New Jersey: 2003. [Google Scholar]
- 34.Fagan A, Roe C, Xiong C, et al. Cerebrospinal fluid tau/Aβ42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 35.Jack CR, Jr., Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagy Z, Jobst KA, Esiri MM, et al. Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer's disease: Clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia. 1996;7:76–81. doi: 10.1159/000106857. [DOI] [PubMed] [Google Scholar]
- 37.Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003;61:487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- 38.Josephs KA, Whitwell JL, Ahmed Z, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008;63:204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Ingelsson M, Fukumoto H, Newell KL, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 41.Archer H, Edison P, Brooks D, et al. Amyloid load and cerebral atrophy in Alzheimer's disease: An 11C-PIB positron emission tomography study. Ann Neurol. 2006;60:145–147. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- 42.de Leon MJ, DeSanti S, Zinkowski R, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Hampel H, Burger K, Pruessner J, et al. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol. 2005;62:770–773. doi: 10.1001/archneur.62.5.770. [DOI] [PubMed] [Google Scholar]
- 44.Wahlund LO, Blennow K. Cerebrospinal fluid biomarkers for disease stage and intensity in cognitively impaired patients. Neurosci Lett. 2003;339:99–102. doi: 10.1016/s0304-3940(02)01483-0. [DOI] [PubMed] [Google Scholar]
- 45.Schonknecht P, Pantel J, Hartmann T, et al. Cerebrospinal fluid tau levels in Alzheimer's disease are elevated when compared with vascular dementia but do not correlate with measures of cerebral atrophy. Psychiatry Res. 2003;120:231–238. doi: 10.1016/s0165-1781(03)00197-5. [DOI] [PubMed] [Google Scholar]
- 46.Schoonenboom NS, van der Flier WM, Blankenstein MA, et al. CSF and MRI markers independently contribute to the diagnosis of Alzheimer's disease. Neurobiol Aging. 2008;29:669–675. doi: 10.1016/j.neurobiolaging.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Schroder J, Pantel J, Ida N, et al. Cerebral changes and cerebrospinal fluid beta-amyloid in Alzheimer's disease: A study with quantitative magnetic resonance imaging. Mol Psychiatry. 1997;2:505–507. doi: 10.1038/sj.mp.4000313. [DOI] [PubMed] [Google Scholar]
- 48.Dickerson B, Bakkour A, Salat D, et al. The cortical signature of Alzheimer's Disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2008 Jul 16; doi: 10.1093/cercor/bhn113. [Epub ahead of print] PMID: 18632739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West MJ, Kawas CH, Stewart WF, et al. Hippocampal neurons in pre-clinical Alzheimer's disease. Neurobiol Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Li G, Sokal I, Quinn J, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: A follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 51.Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]