Abstract
Reservosomes are the endpoint of the endocytic pathway in Trypanosoma cruzi epimastigotes. These organelles have the particular ability to concentrate proteins and lipids obtained from medium together with the main proteolytic enzymes originated from the secretory pathway, being at the same time a storage organelle and the main site of protein degradation. Subcellular proteomics have been extensively used for profiling organelles in different cell types. Here, we combine cell fractionation and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis to identify reservosome-resident proteins. Starting from a purified reservosome fraction, we established a protocol to isolate reservosome membranes. Transmission electron microscopy was applied to confirm the purity of the fractions. To achieve a better coverage of identified proteins we analyzed the fractions separately and combined the results. LC-MS/MS analysis identified in total 709 T. cruzi-specific proteins; of these, 456 had predicted function and 253 were classified as hypothetical proteins. We could confirm the presence of most of the proteins validated by previous work and identify new proteins from different classes such as enzymes, proton pumps, transport proteins and others. The definition of the reservosome protein profile is a good tool to assess their molecular signature, identify molecular markers, and understand their relationship with different organelles.
Keywords: mass spectrometry, proteomic analysis, reservosomes, Trypanosoma cruzi
INTRODUCTION
The protozoan parasite Trypanosoma cruzi, the etiologic agent of Chagas’ disease, has a complex life cycle, which includes three different developmental stages [1]. Like other eukaryotic cells, T. cruzi needs to ingest macromolecules by endocytosis, which constitutes one of the main challenges during its life cycle. In T. cruzi, significant nutrient uptake takes place only in epimastigotes and is low or absent in both trypomastigotes and amastigotes [2]. Epimastigotes can ingest exogenous macromolecules by the flagellar pocket and mainly by the cytostome [3, 4]. From the entry sites, endocytic vesicles bud off and fuse with branched tubular-vesicular early endosomes. Subsequently, the macromolecules are delivered to T. cruzi storage organelles, the reservosomes [4].
Reservosomes are the main site for the storage of ingested proteins and lipids, as well as of secretory proteins synthesized by the protozoan [2, 5]. Based on characteristics found in mammalian lysosome-related organelles (LROs) [6], we recently proposed that T. cruzi reservosomes are members of the LRO group [7]. They have been considered as pre-lysosomal organelles due to the absence of bona fide lysosomal molecular markers and to pH evaluation at 6.0 [8]. Reservosomes are round organelles (average diameter of 400 – 600 nm) surrounded by a membrane and mainly localized at the posterior region of epimastigotes. The core of the organelles is composed of an electrondense protein matrix and electronlucent lipid inclusions [2]. Recently, we have demonstrated the presence of inner membranes and described unusual rod-shaped bodies, which are presumably lipids [9]. We have also determined the presence and distribution of transmembrane proteins in the organelle membranes by freeze-fracture. Furthermore, we have described organelles that share typical reservosome properties in trypomastigotes and amastigotes, also characterized as LROs [7]. This finding has inferred that the reservosomes may be a potential chemotherapy target against Chagas’ disease.
Few proteins have been identified and characterized in reservosomes. Among them, there are two lysosomal proteases, cruzipain [8, 10, 11] and serine carboxipeptidase [7, 12]. Due to the concentration of these proteases, reservosomes have been hypothesized to be the main site of protein degradation. Chagasin, a tight-binding natural inhibitor of cruzipain, was also localized in the reservosomes, suggesting modulation of proteolytic activity inside the organelle [13]. In addition, two P-type H+-ATPase isoforms (TcHA1 and TcHA2), usually found in the plasma membrane of plant and yeast cells [14], were shown to be responsible for generating the acidic character of reservosomes [15]. Unexpectedly, TcRab11, a homologue of mammalian rab11, generally found in recycling endosomes [16] was also suggested to be localized in the reservosomes [17].
Even though some reservosome proteins have been identified, a molecular marker for this organelle has not yet been characterized. Seeking a better understanding of the function of this organelle and the determination of possible molecular markers, we performed a comprehensive subcellular proteomic analysis of the purified epimastigote reservosome fraction by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
MATERIALS AND METHODS
Parasites
T. cruzi epimastigotes from the Dm28c clone were cultivated for 4 days at 28°C in liver infusion tryptose (LIT) medium [18] supplemented with 10% fetal calf serum.
Reservosome fractionation
The reservosome fraction was obtained according to [11]. Briefly, epimastigotes in TMS buffer (20 mM Tris-HCl, pH 7.2, 2 mM MgCl2, 250 mM sucrose) were disrupted by sonication on ice, in an ultrasonic apparatus (Sigma, GEX 600 Model) using a standard probe. After centrifugation at 2,450g for 10 min, the supernatant was mixed with an equal volume of 2.3 M sucrose in TMS buffer, deposited into a Beckman SW28 centrifuge tube, overlaid with 10 mL of 1.2 M, 10 mL of 1.0 M and 5 mL of 0.8 M sucrose (in TMS buffer) and centrifuged at 97,000 g for 150 min. The interface 0.8 M/1.0 M was collected, diluted in TMS buffer and centrifuged at 120,000g for 30 min. The pellet, named B1 reservosome fraction, was resuspended in TMS, characterized by transmission electron microscopy (TEM) and submitted to LC-MS/MS.
Isolation of reservosome membranes
Isolated reservosome fraction (B1) was disrupted by 5 cycles of freezing in liquid nitrogen and thawing in a water bath at 37°C. Subsequently, the sample was extracted with 200 mM sodium carbonate, pH 11.5, at 4°C for 30 minutes, under mild agitation. The membrane fraction (B1M) was obtained by centrifugation at 120,000g for 2 h at 4°C, characterized by TEM and submitted to LC-MS/MS.
Transmission electron microscopy
Isolated fractions were processed according to [11] and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and examined in a Jeol 1200EX electron microscope.
Protein digestion and peptide fractionation
Total proteins from B1 and B1M fractions were digested with trypsin (TU strategy) or with trypsin and endoproteinase Glu-C (TG strategy). For the TU strategy, the proteins were digested as described [19]. For the TG strategy, samples were digested with the same procedure as for the TU strategy but, after the trypsin digestion, two micrograms sequencing-grade endoproteinase Glu-C were added, and the reaction was allowed to proceed for 24 h at 37°C. The reaction was terminated by adding 0.05% trifluoroacetic acid (TFA), and the digested proteins were desalted using reverse-phase ZipTip columns (POROS R2 50, Applied Biosystems, Foster City, CA) as described [20]. The resulting peptides were fractionated in a strong cation-exchange (SCX) SCX ZipTip (POROS HS 50, Applied Biosystems) by eluting with increasing concentrations of NaCl (25, 50, 100, 200, and 500 mM) [21]. The peptide fractions were dried to remove the acetonitrile (ACN) and desalted in reverse-phase ZipTips.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
The LC-MS/MS analysis was performed in a electrospray ionization-linear ion-trap mass spectrometer (ESI-LIT-MS) equipped with a nanospray source (LTQXL, Thermo Fisher Scientific, San Jose, CA). Each SCX fraction was resuspended in 20 μl 0.05% TFA, and 8-μl aliquot from each sample was loaded onto a C18 trap column (0.25 μL C18, OPTI-PAK. Oregon City, OR). Tryptic peptide separation was performed on a capillary reverse-phase column (Acclaim, 3-μm C18, 75 μm × 25 cm, LC Packings/Waters, Amsterdam, The Netherlands) connected to a nanoHPLC system (nanoLC 1D plus, Eksigent, Dublin, CA). The peptides were eluted using a linear gradient from 0 to 40% ACN in 0.1% FA for 200 min and directly analyzed in the ESI-LIT-MS. MS spectra were collected in centroid mode in the 400 to 1700 m/z range, and the five most abundant ions of each spectrum were subjected twice to collision-induced dissociation (CID) using 35% normalized collision energy, before dynamic exclusion for 120 sec.
Database search and peptide identification/validation
MS/MS spectra from peptides with 600–3500 Da and at least 100 counts and 15 fragments were converted into DTA files using Bioworks v.3.3.1 (Thermo Scientific). The DTA files were submitted to a database search using TurboSequest [22] (available in Bioworks) against a database composed of T. cruzi, bovine, human keratin and porcine trypsin sequences (downloaded March 17th, 2008 from GenBank), in the forward and reverse orientations, forming a dataset of 191,762 sequences. Database search parameters included: i) trypsin or endoproteinase Glu-C cleavage in both peptide termini with one allowed missed cleavage site; ii) carbamidomethylation of cysteine residues as a fixed modification; iii) oxidation of methionine residues as a variable modification; and iv) 2.0 Da and 1.0 Da for peptide and fragment mass tolerances, respectively. The following filters were applied in Bioworks: DCn ≥ 0.085; protein probability ≤1E-3; consensus score; and Xcorr ≥1.5, 2.2, and 2.7 for singly-, doubly-, and triply-charged peptides, respectively. The false-positive rate (FPR) was calculated as previously described [21].
Bioinformatic analysis of identified protein sequences
All valid T. cruzi-specific protein sequences were compared to sequences deposited in the GenBank using the Blast tool (http://www.ncbi.nlm.nih.gov/BLAST/). Gene ontology (GO) annotation was assigned by similarity searches against the Swiss-Prot and TrEMBL databases (invertebrate taxonomy, which includes all eukaryotic entries, except those from vertebrates, fungi, and plants) using GOblet tool [23], which is available online at http://goblet.molgen.mpg.de. Only GOs from proteins with evalues ≤1e-10 for database search were accepted. This analysis was performed on October 3rd, 2008. The sequences were also analyzed to determine potential transmembrane domains using the TMHMM server v2.0 software (http://www.cbs.dtu.dk/services/TMHMM-2.0/).
RESULTS
Ultrastructure of isolated reservosomes and their isolated membranes
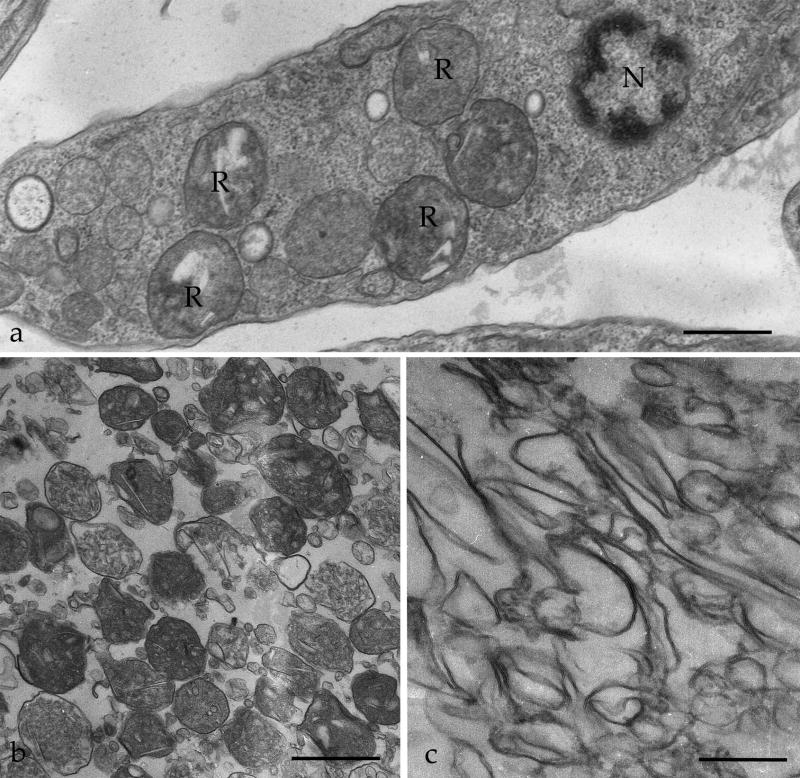
In order to identify reservosome-resident proteins, we initially purified reservosomes to obtain the intact organelles (B1). Subsequently, we established a new protocol to isolate reservosome membranes. Isolated organelles were disrupted by freezing and thawing, the associated proteins were removed with sodium carbonate buffer, and the membrane (B1M) fraction was recovered by centrifugation. To assess the purity and preservation of isolated fractions, B1 and B1M fractions were analyzed by TEM (Fig. 1b,c). The B1 fraction displayed intact reservosomes with a characteristic morphology observed for in situ reservosomes (Fig. 1a). Some disrupted organelles were also visualized, as previously reported by Cunha-e-Silva and co-workers [11], probably due to the fractionation procedures. While examining several randomly collected ultrathin sections, the presence of other structures such as mitochondria, kinetoplast, endoplasmic reticulum cisternae, glycosomes, acidocalcisomes, nuclei or flagella were not detected, thus indicating that the preparation was virtually free of these contaminating organelles (data not shown). The B1M fraction showed highly purified total reservosome membranes (Fig. 1c) because no intact organelle was detected. The B1 and B1M fractions were then separately subjected to LC-MS/MS analysis.
Figure 1.
Transmission electron microscopy of T. cruzi reservosomes. (a) Ultrathin section of an epimastigote showing reservosomes (R) in situ, with their typical morphology and position, between nucleus (N) and posterior end of the cell. (b) Purified reservosome fraction (B1). (c) subcellular fraction containing reservosome membranes (B1M). Bars represent 0.3 μm (a) and 1 μm (b,c).
Identification of reservosome proteins by LC-MS/MS
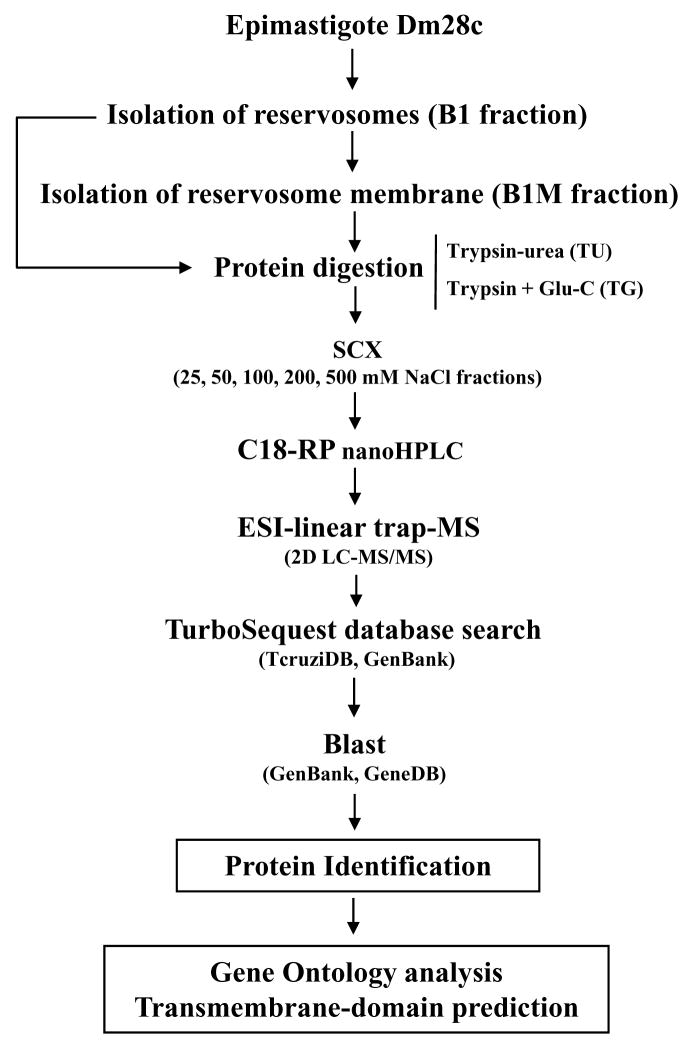
B1 and B1M fractions were digested with two distinct strategies: i) trypsin, with urea as a denaturing agent (TU), and ii) double digestion with trypsin and endoproteinase Glu-C (TG). After digestion, resulting peptides were fractionated by SCX chromatography, analyzed LC-MS/MS, and submitted to search through a database containing forward and reverse sequences of T. cruzi, bovine, keratin and porcine trypsin sequences. The FPR was determined as described in the Materials and Methods. Our data were validated with a FRP = 2.41%. Figure 2 shows a schematic summary of the approach used in our analyses.
Figure 2.
Schematic representation of the strategy used for the identification of proteins of the reservosomes of T. cruzi epimastigotes.
In total, LC-MS/MS data allowed the unambiguous identification of 869 proteins, with 709 being T. cruzi-specific proteins and 160 as proteins from the culture medium and contaminants from the sample preparation (see complete list in Supplementary Table 1). In the case of T. cruzi, 456 proteins were assigned as having a predicted function, and 253 were assigned as having an unknown function or being hypothetical proteins. Of these proteins, cruzipain [8, 10, 11], serine carboxipeptidase [7, 12], ABC transporters [24], and a protein tyrosine phosphatase [25] have already been previously described to be present in reservosomes. Our proteomic analysis of reservosomes allowed the identification of a novel isoform of P-type H+-ATPase, TcHA3. We also confirmed the presence of TcHA1 and TcHA2, the other proton pump described in reservosomes [15]. We could identify several additional hydrolases, such as cysteine peptidases, α-mannosidases, acid phosphatase, acid phosphatase 2, calpain cysteine peptidases, lipase, and serine carboxypeptidase S28. In addition, a calcium translocation pump and calcium-binding proteins were found. A chloride channel protein, also identified in mammalian endosomes [26], was detected. Members of the ABC family, which act in lipid metabolism [27], also appeared, as well as a P-glycoprotein. Surprisingly, a multidrug resistance (MDR) protein was identified. An interesting finding was the presence of p67, a glycoprotein molecular marker of T. brucei lysosome [28]. In addition, we detected a glycoprotein, which thus far has an undefined function, which is also present in the Golgi complex and lysosomes.
In our analysis, the reservosomes exhibited endosomal integral membrane proteins and proteins involved in vesicular traffic, such as COP-coated vesicle membrane proteins erv25 and gp25L, mu-adaptin 1, huntingtin-interacting protein (HIP), vesicle-associated membrane proteins (VAMPs), and the GTP-binding proteins Rab1, Rab2a, Rab7, and Rab18.
Also, our reservosome proteomic data showed proteins related to lipid metabolism, including sterol 24-c-methyltransferase, fatty acyl CoA synthetase, phospholipid-translocating ATPase, C-8 sterol isomerase, fatty acyl CoA syntetase 1, and phosphatidylcholine:ceramide cholinephosphotransferase, which suggests that these organelles play a role in lipid synthesis in addition to storing lipids ingested by the endocytic pathway [11]. We also found enzymes that act as regulators of signal transduction pathways in most eukaryotic cell types, e. g., protein tyrosine phosphatase, regulatory subunit of protein kinase A, casein kinase, MCAK-like kinesin, activated protein kinase C receptor, protein kinase, and serine/threonine protein kinase.
Transmembrane proteins from the plasma membrane, such as dispersed gene family protein 1 (DGF-1), ferric reductase, hexose transporter, folate/pteridine transporter and GPI-anchored p63, were identified in the reservosome proteome, confirming a membrane-trafficking relationship between the plasma membrane and reservosomes. We also identified cytoskeleton components essential for vesicular traffic, among other functions, such as alpha and beta tubulin, cytoskeleton-associated protein CAP5.5, actin, and cofilin/actin depolymerizing factor.
Other proteins that were detected can be characterized as non-reservosomal, including mitochondrial, glycosomal or flagellar proteins. This suggests a functional relationship between the organelles, a recycling process of these proteins in the reservosomes, or a possible contamination, commonly observed in subcellular fractionation protocols. However, only very few of these presumed contaminants are transmembrane proteins, thus suggesting a low level of contamination with other organelle membranes. Predictably, we could also identify bovine serum proteins, which are supposed to be derived from the culture medium and likely reached the reservosomes by an endocytic process.
Gene ontology analysis
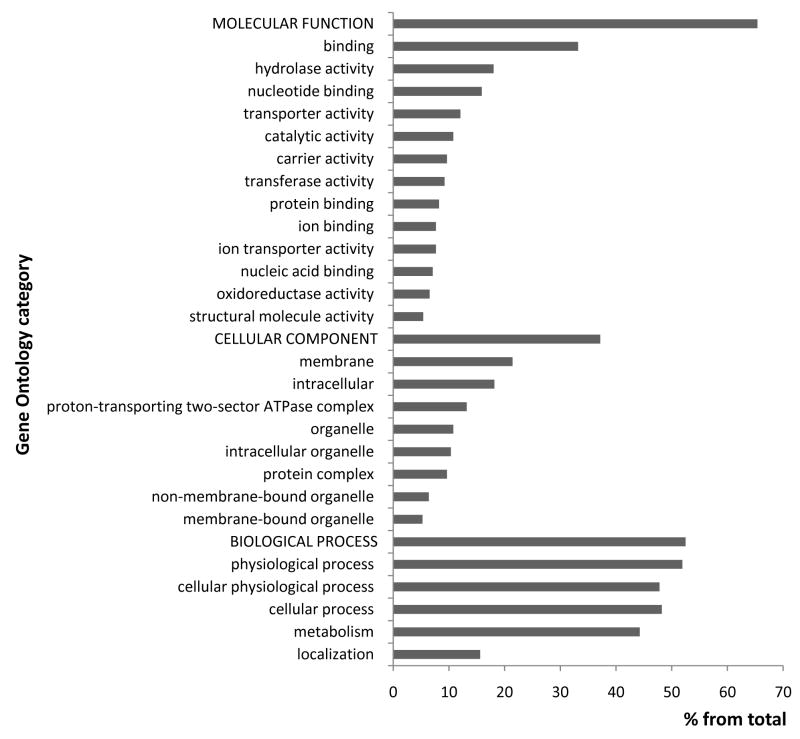
In order to understand their function and to identify processes related to reservosomes, we performed gene ontology (GO) annotation of all proteins identified in the proteome analysis (Fig. 3 and Supplemental material 1). Catalytic activity was prevalent in the reservosome molecular function. Reservosomes are also rich in proteins involved in binding and hydrolase activity. Proteins found in the reservosome proteome are involved in transporter, carrier and ion-transporter activities, each representing about 12% of the proteins identified. Our analysis found significant relationships to nucleotide-binding, transferase activity, ion-binding, and oxidoreductase activity. A large number of reservosomal proteins were related to metabolism as well as to physiological and cellular processes. Membrane localization of the proteins identified by LC-MS/MS analysis was remarkable. Reservosome proteins were also predicted to be localized in the proton-transporting complex.
Figure 3.
Functional classification of T. cruzi reservosome proteins by gene ontology. The gene ontology prediction was performed using GOblet and the most representative categories (≥3% from the total sequences) were plotted in the graph. For the complete list see Supplemental Table 1.
DISCUSSION
Subcellular proteomics have been extensively used to identify the molecular composition of several cytoplasmic organelles and has been considered an effective approach to understand cellular processes and integrated cell function. It takes advantage of the subcellular fractionation strategies, which are based on sequential and/or density-gradient centrifugation, allowing the separation of different population of organelles based on their size, density and charge, in combination with mass spectrometry analysis [29]. This strategy greatly reduces sample complexity in comparison with whole cell proteomic analysis.
Sub-cellular fractionation methodologies have been widely employed in protozoan parasites [30] in order to carry out parasite-specific organelles characterization and understand their role in parasite cell biology. These organelles have particular interest since their absence in higher eukaryotes may comprise a potential chemotherapeutic target against human and animal parasitism. By centrifugation in a sucrose gradient, our group reported an accurate and reproducible protocol to obtain a highly purified T. cruzi epimastigote reservosome fraction [11]. Afterwards, the same protocol was successfully used to measure proton transport in isolated reservosomes [15]. The purity of the fraction was assessed using three strategies: (i) enzymatic assays by means of enzymes recognized as markers of cell organelles in mammal cells and protozoa such as acid phosphatase (lysosomes), hexokinase (glycosomes), vacuolar H+-pyrophosphatase (acidocalcisomes) and succinate-cytochrome c reductase (mitochondria); (ii) Western blot, using antibodies against cruzipain (reservosomes) and lipopeptidephosphoglycan (plasma membrane) and (iii) ultrastructure analysis by transmission electron microcopy, a powerful mean for judging the efficiency of the method. In conclusion, we could essentially assure the high degree of purity and reproducibility of the reservosome fraction obtained.
Taking advantage of efficiency of the cell fractionation of reservosome [11], we isolated for the first time a highly purified reservosome fraction from Dm28c epimastigotes (Fig. 1b). In addition, to enrich the membrane protein identification and avoid the contamination of the proteomic analysis with a great number of medium-derived peptides, we developed a methodology to purify total reservosome membranes (Fig. 1c). Our morphological analysis did not allow discrimination between reservosome-surrounding membranes and membranes derived from inside the organelle. Reservosomes had been described as an organelle where few or no inner membranes were seen [2]. However, we have recently demonstrated, using different electron microscopy approaches, the presence of vesicles and planar membranes in the lumen of reservosomes [9]. Nevertheless, the composition and function of these structures need to be clarified and are objects of our studies. Intending to achieve better proteome profiling of reservosomes, we used both B1 and B1M fractions in our proteomic analysis.
Proteomic analysis of B1 and B1M fractions led to the identification of 709 T. cruzi-specific proteins. Several identified proteins are expected components for endocytic organelles, which interact and fuse with vesicles coming from the secretory pathway and/or the plasma membrane as part of the endocytic process.
We also found cytoskeleton proteins, as did other proteomic analyses of T. cruzi subcellular fractions [31]. Since the pool of unpolymerized tubulin is very small in epimastigote forms [32], this finding may represent an association of reservosomes with microtubules, which are responsible for traffic and intracellular organelle positioning.
Rab 1, Rab 2a, Rab 7, and Rab 18, small GTPases of the Rab family, which are crucial regulators of vesicle traffic in endocytic and secretory pathways [33], were another important class of proteins found in our proteomic analysis. Rab 1 and Rab 2 have been implicated in the transport from the ER to the Golgi complex [34]. Orthologues of mammalian Rab 1 and Rab 2 were characterized in T. brucei [35], and it was shown that TbRab1 and TbRab2 play a role in the early events of the secretory pathway, suggesting a conserved function. Rab 7 has been regarded as a mediator of early to late endosome transport [36], and evidence has accumulated suggesting that Rab 7 may work in the transport from late endosomes to lysosomes [37]. In Leishmania [38] and T. brucei [39], the orthologue of mammalian Rab 7 was localized in the endosomal/lysosomal system. Surprisingly, T. cruzi Rab 7 was found in high concentration in the Golgi complex by ultrastructural localization [40]. Our analysis detected TcRab 7 in the reservosome proteome, which suggests a possible role of this small GTPase in the membrane traffic between Golgi complex/reservosomes. Unexpectedly, TcRab 11, previously suggested to be localized in the reservosomes [17], was not detected in our proteomic analysis. The suggestion of TcRab11 localization in reservosomes was based exclusively on immunofluorescence images. No further confirmation by immunoelectronmicroscopy or western blot using isolated reservosome fraction was published since then. We cannot exclude that the absence of this protein in reservosome proteomic profile be a consequence of sample preparation, but this possibility does not seem to be probable, as sample processing did not affect other Rab proteins.
While mammalian Rab 18 has been involved in endocytic transport and in association with lipid droplets [41], T. brucei Rab 18 seems to function in Golgi transport [42]. The presence of Rab18 in the reservosome proteomic profile may point to its role in the mobilization of the abundant lipid storage [9, 11], similar to Rab18 function in adipocytes [41]. Undoubtedly, the presence of several Rab proteins associated with reservosomes argues for the dynamic nature of this organelle and is compatible with the nature of a LRO [7] with a secretory character, sending molecules to other cytoplasmic compartments or the extracellular medium, while simultaneously receiving molecules from their cell “progenitors” [43], the Golgi complex, and endocytic pathway [44]. Furthermore, proteins that belong to the fusion machinery, such as syntaxyn, NSF, SNAP and others whose genes were found in the T. cruzi genome, were not identified during our analysis. This virtual absence may be justified by the low level of protein expression, differences in the sequences from different strains (the T. cruzi genome project was carried out with the CL Brener strain, while we used the Dm28c clone), or protein modifications that cannot or are difficult to be identified by LC-MS/MS. We also cannot exclude the possibility of losing this sort of protein during the subcellular fractionation procedure.
We also found isoforms of ABC transporters that, in mammalian cells, work in lipid metabolism, and P-glycoprotein (Table 1). Recently, our group reported the intracellular trafficking of heme [45]. Using a specific inhibitor, cyclosporin A, it was hypothesized that a P-glycoprotein transporter is likely to be the responsible for heme transport through the plasma membrane. In addition, heme is stored in reservosomes, probably by the action of a second transporter. The identification of P-glycoprotein in the proteomic profile of reservosomes reinforces this hypothesis. Moreover, reservosomes store high levels of neutral lipids, such as cholesteryl esters [11], as a result of the endocytic process. The high concentration of lipids in the reservosome lumen seems to be involved in the formation of rectangular lipid bodies [9]. Like in mammals, ABCA1 could serve to control the rate of exogenous cholesterol internalized into early and late endosomes, back to plasma membrane or, yet, out of the cell [27].
Table 1.
Proteins of T. cruzi epimastigote reservosomes identified by proteomic analysis and with annotated function or cellular localization
| Hit number |
Protein group | GenBank accession number |
Function/Cellular localization | Transmembrane domain (number of domains)1 |
|---|---|---|---|---|
| (1) Protein metabolism | ||||
| 1 | Serine carboxypeptidase | XP_803003 | Lysosomal, peptidase family S10 | N |
| 2 | Lysosomal alpha-mannosidase | XP_819105 | Lysosomal, carbohydrate metabolism | N |
| 3 | Histidine ammonia-lyase | XP_820336 | Convert histidine into ammonia and urocanic acid | N |
| 4 | Cysteine proteinase | XP_811957 | Lysosomal, similar to endopeptidase papain | Y (1) |
| 5 | Cysteine peptidase | XP_821954 | Lysosomal integral to membrane, proteolysis | Y (1) |
| 6 | S-Adenosylhomocysteine hydrolase | XP_809153 | Adenosylhomocysteine hydrolase activity | N |
| 7 | Cysteine peptidase C | XP_812694 | Lysosomal, proteolysis | N |
| 8 | Calpain cysteine peptidase | XP_816696 | Calcium-dependent cysteine-type endopeptidase activity | N |
| 9 | Membrane-bound acid phosphatase | XP_808329 | Catalytic activity | N |
| 10 | Membrane-bound acid phosphatase 2 | XP_815570 | Lysosomal, acid phosphatase activity | Y (1) |
| 11 | Serine carboxypeptidase S28 | XP_818592 | Peptidase S28 | N |
| 12 | Cruzipain | AAF75547 | Cysteine peptidase, clan CA, family C1 | N |
| 13 | Tartrate-resistant acid phosphatase type 5 | XP_811082 | Metallophosphoesterase | Y (2) |
| 14 | Ubiquitin hydrolase | XP_821344 | Protein deubiquitination | N |
| 15 | Glutamamyl carboxypeptidase | XP_804169 | Metallopeptidase activity, proteolysis | N |
| 16 | Peptidase M20/M25/M40 | XP_813635 | Metallopeptidase activity | N |
| 17 | Chagasin | 2FO8 | Cysteine Protease Inhibitor | N |
| (2) Endosomal/lysosomal membrane proteins | ||||
| 18 | Golgi/lysosome glycoprotein | XP_817916 | Golgi/lysosome membrane protein | Y (1) |
| 19 | Lysosomal/endosomal membrane protein p67 | XP_803464 | Lysosomal/endosomal membrane protein | Y (1) |
| 20 | Endosomal integral membrane protein | XP_817908 | Integral to endosomal membrane | Y (9) |
| 21 | Endosomal integral membrane protein | XP_813892 | Endosomal protein | Y (11) |
| 22 | Huntingtin interacting protein (HIP) | XP_816878 | Endocytosis, metal ion binding | Y (4) |
| (3) Vesicular traffic | ||||
| 23 | Vesicle-associated membrane protein | XP_816323 | Integral to plasma membrane, vesicle targeting | Y (1) |
| 24 | Vesicle-associated membrane protein | XP_812489 | Exocytosis | Y (1) |
| 25 | Rab1 | XP_805990 | ER, small GTPase mediated signal transduction | N |
| 26 | Rab-2a | XP_809735 | ER, Small GTPase mediated signal transduction | N |
| 27 | COP-coated vesicle membrane protein erv25 | XP_821489 | Coated vesicle membrane protein | Y (1) |
| 28 | COP-coated vesicle membrane protein gp25l | XP_805665 | ER to Golgi vesicle-mediated transport | Y (1) |
| 29 | Small GTP-binding protein | XP_808772 | ER to Golgi vesicle-mediated transport | N |
| 30 | Rab7 | XP_821619 | Small GTPase mediated signal transduction | N |
| 31 | Rab18 | XP_818013 | Small GTPase mediated signal transduction | N |
| 32 | Mu-adaptin 1 | XP_818899 | Adaptor complexes 1 subunit | N |
| (4) pumps and channel proteins | ||||
| 33 | P-type H+-atpase (tcha3) | XP_812631 | ATPase, transmembrane proton transport | Y (6) |
| 34 | P-type H+-atpase (tcha1) | XP_804256 | ATPase, transmembrane proton transport | Y (3) |
| 35 | P-type H+-atpase (tcha2) | AAL87542.1 | ATPase, transmembrane proton transport | Y (8) |
| 36 | ABC transporter | XP_818638 | ATPase, transmembrane transport of substances | Y (12) |
| 37 | P-glycoprotein | AAC09044.1 | ABC transporter protein | Y (8) |
| 38 | Multidrug resistance protein E | XP_815145 | Xenobiotic-transporting ATPase activity | Y (4) |
| 39 | Proton motive atpase | AAB70152 | Cation transport ATPase, P-type ATPase | N |
| 40 | Vacuolar-type proton pyrophosphatase | XP_813460 | Hydrogen-translocating pyrophosphatase activity | Y (15) |
| 41 | Ferric reductase | XP_808251 | Electron transport, oxidoreductase activity | Y (12) |
| 42 | Chloride channel protein | XP_816207 | Voltage-gated chloride channel activity | Y (10) |
| 43 | Hexose transporter | XP_814821 | Glucose transmembrane transporter activity | Y (12) |
| 44 | V-type atpase, A subunit | XP_807670 | Lysosomal proton-transporting V-type ATPase complex | N |
| 45 | Folate/pteridine transporter | XP_810759 | Biopterin transport | Y (12) |
| 46 | Vacuolar ATP synthase | XP_808900 | Hydrogen-exporting ATP activity, phosphorylative mechanism | N |
| 47 | Amino acid permease | XP_802539 | Transmembrane amino acid transporter protein | Y (10) |
| 48 | MFS transporter | XP_804323 | Transport small solutes across membranes | Y (7) |
| 49 | Amino acid permease/transporter | XP_819801 | Amino acid transport | Y (10) |
| 50 | Calcium-translocating P-type atpase | XP_814228 | Calcium-transporting ATPase activity | Y (3) |
| (5) Cell surface proteins | ||||
| 51 | Surface protease GP63 | XP_821289 | Neutral zinc metallopeptidases | Y (1) |
| 52 | Dispersed gene family protein 1 (DGF-1) | XP_820404 | Cell surface protein | Y (9) |
| 53 | Procyclic form surface glycoprotein | XP_803886 | Y (3) | |
| 54 | Kinetoplastid membrane protein KMP-11 | XP_808865 | kinetoplastid membrane protein | N |
| (6) Lipid metabolism | ||||
| 55 | Sterol 24-c-methyltransferase | XP_802864 | Biosynthesis of ergosterol | N |
| 56 | Fatty acid elongase | XP_813971 | Long-chain fatty acid biosynthetic process | Y (7) |
| 57 | 3-oxo-5-alpha-steroid 4-dehydrogenase | XP_802815 | Very-long-chain fatty acid metabolic process | Y (4) |
| 58 | Fatty acyl coa synthetase | XP_817096 | Long-chain-fatty-acid-CoA ligase activity | N |
| 59 | Serine-palmitoyl-coa transferase | XP_812739 | Sphingolipid metabolic process | N |
| 60 | Sterol C-24 reductase | XP_816888 | Ergosterol biosynthesis | Y (9) |
| 61 | Phospholipid-translocating atpase | XP_817305 | Phospholipid-translocating P-type ATPase, flippase | Y (12) |
| 62 | Fatty acyl coa synthetase 2 | XP_802708 | Long-chain fatty acid activation | N |
| 63 | C-8 sterol isomerase | XP_821186 | Ergosterol biosynthetic process | Y (1) |
| 64 | Fatty acyl coa syntetase 1 | XP_812351 | Fatty acid metabolic process | N |
| 65 | Phosphatidylcholine:ceramide cholinephosphotransferase | XP_821506 | Sphingomyelin biosynthetic process | Y (5) |
| 66 | Phosphatidic acid phosphatase protein | XP_814714 | Phospholipid metabolic process | Y (5) |
| 67 | Lipase domain protein | XP_814907 | Lipid metabolic process | Y (7) |
| (7) Carbohydrate metabolism | ||||
| 68 | UDP-Gal or UDP-glcnac-dependent glycosyltransferase | XP_814599 | Transfer galactose to GlcNAc terminal chains | N |
| 69 | Enolase | XP_819700 | Glycolysis, phosphopyruvate hydratase activity | N |
| 70 | Hexokinase | XP_808992 | Carbohydrate metabolic process | N |
| 71 | Mannosyl-oligosaccharide 1,2-alpha-mannosidase IB | XP_811964 | Protein amino acid N-linked glycosylation | N |
| 72 | Beta galactofuranosyl glycosyltransferase | XP_810392 | N | |
| 73 | Lectin | XP_807732 | Mannose binding | N |
| 74 | Glucokinase 1 | XP_821474 | Glucokinase activity | N |
| 75 | Glucosamine-6-phosphate isomerase | XP_807857 | N-acetylglucosamine catabolic process | N |
| (8) Cell signaling | ||||
| 76 | Protein kinase | XP_807538 | Protein amino acid phosphorilation | N |
| 77 | Protein tyrosine phosphatase | XP_816679 | Protein tyrosine phosphatase activator factor | N |
| 78 | Serine/threonine protein kinase | XP_822060 | Protein amino acid phosphorilation | N |
| 79 | Serine/threonine protein phosphatase | XP_809469 | Calcium-dependent protein serine/threonine phosphatase | Y (2) |
| 80 | Rac serine-threonine kinase | XP_815140 | N | |
| 81 | Regulatory subunit of protein kinase a | XP_810045 | Protein kinase activity | N |
| 82 | Casein kinase delta isoform | XP_803179 | Casein kinase activity | N |
| 83 | Activated protein kinase C receptor | XP_817733 | Protein kinase C binding | N |
| 84 | Casein kinase | XP_803179 | Protein amino acid phosphorylation | N |
| (9) Cytoskeleton-associated proteins | ||||
| 85 | Beta tubulin | XP_816690 | Microtubule cytoskeleton organization and biogenesis | N |
| 86 | Alpha tubulin | XP_802499 | Microtubule cytoskeleton organization and biogenesis | N |
| 87 | Actin | XP_807262 | Microfilament organization and biogenesis | N |
| 88 | MCAK-like kinesin | XP_813221 | Microtubule associated complex | N |
| 89 | Cytoskeleton-associated protein CAP5.5 | XP_809814 | Cytoskeleton-associated protein | N |
| 90 | Cofilin/actin depolymerizing factor | XP_806100 | Actin filament organization | |
| 91 | I/6 autoantigen | XP_813169 | Calcium ion binding, structural constituent of cytoskeleton | N |
| (10) Cytosolic proteins | ||||
| 92 | Elongation factor alpha G5 | AAU47272 | Calcium mediated signaling, microtubule-based process | N |
| 93 | Tryparedoxin peroxidase | XP_802393 | Trypanothione-disulfide reductase activity | N |
| 94 | Hypoxanthine-guanine phosphoribosyltransferase | XP_813397 | Nucleotide transport and metabolism | N |
| 95 | Methylthioadenosine phosphorylase | XP_819927 | Amino acid salvage | N |
| 96 | 6-phosphogluconate dehydrogenase | XP_808031 | Pentose-phosphate shunt | N |
| 97 | Disulfide isomerase | XP_821173 | Protein folding | N |
| (11) Glycosomal proteins | ||||
| 98 | Glutamate dehydrogenase | XP_818855 | Glutamate biosynthesis, using glutamate synthase (NADPH) | N |
| 99 | Glycosomal phosphoenolpyruvate carboxykinase | XP_810815 | Gluconeosenesis, microtubule binding | N |
| 100 | Glyceraldehyde 3-phosphate dehydrogenase | XP_812137 | Glycolysis | N |
| 101 | NADH-dependent fumarate reductase | XP_810232 | Glucose metabolic process | N |
| 102 | Gim5A protein | XP_821649 | Glycosomal membrane | N |
| 103 | Glycosomal malate dehydrogenase | XP_812467 | N | |
| 104 | Fructose-bisphosphate aldolase | XP_809369 | Fructose-bisphosphate aldolase activity | N |
| 105 | Glucose-6-phosphate isomerase | XP_821969 | Gluconeogenesis, glycolysis | N |
| 106 | 6-phospho-1-fructokinase | XP_822053 | Glycolysis | N |
| (12) Mitochondrial proteins | ||||
| 107 | Heat shock 70 kda protein | XP_806221 | Membrane protein folding | N |
| 108 | Malate dehydrogenase | XP_809210 | Tricarboxilyc acid cycle | N |
| 109 | Mitochondrial processing peptidase, beta subunit | XP_816361 | Mitochondrial processing peptidase activity | N |
| 110 | Pyruvate dehydrogenase E1 beta subunit | XP_811646 | Pyruvate dehydrogenase (acetyl-transferring) activity | N |
| 111 | Succinyl-coa ligase [GDP-forming] beta-chain | XP_802641 | Succinate-CoA ligase (GDP-forming) activity | N |
| 112 | P22 protein precursor | XP_813311 | Transcription regulator activity | N |
| 113 | ADP, ATP carrier protein 1 | XP_812264 | Transport, mitochondrial inner membrane | Y (3) |
| 114 | RNA-binding protein 2 | XP_818766 | Mitochondrial RNA binding | N |
| 115 | Acyl carrier protein | XP_814961 | Fatty acid biosynthetic process | N |
| 116 | Oligo_U binding protein TBRGG1 | XP_809536 | N | |
| 117 | ATP synthase, epsilon chain | XP_818987 | Proton-transporting ATP synthase complex | N |
| 118 | Chaperonin HSP60/CNP60 | XP_804030 | Protein import into mitochondrial matrix | N |
| 119 | Succinyl-coa ligase [GDP-forming] beta-chain | XP_818583 | Tricarboxylic acid cycle | N |
| 120 | Aldehyde dehydrogenase | XP_813115 | Oxidation of aldehydes | N |
| 121 | ADP, ATP carrier protein 1 | XP_819458 | Mitochodria inner membrane, transport | Y (3) |
| (13) others | ||||
| 122 | Glucose-regulated protein 78 | XP_809817 | Endoplasmic reticulum, protein folding | N |
| 123 | Calreticulin | XP_812571 | Protein folding, sugar binding | N |
| 124 | Calcium-binding protein | XP_808304 | Flagellar calcium binding protein | N |
| 125 | Malic enzyme | XP_814410 | Malate metabolic process | N |
| 126 | Cyclophilin A | XP_821578 | Protein folding | N |
| 127 | S-phase kinase-associated protein | XP_806972 | Ubiquitin-dependent protein catabolic process | N |
| 128 | Calmodulin | XP_874377 | Calcium ion binding | N |
| 129 | Centrin | XP_813165 | Basal body, calcium ion binding | N |
N, no; Y, yes
LC-MS/MS analysis confirmed the reservosomal localization of cruzipain and its inhibitor chagasin and serine carboxypeptidase, shown by previous works to be present [7, 10,13] and functional [11, 12] in reservosomes. Novel hydrolases were found, such as lysosomal α-mannosidase, cysteine proteases, calpain cysteine peptidase, cysteine protease C, and membrane-bound acid phosphatase. Interestingly, in a previous paper, acid phosphatase was not detected in reservosomes by ultrastructural cytochemistry. Based on the absence of this enzyme and other lysosomal structural proteins, reservosomes were classified as non-lysosomal endocytic organelles [8]. Recently, a typical lysosome enzyme, aryl sulphatase, was shown to be functional in epimastigote small vesicles but not in reservosomes [46], reinforcing the reservosome non-lysosomal character. Nevertheless, our proteomic results confirmed that reservosomes are the main site of lysosomal hydrolases and are hence working as an important endogenous protein regulator.
On the other hand, reservosomes concentrate lysosomal hydrolases, and they have been considered the main site of protein degradation and regulation. Therefore, it is reasonable that the reservosome constitutes an important site of protein recycling, justifying the presence of such proteins in the proteomic analysis. An autophagic process was recently reported in T. cruzi [47, 48], showing that autophagy is essential for parasite survival during starvation and differentiation. This work suggested that part of the cytoplasm and organelles are degraded in reservosomes.
We could detect some proteins that are not related to endocytic compartments. These proteins are constituents of the cytoplasm, mitochondrion, glycosome, acidocalcisome, nucleus, endoplasmic reticulum or flagellum. The presence of contaminants derived from other organelles is the main challenge faced during the subcellular fractionation, and it is a possible explanation for the presence of several proteins from different origin in the reservosome proteomics. In order to demonstrate the low level of cross-contamination with membranes from different organelles we predicted transmembrane domains in non-reservosomal proteins. In this way, we managed to show that our fractionation protocol led to very low degree of contamination with non-reservosomal membranes.
Subcellular proteomics have contributed to determine protein localization and function [49]. However, finding proteins in multiple locations helps to determine the relationship between different organelles or structures, such as organelles from the endocytic pathway. Recently, it was estimated that up to 39% of organelle proteins can be detected in multiple intracellular compartments [50]. The authors suggested some hypotheses to explain these findings: i) proteins are indeed found in different compartments; ii) contaminants are created during the fractionation process; and iii) proteins are mistakenly identified. It is important to point out that reservosomes result from the fusion of vesicles originating in the plasma membrane during the endocytic pathway with vesicles from the endoplasmic reticulum-Golgi complex via the secretory pathway [44]. Therefore, the protein profile of the reservosomes may reflect the dynamic traffic between these structures.
Among the transmembrane proteins identified in the reservosomal proteome, we can suggest P-type H+-ATPase (TcHA3) and p67 as possible molecular markers. In T. cruzi, only two isoforms of P-type H+-ATPases, TcHA1 and TcHA2, were characterized and had their localization determined [15]. TcHA1 was found in the plasma membrane and also in the endocytic pathway, while TcHA2 plays a role exclusively in the acidification of reservosomes. Besides TcHA1 and TcHA2, our proteomic data using Dm28c clone detected the presence of TcHA3 in the reservosome membranes with a high coverage, what may imply a high abundance. Due to the absence of these proteins in mammalian cells and their presence in fungi and trypanosomatids, these pumps may be considered as potential chemotherapeutic targets.
p67 is a type I transmembrane glycoprotein that has been described as a T. brucei lysosome molecular marker [28]. It presents a general structure similar to LAMPs (lysosome-associated membrane proteins) in mammalian cells, although without sequence homology. In African trypanosomes, p67 has been related to the formation of lysosomal glycocalyx, protecting against hydrolases. The p67 gene was found in T. cruzi, and its reservosomal localization was demonstrated in our proteomic analysis. Because orthologues of p67 are not found in mammals, it might be a candidate for chemotherapy as well.
In protozoan parasites, subcellular proteomic analyses were successfully applied to the characterization of Entamoeba histolytica [51] and Dictyostelium discoideum [52] phagosome. In trypanosomatids, we can highlight the proteome of flagella [53], glycosomes [54], and the comparative proteomic analysis of glycosomes and mitochondria [55] of T. brucei. Recently, Ferella and co-workers [31] prepared a subcellular fraction from T. cruzi CL Brener epimastigotes that was enriched in acidocalcisomes and glycosomes but also contained endoplasmic reticulum and Golgi components. Using 1D and 2D electrophoresis followed by MS analysis, they identified proteins corresponding to 396 genes, 258 of which were annotated as previously described and 138 were hypothetical proteins. Among the identified proteins, only three are presumably reservosomal: glutathione S-transferase [56], cysteine peptidase [10], and serine carboxypeptidase [7, 12]. These studies yielded valuable information about protein localization and reported the identification of novel proteins in these organelles. In the current paper, we performed the proteomic analysis of T. cruzi epimastigote reservosomes, which is the first protein profiling of an endocytic organelle in a trypanosomatid family member. This analysis offers a new perspective in the identification of reservosomal molecular markers and contributes to the understanding of the biogenesis and dynamic interactions of these organelles with other T. cruzi structures.
Supplementary Material
Acknowledgments
The authors would like to thank Mr. Antonio Bosco Carlos for technical support. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) e Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and National Institutes of Health (NIH). ICA was supported by the grants 1R01AI070655, 2S06GM008012-37, and 5G12RR008124 from the NIH. E.S.N. was supported by the George A. Krutilek memorial graduate scholarship from Graduate School, UTEP. We thank the Biomolecule Analysis Core Analysis at the Border Biomedical Research Center/UTEP (NIH grant 5G12RR008124), for the access to the LC-MS instrumentation.
Footnotes
The authors declare that this work does not represent any conflicts of interest of commercial or financial nature.
References
- 1.De Souza W. From the cell biology to the development of new chemotherapeutic approaches against trypanosomatids: dreams and reality. Kinetoplastid Biol Dis. 2002;1:3. doi: 10.1186/1475-9292-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soares MJ, De Souza W. Cytoplasmic organelles of trypanosomatids: a cytochemical and stereological study. J Submicrosc Cytol Pathol. 1988;20:349–361. [PubMed] [Google Scholar]
- 3.Soares MJ, de Souza W. Endocytosis of gold-labeled proteins and LDL by Trypanosoma cruzi. Parasitol Res. 1991;77:461–468. doi: 10.1007/BF00928410. [DOI] [PubMed] [Google Scholar]
- 4.Porto-Carreiro I, Attias M, Miranda K, De Souza W, Cunha-e-Silva N. Trypanosoma cruzi epimastigote endocytic pathway: cargo enters the cytostome and passes through an early endosomal network before storage in reservosomes. Eur J Cell Biol. 2000;79:858–869. doi: 10.1078/0171-9335-00112. [DOI] [PubMed] [Google Scholar]
- 5.Cunha-e-Silva N, Sant’Anna C, Pereira MG, Porto-Carreiro I, et al. Reservosomes: multipurpose organelles? Parasitol Res. 2006;99:325–327. doi: 10.1007/s00436-006-0190-3. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sant’Anna C, Parussini F, Lourenço D, de Souza W, et al. All Trypanosoma cruzi developmental forms present lysosome-related organelles. Histochem Cell Biol. 2008 doi: 10.1007/s00418-008-0486-8. [DOI] [PubMed] [Google Scholar]
- 8.Soares MJ, Souto-Padron T, De Souza W. Identification of a large pre-lysosomal compartment in the pathogenic protozoon Trypanosoma cruzi. J Cell Sci. 1992;102:157–167. doi: 10.1242/jcs.102.1.157. [DOI] [PubMed] [Google Scholar]
- 9.Sant’Anna C, Pereira MG, Lemgruber L, de Souza W, Cunha e Silva NL. New insights into the morphology of Trypanosoma cruzi reservosome. Microsc Res Tech. 2008;71:599–605. doi: 10.1002/jemt.20592. [DOI] [PubMed] [Google Scholar]
- 10.Souto-Padron T, Campetella OE, Cazzulo JJ, de Souza W. Cysteine proteinase in Trypanosoma cruzi: immunocytochemical localization and involvement in parasite-host cell interaction. J Cell Sci. 1990;96:485–490. doi: 10.1242/jcs.96.3.485. [DOI] [PubMed] [Google Scholar]
- 11.Cunha-e-Silva NL, Atella GC, Porto-Carreiro IA, Morgado-Diaz JA, et al. Isolation and characterization of a reservosome fraction from Trypanosoma cruzi. FEMS Microbiol Lett. 2002;214:7–12. doi: 10.1111/j.1574-6968.2002.tb11317.x. [DOI] [PubMed] [Google Scholar]
- 12.Parussini F, Garcia M, Mucci J, Aguero F, et al. Characterization of a lysosomal serine carboxypeptidase from Trypanosoma cruzi. Mol Biochem Parasitol. 2003;131:11–23. doi: 10.1016/s0166-6851(03)00175-0. [DOI] [PubMed] [Google Scholar]
- 13.Santos CC, Sant’Anna C, Terres A, Cunha-e-Silva NL, et al. Chagasin, the endogenous cysteine-protease inhibitor of Trypanosoma cruzi, modulates parasite differentiation and invasion of mammalian cells. J Cell Sci. 2005;118:901–915. doi: 10.1242/jcs.01677. [DOI] [PubMed] [Google Scholar]
- 14.Serrano R, Villalba JM, Palmgren MG, Portillo F, et al. Studies of the plasma membrane H(+)-ATPase of yeast and plants. Biochem Soc Trans. 1992;20:562–566. doi: 10.1042/bst0200562. [DOI] [PubMed] [Google Scholar]
- 15.Vieira M, Rohloff P, Luo S, Cunha-e-Silva NL, et al. Role for a P-type H+-ATPase in the acidification of the endocytic pathway of Trypanosoma cruzi. Biochem J. 2005;392:467–474. doi: 10.1042/BJ20051319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendonca SM, Nepomuceno da Silva JL, Cunha e-Silva N, de Souza W, Gazos Lopes U. Characterization of a Rab11 homologue in Trypanosoma cruzi. Gene. 2000;243:179–185. doi: 10.1016/s0378-1119(99)00480-1. [DOI] [PubMed] [Google Scholar]
- 18.Camargo EP. Growth and Differentiation in Trypanosoma cruzi. I. Origin of Metacyclic Trypanosomes in Liquid Media. Rev Inst Med Trop Sao Paulo. 1964;12:93–100. [PubMed] [Google Scholar]
- 19.Stone KL, Williams KR. Enzymatic digestion of proteins in solution and in SDS polyacrylamide gel. In: Walker JM, editor. The protein protocol handbook. Humana Press; New Jersey: 1996. pp. 415–425. [Google Scholar]
- 20.Jurado JD, Rael ED, Lieb CS, Nakayasu E, et al. Complement inactivating proteins and intraspecies venom variation in Crotalus oreganus helleri. Toxicon. 2007;49:339–350. doi: 10.1016/j.toxicon.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng JK, McCormack AL, Yates JR., III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 23.Groth D, Lehrach H, Hennig S. GOblet: a platform for Gene Ontology annotation of anonymous sequence data. Nucleic Acids Res. 2004;32:W313–317. doi: 10.1093/nar/gkh406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres C, Perez-Victoria FJ, Parodi-Talice A, Castanys S, Gamarro F. Characterization of an ABCA-like transporter involved in vesicular trafficking in the protozoan parasite Trypanosoma cruzi. Mol Microbiol. 2004;54:632–646. doi: 10.1111/j.1365-2958.2004.04304.x. [DOI] [PubMed] [Google Scholar]
- 25.Cuevas IC, Rohloff P, Sanchez DO, Docampo R. Characterization of farnesylated protein tyrosine phosphatase TcPRL-1 from Trypanosoma cruzi. Eukaryot Cell. 2005;4:1550–1561. doi: 10.1128/EC.4.9.1550-1561.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol. 2008;43:3–36. doi: 10.1080/10409230701829110. [DOI] [PubMed] [Google Scholar]
- 27.Attie AD. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem Sci. 2007;32:172–179. doi: 10.1016/j.tibs.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Alexander DL, Schwartz KJ, Balber AE, Bangs JD. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J Cell Sci. 2002;115:3253–3263. doi: 10.1242/jcs.115.16.3253. [DOI] [PubMed] [Google Scholar]
- 29.Dreger M. Subcellular proteomics. Mass Spectrom Rev. 2003;22:27–56. doi: 10.1002/mas.10047. [DOI] [PubMed] [Google Scholar]
- 30.De Souza W, Cunha-e-Silva N. Cell fractionation of parasitic protozoa – a review. Mem Inst Oswaldo Cruz. 2003;98:151–170. doi: 10.1590/s0074-02762003000200001. [DOI] [PubMed] [Google Scholar]
- 31.Ferella M, Nilsson D, Darban H, Rodrigues C, et al. Proteomics in Trypanosoma cruzi -localization of novel proteins to various organelles. Proteomics. 2008;8:2735–2749. doi: 10.1002/pmic.200700940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva RA, Bartholomeu DC, Teixeira SM. Control mechanisms of tubulin gene expression in Trypanosoma cruzi. Int J Parasitol. 2006;36:87–96. doi: 10.1016/j.ijpara.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 34.Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhir V, Goulding D, Field MC. TbRAB1 and TbRAB2 mediate trafficking through the early secretory pathway of Trypanosoma brucei. Mol Biochem Parasitol. 2004;137:253–265. doi: 10.1016/j.molbiopara.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denny PW, Lewis S, Tempero JE, Goulding D, et al. Leishmania RAB7: characterisation of terminal endocytic stages in an intracellular parasite. Mol Biochem Parasitol. 2002;123:105–113. doi: 10.1016/s0166-6851(02)00133-0. [DOI] [PubMed] [Google Scholar]
- 39.Morgan GW, Hall BS, Denny PW, Field MC, Carrington M. The endocytic apparatus of the kinetoplastida. Part II: machinery and components of the system. Trends Parasitol. 2002;18:540–546. doi: 10.1016/s1471-4922(02)02392-9. [DOI] [PubMed] [Google Scholar]
- 40.Araripe JR, Cunha e Silva NL, Leal ST, de Souza W, Rondinelli E. Trypanosoma cruzi: TcRAB7 protein is localized at the Golgi apparatus in epimastigotes. Biochem Biophys Res Commun. 2004;321:397–402. doi: 10.1016/j.bbrc.2004.06.159. [DOI] [PubMed] [Google Scholar]
- 41.Martin S, Parton RG. Characterization of Rab18, a lipid droplet-associated small GTPase. Methods Enzymol. 2008;438:109–129. doi: 10.1016/S0076-6879(07)38008-7. [DOI] [PubMed] [Google Scholar]
- 42.Jeffries TR, Morgan GW, Field MC. TbRAB18, a developmentally regulated Golgi GTPase from Trypanosoma brucei. Mol Biochem Parasitol. 2002;121:63–74. doi: 10.1016/s0166-6851(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 43.Hu ZZ, Valencia JC, Huang H, Chi A, et al. Comparative Bioinformatics Analyses and Profiling of Lysosome-Related Organelle Proteomes. Int J Mass Spectrom. 2007;259:147–160. doi: 10.1016/j.ijms.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sant’Anna C, de Souza W, Cunha-e-Silva N. Biogenesis of the reservosomes of Trypanosoma cruzi. Microsc Microanal. 2004;10:637–646. doi: 10.1017/S1431927604040863. [DOI] [PubMed] [Google Scholar]
- 45.Lara FA, Sant’anna C, Lemos D, Laranja GA, et al. Heme requirement and intracellular trafficking in Trypanosoma cruzi epimastigotes. Biochem Biophys Res Commun. 2007;355:16–22. doi: 10.1016/j.bbrc.2006.12.238. [DOI] [PubMed] [Google Scholar]
- 46.Adade CM, de Castro SL, Soares MJ. Ultrastructural localization of Trypanosoma cruzi lysosomes by aryl sulphatase cytochemistry. Micron. 2007;38:252–256. doi: 10.1016/j.micron.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez VE, Kosec G, Sant Anna C, Turk V, et al. Blocking autophagy to prevent parasite differentiation: a possible new strategy for fighting parasitic infections? Autophagy. 2008;4:361–363. doi: 10.4161/auto.5592. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez VE, Kosec G, Sant’Anna C, Turk V, et al. Autophagy is involved in nutritional stress response and differentiation in Trypanosoma cruzi. J Biol Chem. 2008;283:3454–3464. doi: 10.1074/jbc.M708474200. [DOI] [PubMed] [Google Scholar]
- 49.Andersen JS, Mann M. Organellar proteomics: turning inventories into insights. EMBO Rep. 2006;7:874–879. doi: 10.1038/sj.embor.7400780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster LJ, de Hoog CL, Zhang Y, Zhang Y, et al. A mammalian organelle map by protein correlation profiling. Cell. 2006;125:187–199. doi: 10.1016/j.cell.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Okada M, Huston CD, Mann BJ, Petri WA, Jr, et al. Proteomic analysis of phagocytosis in the enteric protozoan parasite Entamoeba histolytica. Eukaryot Cell. 2005;4:827–831. doi: 10.1128/EC.4.4.827-831.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotthardt D, Warnatz HJ, Henschel O, Brückert F, et al. High-resolution dissection of phagosome maturation reveals distinct membrane trafficking phases. Mol Biol Cell. 2002;13:3508–3520. doi: 10.1091/mbc.E02-04-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broadhead R, Dawe HR, Farr H, Griffiths S, et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–227. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- 54.Colasante C, Ellis M, Ruppert T, Voncken F. Comparative proteomics of glycosomes from bloodstream form and procyclic culture form Trypanosoma brucei brucei. Proteomics. 2006;6:3275–3293. doi: 10.1002/pmic.200500668. [DOI] [PubMed] [Google Scholar]
- 55.Vertommen D, Van Roy J, Szikora JP, Rider MH, et al. Differential expression of glycosomal and mitochondrial proteins in the two major life-cycle stages of Trypanosoma brucei. Mol Biochem Parasitol. 2008;158:189–201. doi: 10.1016/j.molbiopara.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 56.Ouaissi MA, Dubremetz JF, Schöneck R, Fernandez-Gomez R, et al. Trypanosoma cruzi: a 52-kDa protein sharing sequence homology with glutathione S-transferase is localized in parasite organelles morphologically resembling reservosomes. Exp Parasitol. 1995;81:453–461. doi: 10.1006/expr.1995.1138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.