Abstract
Objective
Measurements of cell proliferation and matrix synthesis in cartilage explants have identified regulatory factors (e.g., interleukin 1, IL-1) that contribute to osteoarthritis and anabolic mediators (e.g., BMP-7) that may have therapeutic potential. The objective of this study was to develop a robust method for measuring cell proliferation and glycosaminoglycan synthesis in articular cartilage that could be applied in vivo.
Methods
A stable isotope-mass spectrometry approach was validated by measuring the metabolic effects of IL-1 and BMP-7 in cultures of mature and immature bovine cartilage explants. The method was also applied in vivo to quantify physiologic turnover rates of matrix and cells in the articular cartilage of normal rats. Heavy water was administered to explants in the culture medium and to rats via drinking water, and cartilage was analyzed for labeling of chondroitin sulfate (CS), hyaluronic acid (HA) and DNA.
Results
As expected, IL-1 inhibited the synthesis of DNA and CS in cartilage explants. However, IL-1 inhibited HA synthesis only in immature cartilage. Futhermore, BMP-7 was generally stimulatory, but immature cartilage was significantly more responsive than mature cartilage, particularly in terms of HA and DNA synthesis. In vivo, labeling of CS and DNA in normal rats for up to a year indicated half-lives of 22 and 862 days, respectively, in the joint.
Conclusions
We describe a method by which deuterium from heavy water is traced into multiple metabolites from a single cartilage specimen to profile its metabolic activity. This method was demonstrated in tissue culture and rodents but may have significant clinical applications.
Introduction
Chief among the degenerative changes associated with osteoarthritis (OA) is the degradation of articular cartilage, the weight-bearing material found at the end of long bones that facilitates the motion of joints. On a molecular level, articular cartilage is a structurally organized connective tissue whose extracellular matrix consists of a cross-linked network of collagen fibers expanded by water and proteoglycans1. The primary proteoglycan in articular cartilage is aggrecan, a macromolecule composed of a core protein covalently attached to up to 150 glycosaminoglycan (GAG) chains, primarily chondroitin sulfate (CS) and keratan sulfate2. Up to 200 aggrecan molecules associate on a backbone of hyaluronic acid (HA) to form aggregates reaching molecular weights of over 200 MDa3.
A homeostatic balance between matrix synthesis and degradation is maintained in healthy cartilage by a sparse population of chondrocytes4, but these metabolic processes are disrupted in degenerative joint diseases, in which a net loss of extracellular matrix components is observed5. Measurements of critical metabolic pathways, including proliferation of chondrocytes and synthesis of extracellular matrix components, have identified regulatory factors that contribute to degenerative joint disease, such as the pro-inflammatory cytokine interleukin 1 (IL-16), as well as anabolic mediators that have therapeutic utility, such as the growth factor BMP-77.
Kinetic measurements of these metabolic pathways have traditionally employed radioactively-tagged precursors. Methods based on radioactive tracers are sensitive and relatively cost-effective, but their use in vivo is limited by safety issues, particularly in humans. In addition to safety advantages over radiotracers, stable isotope labeling with mass spectrometric analysis generates unique information based on patterns of isotopic isomers (e.g., mass isotopomer distribution analysis8,9) that can provide critical information about biosynthetic processes. Stable isotope labeling with heavy water (2H2O) has been used to label a wide range of metabolites in vivo10-14, including DNA15, lipids14 and protein16. Hydrogen-labeled water is an attractive tracer because it equilibrates quickly and uniformly with body water and the body water pool turns over relatively slowly (∼8% a week17,18), allowing 2H2O to be maintained easily at relatively constant label concentrations15,18. Moreover, protons from water enter C-H bonds at specific enzyme-mediated steps in nearly every metabolic pathway, making 2H2O a near universal tracer. We describe here the first implementation of stable isotopes and mass spectrometry, specifically heavy water, for quantifying the synthetic rates of multiple products, including GAGs and cellular DNA, within articular cartilage in vivo.
Relatively few studies have attempted to perform direct metabolic measurements on articular cartilage in vivo. Measurements in living animals are important because articular cartilage interacts with other tissues that comprise the synovial joint and are important in the pathology of OA, but such interactions are difficult to reproduce in vitro19. Here, we present a stable isotope- mass spectrometric technique through which a single tracer can be used to quantify multiple aspects of extracellular matrix synthesis and cell proliferation reliably and accurately in a sample of articular cartilage. To validate the method in a well characterized model, we quantified CS, HA, and DNA synthesis rates in bovine cartilage explants maintained in tissue culture and examined the effects of IL-1 and BMP-7. The methods were then extended to articular cartilage in vivo to measure physiologic rates of extracellular matrix synthesis and articular chondrocytes proliferation in normal, growing rats.
Materials and Methods
Tissue culture of bovine cartilage explants
Bovine stifle joints with intact joint capsules were obtained within 24 hours after slaughter from three 1 to 3 week old calves (San Jose Valley Veal, San Jose, CA) and three 12 to 18 month old steers (Animal Technologies, Dallas, TX). Osteochondral blocks were harvested under sterile conditions from the distal femur using a reciprocating saw (Johnson and Johnson, New Brunswick, NJ). Tissue samples were secured to a sledge microtome (Microm GmbH, Walldorf, Germany), and plane-parallel 1 mm thick sections of articular cartilage were obtained after discarding at least 200 μm from the articular surface. For calf tissue, two sequential 1 mm thick cartilage sections were obtained, whereas the adult articular cartilage layer was only thick enough to yield one section of 1 mm thickness. From each of the cartilage sections, 3 mm diameter disks were obtained using a stainless-steel dermal punch (Miltex GmbH, Tuttlingen, Germany). Cartilage disks were maintained at 37° C and 5% CO2 in a basal medium consisting of Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 25 μg/mL ascorbate, antibiotics, and either 4 or 8% 2H20 (Spectra Stable Isotopes, Columbia, MD). In some samples, recombinant human IL-1α (R&D Systems, Minneapolis, MN) was added to the medium at a final concentration of either 0.5 or 5 ng/ml. IL-1α was chosen because bovine chondrocytes are more responsive to the α- than the β-isoform20,21. This concentration range induces a significant loss of tissue sulfated GAG (sGAG) and inhibits sGAG biosynthesis in adult bovine cartilage explants21,22. Some cultures included 100 ng/ml recombinant human BMP-7 (R&D Systems, Minneapolis, MN) throughout the culture period, previously shown to stimulate the biosynthesis of sGAG in adult bovine cartilage in the presence of serum23-25. Individual cartilage disks were incubated in 1 mL of medium and transferred to new culture wells containing fresh medium every 2 days to minimize the impact of cell migration onto tissue culture plastic. Explant disks were maintained in culture for 5 or 10 days. Spent medium was frozen and analysed for released extracellular matrix molecules.
Time course of label incorporation in articular cartilage of normal rats
Fifty male Sprague Dawley rats (initial weights 254-279 g, Charles River, Wilmington, MA) were administered 2H20 under isoflurane anesthesia. Intraperitoneal injection of a priming bolus (35 μL/g body weight) consisted of 100% 2H20 in sterile 0.9% saline. Drinking water was replaced with water containing 8% 2H20, and animals drank ad libitum. Plasma was collected at sacrifice via cardiac puncture. The hind legs were removed, stored at -20°C until processing and articular cartilage was then carefully scraped from the surface of the medial tibia plateaus (MTP) using a scalpel, taking care not to penetrate the subchondral bone. All animal experiments received IACUC approval.
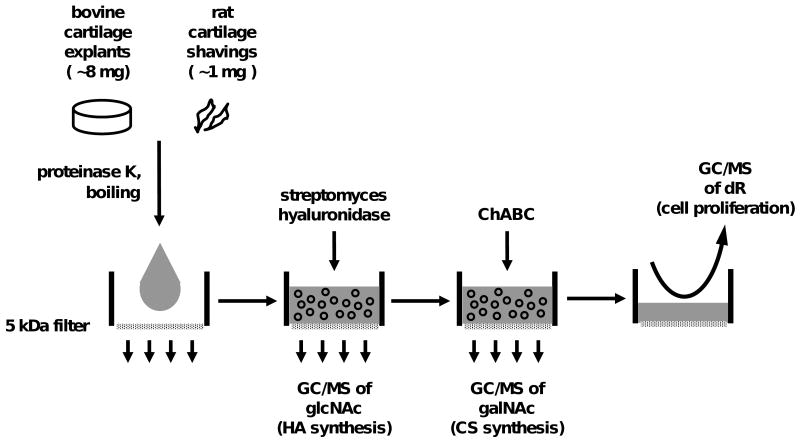
Isolation of DNA, CS, and HA (Fig. 1)
Figure 1.
Protocol for isolating several metabolites from a single specimen of articular cartilage and analyzing by gas chromatography/ mass spectrometry (GC/MS). Proteinase K-solubilized cartilage is subjected to sequential digestion steps with hyaluronidase and chondroitinase ABC to separate the molecular constituents of hyaluronic acid (HA), chondroitin sulfate (CS), and DNA. Further hydrolysis and derivatization steps (not shown) are used to prepare glcNAc, galNAc, and deoxyribose (dR) for GC/MS analysis, providing indices of the fractional synthesis HA, CS and DNA, respectively.
Cartilage was solubilized by overnight digestion with 500 μg/ml proteinase K at 55°C. The digest was then boiled for 10 minutes to deactivate proteinase K26 and portions were transferred to microfiltration tubes with 5 kDa cellulose filters (Millipore, Billerica, MA) and centrifuged (60 minutes, 5,000 g) to retain macromolecular material. 50 mM acetate (pH 6) including 10 TRU/ml streptomyces hyaluronidase (Seikagaku, Tokyo, JPN) and 0.02% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) was added, allowed to incubate at 55°C for 2 hours, centrifuged (60 minutes, 5,000 g) and the filtrate was collected. Residual macromolecular material was digested overnight with 0.1 U chondroitinase ABC (Sigma-Aldrich, St. Louis, MO) in 200 mL 50 mM Tris-acetate (pH 8) including 0.01% BSA, and centrifuged. The two solutions containing hyaluronidase or chondroitinase digestion products were hydrolyzed separately by incubation with equal parts 3N methanolic HCl (1 hour, 20°C). DNA was collected by reconstituting the filter retentate with water, then digested to free deoxyribonucleosides as described previously10.
Measurement of mass isotopomer abundances by gas chromatography/ mass spectrometry (GC/MS)
CS-, HA- and DNA-hydrolysis products were derivatized to the pentofluorobenzyl derivatives and acetylated, as described elsewhere10. N-acetyl glucosamine (glcNAc), N-acetyl galactosamine (galNAc) and deoxyribose (dR) were analyzed by GC/MS as representative analytes of each polymer (CS, HA, and DNA, respectively). GC retention times were established with unlabeled standards, and the abundances of M0, M1, and M2 mass isotopomers were analyzed by selected ion monitoring, using a model 5973 mass spectrometer attached to a 6890 gas chromatograph (Agilent, Palo Alto, CA) and negative chemical ionization (NCI). GC column was DB17 (30 m, 0.25 mm i.d., 0.25 μm film thickness, J&W Scientific, Folsom, CA), the helium gas flow rate was adjusted to 1.0 ml/min., and the temperature was programmed, following an initial hold at 140 °C for 1 min, to rise from 140 °C to 300 °C at 15°C/min. Isotope enrichments of the M1 and M2 mass isotopomers of 2H-labeled samples (EM1 and EM2, respectively) were calculated by subtracting the fractional abundances of unlabeled standards.
Measurement of 2H2O-enrichments in culture medium and terminal rat plasma
Aliquots of plasma (100 μl), placed into the caps of tightly sealed, inverted screw-capped vials, were kept at 60°C in a heating block overnight. The 2H enrichment of the condensate collected in the vial was measured by reacting with calcium carbide to form acetylene. Acetylene samples were then analyzed using a Series 3000 cycloidal mass spectrometer (Monitor Instruments, Cheswick, PA), modified to record ions at m/z 26 and 27 (M0 and M1) and calibrated against a standard curve prepared by mixing 99.9% 2H2O with unlabeled water.
Calculations
In order to interpret label incorporation from 2H2O into an end-product, it is necessary to determine the number of hydrogen atoms (n) in the molecule that originated from body H2O during biosynthesis. While n has been established by use of long-term labeling or combinatorial analysis of mass isotopomer labeling patters for DNA15 and other metabolic products15,27-30, it was not known for GAG molecules, and it may vary under different in vivo and in vitro labeling conditions. Once the number of sites of 2H incorporation has been determined for a molecule, the asymptotic EM1 value corresponding to 100% newly-synthesized molecules (A1*) can be calculated for any 2H2O concentration, as discussed previously16,31. Since the measured EM1 of a labeled molecule represents the weighted average of unlabeled (old) and fully labeled (new) molecules, the fraction of molecules that were newly-synthesized (f) can be calculated. Specifically, n for galNAc and glcNAc was determined to 4.3±0.8 in 10 tissue culture experiments and 6.1±0.7 in 8 rat experiments (additional details available as an Online Supplement). For 2H2O enrichments of 7.2% in vitro and 5% in vivo, A1* for newly-synthesized GAG molecules is 16.0% and 16.3%, respectively. Values of f for CS and HA were calculated by dividing the measured EM1 of galNAc and glcNAc molecules by their asymptotic A1* value. For fractional DNA synthesis, measured EM1 in dR was divided by its A1* value, based on n=5, as established previously10,15,31. Absolute synthesis rates were calculated by multiplying f by pool sizes, measured biochemically (see below).
Biochemistry
Cartilage digest solutions and spent medium were analyzed for sulfated GAG (sGAG) content after incubation with dimethylmethylene blue (DMB, Polysciences, Warrington, PA)32, using type C chondroitin sulfate from shark cartilage (Sigma-Aldrich, St. Louis, MO) as a standard. DNA content in the cartilage digests was assessed by fluorometry after incubation with PicoGreen® (Molecular Probes, Eugene, OR)33 and converted to cell number, assuming 7.7 pg DNA per cell.34
Statistical analysis
All data are expressed as mean ± standard deviation. Statistical analysis was implemented with Systat 5.2 (Systat, Inc., Evanston, IL). For most comparisons, the main treatment effects (IL-1 or BMP-7) were assessed by one- or two-way analysis of variance (ANOVA), with Tukey post-hoc testing. In all statistical tests, independent experiments were introduced as a random variable. For rat studies, deuterium incorporation curves were fit to a first order exponential rise-to-plateau function (SigmaPlot, Systat, Inc., Evanston, IL), and half lives (t1/2) were calculated from the time constant (k) as t1/2 = ln 2 / k. Cumulative f over the first 14 days in rats also was analyzed by linear regression.
Results
Effect of IL-1 and BMP-7 on CS synthesis in bovine explants in vitro
Adult bovine cartilage explants incubated with serum-supplemented control medium (without IL-1 and BMP-7) maintained a relatively constant sGAG content between 5 and 10 days (350±50 and 330±30 μg/disk, respectively; p=0.9, n=8). Under these steady-state culture conditions, the explants released sGAG into the medium at a rate of 7±2 μg per day, representing an sGAG turnover of 2.2±0.7% per day.
For tissue CS, f was determined from deuterium-labeled galNAc molecules in CS chains. In control studies, the measured fractional CS synthesis did not vary when different enrichments of 2H20 were used in the culture medium (f=13.2±1.1% and 13.9±1.9% after 5 days, for 4% and 8% 2H20, respectively; p=0.9, n=4), confirming that heavy water exposure at these concentrations did not affect cartilage physiology. For adult bovine explants maintained under control conditions, 10±1% of the total CS was newly-synthesized over the first 5 days of serum-supplemented culture (Fig. 2A), and f increased over time, reaching 18±2% in the day 10 samples (Fig. 2B). This isotopic result represents an f of 1.9% per day, quantitatively similar to the turnover rate estimated from sGAG release into the medium in the same samples (see above).
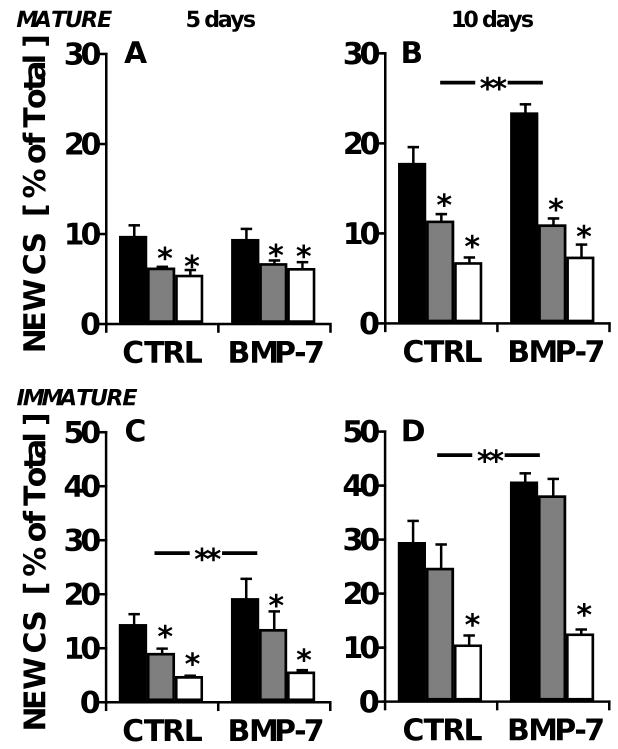
Figure 2.
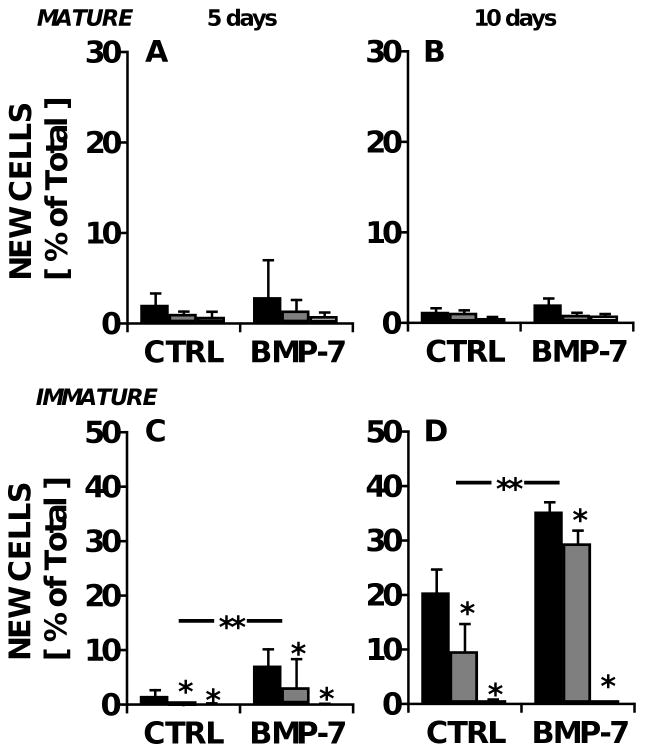
Age-dependent effects of IL-1 and BMP-7 on the biosynthesis of chondroitin sulfate in bovine cartilage explants. Cartilage explants were maintained in medium containing 0 (■), 0.5 ( ), or 5 (□) ng/ml IL-1α, with or without 100 ng/ml BMP-7. Fractional CS synthesis in mature bovine explants after A) five and B) ten days in culture, as well as that in immature bovine explants after C) 5 and D) 10 days is shown. **p<0.05 for BMP-7 main effect; *p<0.05 compared with IL-1-free controls. n=8 from triplicate experiments.
), or 5 (□) ng/ml IL-1α, with or without 100 ng/ml BMP-7. Fractional CS synthesis in mature bovine explants after A) five and B) ten days in culture, as well as that in immature bovine explants after C) 5 and D) 10 days is shown. **p<0.05 for BMP-7 main effect; *p<0.05 compared with IL-1-free controls. n=8 from triplicate experiments.
To induce catabolic changes in the cartilage explants, cultures were stimulated with IL-1. The inclusion of IL-1 did not detectably alter the sGAG content of adult explants at day 5 (p=0.7), but by day 10, tissue sGAG content was diminished by 27% with 0.5 ng/ml IL-1 (p=0.02) and by 86% with 5 ng/ml (p=<0.001; data not shown). The addition of BMP-7 at 100 ng/ml had no effect on total sGAG content at either timepoint (p=0.8 and 0.7 for days 5 and 10, respectively).
Heavy water labeling revealed that IL-1 dose-dependently inhibited the synthesis of CS in the adult explants maintained for 5 and 10 days by up to 63%, down to f of 1.2% per day (Fig. 2A-B, p<0.001 at both timepoints). The inclusion of 100 ng/ml BMP-7 stimulated fractional CS synthesis by 32% at day 10 (Tukey p=0.01), unless IL-1 was present (interaction p=0.03).
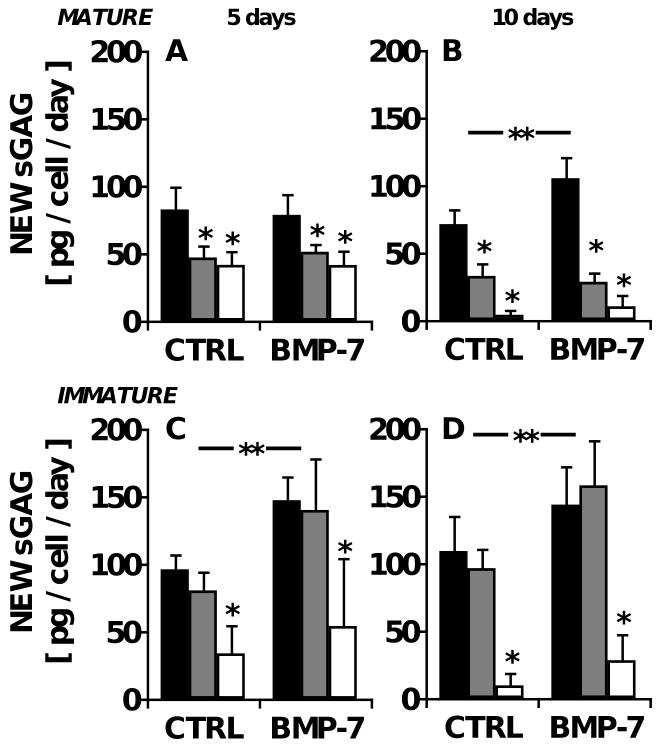
Biosynthesis of CS was also measured in explants of immature, growing articular cartilage, harvested from knee joints of 1-3 week old newborn calves. Like the adult explants, calf explants maintained in basal medium exhibited constant levels of tissue sGAG content between 5 and 10 days of culture (531±42 μg and 538±74 μg, respectively). In these immature cartilage explants, the cumulative f increased over time, from 14±2% new CS at day 5 to 29±4% at day 10 (Fig. 2C-D, n=8). The daily f was 2.9±0.4% per day, which was similar to the rate at which sGAG was released into the medium (2.6±0.9% per day), but 53% higher than that in mature tissue (Fig. 2A-B; p<0.01). In contrast, the absolute synthesis rate of sGAG was only 15% higher when expressed on a per cell basis (80±19 and 98±11 pg per day over the first 5 days for adult and calf chondrocytes, respectively; Fig. 3A, C). The highest dose (5 ng/ml) of IL-1 inhibited f by 60-61% in the calf-derived explants labeled for 5 or 10 days (Fig. 2C-D; p<0.01 at both timepoints), similar to the response of adult cartilage, but inhibition of f by the intermediate dose of IL-1 (0.5 ng/ml) was modest at day 5 (Tukey p<0.05) and undetectable at day 10 (Tukey p=0.3; Fig. 2D). The inclusion of 100 ng/ml BMP-7 stimulated f by an average of 30% (ANOVA p<0.05 for both timepoints) without a statistically significant interaction with IL-1 (interaction p=0.13-0.15) (Fig. 2C-D).
Figure 3.
Age-dependent effects of IL-1 and BMP-7 on the biosynthesis rate of sulfated glycosaminoglycan (sGAG) in bovine cartilage explants, expressed as sGAG synthesized per day. Cartilage explants were maintained in medium containing 0 (■), 0.5 ( ), or 5 (□) ng/ml IL-1α, with or without 100 ng/ml BMP-7. Absolute CS deposited is determined from the product of fractional CS synthesis and total tissue content of sGAG and presented normalized to cell number. CS biosynthesis was determined for mature bovine explants maintained for A) 5 and B) 10 days and for immature bovine explants maintained for C) 5 and D) 10 days. **p<0.05 for BMP-7 main effect; *p<0.05 compared with IL-1-free controls. n=8 from triplicate experiments.
), or 5 (□) ng/ml IL-1α, with or without 100 ng/ml BMP-7. Absolute CS deposited is determined from the product of fractional CS synthesis and total tissue content of sGAG and presented normalized to cell number. CS biosynthesis was determined for mature bovine explants maintained for A) 5 and B) 10 days and for immature bovine explants maintained for C) 5 and D) 10 days. **p<0.05 for BMP-7 main effect; *p<0.05 compared with IL-1-free controls. n=8 from triplicate experiments.
Effect of IL-1 and BMP-7 on HA synthesis in bovine explants in vitro
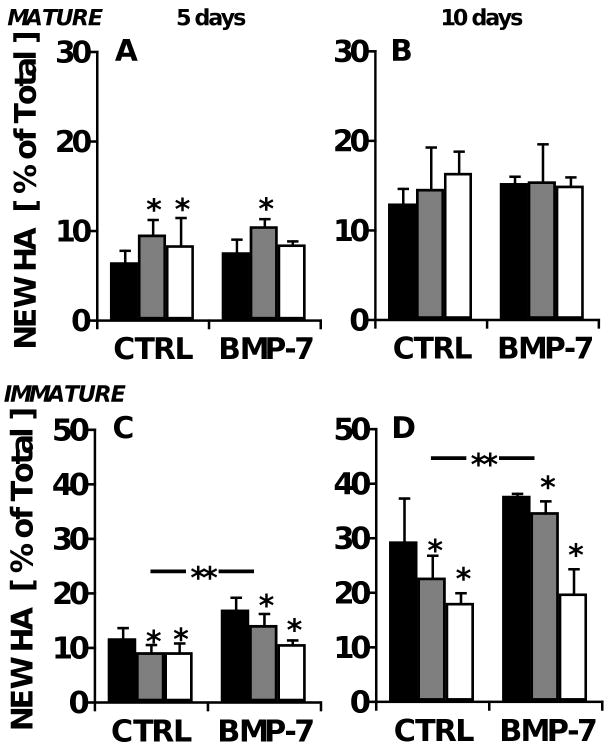
Fractional synthesis of tissue HA was determined by [2H]-label incorporation into glcNAc molecules in HA chains. For HA, f was 1.3±0.2 % per day in adult cartilage explants maintained under control conditions, 32% slower than f for CS. HA synthesis increased by an average of 32% (p=0.03) by addition of IL-1 at the early timepoint (Fig. 4A), but by day 10, the stimulation had dissipated (Fig. 4B; p=0.6). BMP-7 had no effect on HA synthesis at either timepoint (p=0.7-0.8).
Figure 4.
Age-dependent effects of IL-1 and BMP-7 on the biosynthesis of hyaluronic acid in bovine cartilage explants. Cartilage explants were maintained in medium containing 0 (■), 0.5 ( ), or 5 (□) ng/ml IL-1α, with or without 100 ng/ml BMP-7. Fractional HA synthesis in mature bovine explants after A) five and B) ten days in culture, as well as that in immature bovine explants after C) 5 and D) 10 days is shown. **p<0.05 for BMP-7 main effect; *p<0.05 compared with IL-1-free controls. n=8 from triplicate experiments.
), or 5 (□) ng/ml IL-1α, with or without 100 ng/ml BMP-7. Fractional HA synthesis in mature bovine explants after A) five and B) ten days in culture, as well as that in immature bovine explants after C) 5 and D) 10 days is shown. **p<0.05 for BMP-7 main effect; *p<0.05 compared with IL-1-free controls. n=8 from triplicate experiments.
In contrast, in immature bovine cartilage, f for HA was 2.3 times higher than in the adult cartilage (∼3% per day; Fig. 4C-D), but regulation by IL-1 and BMP-7 more closely resembled the CS response, in that IL-1 was markedly inhibitory (p=0.02 at both timepoints) and BMP-7 was markedly stimulatory (p=0.02-0.03).
Effect of IL-1 and BMP-7 on DNA synthesis in bovine explants in vitro
In adult cartilage explants, chondrocyte proliferation was generally low, with 0.2-0.4% of the total cell population proliferating per day. This low basal level of cell turnover was not detectably affected by either BMP-7 or IL-1 (Fig. 5A-B, p=0.2-0.4).
Figure 5.
Age-dependent effects of IL-1 and BMP-7 on cell proliferation in bovine cartilage explants. Cartilage explants were maintained in medium containing 0 (■), 0.5 ( ), or 5 (□) ng/ml IL-1α, with or without 100 ng/ml BMP-7. Fractional DNA synthesis in mature bovine explants after A) five and B) ten days in culture, as well as that in immature bovine explants after C) 5 and D) 10 days is shown. **p<0.05 for BMP-7 main effect; *p<0.05 compared with IL-1-free controls. n=8 from triplicate experiments.
), or 5 (□) ng/ml IL-1α, with or without 100 ng/ml BMP-7. Fractional DNA synthesis in mature bovine explants after A) five and B) ten days in culture, as well as that in immature bovine explants after C) 5 and D) 10 days is shown. **p<0.05 for BMP-7 main effect; *p<0.05 compared with IL-1-free controls. n=8 from triplicate experiments.
In contrast, cell proliferation in newborn cartilage exhibited a temporal lag, with the average f for DNA initially similar to that in the adult (0.55±0.25% per day; n=8; Fig. 5C), but increasing 6.5-fold between days 5 and 10 to an average of 2% per day (Fig. 5D). Proliferation was inhibited by IL-1 at both timepoints, (p=0.03 on day 5 and p<0.001 on day 10) and there was an overall 2-fold stimulation by BMP-7 (p=0.02 on day 5 and p<0.001 on day 10), except at the highest IL-1 dose (interaction p=0.001 on day 10).
In vivo measurements of CS synthesis and cell proliferation in articular cartilage
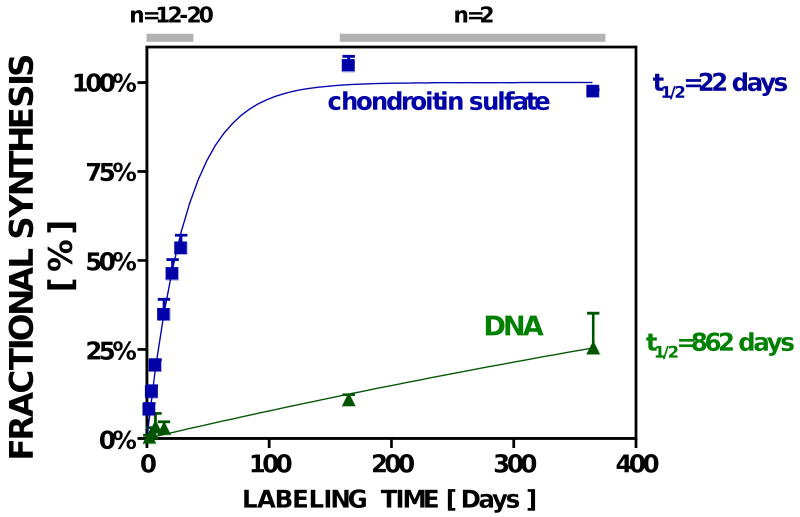
After receiving 2H2O, the average body water enrichment of rats sacrificed at timepoints ranging from 2 to 365 days was 5.2±0.5% (n=50). Based on the measured value of n=6 C-H bonds (see Online Supplement), this 2H2O enrichment (p) corresponds to an asymptotic EM1 (A1*) of 16.3% for newly-synthesized galNAc in CS. After 365 days of 2H2O, enrichment of CS from the cartilage of the MTP reached 95±0.2% of this asymptote, confirming the calculated theoretical maximum enrichment and indicating virtually complete CS turnover (Fig. 6). Over the initial 17 days of 2H2O labeling, fractional CS synthesis in the rat MTP appeared to be relatively linear (r2=0.94, p<0.01, n=48), averaging 2.6±0.5% /day, similar to the turnover rate of CS seen in adult and immature bovine cartilage in vitro over 10 days in culture. Overall, the kinetics of CS in the rat MTP exhibited a first-order half-life of 22 days.
Figure 6.
Fractional synthesis of CS & DNA (cells) in vivo in rat knee cartilage.
In contrast to the CS turnover rate in articular cartilage of the rat, chondrocyte proliferation was only 44±4% after 1 year (t1/2=862 days), and the initial rate of cell proliferation (0.68±0.09% /day; n=2) was comparable to values in the explants from newborn calves at the onset of culture. Of note, articular cartilage in vitro in rats and from newborn calf explants both exhibited detectable cell proliferation, unlike adult bovine explants.
Discussion
We describe here a method for direct measurement of the synthesis rates of multiple metabolites simultaneously from a single specimen of cartilage that is also applicable in vivo. The data presented here validate in several ways the stable isotope-mass spectrometric techniques for monitoring articular biology in vivo. First, a widely-used in vitro model system was characterized kinetically, followed by the in vivo application of the method over an extended period of time. The similarities between isotopically measured CS synthesis rates and CS release in steady-state culture of cartilage explants and the finding that rat cartilage-derived CS was enriched with deuterium to 95% of the theoretical maximum value after a year on heavy water both support the quantitative nature of this method.
Matrix and cell turnover in articular cartilage were quantified after the administration of 2H2O from the labeling of N-acetyl glucosamine, N-acetyl galactosamine and deoxyribose derived from HA, CS and DNA, respectively. Sufficient yields of the latter two analytes were obtained from a single specimen of cartilage at least 0.3 mg (by wet weight); ca. 1 mg of cartilage was necessary to reproducibly analyze HA. While the enzyme-based methods used to isolate these metabolites do not achieve purity, the subsequent GC/MS analysis (which involves both GC separation and mass identification) ensured the purity of the analytes. The degree to which cultured cartilage explants incorporated 2H into newly-synthesized HA, CS, and DNA was quantified, enabling measurements of matrix synthesis and cell proliferation that were modulated positively by BMP-7 and negatively by IL-1.
IL-1 is a proinflammatory cytokine that contributes to the disruption of matrix homeostasis in joint diseases35, in part by exerting inhibitory effects on aggrecan gene expression36 and the deposition of newly-synthesized sGAG6,20,22,37. In bovine explant cultures, we show that IL-1 inhibits the fractional synthesis of CS in both adult and immature cartilage by as much as 63% at a concentration of 5 ng/ml. This result is quantitatively similar to previous studies on cartilage metabolism, in which bovine cartilage maintained under similar culture conditions exhibited a 50-65% inhibition in radiosulfate incorporation when exposed to such high doses of IL-121,38. Furthermore, we found that under the serum-supplemented conditions used here, 0.5 ng/ml IL-1 inhibited fractional CS synthesis in mature, but not immature, bovine cartilage, indicating higher sensitivity of mature cartilage to degenerative changes. This is consistent with in vivo studies in mice39,40 and rabbits37 but other in vitro studies that directly compare the inhibition of GAG by IL-1 on young and old cartilage have shown mixed results40-43, perhaps due to differences in culture conditions. Indeed, van Beuningen and co-authors reported that age-related differences in IL-1 sensitivity dissipated when using defined, rather than serum-supplemented, medium40, a finding that underscores that the counteracting response to anabolic growth factors modulates the effect of IL-1. In our study, IL-1 also suppressed the rates of fractional HA synthesis and cell proliferation in immature bovine cartilage, whereas adult cartilage exhibited a moderate upregulation of HA synthesis in response to IL-1, which has been demonstrated previously for chondrocytes isolated from bovine animals44,45. In immature cartilage especially, the stimulation of DNA and GAG synthesis by BMP-7 was sufficient to offset the inhibitory effects of IL-1, consistent with the chondroprotective role of BMP-7 shown in vitro46 and in animal studies.47 Although BMP-7 had been thought to exert negligible effects on chondrocyte proliferation,7 our data indicate that BMP-7, in combination with serum, induced a marked stimulation of DNA synthesis in immature chondrocytes. This finding extends a recent report in which the proliferation of adult chondrocytes, isolated from their native cartilage matrix, was enhanced by the synergistic effects of BMP-7 combined with the growth factor IGF-1.48 Age-dependent variations in the response to regulatory factors such as IL-1, BMP-7 and those found in serum, as demonstrated here and elsewhere6, may clarify why young cartilage is less susceptible to the degenerative changes associated with osteoarthritis. Of interest, we found that the fractional synthesis rates of CS were 53% higher in calf cartilage than in adult cartilage, but that the absolute synthesis rate was only 15% higher when expressed on a per cell basis, suggesting that reduced cellularity at later stages of growth accounts for the majority of the age-related decline.
Stable isotope- mass spectrometric approaches are particularly well-suited for kinetic studies that require in vivo measurements. In 1952, 16-day pulse-chase studies using adult rats estimated the half-life of incorporated radiosulfate to be 17 days in rib cartilage49, comparable to the physiological turnover rate of articular cartilage CS reported here (t1/2 = 22 days). More recently, ex vivo tissue culture models have been more commonly used. Maroudas and coworkers demonstrated in rabbits and dogs that in vitro labeling of freshly harvested cartilage corresponded with in vivo labeling50, suggesting that under controlled conditions, it is possible to obtain a quantitative measure of sGAG synthesis on the basis of ex vivo experiments. When interpreting data from such assays in vitro, however, it is important to consider the injurious effects of tissue lacerations on the viability of adjacent cells51, the diffusivity of the tracer in short-term labeling protocols, and the presence or absence of confounding medium components, such as serum. Moreover, osteoarthritis, in particular, is a disease that involves interaction between multiple tissues, and as such, many relevant aspects of the pathology are difficult to reproduce in vitro.
Measurement of cartilage-related kinetics using heavy water allows for in vivo metabolic measurements over a range of labeling intervals. The incorporation of deuterium into newly-synthesized CS in rat articular cartilage was approximately linear for the first 2 weeks when heavy water was administered, with cumulative values of f reaching 35±4% after 14 days. On the other hand, mitosis occurred in vivo on a much slower timescale, with only 44% of the chondrocytes labeled as new after 1 year. We began labeling in rats at an age that is considered skeletally mature52. In rats53 and in other skeletally-mature animal models 54-56, chondrocyte proliferation has been reported to be a rare event when tritiated thymidine was used as a label. Since those studies involved short-term labeling periods (e.g., 4 hours56), low labeling indices on the order of 1 mitotic cell per 1000 would result if only 0.68% of chondrocytes turn over per day, as we measured, which may be difficult to detect with autoradiography. The ability of heavy water labeling techniques to make metabolic measurements over an extended period of time is a potential advantage for monitoring the effects of regulatory factors that are expected to exert their influence over long periods of time, such as the cumulative, time-averaged effects of exercise (joint loading), the chronic effects of osteoarthritis, or the in vivo pharmacodynamics of therapeutic interventions.
This kinetic approach has potential clinical applications. Heavy water has been studied in animal models as well as humans for over 60 years. Extensive studies with long-term administration in animal models indicate that there are no adverse effects on normal physiology, including growth, appetite, reproduction, neoplasia or hematological parameters, at 2H2O levels several-fold higher than in the present studies57,58. Indeed, at levels of deuterium enrichment in body water below 15%, harmful effects in mammals have not been detected58,59. Moreoever, maintenance of >15% deuterium enrichment in body water requires ongoing intake of drinking water at even higher enrichments (20-25% or higher). These enrichments of body water or drinking water are several-fold higher than those in the present studies, or in any human studies carried out with this method. Heavy water has also been given alone10,16,18,60-63, or as part of doubly labeled water protocols for measuring energy expenditure64-68, to thousands of human subjects without observable adverse effects. The heavy water labeling approach described here has been successfully applied in preliminary studies of non-load-bearing articular cartilage sampled in humans at the time of routine notchplasty surgery, following a few weeks of oral heavy water intake (unpublished observations). Moreover, it may be possible to develop synovial fluid biomarkers of cartilage metabolism following heavy water intake, raising the prospect of using less invasive methods.
Supplementary Material
Acknowledgments
The authors are grateful for financial support from the NIH (Grant #R21AG24120).
Funding source: NIH R21AG24120
Footnotes
Conflict of interest statement: Some authors (KWL, SAS, EWC, MA, SMT) were full-time employees of KineMed, Inc. while contributing to this research. MKH is Chief of the Scientific Advisory Board of KineMed, Inc. and has received consulting income and stock considerations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muir IHM. Articular Cartilage: Biochemistry. In: Freeman MAR, editor. Adult Articular Cartilage. Tunbridge Wells, England: Pitman Medical; 1979. pp. 145–214. [Google Scholar]
- 2.Lohmander LS, Kimura J. Biosynthesis of Cartilage Proteoglycan. In: Kuettner K, Schleyerbach R, Hascall VC, editors. Articular Cartilage Biochemistry. New York: Raven Press; 1986. [Google Scholar]
- 3.Mow VC, Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. New York: Raven Press; 1997. pp. 113–178. [Google Scholar]
- 4.Stockwell RA, Meachim G. The chondrocytes. In: Freeman MAR, editor. Adult Articular Cartilage. Tunbridge Wells, England: Pitman Medical; 1979. pp. 69–144. [Google Scholar]
- 5.Buckwalter JA, Mankin HJ. Articular cartilage. Part II: degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg. 1997;79-A:612–632. [Google Scholar]
- 6.Morris EA, Treadwell BV. Effect of interleukin 1 on articular cartilage from young and aged horses and comparison with metabolism of osteoarthritic cartilage. Am J Vet Res. 1994;55:138–146. [PubMed] [Google Scholar]
- 7.Chubinskaya S, Kuettner KE. Regulation of osteogenic proteins by chondrocytes. Int J Biochem Cell Biol. 2003;35:1323–1340. doi: 10.1016/s1357-2725(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 8.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol. 1999;276:E1146–1170. doi: 10.1152/ajpendo.1999.276.6.E1146. [DOI] [PubMed] [Google Scholar]
- 9.Hellerstein MK. Relationship between precursor enrichment and ratio of excess M2/excess M1 isotopomer frequencies in a secreted polymer. J Biol Chem. 1991;266:10920–10924. [PubMed] [Google Scholar]
- 10.Busch R, Cesar D, Higuera-Alhino D, Gee T, Hellerstein MK, McCune JM. Isolation of peripheral blood CD4(+) T cells using RosetteSep and MACS for studies of DNA turnover by deuterium labeling. J Immunol Methods. 2004;286:97–109. doi: 10.1016/j.jim.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Gardner JL, Turner SM, Bautista A, Lindwall G, Awada M, Hellerstein MK. Measurement of liver collagen synthesis by heavy water labeling: effects of profibrotic toxicants and antifibrotic interventions. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1695–1705. doi: 10.1152/ajpgi.00209.2006. [DOI] [PubMed] [Google Scholar]
- 12.Hellerstein MK. New stable isotope-mass spectrometric techniques for measuring fluxes through intact metabolic pathways in mammalian systems: introduction of moving pictures into functional genomics and biochemical phenotyping. Metab Eng. 2004;6:85–100. doi: 10.1016/j.ymben.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Dufner D, Previs SF. Measuring in vivo metabolism using heavy water. Curr Opin Clin Nutr Metab Care. 2003;6:511–517. doi: 10.1097/00075197-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Turner SM, Roy S, Sul HS, Neese RA, Murphy EJ, Samandi W, et al. Dissociation between adipose tissue fluxes and lipogenic gene expression in ob/ob mice. Am J Physiol Endocrinol Metab. 2007;292:E1101–1109. doi: 10.1152/ajpendo.00309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neese RA, Misell LM, Turner S, Chu A, Kim J, Cesar D, et al. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci U S A. 2002;99:15345–15350. doi: 10.1073/pnas.232551499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busch R, Kim YK, Neese RA, Schade-Serin V, Collins M, Awada M, et al. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta. 2006;1760:730–744. doi: 10.1016/j.bbagen.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Shimamoto H, Komiya S. The turnover of body water as an indicator of health. J Physiol Anthropol Appl Human Sci. 2000;19:207–212. doi: 10.2114/jpa.19.207. [DOI] [PubMed] [Google Scholar]
- 18.Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115:755–764. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson F, Dahlberg L, Laverty S, Reiner A, Pidoux I, Ionescu M, et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115–2125. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aydelotte MB, Raiss RX, Caterson B, Kuettner KE. Influence of interleukin-1 on the morphology and proteoglycan metabolism of cultured bovine articular chondrocytes. Connect Tissue Res. 1992;28:143–159. doi: 10.3109/03008209209014233. [DOI] [PubMed] [Google Scholar]
- 21.Smith RJ, Rohloff NA, Sam LM, Justen JM, Deibel MR, Cornette JC. Recombinant human interleukin-1 alpha and recombinant human interleukin-1 beta stimulate cartilage matrix degradation and inhibit glycosaminoglycan synthesis. Inflammation. 1989;13:367–382. doi: 10.1007/BF00914921. [DOI] [PubMed] [Google Scholar]
- 22.Billinghurst RC, Wu W, Ionescu M, Reiner A, Dahlberg L, Chen J, et al. Comparison of the degradation of type II collagen and proteoglycan in nasal and articular cartilages induced by interleukin-1 and the selective inhibition of type II collagen cleavage by collagenase. Arthritis Rheum. 2000;43:664–672. doi: 10.1002/1529-0131(200003)43:3<664::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 23.Koepp HE, Sampath KT, Kuettner KE, Homandberg GA. Osteogenic protein-1 (OP-1) blocks cartilage damage caused by fibronectin fragments and promotes repair by enhancing proteoglycan synthesis. Inflamm Res. 1999;48:199–204. doi: 10.1007/s000110050446. [DOI] [PubMed] [Google Scholar]
- 24.Nishida Y, D'Souza AL, Thonar EJ, Knudson W. Stimulation of hyaluronan metabolism by interleukin-1alpha in human articular cartilage. Arthritis Rheum. 2000;43:1315–1326. doi: 10.1002/1529-0131(200006)43:6<1315::AID-ANR14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Nishida Y, Knudson CB, Knudson W. Osteogenic Protein-1 inhibits matrix depletion in a hyaluronan hexasaccharide-induced model of osteoarthritis. Osteoarthritis Cartilage. 2004;12:374–382. doi: 10.1016/j.joca.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Calabro A, Hascall VC, Midura RJ. Adaptation of FACE methodology for microanalysis of total hyaluronan and chondroitin sulfate composition from cartilage. Glycobiology. 2000;10:283–293. doi: 10.1093/glycob/10.3.283. [DOI] [PubMed] [Google Scholar]
- 27.Turner SM, Murphy EJ, Neese RA, Antelo F, Thomas T, Agarwal A, et al. Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am J Physiol Endocrinol Metab. 2003;285:E790–803. doi: 10.1152/ajpendo.00402.2002. [DOI] [PubMed] [Google Scholar]
- 28.Kurland IJ, Alcivar A, Bassilian S, Lee WN. Loss of [13C]glycerol carbon via the pentose cycle. Implications for gluconeogenesis measurement by mass isotoper distribution analysis. J Biol Chem. 2000;275:36787–36793. doi: 10.1074/jbc.M004739200. [DOI] [PubMed] [Google Scholar]
- 29.Chen JL, Peacock E, Samady W, Turner SM, Neese RA, Hellerstein MK, et al. Physiologic and pharmacologic factors influencing glyceroneogenic contribution to triacylglyceride glycerol measured by mass isotopomer distribution analysis. J Biol Chem. 2005;280:25396–25402. doi: 10.1074/jbc.M413948200. [DOI] [PubMed] [Google Scholar]
- 30.Lee WN, Bassilian S, Ajie HO, Schoeller DA, Edmond J, Bergner EA, et al. In vivo measurement of fatty acids and cholesterol synthesis using D2O and mass isotopomer analysis. Am J Physiol. 1994;266:E699–708. doi: 10.1152/ajpendo.1994.266.5.E699. [DOI] [PubMed] [Google Scholar]
- 31.Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK. Measurement of cell proliferation by heavy water labeling. Nat Protoc. 2007;2:3045–3057. doi: 10.1038/nprot.2007.420. [DOI] [PubMed] [Google Scholar]
- 32.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 33.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen® and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 34.Kim YJ, Sah RLY, Doong JYH, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 35.Heinegard D, Inerot S, Olsson SE, Saxne T. Cartilage proteoglycans in degenerative joint disease. J Rheumatol. 1987;14:110–112. Spec No: [PubMed] [Google Scholar]
- 36.Fan Z, Bau B, Yang H, Soeder S, Aigner T. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1beta. Arthritis Rheum. 2005;52:136–143. doi: 10.1002/art.20725. [DOI] [PubMed] [Google Scholar]
- 37.Arner EC. Effect of animal age and chronicity of interleukin-1 exposure on cartilage proteoglycan depletion in vivo. J Orthop Res. 1994;12:321–330. doi: 10.1002/jor.1100120304. [DOI] [PubMed] [Google Scholar]
- 38.Pratta MA, Di Meo TM, Ruhl DM, Arner EC. Effect of interleukin-1-beta and tumor necrosis factor-alpha on cartilage proteoglycan metabolism in vitro. Agents Actions. 1989;27:250–253. doi: 10.1007/BF01972788. [DOI] [PubMed] [Google Scholar]
- 39.van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: age-related differences. Ann Rheum Dis. 1994;53:593–600. doi: 10.1136/ard.53.9.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Beuningen HM, Arntz OJ, van den Berg WB. In vivo effects of interleukin-1 on articular cartilage. Prolongation of proteoglycan metabolic disturbances in old mice. Arthritis Rheum. 1991;34:606–615. doi: 10.1002/art.1780340513. [DOI] [PubMed] [Google Scholar]
- 41.Hickery MS, Bayliss MT, Dudhia J, Lewthwaite JC, Edwards JC, Pitsillides AA. Age-related changes in the response of human articular cartilage to IL-1alpha and transforming growth factor-beta (TGF-beta): chondrocytes exhibit a diminished sensitivity to TGF-beta. J Biol Chem. 2003;278:53063–53071. doi: 10.1074/jbc.M209632200. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald MH, Stover SM, Willits NH, Benton HP. Regulation of matrix metabolism in equine cartilage explant cultures by interleukin 1. Am J Vet Res. 1992;53:2278–2285. [PubMed] [Google Scholar]
- 43.Nietfeld JJ, Wilbrink B, Den Otter W, Huber J, Huber-Bruning O. The effect of human interleukin 1 on proteoglycan metabolism in human and porcine cartilage explants. J Rheumatol. 1990;17:818–826. [PubMed] [Google Scholar]
- 44.Kolibas LM, Goldberg RL. Effect of cytokines and anti-arthritic drugs on glycosaminoglycan synthesis by bovine articular chondrocytes. Agents Actions. 1989;27:245–249. doi: 10.1007/BF01972787. [DOI] [PubMed] [Google Scholar]
- 45.D'Souza AL, Masuda K, Otten LM, Nishida Y, Knudson W, Thonar EJ. Differential effects of interleukin-1 on hyaluronan and proteoglycan metabolism in two compartments of the matrix formed by articular chondrocytes maintained in alginate. Arch Biochem Biophys. 2000;374:59–65. doi: 10.1006/abbi.1999.1626. [DOI] [PubMed] [Google Scholar]
- 46.Huch K, Wilbrink B, Flechtenmacher J, Koepp HE, Aydelotte MB, Sampath TK, et al. Effects of recombinant human osteogenic protein 1 on the production of proteoglycan, prostaglandin E2, and interleukin-1 receptor antagonist by human articular chondrocytes cultured in the presence of interleukin-1beta. Arthritis Rheum. 1997;40:2157–2161. doi: 10.1002/art.1780401209. [DOI] [PubMed] [Google Scholar]
- 47.Cook SD, Patron LP, Salkeld SL, Rueger DC. Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg Am. 2003;85-A 3:116–123. doi: 10.2106/00004623-200300003-00018. [DOI] [PubMed] [Google Scholar]
- 48.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–2196. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 49.Bostrom H. On the metabolism of the sulfate group of chondroitinsulfuric acid. J Biol Chem. 1952;196:477–481. [PubMed] [Google Scholar]
- 50.Maroudas A. Glycosaminoglycan turn-over in articular cartilage. Philos Trans R Soc Lond B Biol Sci. 1975;271:293–313. doi: 10.1098/rstb.1975.0054. [DOI] [PubMed] [Google Scholar]
- 51.Maroudas A. Physico-chemical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Tunbridge Wells, England: Pitman Medical; 1979. pp. 215–290. [Google Scholar]
- 52.Hughes PC, Tanner JM. The assessment of skeletal maturity in the growing rat. J Anat. 1970;106:371–402. [PMC free article] [PubMed] [Google Scholar]
- 53.Tonna EA, Cronkite EP. A Study of the Persistence of the H3-Thymidine Label in the Femora of Rats. Lab Invest. 1964;13:161–171. [PubMed] [Google Scholar]
- 54.Rotzer A, Mohr W. 3H-thymidine incorporation into chondrocytes of arthritic cartilage. Z Rheumatol. 1992;51:172–176. [PubMed] [Google Scholar]
- 55.Mankin HJ. Mitosis in articular cartilage of immature rabbits. A histologic, stathmokinetic (colchicine) and autoradiographic study. Clin Orthop Relat Res. 1964;34:170–183. [PubMed] [Google Scholar]
- 56.Havdrup T, Telhag H. Mitosis of chondrocytes in normal adult joint cartilage. Clin Orthop Relat Res. 1980:248–252. [PubMed] [Google Scholar]
- 57.Koletzko B, Sauerwald T, Demmelmair H. Safety of stable isotope use. Eur J Pediatr. 1997;156 1:S12–17. doi: 10.1007/pl00014267. [DOI] [PubMed] [Google Scholar]
- 58.Jones PJ, Leatherdale ST. Stable isotopes in clinical research: safety reaffirmed. Clin Sci (Lond) 1991;80:277–280. doi: 10.1042/cs0800277. [DOI] [PubMed] [Google Scholar]
- 59.Thomson JF. Biological effects of deuterium. NY: Pergannon Press; 1963. [Google Scholar]
- 60.Schwartz GN, Vance BA, Levine BM, Fukazawa M, Telford WG, Cesar D, et al. Proliferation kinetics of subpopulations of human marrow cells determined by quantifying in vivo incorporation of [2H2]-glucose into DNA of S-phase cells. Blood. 2003;102:2068–2073. doi: 10.1182/blood-2003-01-0139. [DOI] [PubMed] [Google Scholar]
- 61.Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96:2735–2743. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Misell LM, Hwang ES, Au A, Esserman L, Hellerstein MK. Development of a novel method for measuring in vivo breast epithelial cell proliferation in humans. Breast Cancer Res Treat. 2005;89:257–264. doi: 10.1007/s10549-004-2228-5. [DOI] [PubMed] [Google Scholar]
- 63.Misell LM, Williams N, Turek P, Hellerstein M. Development of an in vivo stable isotope-mass spectrometric method for measuring proliferation of prostate epithelial cells (PEC) from prostate tissue and ejaculated seminal. Preceedings of the American Association for Cancer Research. 2004;45:1032–1033. [Google Scholar]
- 64.Schoeller DA. Recent advances from application of doubly labeled water to measurement of human energy expenditure. J Nutr. 1999;129:1765–1768. doi: 10.1093/jn/129.10.1765. [DOI] [PubMed] [Google Scholar]
- 65.Schoeller DA. Insights into energy balance from doubly labeled water. Int J Obes (Lond) 2008;32 7:S72–75. doi: 10.1038/ijo.2008.241. [DOI] [PubMed] [Google Scholar]
- 66.Ravussin E, Harper IT, Rising R, Bogardus C. Energy expenditure by doubly labeled water: validation in lean and obese subjects. Am J Physiol. 1991;261:E402–409. doi: 10.1152/ajpendo.1991.261.3.E402. [DOI] [PubMed] [Google Scholar]
- 67.Johannsen DL, DeLany JP, Frisard MI, Welsch MA, Rowley CK, Fang X, et al. Physical activity in aging: comparison among young, aged, and nonagenarian individuals. J Appl Physiol. 2008;105:495–501. doi: 10.1152/japplphysiol.90450.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao M, McCrory MA, Ma G, Li Y, Dolnikowski GG, Roberts SB. Energy requirements of urban Chinese adults with manual or sedentary occupations, determined using the doubly labeled water method. Eur J Clin Nutr. 2002;56:575–584. doi: 10.1038/sj.ejcn.1601361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.