Abstract
Hemoglobin digestion in the midgut of hematophagous animals results in the release of its prosthetic group, heme, which is a pro-oxidant molecule. Heme enzymatic degradation is a protective mechanism that has been described in several organisms, including plants, bacteria, and mammals. This reaction is catalyzed by heme oxygenase and results in formation of carbon monoxide, ferrous ion, and biliverdin IXα. During digestion, a large amount of a green pigment is produced and secreted into the intestinal lumen of A. aegypti adult females. In the case of another blood-sucking insect, the kissing-bug Rhodnius prolixus, we have recently shown that heme degradation involves a complex pathway that generates dicysteinyl-biliverdin IX gamma. The light absorption spectrum of the Aedes purified pigment was similar to biliverdin, but its mobility on a reverse-phase chromatography column suggested a compound less hydrophobic than biliverdin IXα. Structural characterization by ESI-MS revealed that the mosquito pigment is the α isomer of biliverdin bound to two glutamine residues by an amide bond. This biglutaminyl-biliverdin is formed by oxidative cleavage of the heme porphyrin ring followed by two subsequent additions of glutamine residues to the biliverdin IXα. The role of this pathway in the adaptation of this insect vector to a blood-feeding habit is discussed.
Keywords: heme, heme oxygenase, biliverdin, detoxification, Aedes aegypti
Dengue fever, associated with dengue hemorrhagic fever (DHF), is the most prevalent vector-borne viral disease affecting humans, transmitted mainly by the mosquito Aedes aegypti (1). The ability of A. aegypti females to transmit these viruses from one vertebrate host to another is strictly related to their hematophagous habit. Ingestion of a blood meal triggers intense protein digestion which takes place in the lumen, providing nutrients required for oogenesis (2) but also leading to the release of high amounts of free heme due to host hemoglobin proteolysis.
Heme is potentially toxic due to its capacity to promote the generation of reactive oxygen species (ROS) (3). Being an amphiphilic molecule, heme can also insert itself into phospholipid bilayers, leading to destabilization of membrane structure and cell lysis (4). Blood-sucking arthropods present a complex array of heme detoxification and antioxidant mechanisms (5) that allow them to counteract free heme cytotoxicity and adapt to hematophagy. These include heme-binding proteins in the hemolymph of kissing-bugs (6) and ticks (7), high levels of antioxidant enzymes in the midgut of Rhodnius prolixus (8), and aggregation of heme in the midgut of this kissing-bug (9) and ticks (10, 11). In A. aegypti, the peritrophic matrix, an extracellular layer that is secreted by the midgut cells and separates the meal from the midgut epithelium, acts as a protective mechanism by binding free heme released during digestion (12). Recently, an Aedes aegypti peritrophin – a protein component of the peritrophic matrix – has been shown to be a heme-binding protein (13).
From mammals and plants to bacteria and yeast, most organisms deal with heme toxicity by means of degradation by heme oxygenase (HO) (14). The conversion of heme into biliverdin IXα, CO and Fe2+ by HO proceeds by a multistep mechanism that involves rapid hydroxylation at the meso-carbon of the porphyrin ring, oxygen-dependent elimination of the hydroxylated meso-carbon as CO producing α-verdoheme, and oxidative cleavage of α-verdoheme to biliverdin IXα, in a reaction dependent on reducing equivalents and O2, with concomitant release of Fe2+ (15). In mammals, biliverdin is further reduced, by biliverdin reductase, to bilirubin (16), which circulates in plasma bound to albumin and is taken up by the hepatocyte and finally is excreted into the bile mainly as bilirubin glucuronide conjugates (17).
We have recently shown that in the blood-sucking hemipteran insect, Rhodnius prolixus, heme oxidative degradation is mechanistically distinct from the well characterized reaction catalyzed by canonical HO. It involves an initial step of modification of heme by addition of two cysteinylglycine residues prior to cleavage – at the gamma position instead of the usual alpha meso-carbon – of the porphyrin ring, followed by proteolytic trimming of these dipeptides, to produce dicysteinyl-BV IX as the main end-product (18). The present work describes the heme degradation pathway in the mosquito A. aegypti and shows that – in contrast to the R. prolixus – this involves oxidative cleavage of heme without previous modification, in the α meso-carbon. However, distinct from all other previously described organisms, the biliverdin produced by this reaction is further modified by conjugation of two glutamine residues, leading to the formation of an excretable biglutaminyl-biliverdin IXα.
EXPERIMENTAL PROCEDURES
Chemicals
Hemin (Fe(III) protoporphyrin IX chloride), Fe(III)-mesoporphyrin IX and biliverdin IXα were purchased from Frontier Scientific (Logan, UT). Biliverdin IXγ was kindly provided by Dr. Boris Dunkov (University of Arizona, Tucson, AZ).
Mosquitoes and pigment extraction
Aedes aegypti (RED strain) were from a colony kept at the Universidade Federal do Rio de Janeiro, Instituto de Biofísica Carlos Chagas Filho. They were maintained at 28 °C ± 1 °C and 75 to 80% relative humidity, with a 12 h light/dark photoperiod. Larvae were fed with dog chow pellets (Purina) and adults were offered 10% sucrose ad libitum. Three to five days after emergence females were used in the experiments.
For pigment identification and purification, females were fed with rabbit blood. In artificial feeding, meals were offered through a Parafilm membrane stretched across the bottom of a water-jacketed glass feeder apparatus kept at 37 °C adapted from (19). Stock 5 mM hemin (Fe(III) protoporphyrin IX chloride), Fe(III)-mesoporphyrin IX or biliverdin IXα solutions were freshly prepared in 0.1 N NaOH. These solutions were neutralized by adding four volumes of 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.4. In order to feed insects, these porphyrin solutions were then mixed with blood or plasma to achieve the desired concentration. Biliverdin IXγ was a gift from Dr. Boris Dunkov.
Female midguts were dissected under 50% ethanol, transferred to 10 mM sodium phosphate, 0.15 M NaCl, pH 7.4 (PBS), homogenized and centrifuged for 15 min at 12,000 X g. Supernatants were dried under vacuum using a speed-vac system (model SC 110, Savant-NY, USA), kept protected from light, and stored at -20 °C until use.
HPLC fractionation and light absorption spectra
HPLC was performed on a Shimadzu CLC-ODS C18 column (15mm × 22cm) using a Shimadzu LC-10AT device (Tokyo, Japan), equipped with a diode array detector (SPD-M10A). Chromatography analysis was performed using 5% acetonitrile with 0.05% trifluoroacetic acid (TFA) as solvent, at a flow rate of 0.4 ml/min. Before injection, dried midguts homogenates were diluted in 20% acetonitrile with 0.05% TFA and centrifuged for 15 min at 12,000 X g. Ten minutes after sample injection a 40 min linear acetonitrile gradient (5–80%) was applied, followed by 20 min of 80% acetonitrile. Light absorption spectra were recorded during the chromatography by the HPLC diode array detector.
Electrospray ionization mass spectrometry (ESI-MS)
Mass spectra were obtained in the positive-ion mode using a Finnigan LCQ-Duo ion trap mass spectrometer (Thermo Electron Co., San Jose, CA). HPLC fractions were prepared in 50% acetonitrile, 0.1% formic acid. Heme stock solutions were made in 100% DMSO and diluted in 50% methanol immediately before use. Samples were introduced into the electrospray source by injection through a 50-Xm internal diameter fused silica capillary at a 5 μl/min flow rate. ESI source and capillary voltages were set at 36-46 V and 4.5 kV, respectively; the capillary temperature was 250 °C. Spectra were acquired at 3 s/scan. Collision-induced fragmentation (tandem ESI-MS) of parent ions was carried out using a relative collision energy of 30-50% (1.5-2.5eV). Source-induced dissociation (SID) (cone fragmentation) was achieved by applying a cone fragmentation voltage of 92 V.
N-acetylation of free amino groups of A. aegypti biliverdin
Authentic biliverdin IXα and purified A. aegypti biliverdin (AaBV) were resuspended in pre-cooled 100 μL 1 M NH4OH. After addition of 2.5 μl acetic anhydride, samples were incubated for 10 min at 4 °C. This step was repeated 2 times. After incubation at 25 °C for 30 min, samples were dried under vacuum and resuspended in 5% acetonitrile, 0.1% formic acid for ESI-MS analysis.
Methylation of carboxyl groups of biliverdin
Biliverdin IXα and purified AaBV were resuspended in 100 μl NH4OH. After addition of 100 μl 100% methanol, samples were incubated for 1 h at 37 °C. Samples were dried under vacuum and washed two times with 100 μl 100% methanol. The precipitates were resuspended in 100 μl of methanol and incubated for 1 h at 75 °C. After addition of tert-butanol (20 μl), samples were dried under vacuum and resuspended in 50% acetonitrile, 0.1% formic acid for ESI-MS analysis.
RESULTS
Purification and identification of green pigment
Digestion in the mosquito midgut lumen shows maximum proteolysis of host blood proteins by 24 h following feeding (20). Interestingly, the water-soluble fraction of the midgut contents shows an intense modification in color during the course of digestion; changing from bright red, typical of hemoglobin, to a green color, suggesting degradation of heme to a bilin pigment (Figures 1A and B).
Figure 1.
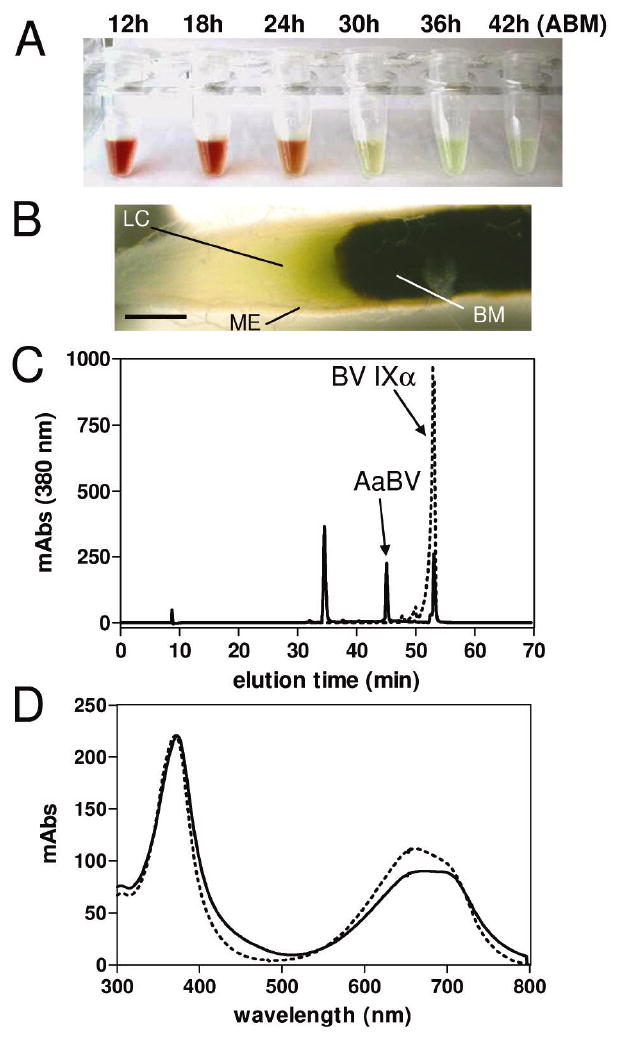
Accumulation of a bilin pigment in the midgut of A. aegypti after a blood meal. (A) Midgut dissected at different times after a blood meal (ABM) were homogenized in PBS and centrifuged. Tubes containing the supernatants are shown. (B) A. aegypti midgut 38 h ABM. Midgut epithelium (ME); lumenal content (LC); blood meal (BM); scale bar = 0.2 mm. (C) A. aegypti pigment was purified by reverse phase HPLC using a C18 column. Chromatograms of A. aegypti midgut PBS extract (solid lines) and standard biliverdin IXα (dashed lines) are shown. (D) Light-absorption spectra of peaks containing AaBV (solid line) and BV IXα (dashed lines).
In order to characterize the A. aegypti green pigment, we analyzed the midgut homogenates by reverse-phase HPLC (Figure 1C). One of the major peaks displayed a light-absorption spectrum similar to that of biliverdin IXα, with two λmax at 379 nm and 690 nm (Figure 1D). However, this component was eluted with a lower retention time than BV IXα, suggesting a compound more hydrophilic than BV IXα (Figure 1C), the product of heme degradation catalyzed by most HO enzymes (14).
Feeding mosquitoes with plasma drastically reduced the amount of green pigment in the midgut when compared to insect fed on blood (Figures 2B and A, respectively). Addition of heme to plasma, in a concentration twenty-fold lower than that found in host blood, partially reversed this effect (Figure 2C). This result strongly suggests that the green component present in the insect midgut is a degradation product of heme from the blood meal.
Figure 2.
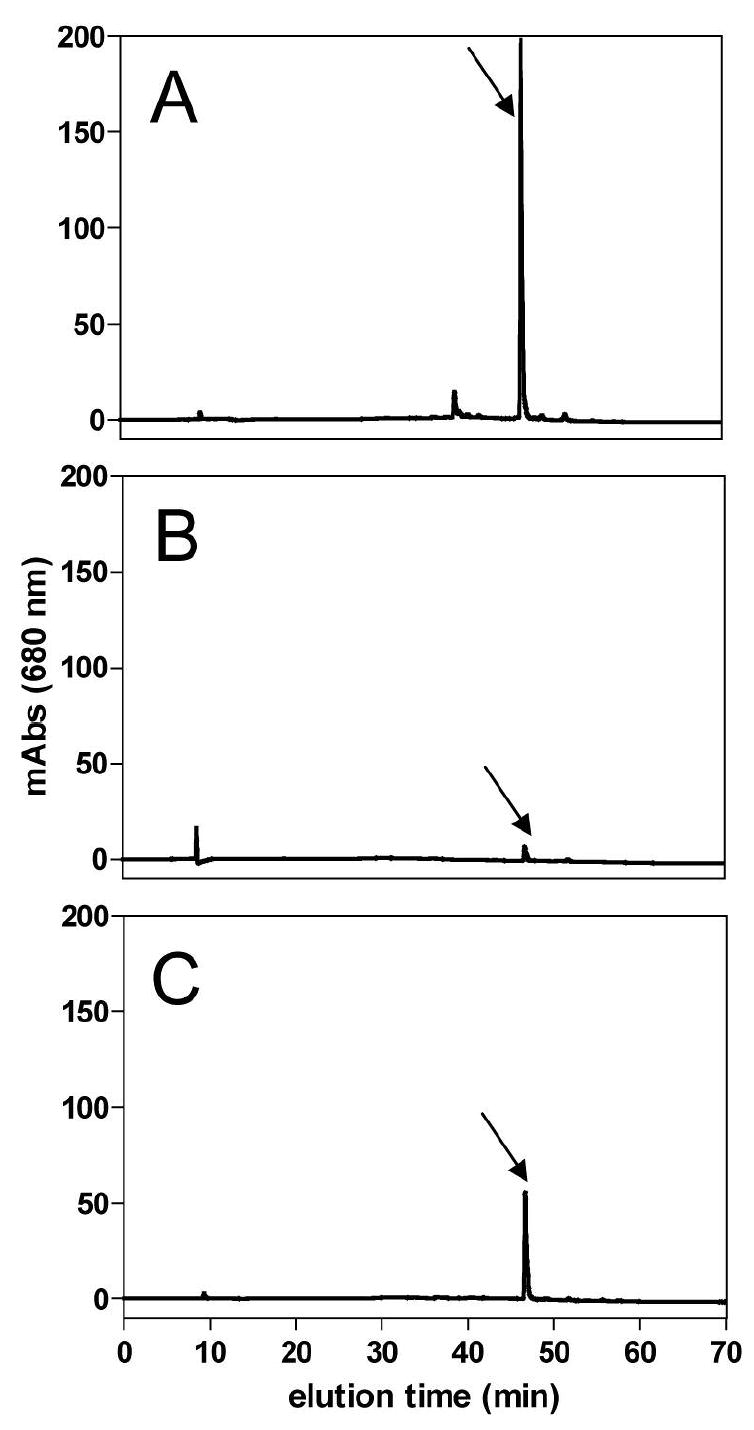
A. aegypti bilin pigment is derived from ingested heme. (A) HPLC profile of midgut homogenate dissected 24 h after meal from 5 females fed on blood, (B) plasma, and (C) plasma supplemented with 0.5 mM hemin. Arrow indicates AaBV peak.
Structural characterization of A. aegypti biliverdin
Purified AaBV was analyzed by ESI-MS. This molecule showed a molecular mass (M) of 838.3 Da ([AaBV + H]+ at m/z 839.3) (Figure 3B), higher than that of biliverdin IXα ([BV IXα + H]+ at m/z 583.2) (Figure 3A). Analysis of AaBV sequential fragmentation by tandem ESI-MS produced an ion species at m/z 711, by removal of a 128 Da residue (Figure 3C). The subsequent fragmentation of this species produced a daughter-ion species with the same molecular mass as BV IXα (m/z 583.2), again by the loss of a 128 Da fragment (Figure 3D). These results demonstrated that AaBV consists of a BV IX coupled to two residues of 128 Da.
Figure 3.
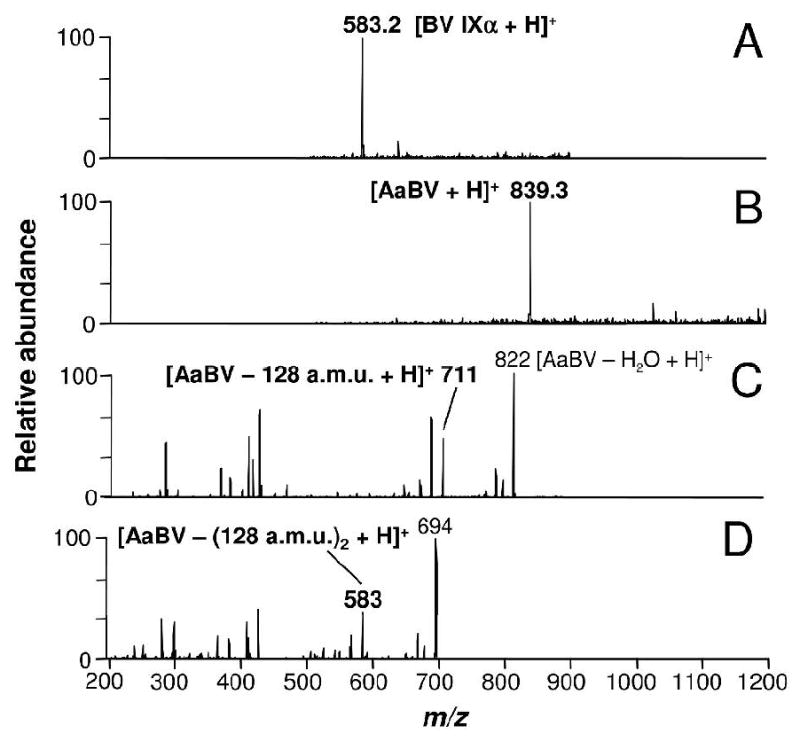
ESI-MS analysis of A. aegypti biliverdin. (A) Mass spectrum of BV IXα [M + H]+ at m/z 583.2 and (B) AaBV [M + H]+ at m/z 839.3, revealed a molecular mass for A. aegypti purified pigment of 838 Da, higher than BV IXα. (C) Fragmentation of AaBV (tandem ESI-MS spectrum) produced a daughter-ion species at m/z 711, by the loss of a 128 Da fragment. (D) MS3 of the species at m/z 711 revealed a 582 Da fragment by the loss again of a 128 Da fragment. a.m.u., atomic mass unit.
In order to definitively prove that the green pigment produced by the mosquito during blood digestion was a BV IXα, we compare the tandem ESI-MS profile of BV IXα with that of the ion species at m/z 583 produced by AaBV sequential fragmentation (Figure 4). A daughter-ion species at m/z 297 was produced by AaBV fragmentation (Figure 4A). This ion species is typically produced by the fragmentation of the α isomer of BV IX (21, 18) (Figure 4B). In contrast, the γ isomer of BV IX, produced by some insects (22), generates a different spectrum, with a characteristic daughter-ion species at m/z 402 (18) (Figure 4C). The structure of both BV isomers and the assumed fragmentation pattern are shown as insets (Figures 4B and C).
Figure 4.
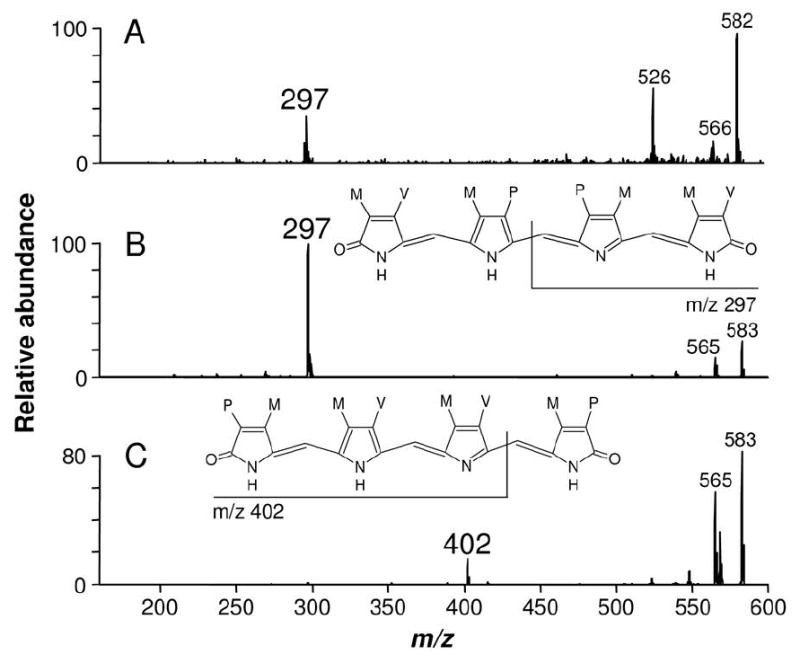
AaBV isomer identification. (A) Fragmentation profiles of AaBV ion species at m/z 583 and (B) BV IXα showed identical daughter-ion species at m/z 297, in contrast to (C) BV IXγ fragmentation profile that presented its typical daughter-ion species at m/z 402. Structure of BV IXα and BV IXγ and respective assignments of their daughter-ions are shown.
Analysis and identification of the two 128 Da fragments
Based on these results, we concluded that the AaBV is the α isomer of BV modified by two fragments of 128 Da. According to the solubility of the pigment and to molecular mass of the residues, it was plausible that these fragments were amino acids, since they have charged groups and compatible molecular mass. Among them, two amino acids presented molecular mass of 128 Da (or 146 Da for the hydrated molecule, m/z 147): glutamine and lysine. The glutamic acid, with molecular mass of 129 Da (or 147 Da for the hydrated molecule, m/z 148), would be a less feasible candidate since the addition of two glutamic acids to BV IXα would result in a compound with molecular mass of 840 Da, higher than that of AaBV.
Cone fragmentation of AaBV produced ion species with m/z at 147 (data not shown). When fragmented and analyzed by tandem ESI-MS, this ion species produced, among other fragments, a daughter-ion at m/z 101 (Figure 5A). The same species was produced by glutamine fragmentation but not from lysine (Figure 5B and C, respectively). In fact, the fragment at m/z 101 is considered a signature of glutamine when analyzed by ESI-MS fragmentation (23). Lysine fragmentation did not share any common major ion with the species at m/z 147 produced from AaBV (Figure 5C). None of the ion species produced by fragmentation of glutamic acid were found in AaBV fragmentation profile (data not shown). This result indicated that AaBV was likely to be produced by the attachment of two glutamine residues, resulting in a biglutaminyl-biliverdin.
Figure 5.
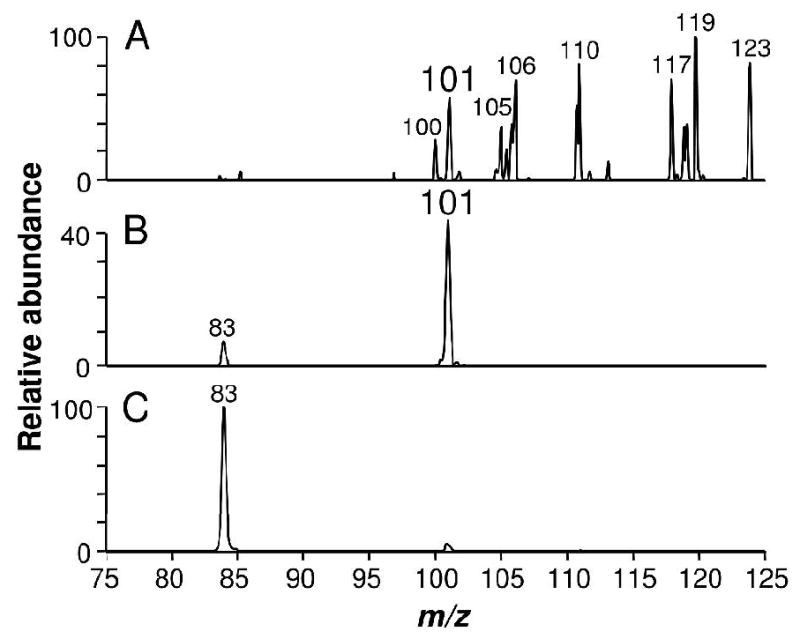
AaBV hydrophilic residues are glutamines. (A) ESI-MS spectrum of the ion species at m/z 147 (hydrated 128 Da) produced by cone fragmentation from AaBV. Fragmentation of (B) authentic glutamine and (C) lysine.
Identification of the chemical groups involved in the covalent attachment of glutamine to biliverdin
To confirm this unusual biliverdin modification and to better investigate the nature of AaBV, females were fed with plasma supplemented with a heme analog, Fe(III)mesoporphyrin IX, whose vinyls are substituted by ethyl groups. The resulting product showed an elution time in the reverse-phase HPLC and light absorption spectrum (Figure 6B) close, but not identical, to AaBV, the molecule produced by feeding insects with blood or plasma plus heme (Figure 6A). ESI-MS analysis of mesoheme derived molecule showed ions species at m/z 843.3 (M = 842.3 Da) that corresponds to mesoAaBV (Figure 6D). The differences observed in the molecular mass between mesoAaBV and AaBV were due to the four extra hydrogens from the ethane residues present in the former molecule (Figure 6D and C, respectively). These results demonstrated that this insect is able to catalyze the oxidative cleavage of the mesoheme porphyrin ring generating mesoBV, and further modify this molecule by addition of the two glutamines. Furthermore, they showed that glutamine linkages in AaBV do not involve the vinyl groups of BV IX tetrapyrrol. Based on the previous results, we hypothesized that BV IX carboxyl propionates were involved in glutamine linkage.
Figure 6.
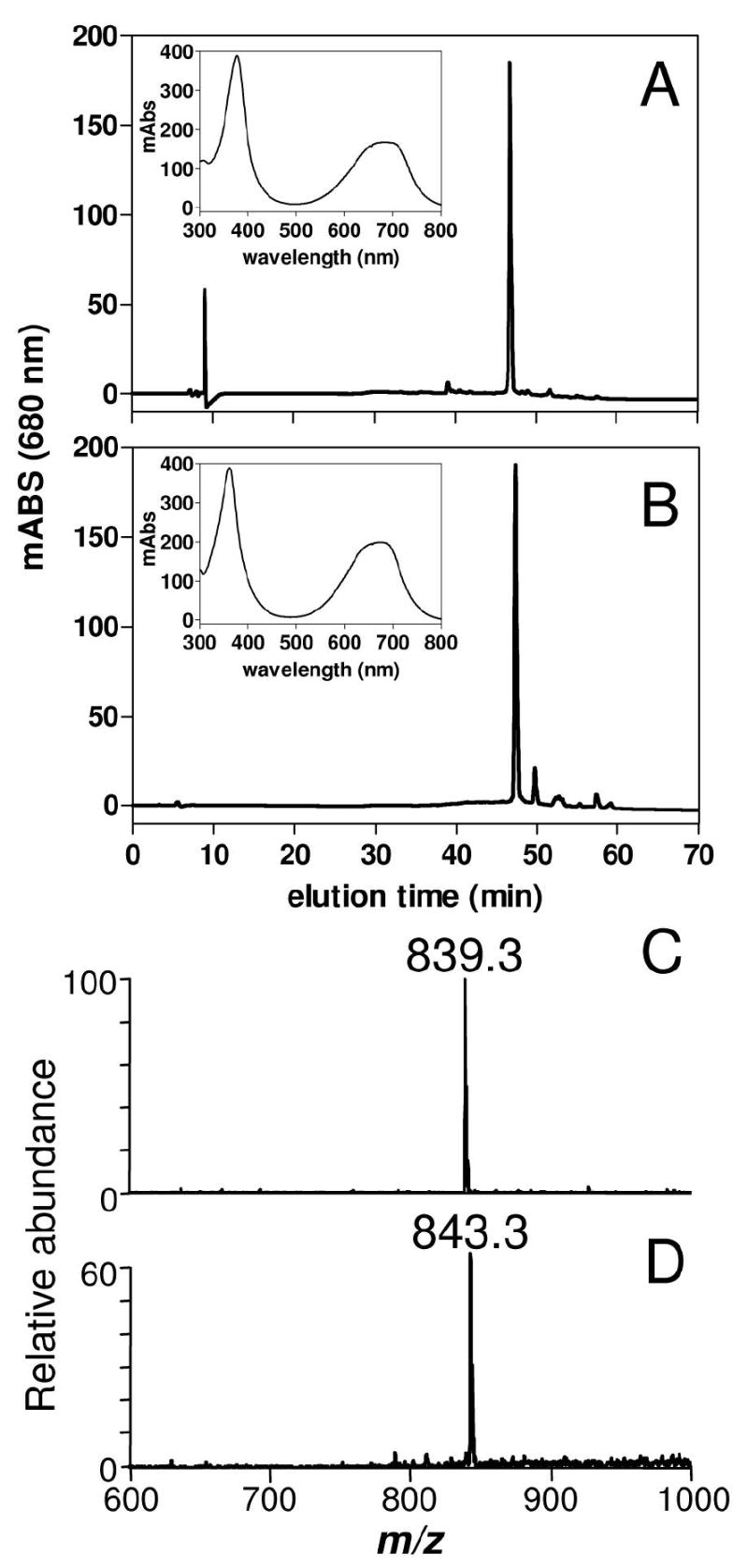
Degradation of mesoheme and production of mesoAaBV by A. aegypti midgut. (A) HPLC profile of midgut homogenates dissected 24 h after meal from females fed on plasma supplemented with 1.0 mM hemin or with (B) 1.0 mM Fe(III) mesoporphyrin IX. Light-absorbance spectra of the bilin peak are shown as insets in panels (A) and (B). ESI-MS spectra of modified bilin produced by glutamine addition after feeding on plasma supplemented with (C) hemin or (D) with Fe(III) mesoporphyrin IX.
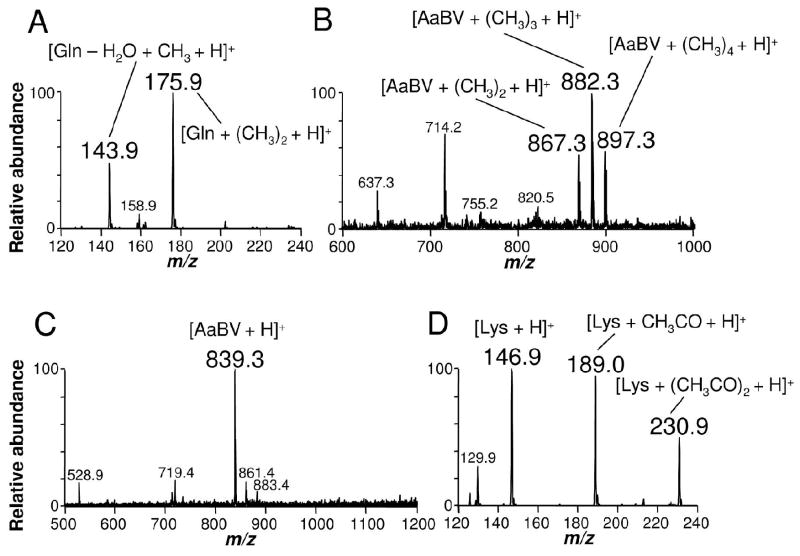
In order to definitely characterize residues of BV IXα involved in the binding of glutamine residues, methylation and acetylation reactions were carried out to identify free carboxyl and amine groups, respectively (Figure 7). Methylation of glutamine increased its molecular mass by addition of two methyl groups, resulting in m/z at 175.9 (Figure 7A). This was expected, since under the methylation assay conditions – high temperature in acid medium – the amide groups can be converted into carboxyl groups (24). When submitted to methylation, only two methyl groups were added to BV IXα, corresponding to the modification of the two propionate carboxyl free groups (data not shown). In contrast, methylation of AaBV produced four methyl additions (m/z at 897.3): two bound to the glutamine carboxyl groups and two bound to the amide-derived carboxyls of both glutamines (Figure 7B). If propionate carboxyl groups were also free in AaBV, as they are in BV IXα, this reaction would result into addition of six methyl groups. These data supported the involvement of biliverdin propionate groups in glutamine attachment. No modification of AaBV molecular mass after acetylation reaction was observed, indicating absence of free amine groups (Figure 7C). This result confirmed that amine groups of glutamine were involved in the binding of BV to these aminoacid residues through an amide bond, and discards definitively the participation of lysine in AaBV structure. A positive control of the reaction was performed by acetylation of the aminoacid lysine (Figure 7D). The proposed structure for AaBV is shown in Figure 9.
Figure 7.
Identification of the chemical groups involved in the covalent attachment of glutamine to BV IXα in AaBV. AaBV, glutamine and lysine were submitted to methylation or acetylation reactions to identify free COOH and amino groups, respectively. ESI-MS analysis of (A) glutamine and (B) AaBV after methylation or (C) AaBV and (D) lysine after acetylation.
Figure 9.
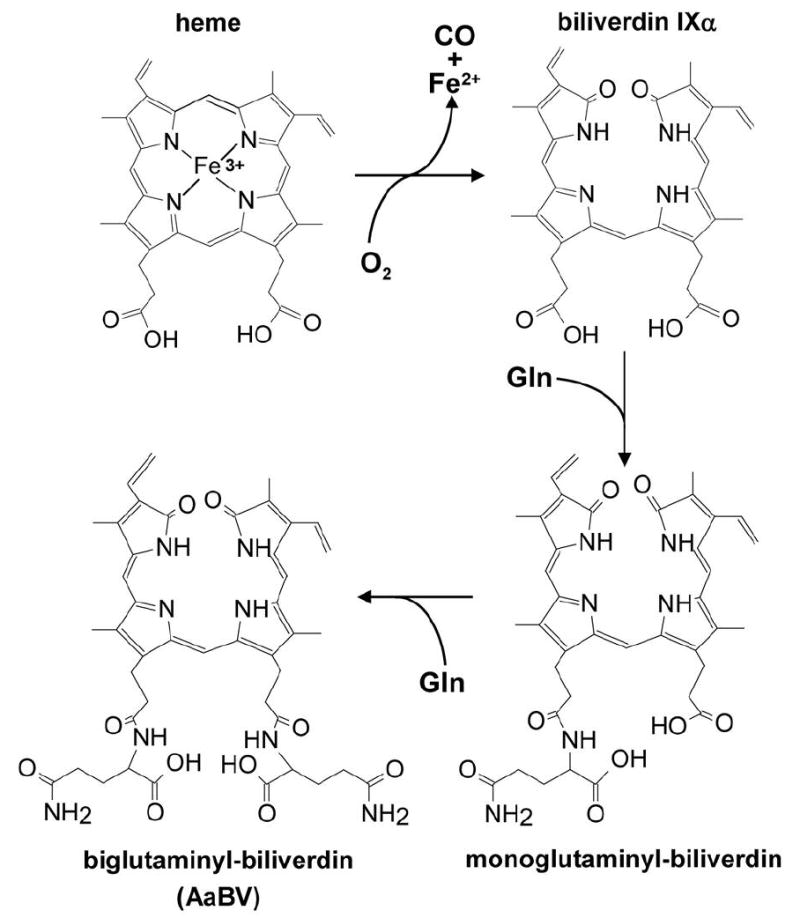
Biglutaminyl-biliverdin generation pathway in Aedes aegypti. Heme degradation into BV IXα is followed by two sequential additions of glutamine residues.
AaBV formation pathway
In order to observe the addition of the two glutamine residues to the BV in the mosquito midgut, adult females were fed on plasma supplemented with BV IXα. Reverse phase-HPLC fractionation of insect midgut homogenates revealed the presence of a molecule with the same elution time as AaBV (Figure 8A). Both molecules have the same light absorption (data not shown) and ESI-MS spectra (Figures 3B and 8B). Moreover, ESI-MS analysis of a minor peak that was eluted between AaBV and BV IXα revealed a compound with m/z at 711 (M = 710 Da) (Figure 8C), which corresponds to the precise molecular mass of a biliverdin IX linked to one residue of glutamine (monoglutaminyl-BV). Its tandem ESI-MS profile revealed a daughter-ion species at m/z 583 (identical to the singly-charged BV IXα species; Figure 3A), generated by the loss of a 128 Da fragment that corresponds to a glutamine residue (Figure 8D). The ion species at m/z 583 was identified as the α isomer of BVIX by subsequent fragmentation (MS3) (Figure 8E). These data demonstrated that BV IXα is modified by two sequential additions of glutamine residues. The proposed AaBV biosynthesis pathway is represented schematically in Figure 9. The posibility that the two glutamine residues are tandemly linked to one of the propionate of the biliverdin IX can not be excluded. However, this structure seems to be less plausible due to the fact that we could not found any of the specific fragments that would be generated by fragmentation of this molecule by ESI-MS (data not shown).
Figure 8.
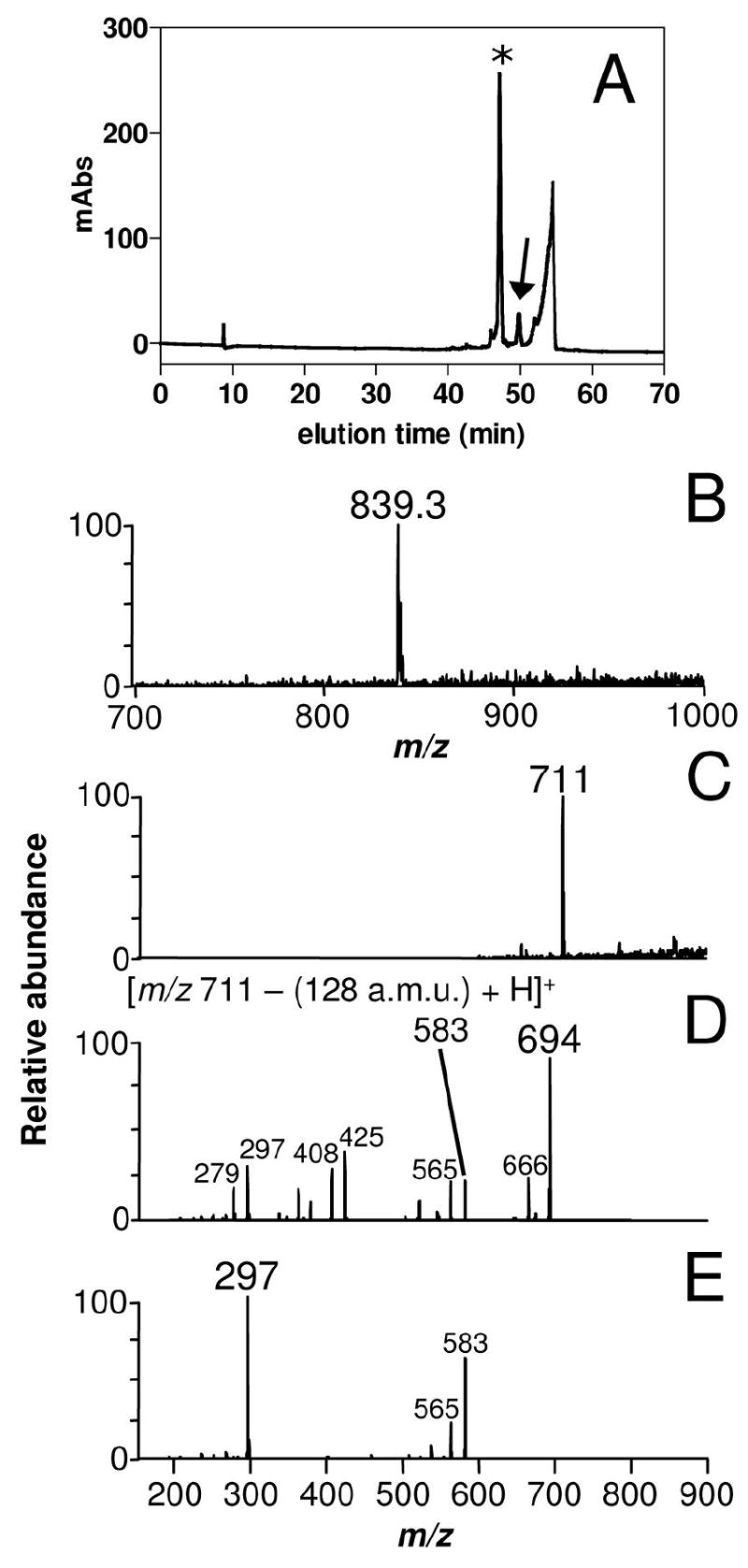
Identification of monoglutaminyl-BV as an intermediate of the pathway. (A) HPLC profile of midgut homogenates dissected 24 h after meal from females fed on plasma supplemented with 0.5 mM BV IXα. (B) ESI/MS analysis of AaBV produced upon feeding with BV IXα (indicated by an asterisk in the chromatogram). (C) ESI/MS analysis of a minor peak (arrow in Panel A) eluted between AaBV and BV IXα. (D) Fragmentation (tandem ESI-MS) spectrum of monoglutaminyl-BV IXα (m/z 711) produced a daughter-ion species at m/z 583, which, by further fragmentation (MS3) (E) gave origin to similar daughter-ion species as BV IXα (Figure 4B).
DISCUSSION
Here, we have characterized a unique heme degradation pathway found in the mosquito A. aegypti, one of the major vectors of dengue virus. We show that, in this mosquito, heme detoxification initiates by the HO reaction, followed by sequential addition of two glutamines to the biliverdin IX α produced by HO. This pathway is clearly distinct from the one identified by our group in another hematophagous insect, the hemipteran R. prolixus (18), as well as it is different from the mammalian mechanism of disposal of excess bilin. The end product of heme degradation in R. prolixus is a γ-biliverdin with two cysteine residues attached to the vinyl groups and the heme molecule is initially modified prior oxidative cleavage of the porphyrin. In mammals, biliverdin is eventually converted to the hydrophobic bilirubin that, when found in high concentrations, can be extremely toxic. The insoluble bilirubin, is modified by mono- and bi-glucuronidation in the liver, to make a soluble compound that can be excreted along with the feces and the bile. Deleterious consequences of accumulation of these pigments are illustrated by diseases such as the Crigler-Najar Syndrome, where failure in bilirubin conjugation to glucuronic acid causes jaundice and severe neurological disorders (3,25). The common trend between biliverdin modifications produced by A. aegypti and R. prolixus – and mammals as well – is the necessity to turn these molecules more soluble and easier to be excreted. Considering that the habit of blood feeding evolved independently several times in different groups of hematophagous arthropods (26), these modifications could represent an evolutive convergence to avoid membrane disturbance and cytotoxicity caused by hydrophobic bile pigments. Taken all together, these results point out that the toxicity of high amounts of bilin pigments may be seen as a selective pressure that ultimately led to the development of mechanisms to allow efficient disposal of these compounds as part of the adaptation of these animals to a blood feeding way of life.
Although we were not able to find BV IXα in the midgut of Aedes, the fact that feeding A. aegypti female with plasma and BV IXα led to a monoglutaminyl-BV accumulation – besides biglutaminyl-BV – suggests that BV IXα is an intermediate in the mosquito pathway. This result supported the conclusion that this pathway has a regular heme oxygenase activity as its initial step, implying an enzyme similar to that found in most organisms. This is consistent with the finding of a gene with high degree of similarity with a heme oxygenase in the recently released Aedes genome (GenBank accession number: AY433250). Feeding with plasma and Fe(III)mesoporphyrin IX demonstrated that the enzyme responsible for the heme degradation in A. aegypti was able to catalyze this reaction in the absence of vinyl groups. Crystal structure of mammalian heme oxygenase-1 shows that charge interactions of propionate groups are very important in the correct orientation of the heme, placing the α-meso carbon in the position for hydroxylation. In contrast, vinyl and methyl heme substituents appeared not to be important in the alignment of heme in the enzyme catalytic site (27). These data reinforce the conclusion that a typical HO activity is responsible for the enzymatic degradation of heme in the mosquito midgut. In fact, most of the human HO residues (Lys 18, Lys 22, Tyr 134, Lys 179, Arg 183) known to surround the propionates of heme seem to be conserved in the insect enzyme (Arg 8, Arg 12, Lys 114, Lys 163, Arg 167 respectively) (data not shown).
We observed that Aedes aegypti is able to modify a α-biliverdin isomer by two glutamine covalent additions. This amino acid can be supplied by proteolysis of blood meal proteins, or, alternatively, by synthesis from glutamate and ammonia. Increased glutamine synthetase mRNA expression by A. aegypti midgut post blood feeding has been reported (28). It was proposed that this reaction provides the glutamine needed for the initial steps of biosynthesis of chitin, the main component of peritrophic matrix, which is synthesized in response to a blood meal (29). Likewise, glutamine hemolymphatic levels increase after protein feeding and remain high during ovary development (30).
A situation that resembles the production of AaBV concerning the type of chemical linkages that are formed is the synthesis of plant volatiles formation elicitors in the midgut of plant-feeding lepidoteran. Plant volatiles are compounds synthesized by plants in response to herbivorism. Formation of plant volatiles can be triggered by N-linolenoyl-L-glutamine, formed by a microsomal fraction of digestive apparatus from the moth Manduca sexta (31). The type of linkage between glutamine and linolenate, a hydrophobic molecule such as biliverdin, is an amide bond between the α-amino group of the glutamine and the carboxyl group of the fatty acid, the same linkage observed in AaBV. In the midgut of Spodoptera exigua the linolenic acid portion is derived from larval food while the glutamine is provided by the caterpillar (32). A similar situation may be occurring in the production of AaBV.
Free heme has been shown to have harmful effects on cell physiology either by acute effects that lead to immediate lysis of certain cell types upon exposure to this molecule or, alternatively, heme toxicity can be exerted by inducing alterations of cell metabolism that eventually led to cell death (3). Heme oxygenase has been attributed an important role in cellular protection as a consequence of its capacity to ameliorate toxic effects of heme (33). We have proposed that blood-feeding organisms face a special situation regarding heme toxicity, as a consequence of digestion of their blood meal (5). Therefore, heme degradation in A. aegypti by the pathway described here is certainly an adaptation to a blood feeding way of life. In addition to lowering heme concentration in midgut cells, HO can be protective also due to the fact that BV, one of the products of the enzyme, is a powerful antioxidant (34,35). Moreover, recent reports have shown that both heme and its degradation products (biliverdin and CO) are capable of interfering in gene expression and cell signaling, especially in the modulation of immunity (36-40). A question for future research is whether or not these regulatory roles of CO and biliverdin – described for mammalian immune cells – are also valid for heme degradation in insects, especially in the case of the AaBV.
Besides its role in heme detoxification, this heme degradation pathway may be crucial in the acquisition of free iron - an essential micronutrient required as a cofactor of a variety of proteins and in hemeproteins biosynthesis - as it has been shown for some pathogenic bacterias (41-43). In fact, it has already been shown that, in A. aegypti, the blood meal induces the expression of ferritin (44-45), the main protein involved in storage and transport of iron in insects (reviewed in 46).
Finally, microarray mRNA expression analysis of an Anopheles strain refractory to Plasmodium indicated that these mosquitoes exhibited a chronic state of oxidative stress, which was exacerbated by blood feeding, resulting in increased steady-state levels of ROS, which favored melanization of parasites (47). Considering the scenario of the midgut of a hematophagous insect, the presence of a heme degradation pathway such as the one described here represents a new important factor that may interfere both with signaling cascades that govern innate immunity and with the control of redox balance, two aspects of vector biology that are starting to be recognized as crucial determinants of vectorial competence.
Acknowledgments
We thank Maria Conceição Costa for colony maintenance, S.J. Tadeu, S.R. de Cassia and M. Okada for expert assistance and Boris Dunkov for providing biliverdin IX gamma.
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Coordenação de Aperfeiçoamento do Pessoal de nível Superior (CAPES), Fundação de Amparo à Pesquisa de Estado do Rio de Janeiro (FAPERJ), Fundação Universitária José Bonifácio (FUJB), Programa de Núcleos de Excelência (PRONEX), Howard Hughes Medical Institute (HHMI) and by Fundação de Amparo à Pesquisa de Estado de São Paulo (FAPESP, grant #98/10495-5. The project described was in part supported by Grant Number 5G12RR008124 (to the Border Biomedical Research Center/University of Texas at El Paso) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
The abbreviations used are
- BV
biliverdin
- AaBV
Biliverdin from Aedes aegypti
- ROS
reactive oxygen species
- HO
heme oxygenase
- ABM
after a blood meal
- PBS
phosphate buffered saline
- HPLC
high performance liquid chromatography
- TFA
trifluoroacetic acid
- ESI-MS
electronspray ionization mass spectrometry
- BV IXα
biliverdin IXα
- Gln
glutamine
- Lys
lysine
- Arg
arginine
- tyr
tyrosine
References
- 1.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briegel H, Lea AO. Relationship between protein and proteolytic activity in the midgut of mosquitoes. J Insect Physiol. 1975;21:1597–1604. doi: 10.1016/0022-1910(75)90197-3. [DOI] [PubMed] [Google Scholar]
- 3.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radical Biol Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt TH, Frezzatti WA, Jr, Schreier S. Hemin-induced lipid membrane disorder and increased permeability: a molecular model for the mechanism of cell lysis. Arch Biochem Biophys. 1993;307:96–103. doi: 10.1006/abbi.1993.1566. [DOI] [PubMed] [Google Scholar]
- 5.Graça-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol. 2006;36:322–335. doi: 10.1016/j.ibmb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Dansa-Petretski M, Ribeiro JM, Atella GC, Masuda H, Oliveira PL. Antioxidant role of Rhodnius prolixus heme-binding protein. Protection against heme-induced lipid peroxidation. J Biol Chem. 1995;270:10893–10896. doi: 10.1074/jbc.270.18.10893. [DOI] [PubMed] [Google Scholar]
- 7.Maya-Monteiro CM, Daffre S, Logullo C, Lara FA, Alves EW, Capurro ML, Zingali R, Almeida IC, Oliveira PL. HeLp, a heme lipoprotein from the hemolymph of the cattle tick, Boophilus microplus, J Biol Chem. 2000;275:36584–36589. doi: 10.1074/jbc.M007344200. [DOI] [PubMed] [Google Scholar]
- 8.Paes MC, Oliveira MB, Oliveira PL. Hydrogen peroxide detoxification in the midgut of the blood-sucking insect, Rhodnius prolixus. Arch Insect Biochem Physiol. 2001;48:63–71. doi: 10.1002/arch.1058. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira MF, Silva JR, Dansa-Petretski M, de Souza W, Lins U, Braga CM, Masuda H, Oliveira PL. Haem detoxification by an insect. Nature. 1999;400:517–518. doi: 10.1038/22910. [DOI] [PubMed] [Google Scholar]
- 10.Lara FA, Lins U, Paiva-Silva G, Almeida IC, Braga CM, Miguens FC, Oliveira PL, Dansa-Petretski M. A new intracellular pathway of haem detoxification in the midgut of the cattle tick Boophilus microplus: aggregation inside a specialized organelle, the hemosome. J Exp Biol. 2003;206:1707–1715. doi: 10.1242/jeb.00334. [DOI] [PubMed] [Google Scholar]
- 11.Lara FA, Lins U, Bechara GH, Oliveira PL. Tracing heme in a living cell: hemoglobin degradation and heme traffic in digest cells of the cattle tick Boophilus microplus. J Exp Biol. 2005;208:3093–3101. doi: 10.1242/jeb.01749. [DOI] [PubMed] [Google Scholar]
- 12.Pascoa V, Oliveira PL, Dansa-Petretski M, Silva JR, Alvarenga PH, Jacobs-Lorena M, Lemos FJ. Aedes aegypti peritrophic matrix and its interaction with heme during blood digestion. Insect Biochem Mol Biol. 2002;32:517–523. doi: 10.1016/s0965-1748(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 13.Devenport M, Alvarenga PH, Shao L, Fujioka H, Bianconi ML, Oliveira PL, Jacobs-Lorena M. Identification of the Aedes aegypti peritrophic matrix protein AeIMUCI as a heme-binding protein. Biochemistry. 2006;45:9540–9549. doi: 10.1021/bi0605991. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz de Montellano PR, Wilks A. Heme Oxygenase: Structure and Mechanism. Adv Inorg Chem. 2000;51:359–407. [Google Scholar]
- 15.Ortiz de Montellano PR. The mechanism of heme oxygenase. Curr Opin Chem Biol. 2000;4:221–227. doi: 10.1016/s1367-5931(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 16.Kutty RK, Maines MD. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981;256:3956–3962. [PubMed] [Google Scholar]
- 17.Billing BH, Cole PG, Lathe GH. The excretion of bilirubin as a diglucuronide giving the direct van den Bergh reaction. Biochem J. 1957;65:774–784. doi: 10.1042/bj0650774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paiva-Silva GO, Cruz-Oliveira C, Nakayasu ES, Maya-Monteiro CM, Dunkov BC, Masuda H, Almeida IC, Oliveira PL. A heme-degradation pathway in a bloodsucking insect. Proc Natl Acad Sci USA. 2006;103:8030–8035. doi: 10.1073/pnas.0602224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia ES, Macarini JD, Garcia ML, Ubatuba FB. Feeding of Rhodnius prolixus in the laboratory. An Acad Bras Cienc. 1975;47:537–545. [PubMed] [Google Scholar]
- 20.Noriega FG, Wells MA. A molecular view of trypsin synthesis in the midgut of Aedes aegypti. J Insect Physiol. 1999;45:613–620. doi: 10.1016/s0022-1910(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 21.Niittynen M, Tuomisto JT, Auriola S, Pohjanvirta R, Syrjala P, Simanainen U, Viluksela M, Tuomisto J. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced accumulation of biliverdin and hepatic peliosis in rats. Toxicol Sci. 2003;71:112–123. doi: 10.1093/toxsci/71.1.112. [DOI] [PubMed] [Google Scholar]
- 22.Kayser H. Pigments. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 10. Pergamon Press; Oxford: 1985. pp. 367–415. [Google Scholar]
- 23.Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An Elicitor of Plant Volatiles from Beet Armyworm Oral Secretion. Science. 1997;276:945–949. [Google Scholar]
- 24.Trinh MU, Blake J, Harrison JR, Gerace R, Ranieri E, Fletcher JM, Johnson DW. Quantification of glutamine in dried blood spots and plasma by tandem mass spectrometry for the biochemical diagnosis and monitoring of ornithine transcarbamylase deficiency. Clin Chem. 2003;49:681–684. doi: 10.1373/49.4.681. [DOI] [PubMed] [Google Scholar]
- 25.McDonagh A. Turning green to gold. Nat Struct Biol. 2001;8:198–200. doi: 10.1038/84915. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro JM. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4:143–152. [PubMed] [Google Scholar]
- 27.Schuller DJ, Wilks A, Ortiz de Montellano PR, Poulos TL. Crystal structure of human heme oxygenase-1. Nat Struct Biol. 1999;6:860–867. doi: 10.1038/12319. [DOI] [PubMed] [Google Scholar]
- 28.Smartt CT, Chiles J, Lowenberger C, Christensen BM. Biochemical analysis of a blood meal-induced Aedes aegypti glutamine synthetase gene. Insect Biochem Mol Biol. 1998;28:935–945. doi: 10.1016/s0965-1748(98)00073-3. [DOI] [PubMed] [Google Scholar]
- 29.Shao L, Devenport M, Jacobs-Lorena M. The peritrophic matrix of hematophagous insects. Arch Insect Biochem Physiol. 2001;47:119–125. doi: 10.1002/arch.1042. [DOI] [PubMed] [Google Scholar]
- 30.Goldstrohm DA, Pennington JE, Wells MA. The role of hemolymph proline as a nitrogen sink during blood meal digestion by the mosquito Aedes aegypti, J Insect Physiol. 2003;49:115–121. doi: 10.1016/s0022-1910(02)00267-6. [DOI] [PubMed] [Google Scholar]
- 31.Lait CG, Alborn HT, Tumlinson JH., 3rd Rapid biosynthesis of N-linolenoyl-L-glutamine, an elicitor of plant volatiles, by membrane-associated enzyme(s) in Manduca sexta. Proc Natl Acad Sci USA. 2003;100:7027–7032. doi: 10.1073/pnas.1232474100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paré PW, Alborn HT, Tumlinson JH. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc Natl Acad Sci USA. 1998;95:13971–13975. doi: 10.1073/pnas.95.23.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 34.McDonagh AF. Evidence for singlet oxygen quenching by biliverdin IX-alpha dimethyl ester and its relevance to bilirubin photo-oxidation. Biochem Biophys Res Commun. 1972;48:408–415. doi: 10.1016/s0006-291x(72)80066-4. [DOI] [PubMed] [Google Scholar]
- 35.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 36.Graça-Souza AV, Barja-Fidalgo C, Arruda MAB, de Freitas MS, Oliveira PL. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002;99:4160–4165. doi: 10.1182/blood.v99.11.4160. [DOI] [PubMed] [Google Scholar]
- 37.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 38.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 39.Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid Redox Signal. 2005;7:80–91. doi: 10.1089/ars.2005.7.80. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J. 2004;18:765–767. doi: 10.1096/fj.03-0839fje. [DOI] [PubMed] [Google Scholar]
- 41.Wilks A, Schmitt MP. Expression and characterization of a heme oxygenase (Hmu O) from Corynebacterium diphtheriae. Iron acquisition requires oxidative cleavage of the heme macrocycle. J Biol Chem. 1998;273:837–841. doi: 10.1074/jbc.273.2.837. [DOI] [PubMed] [Google Scholar]
- 42.Zhu W, Wilks A, Stojiljkovic I. Degradation of heme in gram-negative bacteria: the product of the hemO gene of Neisseriae is a heme oxygenase. J Bacteriol. 2000;182:6783–6790. doi: 10.1128/jb.182.23.6783-6790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratliff M, Zhu W, Deshmukh R, Wilks A, Stojiljkovic I. Homologues of neisserial heme oxygenase in gram-negative bacteria: degradation of heme by the product of the pigA gene of Pseudomonas aeruginosa. J Bacteriol. 2001;183:6394–6403. doi: 10.1128/JB.183.21.6394-6403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunkov BC, Georgieva T, Yoshiga T, Hall M, Law JH. Aedes aegypti ferritin heavy chain homologue: feeding of iron or blood influences message levels, lengths and subunit abundance. J Insect Sci. 2002;2:7. doi: 10.1093/jis/2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gêiser DL, Chavez CA, Flores-Munguia R, Winzerling JJ, Pham DQ. Aedes aegypti ferritin. Eur J Biochem. 2003;270:3667–3674. doi: 10.1046/j.1432-1033.2003.03709.x. [DOI] [PubMed] [Google Scholar]
- 46.Law JH. Insects, oxygen, and iron. Biochem Biophys Res Commun. 2002;19:1191–1195. doi: 10.1006/bbrc.2001.2015. [DOI] [PubMed] [Google Scholar]
- 47.Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimopoulos G, Kafatos FC, Barillas-Mury C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci USA. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]