Abstract
Platelets contribute to the development of metastasis, the most common cause of mortality in cancer patients, but the precise role that anti-platelet drugs play in cancer treatment is not defined. Metastatic tumor cells can produce platelet aIIbb3 activators, such as ADP and thromboxane A2 (TXA2). Inhibitors of platelet b3 integrins decrease bone metastases in mice but are associated with significant bleeding. We examined the role of a novel soluble apyrase/ ADPase, APT102, and an inhibitor of TXA2 synthesis, acetylsalicylic acid (aspirin or ASA), in mouse models of experimental bone metastases. We found that treatment with ASA and APT102 in combination (ASA + APT102), but not either drug alone, significantly decreased breast cancer and melanoma bone metastases in mice with fewer bleeding complications than observed with aIIbb3 inhibition. ASA + APT102 diminished tumor cell induced platelet aggregation but did not directly alter tumor cell viability. Notably, APT102 + ASA treatment did not affect initial tumor cell distribution and similar results were observed in b3−/− mice. These results show that treatment with ASA + APT102 decreases bone metastases without significant bleeding complications. Anti-platelet drugs such as ASA + APT102 could be valuable experimental tools for studying the role of platelet activation in metastasis as well as a therapeutic option for the prevention of bone metastases.
Keywords: ADPase, aspirin, bone metastasis, platelets
Bone metastases, the most common type of metastasis for patients with breast and prostate cancer, can result in significant bone loss, fractures, pain, hypercalcemia, and spinal cord compression. Osteoclast mediated bone resorption is a critical component of bone metastasis and can promote tumor growth in bone [Guise, 2000; Mundy, 2002; Hirbe et al., 2006]. Anti-resorptive therapy significantly decreases skeletal complications of cancer and is considered standard of care for patients with bone metastases; however, 50% of patients with bone metastases develop new bone metastases, new skeletal complications and disease progression [Hortobagyi et al., 1996; Roodman, 2004].
Pre-clinical and clinical data support a facilitating role for platelets, another non-malignant host factor, in the metastatic process [Pruemer, 2005; Castelli and Porro, 2006; Zwicker et al., 2007]. As cancer cells travel from a primary site to a distant metastatic site, they co-exist with platelets in thrombi located throughout the organs and circulatory system of patients with metastatic cancer [Gouin-Thibault et al., 2001; Billroth, 2003]. More than 50% of metastatic breast cancer patients have tumor cells detectable in blood termed circulating tumor cells (CTC). CTC number and response to cytotoxic therapy have been shown to predict progression-free and overall survival [Wiedswang et al., 2004; Braun et al., 2005].
Metastatic tumor cell lines induce platelet adhesion and aggregation in vitro through the production of numerous platelet activators such as ADP, thromboxane, thrombin, and von Willebrand factor [Lerner et al., 1983; Gasic, 1984; Karpatkin et al., 1988; Nierodzik et al., 1996; Bakewell et al., 2003; Camerer et al., 2004; Medina et al., 2006; Witz, 2006]. Indeed, the metastatic potential of tumor cell lines is markedly diminished in mice with defective platelet aggregation [Karpatkin et al., 1988; Amirkhosravi et al., 2002; Francis and Amirkhosravi, 2002; Bakewell et al., 2003; Camerer et al., 2004; Hu et al., 2004; Shi et al., 2004; Palumbo et al., 2005]. Moreover, tumor cells engineered to respond to platelet-derived lysophosphatidic acid (LPA) have enhanced bone metastatic potential in mice [Boucharaba et al., 2004]. However, few studies in humans have directly tested the efficacy of anti-platelet agents to inhibit or decrease metastatic tumor growth [Akl et al., 2007; Tagalakis et al., 2007].
Platelet β3 integrins have been implicated in the development of bone metastases [Phillips et al., 1991; Smyth et al., 2001; Bakewell et al., 2003; Camerer et al., 2004]. Mice lacking the β3 integrin are protected from tumor-associated bone osteolysis [Bakewell et al., 2003] and inhibition of platelet specific integrin αIIbβ3 decreases the incidence of bone metastasis in mice. αIIbβ3 plays a central role in the initiation of arterial thrombosis and platelet aggregation [Phillips et al., 1991; Smyth et al., 2001; Bakewell et al., 2003; Camerer et al., 2004]. When resting platelets are activated via ADP, thrombin or epinephrine, αIIbβ3 is activated, thereby enabling binding of its ligand, fibrinogen [Schwartz et al., 1995; Takagi and Springer, 2002]. ADP binding to its G-protein-coupled-receptors (P2Y12 and P2Y1) not only induces αIIbβ3 activation, but also rapidly initiates platelet production of another platelet activator, thromboxane A2 (TXA2)[Murugappan et al., 2004; Maxwell et al., 2006].
Metastatic tumor cells can produce ADP and TXA2 among other factors and can induce β3 integrin activation and platelet aggregation [Boukerche et al., 1994; Gately and Li, 2004]. Aspirin (acetylsalicylic acid or ASA) inhibits cyclooxygenase (COX), a critical enzyme in TXA2 synthesis [Awtry and Loscalzo, 2000; Catella-Lawson and Crofford, 2001; Wang and Dubois, 2006], and effectively decreases platelet aggregation. ASA treatment moderately decreases tumor cell-induced platelet aggregation (TCIPA) in vitro, however when used as a single agent, does not affect pulmonary metastases in mice [Camerer et al., 2004]. ASA has also been used as a chemoprevention agent to prevent the development of invasive cancer from pre-cancerous lesions [Dube et al., 2007], but its role on established tumors and during bone metastasis has not been defined.
APT102 is a novel form of the naturally occurring human apyrase/ADPase, CD39 [Medina et al., 2006]. Apyrases promote the breakdown of extracellular ATP and ADP by catalyzing the removal of the gamma phosphate from ATP and the beta phosphate from ADP [Jin et al., 2005; Marcus et al., 2005]. CD39−/− mice have increased bleeding times and deficient platelet aggregation. APT102 is a soluble, mutated form of the CD39 protein that cleaves ADP more potently than endogenous CD39, and has also been shown to prevent Caco-2 human colon cancer cell induced platelet aggregation in vitro [Medina et al., 2006].
We hypothesized that treating mice with ASA+APT102 in combination would lead to decreased tumor burden in bone and tumor-associated bone loss. We examined the effect of these agents on tumor cell dissemination in two murine bone metastasis models, in which tumor cell lines expressing firefly luciferase and bioluminescence imaging (BLI) are used to assess real-time tumor burden with increased sensitivity at multiple time points.
MATERIALS AND METHODS
Animal Studies
C57BL/6 mice (Harlan Laboratories, Indianapolis, IN) were used for B16 melanoma studies, and BALB/c mice (Taconic, Hudson, NY) and β3 integrin−/− mice on BALB/c background (a generous gift from Dr. Robin Anderson, Peter MacCallum Cancer Institute, East Melbourne, Victoria, Australia[Sloan et al., 2006]) were used for 4T1 breast cancer studies. Mice were housed under pathogen-free conditions according to the guidelines of the Division of Comparative Medicine, Washington University School of Medicine, St. Louis, MO. The Animal Studies Committee of Washington University School of Medicine approved all experiments.
Cell Lines
The osteolytic B16-F10 murine C57BL/6 melanoma cell line was obtained from ATCC (Manassas, VA) and modified to express firefly luciferase (B16-FL) [Hirbe et al., 2006]. The osteolytic 4T1 murine BALB/c breast carcinoma cell line was modified to express GFP and firefly luciferase (4T1-GFP-FL) [Hirbe et al., 2006]. Cell lines were cultured in α-MEM with 10% fetal calf serum.
Expression, Purification, and Pharmacokinetics of APT102
APT102 is a soluble mutant form of human CD39L3, generated by removal of the N-terminal 44amino acids and the C-terminal 43 amino acids. Two point mutations, R67G and T69R, were introduced into the CD39L3 coding sequence as previously described to significantly enhance ADPase activity [Medina et al., 2006]. The mutant gene, APT102, was cloned into an expression plasmid and secreted APT102 protein was purified as described from conditioned medium of transfected HEK293T or CHO cells [Medina et al., 2006].
Pharmacokinetic studies were conducted in rats where single bolus APT102 was intravenously injected (0.75 mg/kg, n = 3 per time-point). Serum samples were examined for both ADPase and ATPase activities. The ADPase and ATPase activities of APT102 were estimated by ADP and ATP hydrolysis assays using malachite green [Baykov et al., 1988]. Enzymatic analysis was initiated by the addition of 5 µl of the enzyme to 495 µl of a mixture containing 50 mM Tris–HCl (pH 7.4), 8 mM CaCl2, and various concentrations of ADP or ATP. Following 30-min incubation at 37°C, 50 µl of the reaction solution was mixed with 900 µl of 50 mM Tris–HCl (pH 7.4) and 50 µl of the malachite working solution. The inorganic phosphate released from the ADP or ATP reacts with the malachite working solution, resulting in a green color. Since the enzymatic activity of apyrases is proportional to the amount of the released inorganic phosphate, apyrase activity can be measured by monitoring the absorbance at 630 nm using an Agilent 8453 UV-Visible spectrophotometer (Agilent, Palo Alto, CA). The kinetics of the enzymatic reaction was determined by measuring the time course of the color development at a wavelength of 630 nm.
The experimental data best fit biphasic exponential curves for either enzyme activity. The distribution phase half-life (T½) of APT102 was calculated to be 40 min and 50% of apyrase activity was cleared from the circulation during this phase. After 24 h the enzyme activity was still significantly elevated above baseline (100 vs. 10 pmol min−1) with a plasma elimination T½ of 20 h. In mice, APT102 (3.1 mg/kg, n = 3 per time point), had a comparable T½ and the dosing interval was chosen to be every 12 h by intravenous injection.
Ex vivo effects of APT102 on ADP-induced platelet aggregation in PRP from APT102 treated mice were also evaluated. Ten minutes after administration of a single bolus of APT102 there was greater than 90% inhibition of ADP-induced platelet aggregation. Inhibition was long lasting, and still significant 6 h after dosing.
Platelet Aggregometry
Platelet aggregation was measured by light aggregometry according to previous methods [Palumbo et al., 2005]. Mouse blood was isolated via cardiac puncture in the presence 5 U/ml heparin. Platelet rich plasma (PRP) and platelet poor plasma (PPP) were obtained by centrifugation of whole blood as previously described [Palumbo et al., 2005]. PRP (1 × 109/ml) was placed in a whole blood ionized calcium lumiaggregometer (Chronolog Corp., Havertown, PA). Light transmission increases as platelets form into aggregates. TCIPA was initiated by the addition of B16-FL cells (106 cells/ml) at time 0 min, and the reaction was monitored and analyzed by the Aggro-link data processing system (Chronolog) for 6 min [Jurasz et al., 2001]. To study the effect of ASA and a potato apyrase (Sigma A6410, St. Louis, MO), platelets were pre-incubated with ASA (10 mM), apyrase (5 U), or ASA+apyrase for 3 min before initiation of aggregation [Alonso-Escolano et al., 2004]. Collagen at 5 µg/ml and 5 µM ADP were used as control agonists.
Platelet-Tumor Adhesion Assay
Wildtype and β3−/− platelets were incubated in a 96-well plate at 37°C for 1 h. Plates were then treated with 1% BSA in PBS for 1 h. The non-adherent cells were washed off 6× with PBS. 105 B16-FL cells were added and incubated for 1 h. The remaining non-adherent cells were then washed off 6× with PBS and binding was measured by Cyquant Cell Proliferation ELISA Kit (Molecular Probes, Eugene, OR).
Bleeding Time Measurements
Bleeding time measurements were performed as described previously with minor modifications [Broze et al., 2001]. Briefly, mice were immobilized and a small transaxial incision was made on the tail. The tail was then immersed in 37°C PBS and blood flow was visually observed and timed for up to 5 min.
MTT Assay
B16-FL or 4T1-GFP-FL cells were plated and allowed to adhere for 24 h in 96-well plates. Vehicle, 0.1 mg/ml ASA, 0.003 mg/ml APT102 or the combination was then added to the media. MTT assay was performed according to manufacturer’s instruction (Sigma).
Administration of Pharmacologic Agents
Mice were treated with 30 mg/kg ASA via oral gavage and/or 3.15 mg/kg APT102 via tail vein injection 30 min prior to all experiments in Figure 1–Figure 3 unless otherwise indicated. For long-term metastasis experiments in Figure 1–Figure 3, ASA and/or APT102 were additionally administered every 12 h post-tumor cell inoculation for a total of 2½ days. For the experiments in Figure 4, ASA+APT102 were given 1 h prior to tumor cell inoculation as a single dose.
Fig. 1.
Aspirin and apyrase decreased tumor cell-induced platelet aggregation and increased bleeding times but did not affect local tumor growth. A: Platelets were pre-incubated with ASA (10 mM), potato apyrase (5 U), the combination or nothing for 3-min priorto addition of B16-FL cells. All four aggregometry tracings of treated platelets were flat prior to tumor cell addition. B16-FL cells were added to the platelets at time 0 min and aggregation was measured for 6 min. ASA, apyrase, and ASA + apyrase treatment decreased B16-FL tumor cell-induced platelet aggregation. B: B16-FL adhesion to a lawn of wild type and β3−/− platelets or BSA (B16-FL alone) was measured by Cyquant Elisa Kit. B16-FL cells adhered to both wildtype and β3−/− platelets in vitro. C: Bleeding times (s = seconds) were measured in C57BL/6 mice treated 30 min prior with vehicle (n = 5), APT102 (n = 5), ASA (n = 5), or ASA + APT102 (n = 5). The treatment groups are denoted on the bottom of the graph. ASA and ASA + APT102 pretreatment elevated bleeding times compared to vehicle (*P = 0.0061, **P = 0.0002, ***P = 0.0001, ****P< 0.0001). D: 4T1-GFP-FL s.c. tumors were established in Balb/c mice for 7 days. ASA +APT102 (n = 5) or vehicle (n = 5) was then administered and BLI was performed at days 7 (pretreatment) and 14. No significant differences in s.c. tumor burden were observed between vehicle and ASA +APT102 (P = 0.23).
Fig. 3.
Aspirin and APT102 in combination significantly decreased metastatic tumor burden in a second murine tumor model. BALB/c mice were treated with either vehicle or ASA + ATP102 30-min prior to i.c. inoculation of 4T1-GFP-FL cells, and drug treatments continued for 2½ days. BLI was performed at days 2 and 9 after tumor inoculation and skeletal tumor burden was measured in a consistent region of interest (ROI). A: 4T1-GFP-FL metastatic tumor burden is significantly decreased in the femur/tibia (day 2, P=0.01; day 9, P=0.02) of mice treated with ASA+APT102 (n=15) compared to control (n = 15). B: Representative images of BLI at day 2.C: Representative histological sections of tibia. T = Tumor, M ¼ Marrow. D: Tumor volume was measured by histomorphometric analysis on paraffin-embedded femurs of mice sacrificed 9 days after tumor inoculation. Tumor volume was decreased in femurs of mice treated with ASA+APT102 compared to vehicle (P = 0.04). (ASA + APT102 treated bones n = 30, vehicle treated bones n = 30.) E: Representative radiographic images of femur/ tibia taken at day 14. Arrows show areas of tumor-associated osteolysis. F: Serial weights were performed on mice starting at day 0 prior to tumor inoculation for 11 days. Average weight loss is plotted in grams. Vehicle treated mice lost significantly moreweightaftertumorinoculationcomparedtoASA+APT102 treated mice (vehicle n = 8, ASA+APT102 n = 5, D0-D11 P = 0.04).
Fig. 4.
Tumor cell dissemination was not altered after ASA + APT102 treatment or in β3−/− mice. A: Wildtype BALB/ cmice were treated with vehicle (n = 5) or ASA + APT102 (n = 5) 1-h prior to i.c. inoculation of 4T1-GFP-FL cells. One hour after tumor cell inoculation, organs were harvested, lysates were prepared, and luciferase activity was measured. At 1 h, 4T1-GFP-FL cell dissemination to all measured organs was not statistically different in ASA + APT102 and vehicle-treated mice (lungs P = 0.17, liver P = 0.44, tibia/femur P = 0.95). B: Platelet-defective β3−/− mice (n = 5) and normal β3+/− mice (n = 5) received i.c. inoculation of 4T1-GFP-FL tumor cells; 1-h post-inoculation, organs were harvested, lysates were prepared, and luciferase activity was measured. At 1 h, 4T1-GFP-FL cell dissemination to all measured organs was equivalent in β3−/− and β3+/− mice (lungs P = 0.72, liver P = 0.58, tibia/femur P = 0.15).
Bone Metastasis Studies
For intra-cardiac (i.c.) injections, operators were blinded to treatment group. Mice were anesthetized with a xylazine-ketamine cocktail and inoculated intra-cardially with 1 × 105 B16-FL or 4T1-GFP-FL cells in 0.05 ml PBS as previously described [Bakewell et al., 2003; Hirbe et al., 2006]. In vivo BLI was performed on days 2 and 9 for 4T1-GFP-FL and day 9 for B16-FL. Each in vivo study was repeated at least once to confirm result significance and treatment groups from separate experiments were pooled. Mice that received 4T1-GFP-FL were euthanized and underwent necropsy on day 9 (for histomorphometric analyses) or day 14 (for radiographic analyses) after tumor cell inoculation. Mice that received B16-FL were euthanized and underwent necropsy on day 12.
Ultrasound-Guided Intra-Cardiac Delivery of 4T1-GFP-FL Cells
Ultrasound-guided injection of 4T1-GFP-FL cells into the left ventricular chamber of the bleeding-prone β3−/− mice was performed using a Vevo770 Ultrasound System (Visual Sonics, Inc, Toronto, Canada). Mice were anesthetized with gaseous isoflurane and secured on an imaging platform in supine position, and the anterior chest was shaved. Ultrasound images of the left ventricle were obtained with a mechanical ultrasound transducer operating at 30 MHz imaging frequency. The cell suspension was injected using a needle secured to the injection arm of the Vevo770 imaging platform. The needle was then advanced through the apex of the left ventricle into the mid cavity, and the cell suspension was injected. After the needle was retracted, mice were monitored for evidence of hemorrhage into the pericardial or pleural space.
In Vivo Bioluminescence Imaging (BLI)
Mice were injected intraperitoneally with 150 mg/kg of D-luciferin in PBS 10 min prior to imaging. Imaging was performed using a charge coupled device (CCD) camera (IVIS 100, Xenogen, Alameda, CA; exposure times 1 s, 10 s, 1 min or 5 min, binning 8, FOV 15 cm, f/stop 1, no filter) in collaboration with the Molecular Imaging Center (Washington University in St Louis, MO). Mice were anesthetized by isoflurane (2% vaporized in O2) and C57BL/6 mice were shaved to minimize attenuation of light by pigmented hair. Total photon flux (photons/s) was measured while the operator was blinded to treatment group from a fixed region-of-interest (ROI) over the tibia/femur area, and mandible using Living Image and IgorPro software (Wavemetrics, Portland, OR) as previously described [Hirbe et al., 2006].
Subcutaneous (s.c) Tumor Cell Implantation
Mice were anesthetized with a xylazine-ketamine cocktail and 2 × 105 4T1-GFP-FL cells were injected into the flank s.c. Mice were treated with vehicle or ASA+APT102 at day 7 post-tumor cell inoculation and treatment continued every 12 h for 2½ days. BLI imaging was performed on day 7 (immediately before drug administration) and day 14. The operator was blinded to treatment group.
Quantification of Luciferase Activity in Organ Lysates
Wildtype BALB/c mice were treated with ASA+APT102 or vehicle as described above 1 h prior to 4T1-GFP-FL inoculation. Untreated β3+/− and β3−/− mice also underwent i.c. tumor cell inoculation under ultrasound guidance. One hour after tumor cell inoculation, vehicle-treated, ASA+APT102-treated, β3+/− and β3−/− mice were sacrificed and lungs, liver, and bones were isolated and snap frozen. The frozen tissues were then weighed and homogenized with the appropriate amount of Glo Lysis Buffer (Promega, Madison, WI). For each gram of tissue, 875 µl of Glo Lysis Buffer was added. The homogenate was vortexed and centrifuged. BLI was performed (IVIS, Xenogen Systems) on 50 µl of the lysate supernatant in black-sided 96-well plates using the Bright-Glo Luciferase Assay System (Promega).
Bone Histomorphometry
Mouse femurs and tibias were fixed in formalin and decalcified in 14% EDTA. Paraffin embedded sections were stained for tartrate resistant acid phosphatase (TRAP). Trabecular bone volume (BV/TV) and osteoclast perimeter (OCPm/TbBPm) were measured from 120 to 1,080 µm distal to the growth plate and tumor area/total area was measured from 120 to 4,800 mm from the growth plate using Bioquant Osteo (Bioquant Image Analysis Corporation, Nashville TN) blinded to treatment group [Hirbe et al., 2006].
Statistical Analysis
For experiments where two populations were compared to each other, group mean values were compared by two-tailed student t-test. In calculating two-tailed significance levels for equality of means, equal variances were assumed for the two populations. For the weight loss analysis in Figure 3, a non-parametric analog to a t-test is used, as the distributions of weights by day are reasonably symmetrical but clearly not gaussian. For the bleeding time analysis in Figure 1 and MTT analysis in Supplemental Figure 1, where more than two populations are present, a mixed linear model was used to determine P values.
RESULTS
Aspirin and Apyrase Decreased Tumor Cell-Induced Platelet Aggregation and Increased Bleeding Times but Did Not Affect Local Tumor Growth
We had previously found that inhibition of platelet β3 integrins blocks B16 tumor cell induced platelet aggregation in vitro [Bakewell et al., 2003]. Cancer cells induce platelet aggregation and secrete the platelet activators ADP and TXA2 [Grignani and Jamieson, 1988; Boukerche et al., 1994; Gately and Li, 2004]. We evaluated the ability of TXA2 inhibitor, ASA, and ADP hydrolyzing agent, potato apyrase, to impair TCIPA. TCIPA was measured in the presence or absence of ASA, apyrase or the combination in vitro (Fig. 1A). Platelets isolated from wildtype C57BL/6 mice were incubated with ASA, apyrase, or ASA+apyrase, and aggregation was induced by addition of B16-FL murine osteolytic melanoma tumor cells. Apyrase treatment reduced B16-FL-induced platelet aggregation by 69% compared to control, while inhibition of platelet aggregation by ASA was more modest. ASA+apyrase treatment reduced platelet aggregation to a similar degree as apyrase treatment alone.
Tumor cells not only induce platelet aggregation, but also platelet adhesion [Karpatkin et al., 1988]. To determine if platelet β3 integrins are necessary for tumor adhesion to platelets, B16-FL cells were incubated with wildtype or β3−/− platelet lawns in vitro and adherent tumor cells were quantitated. B16-FL cells adhered to both wildtype and β3−/− platelets to a similar extent indicating that the β3 integrin was not required for tumor-platelet adhesion (Fig. 1B).
Pharmacokinetic studies in mice showed that a single dose of APT102, soluble mutant form of the human apyrase CD39L3, led to more than 90% inhibition of ADP-induced platelet aggregation by as early as 10 min. The impact of APT102 and ASA on in vivo basal platelet aggregation was quantitated by measurements of bleeding times. Mice were pretreated with ASA, APT102, or ASA+APT102 in combination (ASA+APT102), and standardized bleeding times were measured 30 min after drug administration. ASA administration at doses previously published in mice [Sugimoto et al., 2006], significantly increased bleeding times by an average of 64% compared to vehicle-treated controls whereas APT102 increased bleeding times modestly by 22% compared to vehicle-treated controls. ASA+APT102 increased bleeding times to a similar degree as ASA treatment alone (Fig. 1C).
Direct effects of ASA+APT102 administration on tumor growth in vivo were evaluated on subcutaneously (sc) implanted 4T1-GFP-FL tumors. 4T1-GFP-FL sc tumors were established for 7 days and BLI was performed at day 7, after which ASA+APT102 or vehicle were administered and tumor growth monitored for an additional 7 days. No significant differences in tumor burden as measured by BLI were observed in mice treated with ASA+APT102 (Fig. 1D). ASA, APT102, or ASA+APT102 had no significant effect on 4T1-GFP-FL and B16-FL viability in vitro as measured by the MTT assay (Supplemental Fig. 1). These results demonstrate that ASA and APT102 in combination decreased B16-FL TCIPA in vitro and increased bleeding times in vivo, but had little direct effect on tumor cell viability either in vitro or in vivo.
Aspirin and APT102 in Combination Significantly Decreased Skeletal Metastatic Tumor Burden in Mice
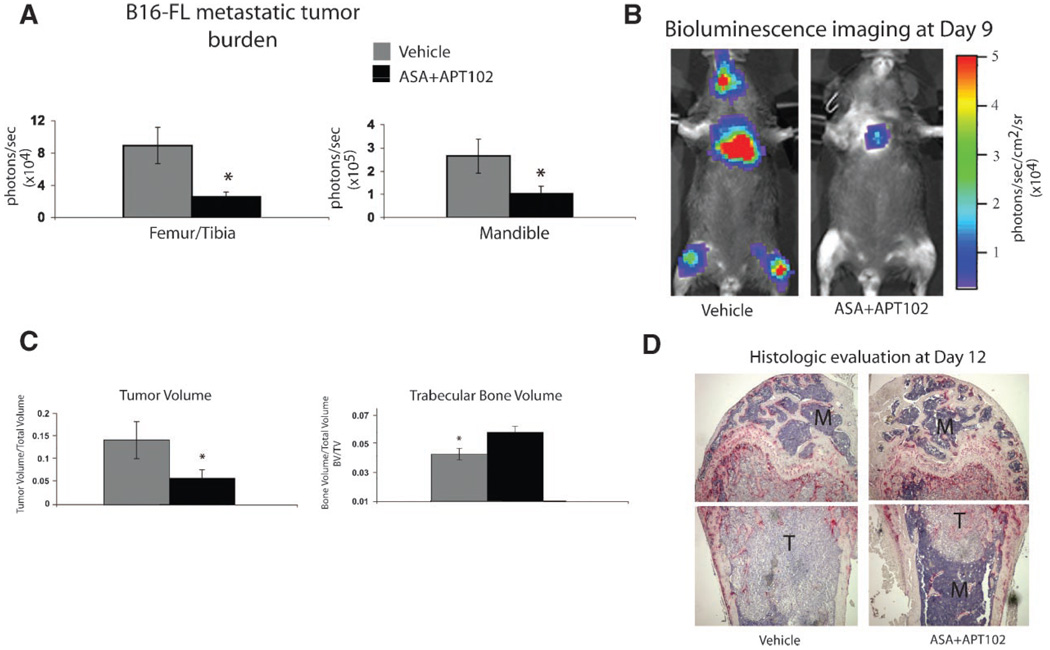
Mice defective in αIIbβ3-mediated platelet aggregation are protected from bone metastasis [Bakewell et al., 2003]. We hypothesized that pharmacologic inhibition of platelet aggregation by ASA and APT102 in combination would provide protection from metastasis. Wildtype C57BL/6 mice were treated with ASA and/or APT102 30-min prior to i.c. inoculation of B16-FL tumor cells, and drug treatments continued for 2½ days. We had previously published that treatment for 2½ days with an inhibitor of platelet αIIbβ3 integrin (ML464) starting at 30-min prior to tumor inoculation significantly decreased tumor metastasis in mice [Bakewell et al., 2003]. Tumor burden was assessed in real time by in vivo BLI 9 days after tumor cell inoculation and was quantitated histologically at the time of sacrifice at day 12. ASA and vehicle-treated mice had comparable levels of tumor luciferase activity in the femur/tibia and mandible 9 days after tumor cell inoculation. A similar result was seen in APT102-treated mice (Supplemental Fig. 2A,B). In contrast, compared to vehicle-treated controls, the combination of ASA+APT102 produced a significant reduction in luciferase activity in the femur/ tibia and mandible 9 days after i.c. tumor cell inoculation (Fig. 2A,B). In addition, there was no increase in i.c. injection procedure mortality in vehicle and treated groups compared to the 50% procedure mortality from hemorrhage reported in the β3 integrin deficient mice [Bakewell et al., 2003].
Fig. 2.
Aspirin and APT102 in combination significantly decreased skeletal metastatic tumor burden in mice. C57BL/6 mice were treated with either vehicle or ASA + APT102 30-min prior to i.c. inoculation of B16-FL tumor cells, and drug treatments continued for 2½ days. Nine days later, BLI was performed and skeletal tumor burden was measured in femurs/ tibiae and mandible in a consistent region of interest (ROI). A: B16-FL skeletal metastatic tumor burden as measured by BLI at day9 was significantly decreasedinthe femur/tibia(P = 0.002) and mandible (P = 0.03) of mice treated with ASA+APT102 (n = 20) compared to vehicle (n = 14). B: Representative images of BLI at day 9 are shown. C: Tumor volume and trabecular bone volume were measured by histomorphometric analysis on paraffin-embedded femurs of mice sacrificed 12 days after B16-FL inoculation (ASA+APT102 treated bones n = 40, vehicle bones n = 28). Tumor volume was decreased in femurs of mice treated with ASA+APT102 compared to vehicle (P = 0.04). Trabecular bone volume was significantly increased in ASA + APT102-treated mice compared to vehicle (P = 0.02). D: Representative histological sections of femurs are depicted. T=Tumor, M=Marrow.
Histomorphometric analysis of the femoral bones of mice treated with ASA+APT102 confirmed decreased tumor volume compared to vehicle-treated controls at day 12 (Fig. 2C,D). Likewise, there was a significant reduction in trabecular bone loss in ASA+APT102-treated mice compared to vehicle controls (Fig. 2C,D) consistent with decreased osteolytic B16-FL tumor burden. Thus, treatment with ASA+APT102 decreased metastatic melanoma tumor burden in bone and bone loss.
Aspirin and APT102 in Combination Significantly Decreased Metastatic Tumor Burden in a Murine Breast Cancer Bone Metastasis Model
We examined the effect of ASA, APT102, and ASA+APT102 treatment on bone metastatic tumor burden following i.c. inoculation of the murine breast cancer cell line, 4T1-GFP-FL, in BALB/c mice to control for the generality of response from cell line to cell line. 4T1-GFP-FL tumor luciferase activity could be detected in bone as early as 2 days after tumor cell inoculation due to increased intrinsic luciferase expression. No detectable differences in metastatic tumor burden were observed in mice treated with ASA alone or APT102 alone compared to vehicle treated controls (data not shown).
However, administration of ASA+APT102 in combination resulted in significantly decreased tumor burden in the femur/tibia (Fig. 3A,B) compared to vehicle controls at days 2 and 9 after tumor cell inoculation. Histologic/histomorphometric analysis of femur/tibia from mice sacrificed at day 9 confirmed that luciferase activity correlated with viable tumor formation (Fig. 3C,D). A cohort of ASA+APT102 and vehicle treated mice were observed for longer times and radiographic images were taken 14 days after tumor inoculation. Representative radiographic images demonstrate visibly decreased bone loss seen as decreased areas of radiolucency in the ASA+APT102 mice compared to vehicle controls (Fig. 3E). In addition, ASA+APT102 treated mice had significantly decreased tumor associated weight loss compared to vehicle controls (Fig. 3F). To evaluate whether the decrease in bone loss observed was a direct effect of ASA+APT102 on bone, non-tumor bearing mice were treated with ASA+APT102 for 2.5 days. There was no significant change in trabecular bone volume or significant changes in osteoclast perimeter compared to vehicle controls (Supplemental Fig. 2C,D) with drug treatment alone. Thus, treatment with ASA+APT102 decreased tumor associated weight loss and 4T1-GFP-FL metastatic tumor burden in bone.
Tumor Cell Dissemination Is Intact in Mice With Defective Platelet Function
The decreased bone tumor burden by ASA+APT102 treatment observed as early as 2 days after i.c. tumor inoculation led us to hypothesize that platelets are important for the initial stages of tumor dissemination to the metastatic organ. To test this hypothesis, we evaluated tumor cell burden 1 h after i.c. inoculation of 4T1-GFP-FL tumor cells in mice with either a genetic or a pharmacologically induced defect in platelet function. One hour after tumor cell inoculation lungs, liver and bones were harvested. Luciferase activity of the lysates was analyzed after being normalized for weight. Lysates were used in this experiment because in vivo real time BLI was not sensitive enough at this early time point.
Tumor burden in the lung, liver, and bones of mice treated with ASA+APT102 1-h prior to 4T1-GFP-FL i.c. inoculation was not significantly different from vehicle-treated controls as measured by luciferase activity (Fig. 4A). Likewise, no difference in initial 4T1-GFP-FL tumor cell dissemination to end organs was observed in the platelet-defective β3−/− mice compared to normal β3+/- ittermates (Fig. 4B). Thus, tumor cell dissemination to metastatic sites including bone in the first hour was intact in mice with defective platelet aggregation.
DISCUSSION
We demonstrated significant inhibition of bone tumor burden by the novel ADPase, APT102, in combination with aspirin (ASA), using two experimental bone metastasis models in separate strains of immunocompetent mice. These agents were not directly cytotoxic to tumor cells and neither ASA+APT102 treatment nor genetic loss of β3 integrin altered initial tumor cell distribution. Finally, we observed decreased tumor associated bone loss and decreased tumor associated weight loss from a 2½-day treatment course of ASA+APT102.
One important implication of these data is that combination rather than single-agent antiplatelet activation therapy may be required for the prevention of metastasis. We found that TCIPA was inhibited in vitro by apyrase alone. However, single agent apyrase APT102 did not result in a decrease in metastatic tumor burden in vivo. One possible explanation for this requirement for combination anti-platelet therapy is that tumor cells activate platelets through a variety of mechanisms [Lerner et al., 1983; Nierodzik et al., 1992; Dorsam et al., 2002; Camerer et al., 2004]. Combination therapy with ASA and the ADP receptor inhibitor, clopidogrel, has been shown to be superior to single agent therapy in the treatment of coronary artery and cerebral vascular disease [Peters et al., 2003]. Many patients with metastatic cancer have detectable CTC in their peripheral blood, and changes in the number of CTCs correlate with response to treatment and disease progression [Seronie-Vivien et al., 2001; Gaforio et al., 2003]. Our results show that combination ASA+APT102 therapy decreased tumor burden in bone after tumor cell inoculation into the circulation. We propose that ASA and ADPase or β3 integrin inhibition could be evaluated in patients with tumor cells in circulation.
Interestingly, we found no difference in end organ localization of tumor cells in the platelet-defective β3−/− mice or ASA+APT102-treated mice at 1 h after tumor cell inoculation compared to controls, despite a marked difference in bone tumor burden 2 and 9 days later. This suggests that tumor cell localization, or the initial organ-specific homing, remained intact even though platelet activation had been inhibited. We cannot exclude the possibility that the luciferase activity observed in lung, liver, and bone lysates was a result of tumor cells circulating in the capillary bed of these organs. It could be possible that platelets do facilitate vessel engagement of tumor cells and migration to specific compartments within the metastatic organ, for example the stem cell niche in the bone marrow (an area rich in growth factors and extracellular calcium)[Adams et al., 2007]. We are currently investigating endosteal lodgment/ adhesion of tumor cells in platelet-defective animals to test this hypothesis.
It has been suggested that platelets decrease metastasis by associating with tumor cells in the bloodstream and contributing self/HLA antigens that could repel attack from antitumor natural killer (NK) or immune cells in the metastatic site. Indeed, Palumbo et al. [2005] found that intact NK cell function was required for the anti-metastatic benefits observed in the platelet activation-defective Gαq−/− mice. APT102 and ASA had the most significant effect on metastatic bone tumor burden, but we also observed some decreases in tumor burden in the lung. It is possible that the treatment with ASA and APT102 would affect all types of metastases but that the models used in these studies favor evaluation of bone metastases. Identifying and targeting non-thrombogenic platelet pathways in the metastatic process could result in therapies that would limit bleeding side effects while maintaining anti-metastatic properties.
Trabecular bone loss was not significantly altered after 2½ days of ASA+APT102 treatment in non-tumor bearing mice. There was also no significant difference in osteoclast perimeter suggesting that short treatment of ASA+APT102 had no dramatic effect on osteoclasts in vivo. However, it is possible that ASA+APT102 treatment might exert some effect on the osteoblasts changing bone formation thus bone turnover. We cannot rule out small effects of ASA+APT102 directly on bone cells but we believe that the magnitude of this change is likely not sufficient to account for the tumor phenotype observed. It is possible that the broader/systemic effect of apyrase treatment, combined with the anti-inflammatory effects of ASA treatment, could act directly on bone to suppress the growth of bone tumors. ADP receptors, such as P2Y1, exist in bone resorbing osteoclasts for example, and treatment of cultured osteoclasts with ADP enhances bone resorption [Hoebertz et al., 2001]. Loss of ADP receptor activation, such as P2Y1 in the bone microenvironment, could prevent bone resorption necessary for tumor growth in bone. Inflammation is well known to increase osteoclast resorption and bone loss [Haynes, 2007]. Tumors produce ADP, TXA2, and other molecules that recruit inflammatory cells, which in addition to recruiting and activating platelets, could contribute to increased osteoclastic resorption thereby creating a more favorable environment for tumor growth in bone. It has been shown that a platelet β3 integrin antagonist decreased the growth of established bone tumors that had been engineered to overexpress the platelet LPA receptor [Boucharaba et al., 2004]. Thus, it is possible that administration with APT102 and ASA for longer times could have a more profound effect on tumor burden in bone and possibly on bone parameters independent of presence of tumor cells.
Pharmacologic targeting of the pathways through which tumor cells activate platelets could decrease or prevent metastatic tumor burden in bone. The use of platelet activation inhibitors in combination could provide a safe way to decrease or prevent the formation of new bone metastatic lesions in high-risk cancer patients, in particular, those with CTC.
Supplementary Material
This article contains supplementary material, which may be viewed at the Journal of Cellular Biochemistry website at http://www.interscience.wiley.com/jpages/0730-2312/suppmat/index.html.
ACKNOWLEDGMENTS
We thank Dr. Paddy Ross, Dr. Michael Tomasson, Dr. David Kipnis, Dr. Bill Frazier, Dr. Deb Novack and Dr. Phil Majerus and Dr. Teitelbaum for helpful discussions. We thank Timothy Mitsky for his participation in the production of APT102. The ultrasound guided tumor cell injections were performed by Dr. Attila Kovacs in the Mouse Cardiovascular Phenotyping Core Facility at Washington University. This study was supported to ACH (Hematology Training Grant 5-T32-HL07088-32), KW (R01-CA097250), MCE (R01-CA097250), ÖU (Cancer Biology Pathway, Kauffman Fellowship, Washington University, P50 CA94056), DF (R01-CA097250, Hematology Training Grant-5-T32-HL07088-32), EAM (MSTP Training Grant-5-T32-GM-07200), Molecular Imaging Center (P50 CA94056).
Grant sponsor: Hematology Training Grant; Grant number: 5-T32-HL07088-32; Grant sponsor: NIH; Grant number: RO1-CA097250; Grant sponsor: Cancer Biology Pathway, Washington University; Grant sponsor: Kauffman Fellowship, Washington University; Grant sponsor: Molecular Imaging Center; Grant number: P50 CA94056; Grant sponsor: MSTP Training Grant; Grant number: 5-T32-GM-07200.
Footnotes
Dr. Jeong and Dr. Chen are employees of APT Therapeutics. All other authors have no conflict of interest.
REFERENCES
- Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, Kronenberg HM, Scadden DT. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- Akl EA, Kamath G, Kim SY, Yosuico V, Barba M, Terrenato I, Sperati F, Schunemann HJ. Oral anticoagulation for prolonging survival in patients with cancer. Cochrane Database Syst Rev. 2007;2:1–27. doi: 10.1002/14651858.CD006466. [DOI] [PubMed] [Google Scholar]
- Alonso-Escolano D, Strongin AY, Chung AW, Deryugina EI, Radomski MW. Membrane type-1 matrix metalloproteinase stimulates tumour cell-induced platelet aggregation: Role of receptor glycoproteins. Br J Pharmacol. 2004;141:241–252. doi: 10.1038/sj.bjp.0705606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirkhosravi A, Meyer T, Chang JY, Amaya M, Siddiqui F, Desai H, Francis JL. Tissue factor pathway inhibitor reduces experimental lung metastasis of B16 melanoma. Thromb Haemost. 2002;87:930–936. [PubMed] [Google Scholar]
- Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, Scarborough R, Kanter J, Abe K, Phillips D, Weilbaecher KN. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci USA. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykov AA, Kasho VN, Avaeva SM. Inorganic pyrophosphatase as a label in heterogeneous enzyme immunoassay. Anal Biochem. 1988;171:271–276. doi: 10.1016/0003-2697(88)90485-x. [DOI] [PubMed] [Google Scholar]
- Billroth T. Pathology and therapeutics, in fifty lectures. 1871. Clin Orthop. 2003;408:4–11. doi: 10.1097/00003086-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukerche H, Berthier-Vergnes O, Penin F, Tabone E, Lizard G, Bailly M, McGregor JL. Human melanoma cell lines differ in their capacity to release ADP and aggregate platelets. Br J Haematol. 1994;87:763–772. doi: 10.1111/j.1365-2141.1994.tb06736.x. [DOI] [PubMed] [Google Scholar]
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, Pantel K. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- Broze GJ, Jr, Yin ZF, Lasky N. A tail vein bleeding time model and delayed bleeding in hemophiliac mice. Thromb Haemost. 2001;85:747–748. [PubMed] [Google Scholar]
- Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- Castelli R, Porro F. Cancer and thromboembolism: From biology to clinics. Minerva Med. 2006;97:175–189. [PubMed] [Google Scholar]
- Catella-Lawson F, Crofford LJ. Cyclooxygenase inhibition and thrombogenicity. Am J Med. 2001;110(Suppl 3A) doi: 10.1016/s0002-9343(00)00683-5. 28S–32S. [DOI] [PubMed] [Google Scholar]
- Dorsam RT, Kim S, Jin J, Kunapuli SP. Coordinated signaling through both G12/13 and G(i) pathways is sufficient to activate GPIIb/IIIa in human platelets. J Biol Chem. 2002;277:47588–47595. doi: 10.1074/jbc.M208778200. [DOI] [PubMed] [Google Scholar]
- Dube C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. The use of aspirin for primary prevention of colorectal cancer: A systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:365–375. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- Francis JL, Amirkhosravi A. Effect of antihemostatic agents on experimental tumor dissemination. Semin Thromb Hemost. 2002;28:29–38. doi: 10.1055/s-2002-20562. [DOI] [PubMed] [Google Scholar]
- Gaforio JJ, Serrano MJ, Sanchez-Rovira P, Sirvent A, Delgado-Rodriguez M, Campos M, de la Torre N, Algarra I, Duenas R, Lozano A. Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer. 2003;107:984–990. doi: 10.1002/ijc.11479. [DOI] [PubMed] [Google Scholar]
- Gasic GJ. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metastasis Rev. 1984;3:99–114. doi: 10.1007/BF00047657. [DOI] [PubMed] [Google Scholar]
- Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: A target for antiangiogenic therapy. Semin Oncol. 2004;31:2–11. doi: 10.1053/j.seminoncol.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol. 2001;106:33–42. doi: 10.1159/000046587. [DOI] [PubMed] [Google Scholar]
- Grignani G, Jamieson GA. Platelets in tumor metastasis: Generation of adenosine diphosphate by tumor cells is specific but unrelated to metastatic potential. Blood. 1988;71:844–849. [PubMed] [Google Scholar]
- Guise TA. Molecular mechanisms of osteolytic bone metastases. Cancer. 2000;88:2892–2898. doi: 10.1002/1097-0142(20000615)88:12+<2892::aid-cncr2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Haynes DR. Inflammatory cells and bone loss in rheumatoid arthritis. Arthritis Res Ther. 2007;9:104. doi: 10.1186/ar2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbe AC, Uluckan O, Morgan EA, Eagleton MC, Prior JL, Piwnica-Worms D, Trinkaus K, Apicelli A, Weilbaecher K. Granulocyte colony-stimulating factor enhances bone tumor growth in mice in an osteoclast-dependent manner. Blood. 2006;109:3424–3431. doi: 10.1182/blood-2006-09-048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebertz A, Meghji S, Burnstock G, Arnett TR. Extracellular ADP is a powerful osteolytic agent: Evidence for signaling through the P2Y(1) receptor on bone cells. FASEB J. 2001;15:1139–1148. doi: 10.1096/fj.00-0395com. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J, Knight RD. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- Hu L, Lee M, Campbell W, Perez-Soler R, Karpatkin S. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood. 2004;104:2746–2751. doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- Jin RC, Voetsch B, Loscalzo J. Endogenous mechanisms of inhibition of platelet function. Microcirculation. 2005;12:247–258. doi: 10.1080/10739680590925493. [DOI] [PubMed] [Google Scholar]
- Jurasz P, Stewart MW, Radomski A, Khadour F, Duszyk M, Radomski MW. Role of von Willebrand factor in tumour cell-induced platelet aggregation: Differential regulation by NO and prostacyclin. Br J Pharmacol. 2001;134:1104–1112. doi: 10.1038/sj.bjp.0704343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S, Ambrogio C, Pearlstein E. The role of tumor-induced platelet aggregation, platelet adhesion and adhesive proteins in tumor metastasis. Prog Clin Biol Res. 1988;283:585–606. [PubMed] [Google Scholar]
- Lerner WA, Pearlstein E, Ambrogio C, Karpatkin S. A new mechanism for tumor induced platelet aggregation. Comparison with mechanisms shared by other tumor with possible pharmacologic strategy toward prevention of metastases. Int J Cancer. 1983;31:463–469. doi: 10.1002/ijc.2910310411. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JH, Olson KE, Islam N, Pinsky DJ, Levi R. Role of CD39 (NTPDase-1) in thromboregulation, cerebroprotection, and cardioprotection. Semin Thromb Hemost. 2005;31:234–246. doi: 10.1055/s-2005-869528. [DOI] [PubMed] [Google Scholar]
- Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a two-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2006;109:566–576. doi: 10.1182/blood-2006-07-028282. [DOI] [PubMed] [Google Scholar]
- Medina C, Jurasz P, Santos-Martinez MJ, Jeong SS, Mitsky T, Chen R, Radomski MW. Platelet aggregation-induced by caco-2 cells: Regulation by matrix metalloproteinase-2 and adenosine diphosphate. J Pharmacol Exp Ther. 2006;317:739–745. doi: 10.1124/jpet.105.098384. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- Murugappan S, Shankar H, Kunapuli SP. Platelet receptors for adenine nucleotides and thromboxane A2. Semin Thromb Hemost. 2004;30:411–418. doi: 10.1055/s-2004-833476. [DOI] [PubMed] [Google Scholar]
- Nierodzik ML, Kajumo F, Karpatkin S. Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res. 1992;52:3267–3272. [PubMed] [Google Scholar]
- Nierodzik ML, Bain RM, Liu LX, Shivji M, Takeshita K, Karpatkin S. Presence of the seven transmembrane thrombin receptor on human tumour cells: Effect of activation on tumour adhesion to platelets and tumor tyrosine phosphorylation. Br J Haematol. 1996;92:452–457. doi: 10.1046/j.1365-2141.1996.d01-1494.x. [DOI] [PubMed] [Google Scholar]
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, Diaz R, Commerford PJ, Valentin V, Yusuf S. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: Observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation. 2003;108:1682–1687. doi: 10.1161/01.CIR.0000091201.39590.CB. [DOI] [PubMed] [Google Scholar]
- Phillips DR, Charo IF, Scarborough RM. GPIIb-IIIa: The responsive integrin. Cell. 1991;65:359–362. doi: 10.1016/0092-8674(91)90451-4. [DOI] [PubMed] [Google Scholar]
- Pruemer J. Prevalence, causes, and impact of cancer-associated thrombosis. Am J Health Syst Pharm. 2005;62:S4–S6. doi: 10.2146/ajhp050431. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: Emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Seronie-Vivien S, Mery E, Delord JP, Fillola G, Tkaczuk J, Voigt JJ, Bugat R. Carcinocythemia as the single extension of breast cancer: Report of a case and review of the literature. Ann Oncol. 2001;12:1019–1022. doi: 10.1023/a:1011184706281. [DOI] [PubMed] [Google Scholar]
- Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2:395–402. [PubMed] [Google Scholar]
- Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth SS, Reis ED, Vaananen H, Zhang W, Coller BS. Variable protection of beta 3-integrin–deficient mice from thrombosis initiated by different mechanisms. Blood. 2001;98:1055–1062. doi: 10.1182/blood.v98.4.1055. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Arai I, Futaki N, Hashimoto Y, Honma Y, Nakaike S. Role of COX-1 and COX-2 on skin PGs biosynthesis by mechanical scratching in mice. Prostaglandins Leukot Essent Fatty Acids. 2006;75:1–8. doi: 10.1016/j.plefa.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Tagalakis V, Blostein M, Robinson-Cohen C, Kahn SR. The effect of anticoagulants on cancer risk and survival: Systematic review. Cancer Treat Rev. 2007;33:358–368. doi: 10.1016/j.ctrv.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Takagi J, Springer TA. Integrin activation and structural rearrangement. Immunol Rev. 2002;186:141–163. doi: 10.1034/j.1600-065x.2002.18613.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedswang G, Borgen E, Karesen R, Qvist H, Janbu J, Kvalheim G, Nesland JM, Naume B. Isolated tumor cells in bone marrow three years after diagnosis in disease-free breast cancer patients predict unfavorable clinical outcome. Clin Cancer Res. 2004;10:5342–5348. doi: 10.1158/1078-0432.CCR-04-0245. [DOI] [PubMed] [Google Scholar]
- Witz IP. The tumor microenvironment: The involvement of the selectin-selectin-ligand axis in tumor-endothelium interactions. Retrovirology. 2006;3(Suppl 1):S91. [Google Scholar]
- Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126–136. doi: 10.1016/j.critrevonc.2007.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplementary material, which may be viewed at the Journal of Cellular Biochemistry website at http://www.interscience.wiley.com/jpages/0730-2312/suppmat/index.html.