Abstract
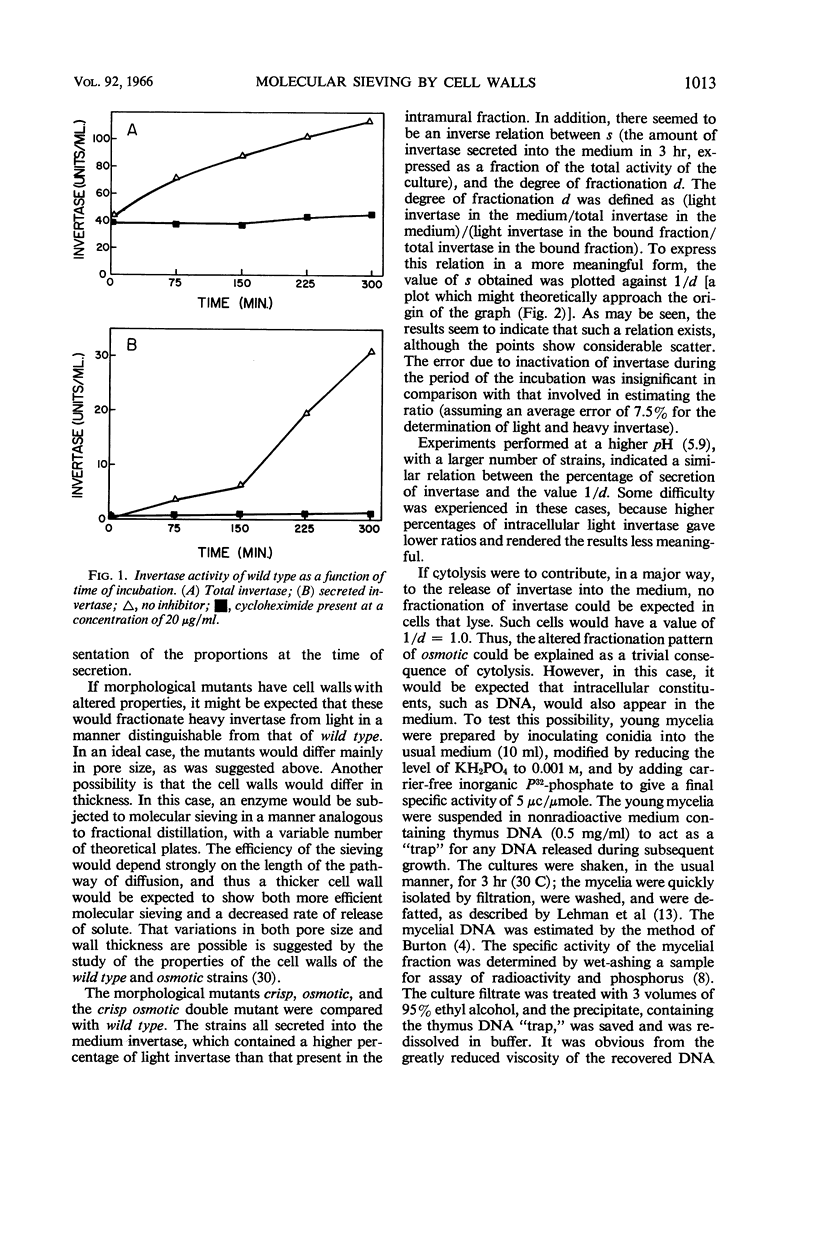
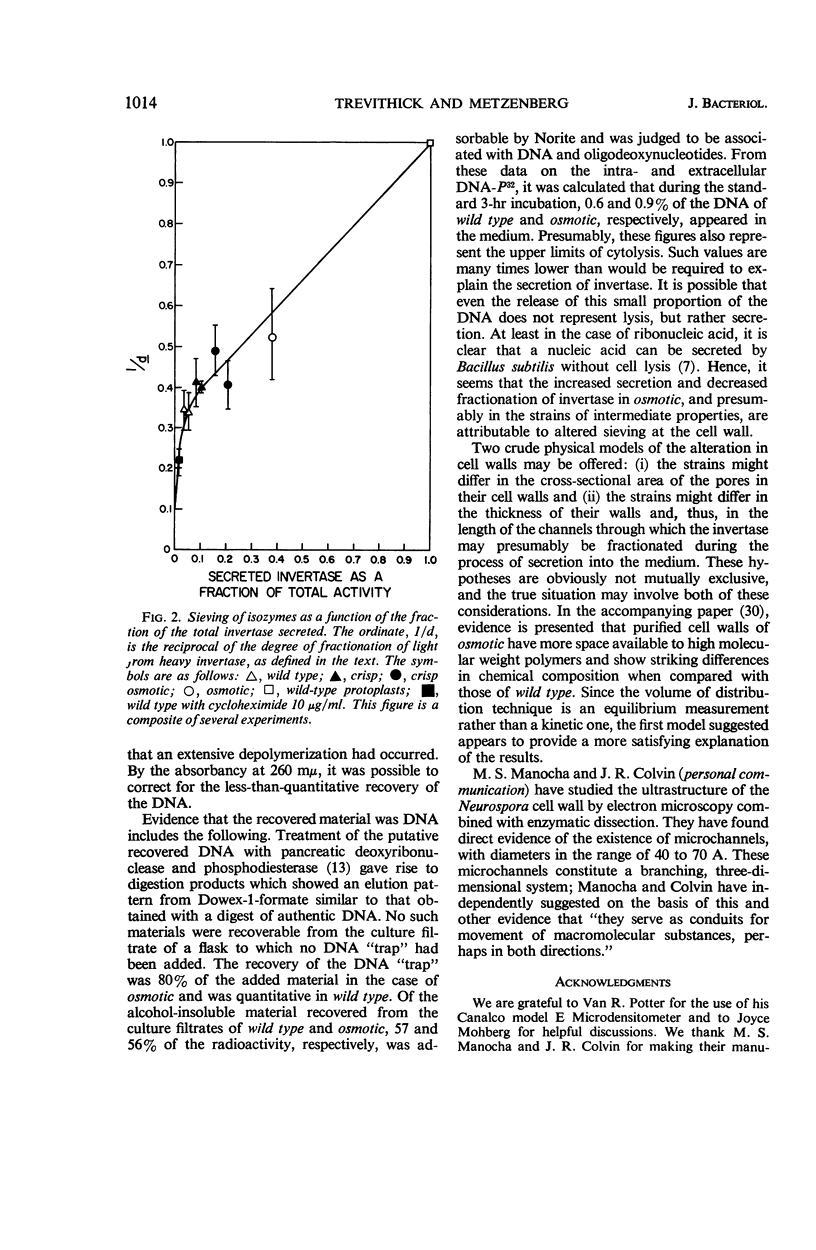
Trevithick, John R. (University of Wisconsin Medical School, Madison), and Robert L. Metzenberg. Molecular sieving by Neurospora cell walls during secretion of invertase isozymes. J. Bacteriol. 92: 1010–1015. 1966.—The secretion of invertase by young mycelia of Neurospora was studied. The process of secretion was found to be dependent upon growth. The results indicate that fractionation of light invertase, the monomer, from heavy invertase, the aggregated form, occurs at the cell wall. Neurospora strains wild type, crisp, osmotic, and the double mutant crisp osmotic were tested. An inverse relation exists between the fraction of the total invertase activity of the culture which the mold secretes into the medium and the degree of fractionation, defined as the ratio of the fraction of the invertase secreted into the medium that is light invertase to the fraction of the invertase remaining associated with the cells that is light invertase. The hypothesis is offered that the increased secretion of invertase and decreased degree of fractionation seen in osmotic mutants, and to a lesser extent in the other mutants, can be explained by an increased porosity of the cell wall.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGER L. S., EBERHART B. M. Extracellular beta-transglucosidase activity from conidia of Neurospora crassa. Biochem Biophys Res Commun. 1961 Oct 23;6:62–66. doi: 10.1016/0006-291x(61)90186-3. [DOI] [PubMed] [Google Scholar]
- BURGER M., BACON E. E., BACON J. S. Some observations on the form and location of invertase in the yeast cell. Biochem J. 1961 Mar;78:504–511. doi: 10.1042/bj0780504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Freese E. Excretion of alkaline phosphatase of Bacillus subtilis. Biochem Biophys Res Commun. 1964 Aug 11;16(6):541–544. doi: 10.1016/0006-291x(64)90189-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DEMAIN A. L., BURG R. W., HENDLIN D. EXCRETION AND DEGRADATION OF RIBONUCLEIC ACID BY BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:640–646. doi: 10.1128/jb.89.3.640-646.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- EBERHART B. M. Exogenous enzymes of Neurospora conidia and mycelia. J Cell Comp Physiol. 1961 Aug;58:11–16. doi: 10.1002/jcp.1030580103. [DOI] [PubMed] [Google Scholar]
- HEREDIA C. F., YEN F., SOLS A. Role and formation of the acid phosphatase in yeast. Biochem Biophys Res Commun. 1963 Jan 18;10:14–18. doi: 10.1016/0006-291x(63)90259-6. [DOI] [PubMed] [Google Scholar]
- HSU K. S. THE GENETIC BASIS OF ACTIDIONE RESISTANCE IN NEUROSPORA. J Gen Microbiol. 1963 Sep;32:341–347. doi: 10.1099/00221287-32-3-341. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., BESSMAN M. J., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. I. Preparation of substrates and partial purification of an enzyme from Escherichia coli. J Biol Chem. 1958 Jul;233(1):163–170. [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- MANDELS G. R. Localization of carbohydrases at the surface of fungus spores by acid treatment. Exp Cell Res. 1953 Sep;5(1):48–55. doi: 10.1016/0014-4827(53)90093-7. [DOI] [PubMed] [Google Scholar]
- MANDELS G. R. Properties and surface location of a sulfhydryl oxidizing enzyme in fungus spores. J Bacteriol. 1956 Aug;72(2):230–234. doi: 10.1128/jb.72.2.230-234.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELS G. R. The properties and surface location of an enzyme oxidizing ascorbic acid in fungus spores. Arch Biochem Biophys. 1953 Jan;42(1):164–173. doi: 10.1016/0003-9861(53)90249-5. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. A gene affecting the repression of invertase and trehalase in Neurospora. Arch Biochem Biophys. 1962 Mar;96:468–474. doi: 10.1016/0003-9861(62)90322-3. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. ENZYMICALLY ACTIVE SUBUNITS OF NEUROSPORA INVERTASE. Biochim Biophys Acta. 1964 Aug 26;89:291–302. doi: 10.1016/0926-6569(64)90217-2. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. THE LOCALIZATION OF BETA-FRUCTOFURANOSIDASE IN NEUROSPORA. Biochim Biophys Acta. 1963 Nov 8;77:455–465. doi: 10.1016/0006-3002(63)90521-3. [DOI] [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Relationship of the major constituents of the Neurospora crassa cell wall to wild-type and colonial morphology. J Bacteriol. 1965 Oct;90(4):1073–1081. doi: 10.1128/jb.90.4.1073-1081.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- REESE E. T., MANDELS M. Use of enzymes in isolation and analysis of polysaccharides. Appl Microbiol. 1959 Nov;7:378–387. doi: 10.1128/am.7.6.378-387.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON D. D., LAMPEN J. O. Localization of sucrose and maltose fermenting systems in Saccharomyces cerevisiae. Biochim Biophys Acta. 1962 Jan 29;56:303–312. doi: 10.1016/0006-3002(62)90567-x. [DOI] [PubMed] [Google Scholar]
- TONOMURA K., TANABE O. LOCALIZATION OF CELL-BOUND ALPHA-AMYLASE IN ASPERGILLUS ORYZAE DEMONSTRATED BY FLUORESCENT-ANTIBODY TECHNIQUE. J Bacteriol. 1964 Jan;87:226–227. doi: 10.1128/jb.87.1.226-227.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevithick J. R., Metzenberg R. L. Genetic alteration of pore size and other properties of the Neurospora cell wall. J Bacteriol. 1966 Oct;92(4):1016–1020. doi: 10.1128/jb.92.4.1016-1020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevithick J. R., Metznberg R. L. The invertase isozyme formed by Neurospora protoplasts. Biochem Biophys Res Commun. 1964 Jul 1;16(4):319–325. doi: 10.1016/0006-291x(64)90033-6. [DOI] [PubMed] [Google Scholar]
- ZAMENHOF S., GRIBOFF G., MARULLO N. Studies on the resistance of desoxyribonucleic acids to physical and chemical factors. Biochim Biophys Acta. 1954 Apr;13(4):459–470. doi: 10.1016/0006-3002(54)90362-5. [DOI] [PubMed] [Google Scholar]



