Abstract
The development of antagonists of the transient receptor potential vanilloid-1 (TRPV1) channel as pain therapeutics has revealed that these compounds cause hyperthermia in humans. This undesirable on-target side effect has triggered a surge of interest in the role of TRPV1 in thermoregulation and revived the hypothesis that TRPV1 channels serve as thermosensors. We review literature data on the distribution of TRPV1 channels in the body and on thermoregulatory responses to TRPV1 agonists and antagonists. We propose that two principal populations of TRPV1-expressing cells have connections with efferent thermoeffector pathways: 1) first-order sensory (polymodal), glutamatergic dorsal-root (and possibly nodose) ganglia neurons that innervate the abdominal viscera and 2) higher-order sensory, glutamatergic neurons presumably located in the median preoptic hypothalamic nucleus. We further hypothesize that all thermoregulatory responses to TRPV1 agonists and antagonists and thermoregulatory manifestations of TRPV1 desensitization stem from primary actions on these two neuronal populations. Agonists act primarily centrally on population 2; antagonists act primarily peripherally on population 1. We analyze what roles TRPV1 might play in thermoregulation and conclude that this channel does not serve as a thermosensor, at least not under physiological conditions. In the hypothalamus, TRPV1 channels are inactive at common brain temperatures. In the abdomen, TRPV1 channels are tonically activated, but not by temperature. However, tonic activation of visceral TRPV1 by nonthermal factors suppresses autonomic cold-defense effectors and, consequently, body temperature. Blockade of this activation by TRPV1 antagonists disinhibits thermoeffectors and causes hyperthermia. Strategies for creating hyperthermia-free TRPV1 antagonists are outlined. The potential physiological and pathological significance of TRPV1-mediated thermoregulatory effects is discussed.
I. Introduction
Life is intimately connected to temperature, and a living organism is constantly responding to changes in ambient and body temperatures (Ta1 and Tb, respectively) with a variety of physiological and behavioral responses. However, the molecular mechanisms of the detection of Ta and Tb signals are largely unknown. A major advance in this area is expected to stem from the discovery and characterization of transient receptor potential (TRP) channels. The superfamily of mammalian TRP channels consists of approximately 30 proteins divided into six subfamilies: ankyrin (TRPA), canonical, melastatin (TRPM), mucolipin, polycystin, and vanilloid (TRPV). Among TRP channels, nine are highly sensitive to temperature and are referred to as the thermo-TRP channels. They include the heat-activated TRPV1 to TRPV4, TRPM2, TRPM4, and TRPM5 as well as the cold-activated TRPA1 and TRPM8 (Patapoutian et al., 2003; Dhaka et al., 2006; Caterina, 2007; Vennekens et al., 2008). Two unique properties of thermo-TRP channels deserve special consideration. First, the activation of all thermo-TRP channels results in an inward, nonselective cationic current and, consequently, membrane depolarization. This electrophysiological mechanism agrees with a possible role of thermo-TRP channels as a molecular substrate of peripheral thermosensitivity (Okazawa et al., 2002). Second, whereas each individual class of thermo-TRP channels is activated within a relatively narrow temperature range, cumulatively, these channels cover a broad span, from noxious cold to noxious heat, which makes them well suited to the detection of thermal signals (Romanovsky, 2007b). These features suggest that at least some thermo-TRP channels may be those long-sought molecules that are responsible for the reception of thermal signals, especially peripheral ones. Indeed, it has been confirmed that TRPM8 (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007), TRPV3 (Moqrich et al., 2005), and TRPV4 (Lee et al., 2005) participate in mechanisms of thermoreception. Even for these channels, however, it is unclear under what conditions and to what extent they contribute to Tb regulation.
This review focuses on the thermoregulatory role of the TRPV1 channel [also known as the vanilloid-1 receptor, or the capsaicin (CAP) receptor]. Long before this channel received its current name, TRPV1, it was suspected to play the roles of both a peripheral thermosensor (Dib, 1983; Donnerer and Lembeck, 1983; Obál et al., 1987) and a central thermosensor (Szolcsányi et al., 1971; Hori and Shinohara, 1979; Dib, 1982) for autonomic and behavioral thermoregulation (i.e., to detect those thermal signals that are used in the control of autonomic and behavioral thermoeffectors). More recently, interest in the thermoregulatory role of TRPV1 has surged because of a serious problem with the development of TRPV1 antagonists, widely regarded as next-generation pain therapeutics. TRPV1 antagonists have been found to cause hyperthermia in experimental animals and in human patients (Gavva et al., 2008). This side effect presents a hurdle for drug development, but it also sheds light on the physiological role of the TRPV1 channel.
In this review, we first describe how Tb is regulated and where TRPV1 channels are located in the body with respect to different elements of the thermoregulatory system. We then analyze data obtained in studies with pharmacological agonists of the TRPV1 channel (conducted over more than a half a century) and in studies with TRPV1 antagonists (which have mushroomed over the past few years). We describe the mechanisms of TRPV1-mediated effects on Tb, identify and critically analyze the contradictions resulting from the use of different pharmacological tools, propose a unifying hypothesis, and answer the primary question of this review: whether TRPV1 channels function as thermosensors under physiological conditions. We also discuss strategies for creating hyperthermia-free TRPV1 antagonists.
II. The Thermoregulatory System
A. Thermoeffectors and Functional Architecture
1. Core and Shell Body Temperatures and Thermoeffectors.
The body can be divided into two compartments: the thermally homogeneous core (the central nervous system and the thoracic and abdominal viscera) and the thermally heterogeneous shell (the rest of the body, including the skin) (Romanovsky, 2007a). It is noteworthy that the core is only relatively homogeneous, and that temperatures of different parts of the core can be different [e.g., when mechanisms of selective brain cooling come into play (section V.C.)]. Nevertheless, it is reasonable to consider that the thermoregulatory system maintains a relatively constant core (deep) Tb under many circumstances. This is achieved by use of multiple effector responses, both behavioral and physiological, that can be activated from multiple thermosensors located within the shell (mostly in the skin) and the core (most importantly in the brain). Thermoregulatory behaviors include heat or cold avoidance and seeking, as well as many others—from simple postural changes to complex behavioral programs. The principal physiological cold-defense responses are autonomic ones [namely, sympathetically controlled skin vasoconstriction and nonshivering thermogenesis in brown adipose tissue (BAT)] and shivering. Although rats exhibit robust shivering responses if core Tb falls, nonshivering thermogenesis is a more important mechanism for heat production in rodents (Cannon and Nedergaard, 2004). Conversely, although humans have significant BAT deposits (Nedergaard et al., 2007), shivering is thought to be a primary mechanism for heat production in humans (Sessler, 1997). Physiological (autonomic) heat defenses include cutaneous vasodilation and species-specific responses aimed at evaporating water from the skin and respiratory tract, such as thermoregulatory salivation in rats and sweating [and perhaps also hyperpnea; see White (2006)] in humans.
2. Body Temperature Control.
How the multisensor, multieffector thermoregulatory system is functionally organized remains a subject of debate, although a consensus concept has been emerging (Romanovsky, 2007b). This concept is based on the idea that, similar to other physiological systems (Partridge, 1982), the thermoregulatory system functions as a federation of relatively independent effector loops (Satinoff, 1978), without a single controller and without a single set point or its equivalent (Werner, 1979, 1988). Each thermoeffector loop includes a unique efferent neural pathway driving the corresponding effector response (Nagashima et al., 2000; Morrison et al., 2008). Each thermoeffector loop uses a negative feedback from the main control variable, core Tb, and a feedback (either negative or positive) from the auxiliary variable, skin temperature (Tsk). The use of auxiliary control allows the body to anticipate the thermal disturbances coming from the environment and to maintain the core Tb at a more stable level. By the same token, each thermoeffector is sensitive to a unique combination of shell and core temperatures (Jessen, 1981, 1985; Roberts, 1988; Sakurada et al., 1993; Ootsuka and McAllen, 2006), and each, therefore, defends a different level of a differently distributed Tb (Romanovsky, 2004, 2007a). Yet the activity of each thermoeffector affects the core Tb, which plays, therefore, an important role in coordinating different thermoeffector responses (Romanovsky, 2007a). In fact, coordination through the common (or overlapping) control variables may be sufficient to explain most, if not all, examples of coordinated recruitment of thermoeffectors in a response. This concept requires neither a neural computation of an integrated Tb nor a comparison with an obvious or hidden set point in a unified system. By acting on the thermoreceptive elements of a thermosensitive neuron, a local Tb (whether Tsk or core Tb) can change the activity of this neuron and, sequentially, of the entire pathway that controls the thermoeffector synaptically linked to this neuron. As explained by Kobayashi and colleagues (Kobayashi, 1989; Okazawa et al., 2002), a thermosensitive neuron does not code its local temperature into an electric signal to be processed by the central control network. Instead, it is an element that generates a signal when the local Tb reaches this element's threshold; this signal then spreads by a neural pathway to drive an effector response. This design emphasizes the significance of the thermoreceptive elements of thermosensory neurons and gives these elements a principal role in determining whether a thermoeffector response will be triggered. Of special importance for this review is the idea that such thermoreceptive elements are likely to be thermo-TRP channels.
The thermoregulatory system also overlaps, or “meshes,” with other control systems in multiple ways. Some relationships within meshed control systems seem “illogical” (Partridge, 1982), perhaps because we often do not understand them. Such relationships can be illustrated with the numerous reflexes in the body that adjust a physiological variable based on the information about a different, sometimes seemingly unrelated, variable. Examples of such reflexes are changes in BAT thermogenesis caused by stomach distension (Pétervári et al., 2005), intraportal glucose (Sakaguchi and Yamazaki, 1988), or intraduodenal hypertonic saline (Osaka et al., 2002). Another example is the skin vasoconstriction response to colorectal distension (Laird et al., 2006). The nonthermal nature of some variables meshed with the thermoregulatory system is important for the discussion of the physiological roles of TRPV1 channels at the end of this review (section V.B.3). Behavioral thermoregulatory responses also involve elements of feed-forward control that use nonthermal signals (e.g., selecting weather-appropriate clothes based on a weather forecast).
B. Neural Pathways
1. Afferent Pathways That Control Autonomic Thermoeffectors
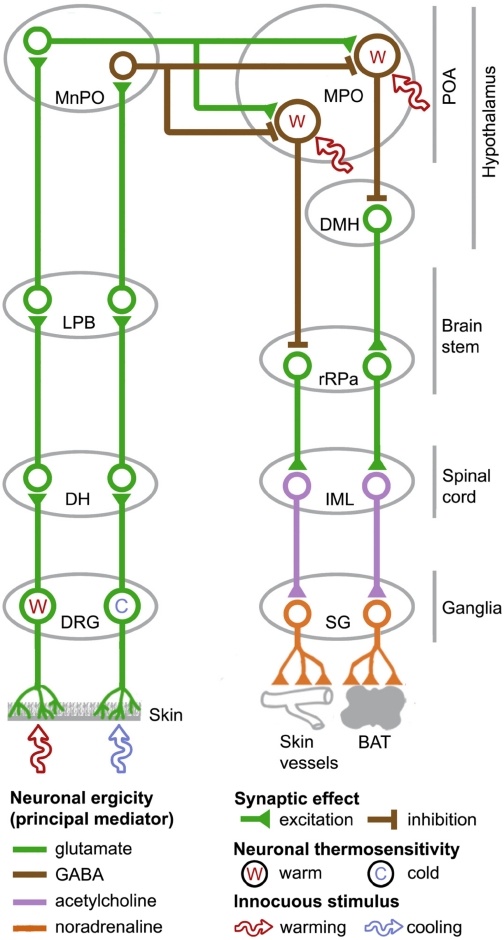
With a few exceptions (Kanosue et al., 2002; Egan et al., 2005; McAllen et al., 2006), human neural thermoregulatory pathways have not been studied and are largely unknown. In the rat, neural pathways for BAT thermogenesis, thermoregulatory skin vasoconstriction and vasodilation, shivering, and thermoregulatory salivation have been identified and characterized to various degrees over the last 2 decades. Although each effector response is controlled independently, neural pathways for different thermoeffectors, especially those for BAT thermogenesis and skin vasoconstriction, follow similar patterns (Fig. 1). The primary afferent neurons within all thermoeffector pathways are sensory neurons with cell bodies located in the dorsal-root ganglia (DRG) and the nodose and trigeminal ganglia (not shown in Fig. 1). Of the three broad physiological types of DRG neurons [i.e., mechanoreceptors, nociceptors, and thermoreceptors (Perl, 1992)], two types respond to thermal stimuli, either noxious (nociceptors) or innocuous (thermoreceptors). Most thermoreceptor endings are located immediately beneath the epidermis and respond to shell temperatures in the skin and in the oral and urogenital mucosa; most (but not all) of these superficial shell receptors are cold-sensitive [for review, see Nomoto et al. (2004)]. Temperature acts on the thermoreceptive elements (at least some of which are probably thermo-TRP channels) in the cutaneous nerve endings of the lightly myelinated Aδ (cold-sensitive) or unmyelinated C (warm-sensitive) fibers of DRG neurons that send their axons to secondary afferent neurons in lamina I of the spinal or medullary (trigeminal) dorsal horn (DH). Craig and colleagues (Andrew and Craig, 2001; Craig et al., 2001) have distinguished thermoreception-specific lamina-I neurons (i.e., those that respond to innocuous cooling or warming of the skin and are important in thermoregulation) from nociception-specific and polymodal nociceptive cells (which respond to noxious heat and cold and are important for pain processing). In addition to neurons that respond to innocuous cold and warmth in the shell, there are peripheral deep-tissue thermoreceptors that respond to core Tb. The sensory endings of these DRG and nodose-ganglion neurons are located on splanchnic and vagal afferents in the esophagus, stomach, large intra-abdominal veins, and other organs (Riedel, 1976; Cranston et al., 1978; Gupta et al., 1979; El Ouazzani and Mei, 1982). Although these deep Tb receptors differ from superficial sensors, they also mainly detect cold rather than warmth (Cranston et al., 1978; Gupta et al., 1979). Nevertheless, warmth-sensitive units certainly exist in the core and are relatively abundant in some organs (e.g., the mesentery) (Adelson et al., 1997).
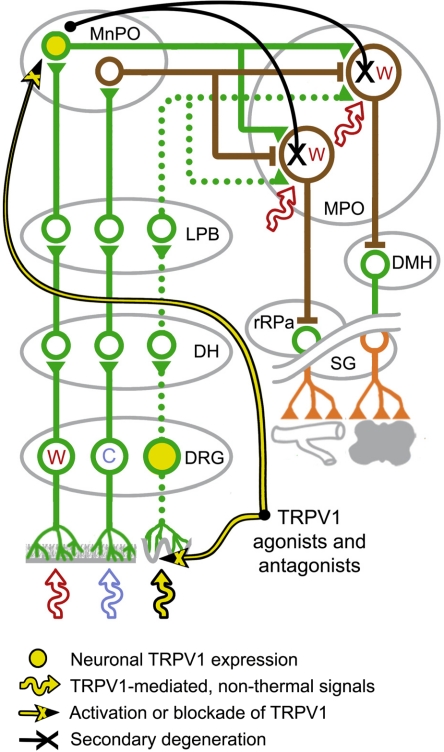
Fig. 1.
A schematic of the afferent (the left portion of the figure) and efferent (the right portion) neural pathways underlying the BAT thermogenesis and cutaneous vasoconstriction responses to innocuous thermal stimulation of the skin. IML, intermediolateral column; SG, sympathetic ganglia. For detailed descriptions, see section II.B.
Lamina-I DH neurons in the rat spinal cord transmit thermal signals from the skin (and potentially from other shell and core tissues) to neurons in the lateral parabrachial nucleus (LPB) (Fig. 1). Within the LPB, most neurons activated by skin cooling are located in the external lateral part of the nucleus and in its central part (Nakamura and Morrison, 2008b). They send glutamatergic projections to GABA-ergic neurons in the median preoptic nucleus (MnPO) of the preoptic hypothalamic area (POA). These MnPO neurons, in turn, project to inhibit warm-sensitive GABA-ergic neurons in another POA structure, the medial preoptic area (MPO) (Nakamura and Morrison, 2008a,b). A parallel pathway for heat-defense responses to innocuous skin warming has been proposed and shown to involve neurons located in the dorsal LPB (Nakamura and Morrison, 2007b). The finding that nanoinjection of glutamate antagonists into the MPO reduces the skin-warming-induced inhibition of spontaneous cutaneous vasoconstrictor activity of the sural nerve (K. Nakamura and S. F. Morrison, unpublished observation) suggests that there is a glutamatergic input to warm-sensitive MPO neurons within this parallel pathway, and we hypothesize (and are currently testing this hypothesis in a direct experiment) that this glutamatergic input comes from an MnPO neuron. Hence, in our current model for a pathway for the facilitation of heat-defense responses initiated by cutaneous warming, neurons in the dorsal LPB drive glutamatergic neurons in the MnPO to excite warm-sensitive GABA-ergic neurons in the MPO. For the control of skin vasomotor tone, the actual location of those neurons that are shown in Fig. 1 as warm-sensitive POA neurons may be in both the caudal MPO and caudal lateral preoptic area (Tanaka et al., 2009); for the sake of simplicity, however, we refer to all warm-sensitive POA cells within thermoeffector pathways as MPO cells.
2. Efferent Pathways to Autonomic Thermoeffectors
The abovementioned warm-sensitive MPO cells tonically suppress BAT thermogenesis and skin vasoconstriction (Osaka, 2004) and can be regarded as the first efferent neurons within the loops controlling these thermoeffectors. They send tonic inhibitory outputs to the dorsomedial hypothalamus (DMH) and the rostral raphe pallidus nucleus (rRPa), both of which provide a sympathoexcitatory drive for thermoeffector activation (Nakamura and Morrison, 2007a; Morrison et al., 2008; Rathner et al., 2008) (Fig. 1). In particular, the rRPa harbors sympathetic premotor neurons that send the essential excitatory input to spinal sympathetic preganglionic neurons for BAT thermogenesis and for cutaneous vasoconstriction (Smith et al., 1998; Cano et al., 2003; Nakamura et al., 2004). The rostral ventrolateral medulla also contains sympathetic premotor neurons influencing the sympathetic outflow to skin blood vessels (not shown in Fig. 1), and these neurons may be involved in cold-induced skin vasoconstriction (Ootsuka and McAllen, 2005). Although these neurons do not receive significant inhibitory input from MPO neurons (Tanaka et al., 2002), they could influence thermoregulatory responses to innocuous thermal stimulation of the skin by affecting the excitability of cutaneous vasoconstrictor sympathetic preganglionic neurons. Neurons in sites other than the DMH or rRPa can also influence BAT thermogenic responses. These sites (not shown in Fig. 1) include the locus ceruleus (LC) (Almeida et al., 2004), the paraventricular nucleus (Horn et al., 1994; Lu et al., 2001; Madden and Morrison, 2009), and some midbrain structures (for review, see Romanovsky et al., 2005; Romanovsky, 2007a).
On the efferent side, separation of the loops controlling BAT thermogenesis and skin vasoconstriction is depicted in Fig. 1. A BAT-specific population of warm-sensitive output neurons in the MPO projects to inhibit BAT-specific sympathoexcitatory neurons in the DMH and possibly in the rRPa (Nakamura et al., 2002, 2005; Nakamura and Morrison, 2007a; Morrison et al., 2008), whereas a distinct, skin vasoconstriction-specific population of warm-sensitive MPO neurons projects to inhibit vasoconstriction-specific sympathetic premotor neurons in the rRPa (Nakamura et al., 2002, 2009; Rathner et al., 2008). Although effector-specific MPO or premotor neurons have yet to be identified, the following are among several observations that argue for the existence of effector-specific thermal efferent pathways. In an anesthetized rat preparation maintained at Tb between 36.5 and 38.5°C, the sympathetic outflow to BAT is silent, whereas that controlling rat tail cutaneous vasoconstriction is significantly activated (Ootsuka and McAllen, 2005; Morrison et al., 2008). Once a common respiration-related periodicity is removed, there is no coherence between the sympathetic activities driving BAT and rat tail cutaneous vasoconstriction (Ootsuka and McAllen, 2006). In addition, the sympathetic outflow to BAT depends on the activity of sympathoexcitatory neurons in the DMH (Zaretskaia et al., 2003; Madden and Morrison, 2004; Dimicco and Zaretsky, 2007), whereas the tail skin vasoconstrictor outflow does not (Rathner et al., 2008). It is also worth mentioning that the vasoconstrictor outflow to different areas of the skin is controlled by independent neural pathways (Tanaka et al., 2007).
3. Pathways for Behavioral Thermoregulation
Although the efferent neural pathways to all autonomic thermoeffectors and to skeletal muscles (shivering) include warm-sensitive MPO neurons (Nagashima et al., 2000; Romanovsky, 2007a), these neurons may not be involved in some thermoregulatory behaviors. The only mammalian thermoregulatory behavior that has been shown to involve the MPO is a relaxed postural extension in response to heat exposure; such postural extension does not occur in MPO-lesioned animals (Roberts and Martin, 1977). It is noteworthy that other thermoregulatory behaviors, such as moving to a “reward” (desired Ta) zone or pressing a lever to trigger warming or cooling of the animal's environment, remain intact in animals with preoptic lesions (Lipton, 1968; Carlisle, 1969; Satinoff and Rutstein, 1970; Schulze et al., 1981). In our study (Almeida et al., 2006b), large bilateral preoptic lesions (including the entire MPO) did not change cold- and warmth-seeking responses of rats to thermal (cold and heat exposure), chemical (TRPV1 and TRPM8 agonists), or inflammatory [low and high doses of bacterial lipopolysaccharide (LPS)] stimuli. Although MPO neurons are unlikely to be involved in most thermoregulatory behaviors, MnPO neurons may be involved in at least some [e.g., in the intensification of an operant thermoregulatory behavior (moving to a reward zone during heat exposure to receive a breeze of cold air) caused by hypertonic saline (Konishi et al., 2007)]. Overall, not much is known about the neural pathways underlying thermoregulatory behaviors (Nagashima et al., 2000; Romanovsky, 2007b).
III. Distribution of Transient Receptor Potential Vanilloid-1 Channels
A. Afferent Nerves
Considering the thermosensor role proposed long ago for the TRPV1 channel (Jancsó-Gábor et al., 1970b), the distribution of TRPV1 in afferent neurons is of particular interest. TRPV1 channels are markedly expressed in peripheral terminals of thin myelinated (Aδ) and unmyelinated (C) fibers of neurochemically heterogeneous primary afferent neurons in the DRG and the trigeminal ganglion and of some peptidergic sensory neurons in the nodose ganglion (Szallasi et al., 1995; Caterina et al., 1997; Tominaga et al., 1998; Mezey et al., 2000). They are also expressed on the central terminals of the DRG, trigeminal, and nodose neurons (Szallasi et al., 1995; Tominaga et al., 1998; Guo et al., 1999). These primary sensory neurons innervate both the skin (of the trunk, limbs, and head) and visceral organs, thus resulting in an extremely wide distribution of TRPV1 channels associated with afferent neurons. Indeed, TRPV1 channels are present in at least 60% of the spinal nerve afferents servicing the upper gastrointestinal tract (GIT), large intestine, and urinary bladder, and in ∼30% of the spinal afferents in the skin or skeletal muscles (Schicho et al., 2004; Hwang et al., 2005; Christianson et al., 2006). At least 20% of the vagal afferents innervating the upper GIT also contain the TRPV1 channel (Schicho et al., 2004; Zhang et al., 2004; Bielefeldt et al., 2006).
Thermoeffector responses are triggered by thermal exposures extensive enough to affect heat exchange between the body and the environment, and the wide distribution of TRPV1 channels throughout the thermal shell and core would be compatible with a physiologically significant thermoreceptor function. However, TRPV1 channels are located predominantly on polymodal nociceptors (Caterina et al., 1997, 2000). Sensory neurons from Trpv1 knockout (KO) mice (i.e., those with a homozygous targeted null mutation in the Trpv1 gene) have deficient responses to noxious stimuli: these mice show no vanilloid-evoked pain behavior, have little thermal hypersensitivity under conditions of inflammation, and are impaired in the detection of painful heat (Caterina et al., 2000). The sensitivity to noxious heat is also reduced in mice that continuously express the short hairpin RNAs that silence the Trpv1 gene (Christoph et al., 2008). Furthermore, TRPV1 is a high-threshold thermo-TRP channel that opens in transfected cells in vitro at ∼43°C (Caterina et al., 1997; Tominaga et al., 1998), which would be considered a very high Tb. Both in rodents (Story et al., 2003; Kobayashi et al., 2005; Hjerling-Leffler et al., 2007) and in humans (Anand et al., 2008), high-threshold heat-sensitive TRPV1 channels are largely coexpressed with high-threshold cold-sensitive TRPA1 channels, and roles for both TRPV1 and TRPA1 gene polymorphisms in cold-pain and heat-pain sensitivity in humans have been proposed (Kim et al., 2004, 2006). All these data make TRPV1-expressing neurons good candidates for detecting noxious thermal stimuli of both “modalities” (i.e., heat and cold) rather than innocuous thermal stimuli that would trigger thermoeffector responses of a particular modality (e.g., heat-defense responses). TRPV1 channels on nociceptors can also be involved in cutaneous vasodilation in response to local warming (Stephens et al., 2001), which is not mediated by the same population of nerve fibers that mediate active vasodilation in response to whole-body warming (Kellogg et al., 1995). Despite all these data, an involvement of TRPV1-expressing polymodal sensory neurons in thermoregulatory responses, especially at a high Tb, cannot be ruled out completely. For example, TRPV1 channels expressed on sensory neural fibers within intercostal nerves have been shown to respond to high (40–44°C) temperatures in the interscapular BAT, the tissue innervated by these fibers, and have been proposed to mediate the inhibition of BAT thermogenesis by high local temperatures (Osaka et al., 1998).
B. The Preoptic Hypothalamus
Early studies by Jancsó-Gábor and colleagues (Jancsó-Gábor et al., 1970a; Szolcsányi et al., 1971) indicated that the POA may be the site of the hypothermic action of CAP, the first and most widely used TRPV1 agonist (section IV.B), and the presence of CAP-responsive structures in the POA was proposed. Later, the TRPV1 channel was shown by some authors to be widely distributed in the brain (Starowicz et al., 2008), with high levels of transcripts of the Trpv1 gene found in the hypothalamus (Sasamura et al., 1998; Mezey et al., 2000). At the protein level, weak TRPV1-like immunoreactivity was detected in rat anterior hypothalamic nuclei (Mezey et al., 2000), whereas resiniferatoxin (RTX), a highly potent TRPV1 agonist, was shown to bind specifically to membranes prepared from rat or human POA tissue (Acs et al., 1996). At least some CAP-sensitive terminals in the POA may be glutamatergic, because CAP causes glutamate release in hypothalamic slices (Sasamura et al., 1998) and enhances the frequency of spontaneous glutamatergic excitatory postsynaptic potentials in MPO neurons by acting both at the immediate presynaptic site and remotely on neurons projecting to the MPO neurons (Karlsson et al., 2005). Because glutamatergic input to warm-sensitive MPO neurons is proposed to come from MnPO glutamatergic interneurons in the afferent pathway stimulated by skin warming (Fig. 1), a potential mechanism through which CAP could induce hypothermia would arise from an action on the MnPO interneurons—if these neurons express the TRPV1 channel. CAP has also been shown to increase the frequency of GABA-ergic inhibitory postsynaptic potentials in MPO neurons by acting presynaptically (Karlsson et al., 2005), but whereas facilitation of excitatory synaptic transmission is typical for CAP (Doyle et al., 2002; Jennings et al., 2003), an enhancement of inhibitory synaptic transmission by this substance is a rare finding. Such an action of CAP would agree with the location of TRPV1 channels on GABA-ergic MnPO neurons within the cutaneous cooling-sensitive pathway that projects to warm-sensitive MPO neurons (Fig. 1). However, the activation of TRPV1 channels on these GABA-ergic MnPO neurons should trigger cold-defense responses and lead to hyperthermia, not hypothermia.
Many authors of the early studies of the thermoregulatory effect of CAP thought that CAP-sensitive neurons in the POA play a thermosensory role (Szolcsányi et al., 1971; Hori and Shinohara, 1979; Dib, 1982), which would mean that warm-sensitive MPO neurons express TRPV1 channels and that these TRPV1 channels determine, at least in part, the neuronal sensitivity to local temperature. However, CAP does not seem to act on MPO neurons directly, because no electrophysiological evidence exists for the presence of postsynaptic CAP-sensitive receptors on these neurons (Karlsson et al., 2005). Furthermore, TRPV1 channels are probably not involved in POA mechanisms of thermosensitivity. Indeed, when POA neurons are exposed to different temperatures, the effects evoked (changes in brief ionic currents of the depolarizing prepotential) are different from the TRP-mediated effects (changes in the resting membrane potential) (Zhao and Boulant, 2005; Boulant, 2006), even though some authors think that mechanisms of central thermosensitivity may be compatible with participation of neuronal thermo-TRP channels (Kobayashi et al., 2006). Moreover, when ruthenium red, a nonselective TRPV1 antagonist, was applied to MPO slices in vitro, it did not reduce the thermosensitivity of warm-sensitive MPO neurons (Unger et al., 2008). Furthermore, a recent study with cDNA from individually characterized warm-sensitive, presumably GABA-ergic (glutamic acid decarboxylase-expressing) neurons from mouse POA found no evidence of expression of thermo-TRP channels in these cells (I. V. Tabarean, J. Eberwine, B. Conti, and T. Bartfai, personal communication).
It should also be mentioned that Tb values close to the in vitro threshold temperature for activation of the TRPV1 channel (>42°C) are very rarely seen in vivo, even in febrile patients (DuBois, 1949). Such temperatures are dangerous: maintaining brain temperature at ∼43°C for more than 1 h results in necrotic lesions and neuronal loss (Britt et al., 1983; Matsumi et al., 1994). Although even higher core Tb values have been reported (on some occasions as high as 45–47°C), such reports are rare and typically refer to measurements at extrabrain sites in severe pathological conditions, including lethal heatstroke (Hart et al., 1982), malignant hyperthermia of anesthesia (Simon, 1993), or certain drug intoxications in humans (Clark and Lipton, 1984). Under normal conditions, brain temperature is at least 5°C lower than the in vitro activation threshold of the TRPV1 channel. For the sake of objectivity, we should add that the in vivo activation threshold temperature for the TRPV1 channel may be substantially lower than that found in vitro (see section IV.E.3).
C. Brain Structures outside the Preoptic Hypothalamus
TRPV1 channels may also be present in the structures that harbor premotor neurons of the thermoregulatory pathways (section II.B.2). Reverse transcriptase-polymerase chain reaction detects TRPV1 mRNA widely throughout the brain (Sasamura et al., 1998), and immunochemistry reveals a TRPV1-like protein in the LC, the raphe area, and (to a lesser extent) the DMH and the paraventricular nucleus (Mezey et al., 2000). Furthermore, CAP has been reported to induce cutaneous vasodilation and hypothermia after microinjection in the dorsal raphe (Hajós et al., 1987). [3H]RTX binds to membranes from both rat and human LC (Acs et al., 1996), and systemic administration of CAP at low doses causes marked excitation of LC neurons (Hajós et al., 1988). However, [3H]RTX autoradiography in rat brain sections (not in membrane preparations) failed to detect any specific labeling (Szallasi et al., 1995), with the exception of the nucleus of the solitary tract, a central termination site for nodose-ganglion neurons. Furthermore, Northern blot hybridization performed with total RNA isolated from the whole rat brain also failed to detect TRPV1 mRNA (Caterina et al., 1997). Overall, the physiological relevance and even the existence of TRPV1 neurons in most extrahypothalamic structures have been debated.
D. Peripheral Non-Neural Tissues
The TRPV1 channel is also expressed in a variety of peripheral non-neural tissues (Szallasi et al., 2006), which raises the possibility of a direct action of TRPV1 agonists at the thermoeffector-tissue level. Indeed, direct action of TRPV1 agonists on vascular smooth muscle cells can cause vasodilation in the skin and vasoconstriction in skeletal muscles (Kark et al., 2008). Recent data from Trpv1 KO mice also suggest that this channel may be involved in the development of obesity by inhibiting thermogenesis (Motter and Ahern, 2008), perhaps nonshivering thermogenesis in BAT. However, the exact opposite role for TRPV1 channels in obesity (i.e., the prevention of adipogenesis) has also been proposed (Zhang et al., 2007). Furthermore, no data have been found that would show a direct involvement of the TRPV1 channel in thermogenesis in brown adipocytes, even though TRPV1 is expressed in preadipocytes and in visceral adipose tissue in mice and humans (Zhang et al., 2007). Because vasodilation in BAT is important for the thermogenic function (Cannon and Nedergaard, 2004), the possibility of a TRPV1 involvement in BAT thermogenesis secondary to a vascular action cannot be ruled out. It should be noted, however, that the level of TRPV1 channel expression in afferent neurons is at least 30 times higher than that in any other cell population (Sanchez et al., 2001), which to some extent questions the physiological relevance of non-neuronal TRPV1 channels. It should also be noted that at least some reports of TRPV1 expression at the protein level in non-neuronal tissues (e.g., the urothelium) are likely to be false-positive, because several anti-TRPV1 antibodies have been shown to cause an aspecific immunostaining in bladder tissue of rats and mice (Everaerts et al., 2009).
IV. Transient Receptor Potential Vanilloid-1 Pharmacology of Thermoregulation
A. Endogenous Ligands and Pharmacological Agonists of the Transient Receptor Potential Vanilloid-1 Channel
In addition to being activated by heat and low pH extracellularly and by high pH intracellularly (Dhaka et al., 2009), the TRPV1 channel is also activated intracellularly by molecular ligands. Some (but not all) of these ligands contain the vanillyl (also known as vanilloyl) functional group and, therefore, are referred to as vanilloids. In fact, the vanilloids gave the name to this channel. Because the most widely known vanilloid is CAP, the principal irritating and pungent constituent of various species of hot peppers (Nelson, 1919), the TRPV1 channel was previously called the “CAP receptor.” Another naturally occurring, exogenous vanilloid TRPV1 agonist—perhaps less well known but 102 to 105 times more potent than CAP—is RTX, a compound found in the plant genus Euphorbia (Szallasi and Blumberg, 1989). Endogenous TRPV1 agonists also exist, but their physiological relevance is still unclear. The list of endogenous agonists includes arachidonoylethanolamide (AEA, or anandamide), oleoylethanolamide (OEA), N-arachidonoyldopamine (NADA), N-oleoyldopamine (OLDA), several lipoxygenase products, and others (Movahed et al., 2005; Pingle et al., 2007). Structures of several endogenous agonists and those of CAP and RTX are shown in Table 1. The EC50 values for most endogenous TRPV1 agonists are high (usually in the micromolar range), but some (e.g., NADA) have a potency in the nanomolar range, which is comparable with that of CAP (Di Marzo et al., 2002; Huang et al., 2002; Chu et al., 2003) (Table 2). Although those endogenous TRPV1 agonists that have higher EC50 values are unlikely to reach effective systemic concentrations, it is still plausible that they can achieve physiologically relevant local concentrations in some tissues. For instance, OEA, a lipid mediator of satiety (Lo Verme et al., 2005), is present in the intestinal wall at concentrations comparable with its EC50 value against the TRPV1 channel (Fu et al., 2007). AEA, NADA, and OEA have been reported to cause hypothermia when administered systemically to rats and mice at milligram doses (Table 2). However, studies of the effects of endogenous TRPV1 agonists on Tb are scarce and typically do not deal with thermoregulatory mechanisms. Most insights into the potential role, or roles, of the TRPV1 channel in thermoregulation came from studies involving the pharmacological TRPV1 agonists CAP and RTX. These studies are discussed in the sections below.
Table 1.
TRPV1 agonists
| Common Name | IUPAC Name | Structure |
|---|---|---|
| N-Arachidonoylethanolamine (anandamide) | (5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)-5,8,11,14-icosa-tetraenamide | 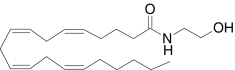 |
| N-Arachidonoyldopamine | (5Z,8Z,11Z,14Z)-N-(2-(3,4-Dihydroxyphenyl)ethyl)-5,8,11,14-icosatetraenamide | 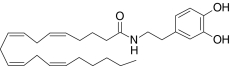 |
| N-Oleoylethanolamide | (9Z)-N-(2-Hydroxy-ethyl)-9-octadecenamide | 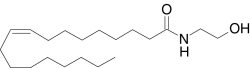 |
| N-Oleoyldopamine | (9Z)-N-(2-(3,4-Dihydroxyphenyl)ethyl)-9-octadecenamide | 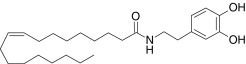 |
| 2-Arachidonoylglycerol | 2-Hydroxy-1-(hydroxylmethyl)ethyl (5Z,8Z,11Z,14Z)-5,8,11,14-icosatetraenoate | 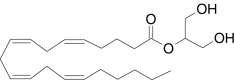 |
| 12(S)-HpETE | (5Z,8Z,10E,12S,14Z)-12-Hydroperoxy-5,8,10,14-icosa-tetraenoic acid | 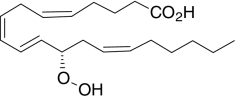 |
| 15(S)-HpETE | (5Z,8Z,11Z,13E,15S)-15-Hydroperoxy-5,8,11,13-icosa-tetraenoic acid | 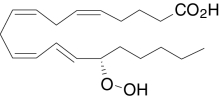 |
| 5(S)-HETE | (5S,6E,8Z,11Z,14Z)-5-Hydroxy-6,8,11,14-icosatetraenoic acid | 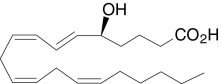 |
| Leukotriene B4 | (5S,6Z,8E,10E,12R,14Z)-5,12-Dihydroxy-6,8,10,14-icosa-tetraenoic acid | 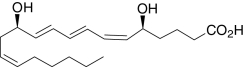 |
| Capsaicin | (6E)-N-(4-Hydroxy-3-methoxybenzyl)-8-methyl-6-nonenamide | 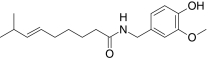 |
| Resiniferatoxin | 4-Hydroxy-3-methoxy-[(2S,3aR,3bS,6aR,9aR,9bR,10R,11aR)-3a,3b,6,6a,9a,10,11,11a-octahydro-6a-hydroxy-8,10-di-methyl-11α-(1-methylethenyl)-7-oxo-2-(phenylmethyl)-7H-2,9β-epoxyazuleno[5,4-e]-1,3-benzo-dioxol-5-yl]-benzeneacetate | 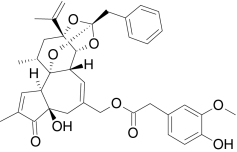 |
Table 2.
TRPV1 agonists: their potencies and their effects on deep Tb in rats and mice.
| Name | In Vitro: EC50 in Different Species |
In Vivo: Effect on Deep Tb |
||||
|---|---|---|---|---|---|---|
| Rat | Mouse | Human | Species | Dose and Route | Effect | |
| nM | nM | nM | mg/kg | |||
| AEA | 350–1560a–c | 3000d | 520–550c,e,f | Rat | 10–20 i.p. | ↓g |
| Mouse | 10–40 i.v. | ↓h,i | ||||
| NADA | 48c | — | 26–63c,e,j | Mouse | 10 i.p. | ↓k |
| OEA | 2000l | — | — | Rat | 20 i.v. | ↓m |
| Mouse | 10 i.v. | ↓i | ||||
| OLDA | 1800n | 258o | 36j | — | — | — |
| CAP | 10–42a–c,n | 9–300d,o> | 19–34c,e,f,j> | Rat | Varied | ↓p |
| Mouse | 1–15 s.c. | ↓q | ||||
| RTX | 0.17a | 0.15o | — | Rat | Varied | ↓p |
| Mouse | 0.0005–0.02 i.p., s.c. | ↓r | ||||
↓, decrease in deep Tb; —, not studied.
Proulx et al. (2005). The effect on deep Tb, although not measured in this study, is an assumption based on the measured effect on heat expenditure.
For review and references, see section IV.B.1.
B. Thermoregulatory Effects of Transient Receptor Potential Vanilloid-1 Agonists: Acute Effects
1. Effect on Body Temperature
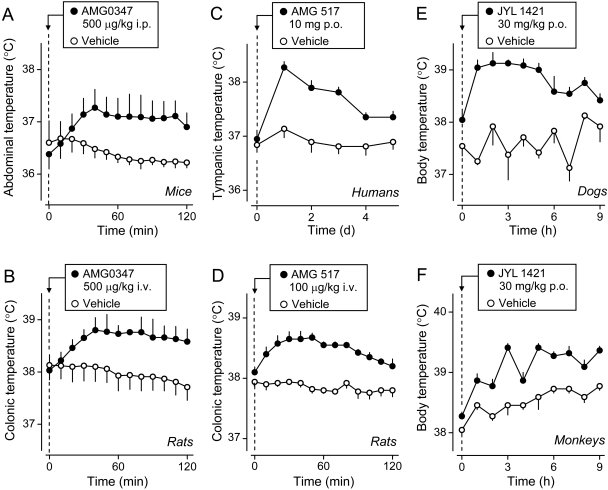
The immediate effects of the first administration of CAP or RTX on Tb regulation (the subject of this section) are different from the delayed effects and from those after repeated administration of these drugs (section IV.C.). Upon the first administration per os, subcutaneously, intramuscularly, intraperitoneally, intravenously, intrathecally, intracerebroventricularly, or into the POA in a variety of species (i.e. mice, rats, guinea pigs, ground squirrels, rabbits, ferrets, goats, dogs, and cats), CAP and RTX cause hypothermia (for review, see Hori, 1984; Szallasi and Blumberg, 1999; Szolcsányi, 2004; Caterina, 2007; see also Table 2 and Figs. 2 and 3). Some species (rabbits and ground squirrels) are less sensitive than others (mice, rats, and cats) (Hori, 1984), and, according to most studies, the avian species are highly insensitive to the hypothermic action (and the nonthermoregulatory actions) of CAP (Mason and Maruniak, 1983; Pierau et al., 1986; Szolcsányi et al., 1986). The insensitivity of the avian TRPV1 channel to CAP allows birds to feed on hot peppers and disseminate pepper seeds (Jordt and Julius, 2002). It has been reported that ducks tolerate a cumulative intravenous dose of CAP as high as 1 g/kg without any apparent thermoregulatory changes or signs of distress (Geisthövel et al., 1986). However, a relatively low dose of CAP (10 mg/kg i.v.) has been shown to cause hypothermia in chickens (Mahmoud et al., 2007).
Fig. 2.
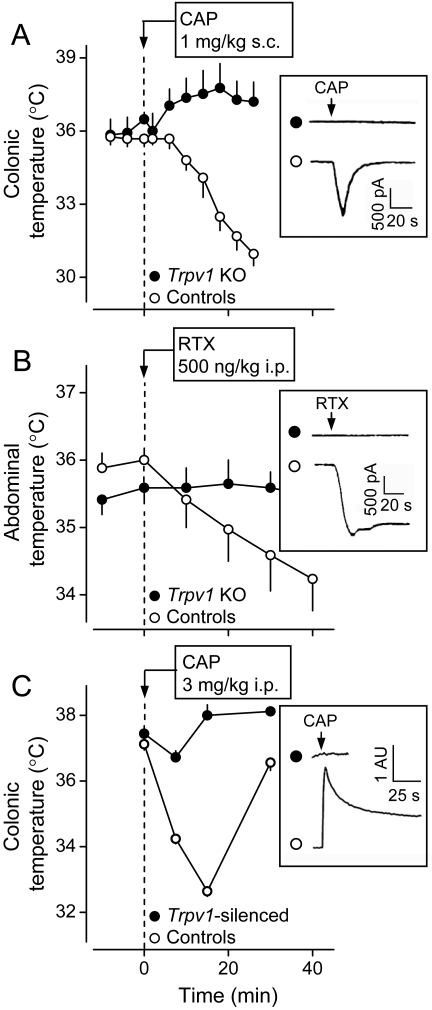
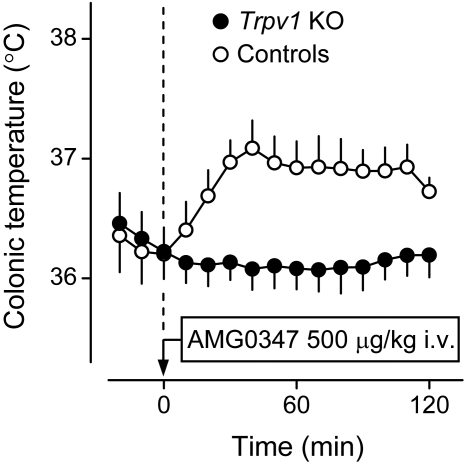
CAP- or RTX-induced hypothermia occurs neither in Trpv1 KO mice nor in transgenic mice with the Trpv1 gene silenced by short hairpin RNAs. A, effects of CAP (1 mg/kg s.c.) on colonic temperature in Trpv1 KO and control mice. B, effects of RTX (500 ng/kg i.p.) on abdominal temperature in Trpv1 KO and control mice. C, effects of CAP (3 mg/kg i.p.) on colonic temperature in transgenic (Trpv1-silenced) and control mice. In A and B, the insets show whole-cell patch-clamp recordings from cultured DRG neurons of Trpv1 KO and control mice. Cells from the KO animals did not respond with a typical inward cationic current to CAP (A) or RTX (B). The inset in C shows changes in the intracellular Ca2+ concentration in cultured DRG neurons from transgenic (Trpv1-silenced) and normal mice. Cells from the transgenic animals did not respond to CAP. [A and the inset in B are modified from Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, and Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313. Copyright © 2000 American Association for the Advancement of Science. The graph in B is modified from Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. (2007) Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 27:7459–7468. Copyright © 2007 Society for Neuroscience. C is modified from Christoph T, Bahrenberg G, De Vry J, Englberger W, Erdmann VA, Frech M, Kögel B, Röhl T, Schiene K, Schröder W, et al. (2008); Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Mol Cell Neurosci 37:579–589. Copyright © 2008 Elsevier. All images used with permission.].
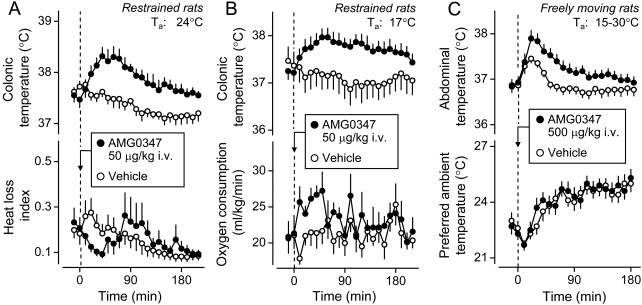
Fig. 3.
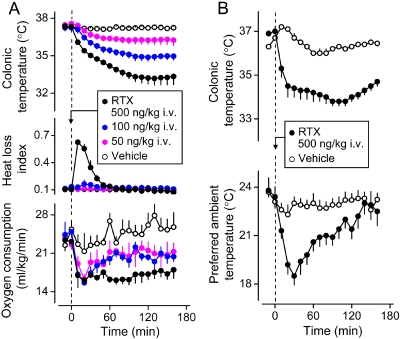
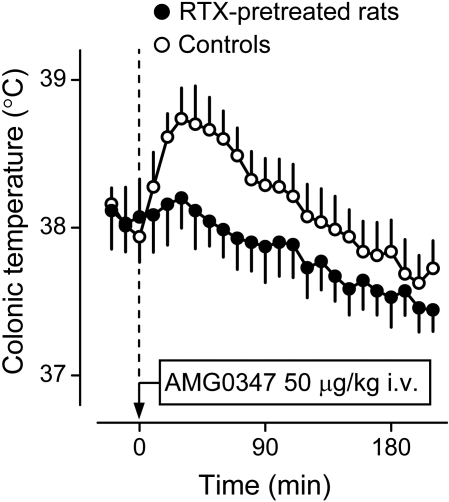
Recruitment of autonomic and behavioral thermoeffectors in the hypothermic response of rats to RTX. A, peripheral administration of RTX (500 ng/kg i.v.) causes hypothermia (a decrease in colonic temperature), tail skin vasodilation (an increase in the HLI), and an inhibition of thermogenesis (a reduction in oxygen consumption). The HLI is calculated according to the formula: HLI = (Tsk − Ta)/(Tb − Ta); it changes between 0 (maximal skin vasoconstriction) and some value that depends on the tail thermocouple position but is smaller than 1.0 (maximal vasodilation) (Romanovsky et al., 2002). A reports original data obtained in catheterized (jugular vein) male Wistar rats placed in a thermocouple-respirometry setup; all the methods involved are described elsewhere (Steiner et al., 2007). The protocol was approved by the St. Joseph's Hospital and Medical Center Animal Care and Use Committee. The data are shown as means ± S.E.; the number of rats in each group was five. B, RTX treatment (500 ng/kg i.v.) lowers abdominal temperature and induces cold-seeking behavior (a decrease in the preferred Ta in a thermogradient apparatus). [B is modified from Almeida MC, Steiner AA, Branco LG, and Romanovsky AA (2006) Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci 23:3359–3367. Copyright © 2006 Wiley-Blackwell. Used with permission.]
Not all effects of CAP are TRPV1-mediated. For example, CAP inhibits prostaglandin (PG) E2 production by LPS-stimulated macrophages in a TRPV1-independent way (Kim et al., 2003), and CAP pretreatment is thought to affect LPS fever in rats (Dogan et al., 2004) and birds (Mahmoud et al., 2007) and LPS hypothermia in birds (Nikami et al., 2008) via a TRPV1-independent mechanism. It was important, therefore, to determine in direct experiments whether the hypothermic responses to CAP and RTX require the presence of the TRPV1 channel. Such experiments have been conducted (Fig. 2) and have shown that the hypothermic responses to CAP (Caterina et al., 2000) and RTX (Steiner et al., 2007) do not occur in mice with the Trpv1 gene knocked out. It has also been shown that CAP-induced hypothermia does not occur in mice that continuously express a short hairpin RNA that permanently silences the Trpv1 gene (Christoph et al., 2008).
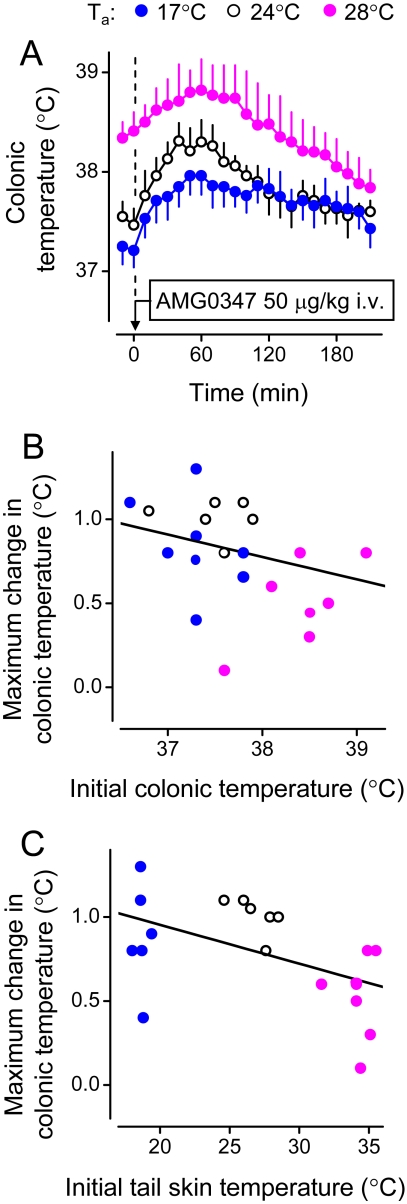
2. Thermoeffector Pattern.
The thermoeffector pattern of hypothermia caused by TRPV1 agonists varies and can involve both autonomic and behavioral thermoeffectors (Hori, 1984; Szolcsányi, 2004). Figure 3 shows that the hypothermic responses to RTX can occur via skin vasodilation [an increase in the heat loss index (HLI); Fig. 3A], suppression of thermogenesis (a decrease in the oxygen consumption; Fig. 3A), and cold-seeking behavior (a decrease in the preferred Ta; Fig. 3B). Likewise, CAP-induced hypothermia can involve skin vasodilation, thermoregulatory salivation, and inhibition of metabolic heat production (Szolcsányi and Jancsó-Gábor, 1973), as well as innate behavioral responses [such as cold-seeking behavior in a thermogradient apparatus (Szekely, 1986)] and learned operant behaviors [such as an increase in the frequency of pressing a lever (if such pressing triggers ambient cooling) or a decrease in the frequency of pressing a lever (if it triggers ambient heating)] (Hori, 1984). It has also been reported that hyperthermia may follow CAP-induced hypothermia (Kobayashi et al., 1998; Osaka et al., 2000), and that both CAP and RTX, even though they cause skin vasodilation to increase heat loss, can also activate metabolism to increase heat production (Watanabe et al., 1988; Kobayashi et al., 1998). This paradoxical hyperthermic effect could be explained by the facilitation of GABA-ergic inhibitory signaling (Karlsson et al., 2005) between MnPO neurons within the cutaneous cooling pathway and warm-sensitive MPO cells (Fig. 1). It is also possible that the paradoxical increase in the metabolic rate is unrelated to TRPV1, because ruthenium red does not block this effect (Okane et al., 2001).
3. Site of Action: The Preoptic Hypothalamus.
There is no consensus in the literature as to whether the hypothermic response to systemically administered TRPV1 agonists is mediated primarily by their action on peripheral or central TRPV1 channels. However, the evidence in support of the central mediation hypothesis is stronger. This hypothesis is feasible because peripherally administered CAP crosses the blood-brain barrier (BBB) (Saria et al., 1982; Miller et al., 1983) and because the mode of action of CAP on POA neurons and the neuromediator profile of neurons affected by CAP (Sasamura et al., 1998; Karlsson et al., 2005) suggest that TRPV1 agonists act on the glutamatergic MnPO neurons within the cutaneous warming pathway (Fig. 1), an action that would cause hypothermia. The following two pieces of evidence unequivocally support the central mediation hypothesis. First, CAP causes hypothermia when administered directly into the POA of rats at doses as low as 200 ng (for references, see Hori, 1984), whereas the lowest intravenous dose reported to cause hypothermia in the same species is ∼5 μg (Donnerer and Lembeck, 1983) (i.e., at least 25 times higher). Second, rats with decreased hypothalamic sensitivity to CAP after the initial intrahypothalamic injection of CAP (see section IV.C.1) show a reduced hypothermic response to a subsequent systemic (subcutaneous) administration of CAP (Jancsó-Gábor et al., 1970b).
Other pieces of evidence that are often cited to support the central mediation hypothesis are less convincing because they leave more room for alternative interpretations. For example, subcutaneous CAP was reported to excite warm-sensitive POA neurons (Nakayama et al., 1978; Hori and Shinohara, 1979; Hori, 1984), but such an excitation could have occurred secondarily to an action on any part of the afferent thermoeffector pathways (Fig. 1). In a study by Szolcsányi and Jancsó-Gábor (1975), electrolytic lesioning of the POA reduced the hypothermic response to subcutaneous CAP, but this finding still does not allow one to distinguish a direct action on POA neurons from a secondary involvement of these cells. Further complicating the interpretation of these data, electrolytic lesioning of the POA in the study by Szolcsányi and Jancsó-Gábor (1975) did not eliminate the response to peripheral CAP completely, possibly because the lesions were partial and did not completely abolish all groups of POA neurons, or because the unrestrained rats used in this study were able to mobilize some behavioral thermoregulatory responses. Indeed, even those MPO lesions that strongly attenuate autonomic thermoeffector responses do not affect cold- and warmth-seeking behaviors, including the cold-seeking behavior caused by intravenous RTX (Almeida et al., 2006b). Finally, the incomplete blockade of CAP-induced hypothermia in POA-lesioned rats can also indicate an action on the thermoeffector pathways downstream from the POA. An involvement of neurons in the midbrain and in some pontomedullary structures, such as the dorsal raphe nucleus, in the thermoregulatory response to CAP has been proposed (Rabe et al., 1980; Hori, 1984; Hajós et al., 1987, 1988), even though such involvement does not necessarily imply a direct action.
Several authors suggest that a contribution of a peripheral action of TRPV1 agonists to the hypothermic response cannot be excluded (Dib, 1983; Donnerer and Lembeck, 1983; Osaka et al., 1998). However, an action in a single thermoeffector tissue (e.g., skin vasculature) cannot explain the involvement of multiple effectors (Fig. 3). A peripheral action that would explain the multiple thermoeffector involvement would fall within the afferent thermoregulatory pathways (e.g., on primary sensory neurons), but a substantial presence of TRPV1 channels on afferents involved in responses to innocuous heat seems questionable (section III.A).
4. Observations in Humans.
It is widely known that the acute effects of CAP self-administration in humans (by consuming hot red pepper) include gustation-evoked sweating (Lee, 1954). This response together with evidence that people living closer to the equator prefer their food hotter than those living in cooler climates (Szallasi and Blumberg, 1999) gave rise to the theory that eating spicy food helps combat the environmental heat via sweating. However, in addition to causing sweating, hot red pepper also exaggerates the thermogenic response to a meal, a phenomenon known as spice-induced thermogenesis (Henry and Emery, 1986). Both hot red pepper and capsiate, a nonpungent TRPV1 agonist, have also been reported to increase oxygen consumption and deep Tb (Ohnuki et al., 2001; Hachiya et al., 2007). The dual effect of TRPV1 agonist consumption on human thermoregulation [i.e., the stimulation of both heat loss (sweating) and heat production (thermogenesis) mechanisms] makes interpretation of these data difficult. An even greater difficulty is that the chronic effects of CAP (TRPV1 desensitization; see section IV.C.1) differ drastically from the acute effects (activation of TRPV1 channels). Hence, even if the consumption of hot pepper is indeed driven by the thermoregulatory effects of CAP, it is unclear whether the driving mechanism is an acute increase in heat loss or a chronic deactivation of neural pathways for heat-defense mechanisms. Furthermore, CAP is metabolized by the liver, and the oral administration of this vanilloid (which delivers CAP to the liver via the portal circulation) is poorly suited for achieving high concentrations in the nervous system (Donnerer et al., 1990; Reilly et al., 2003). It is plausible that people living in hot climates consume more spicy foods simply because more spicy plants grow in such climates and are, therefore, available for greater consumption.
C. Thermoregulatory Effects of Transient Receptor Potential Vanilloid-1 Agonists: Chronic Effects
1. Desensitization.
The delayed effects and the effects of repeated administration of TRPV1 agonists were termed by Jancsó and Jancsó (1949) as desensitization. In general, this term is used to describe a state of CAP- or RTX-induced neuronal insensitivity to exogenous or endogenous vanilloids, and to other stimuli that would normally activate TRPV1-expressing neurons (e.g., noxious heat) (Szallasi and Blumberg, 1999). Different authors apply this term not only to the TRPV1-expressing neurons, but also to the TRPV1 channels responsible for the desensitization phenomenon, to the neural structures that contain the desensitized neurons, or to the entire animal possessing such desensitized neural structures. Having survived many attempts to introduce more accurate terminology, the term continues to unite several different conditions varying from TRPV1 agonist-induced conformational changes in the channel to the death of TRPV1-expressing neurons (Szallasi and Blumberg, 1999). Regardless of the underlying mechanisms, TRPV1 channels pretreated with CAP or RTX no longer respond to stimuli that normally activate them. Hence, the administration of TRPV1 agonists and consequent desensitization can be used as a tool to assess the functional importance of TRPV1 channels (as well as of the neurons or neural structures that express TRPV1).
To cause TRPV1 desensitization in laboratory animals, CAP or RTX is administered systemically (subcutaneously or intraperitoneally) often by use of several escalating doses, at a cumulative dose of 30 to 900 mg/kg for CAP or 200 μg/kg for RTX. Some effects of desensitization, e.g., responses to POA heating, depend on the animal's age at the time of the administration of the desensitizing dose of a TRPV1 agonist and on the desensitizing dose itself. In rats desensitized with lower (e.g., 50 mg/kg) doses of CAP as adults or with lower (50 mg/kg) or higher (∼735 mg/kg) doses as neonates, the responses to localized POA heating are normal (Dib, 1983; Obal et al., 1983). However, in rats desensitized with higher (120–300 mg/kg) doses of CAP as adults, the responses to POA heating are abolished (Jancsó-Gábor et al., 1970b; Obal et al., 1983). These data suggest that the difference between animals desensitized at different ages and with different doses of systemic CAP is in the functional preservation or impairment of POA neurons that respond to local warming (see also section IV.C.5). It is unknown why some POA neurons are functional in animals treated with CAP as neonates, and it has been proposed that these neurons either “escape” or “survive” the neonatal CAP treatment or that they develop at later stages of the ontogenesis (Szolcsányi, 1990; Holzer, 1991). However, because POA neurons of rat pups treated with even lower doses of systemic CAP (75–100 mg/kg) show marked signs of degeneration 6 h after treatment (Ritter and Dinh, 1992), and because neurons throughout the brain are more sensitive to CAP in the neonatal period than in adulthood (Joó et al., 1969; Jancsó and Király, 1981; Buck and Burks, 1986; Ritter and Dinh, 1990, 1992), the de novo development scenario seems more likely. Proliferation and differentiation of neuronal progenitor cells and their migration to the POA occur even in the brain of adult rats (Matsuzaki et al., 2009), and these processes can be expected to be more intensive during the neonatal period.
2. Effect of Transient Receptor Potential Vanilloid-1 Desensitization on Basal Body Temperature.
Delayed effects of treatment with large doses of systemic CAP on deep Tb of laboratory animals were investigated in many studies (Table 3). Whenever experiments were performed within the first few days after CAP administration in adult rats, an increased deep Tb (by up to 1.9°C, but usually by just a few tenths of a degree) and skin vasoconstriction (whenever Tsk was measured) were found (Jancsó-Gábor et al., 1970a; Szolcsányi and Jancsó-Gábor, 1973; Székely and Szolcsányi, 1979; Szikszay et al., 1982). However, no consistent changes in either deep Tb or Tsk were found when experiments were performed 10 days or later after CAP administration to adult or newborn animals: Tb either decreased, increased, or did not change at all, and either vasodilation, vasoconstriction, or normal cutaneous vasomotor tone was revealed (Table 3). One way to explain these contradictory findings is to propose that some neurons expressing TRPV1 channels are tonically activated in a naive, not desensitized animal, and that this activation leads to an inhibition of skin vasoconstriction (and possibly other thermoeffector responses), thus suppressing deep Tb. When these tonically active neurons become silent as the result of desensitization, skin vasoconstriction and hyperthermia occur, as in those studies that were conducted during the first few days after CAP administration (Jancsó-Gábor et al., 1970a; Szolcsányi and Jancsó-Gábor, 1973; Székely and Szolcsányi, 1979; Szikszay et al., 1982). Over the long term, however, compensatory mechanisms can develop (Yamashita et al., 2008), and TRPV1-mediated functions can partially or even completely recover (Jancsó et al., 1977; Hajós et al., 1983), in agreement with the fact that desensitization produces no consistent long-term effect on basal Tb.
Table 3.
The effects of desensitization with subcutaneous or intraperitoneal CAP on basal deep Tb and basal Tsk at different times after CAP administration
| Time of Testing (days after CAP) | Desensitization Model |
Effects |
|||
|---|---|---|---|---|---|
| Age Group | Species | CAP Dose | Basal Deep Tb | Basal Tsk | |
| mg/kg | |||||
| 1–9 | Adult | Rat | 20–160 | ↑a–d | ↓cd |
| 10–100 | Adult | Guinea pig | 30–40 | ↑c | — |
| Rat | 20–310 | ↕a,c,e | ↕e | ||
| ↑df | ↑d | ||||
| ↓bg | ↓b | ||||
| Guinea pig | 30–40 | ↕a,c,c | — | ||
| Mouse | 45 | ↕dh | — | ||
| ↑h | |||||
| ↓h | |||||
| 60–120 | Neonate | Rat | 50–450 | ↕eil | ↕ek |
| ↑m | ↑l | ||||
| ↓j | ↓j,k | ||||
↑, increase; ↓, decrease; ↕, none; —, not studied.
Szelényi et al. (2004). In this study, the daily mean deep Tb was unchanged, whereas the mean daytime (light-phase) Tb was decreased, and the mean nighttime (dark-phase) Tb was increased.
3. Effects on Responses to Thermal Challenges.
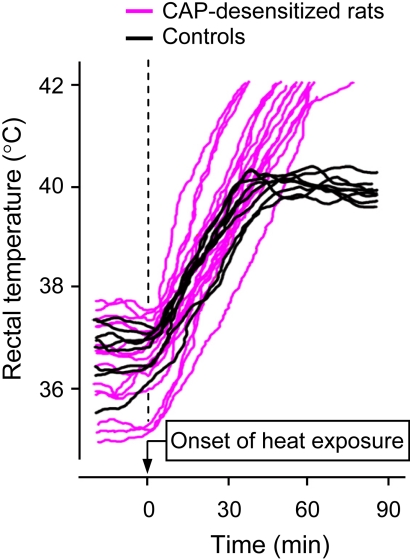
Whereas the effects of CAP desensitization on basal Tb are typically small, relatively short-lived (a few days), and difficult to find, the effects on the responses to heat exposure are profound (Fig. 4), reproducible, and occur in many species, including rats, guinea pigs, and mice (Table 4). Upon exposure to heat, animals desensitized with high systemic doses of CAP, administered either during the neonatal period or in adulthood, develop severe hyperthermia, whereas their nondesensitized counterparts successfully defend their deep Tb against the same challenges (Jancsó-Gábor et al., 1970a,b; Szolcsányi and Jancsó-Gábor, 1975; Cabanac et al., 1976; Obál et al., 1980; Hori and Tsuzuki, 1981; Szikszay et al., 1982; Benedek et al., 1983; Obal et al., 1983; Szolcsányi, 1983; Szelényi et al., 2004) (also see Fig. 4). Obál et al. (1980) reported that, for the same value of deep Tb, heat-exposed CAP-pretreated rats have a substantially lower tail Tsk than heat-exposed nondesensitized rats, thus suggesting that TRPV1-desensitization increases the threshold Tb for tail-skin vasodilation. Other heat-defense responses, both autonomic [e.g., salivation (Cabanac et al., 1976)] and behavioral [e.g., the heat-escape locomotor (Szolcsányi and Jancsó-Gábor, 1975; Obál et al., 1979, 1987) or operant responses (Hori and Tsuzuki, 1981)], can also be diminished in animals desensitized with systemic CAP.
Fig. 4.
The effect of heat exposure (41°C) on rectal temperature of adult rats that received a high dose of CAP (200–300 mg/kg s.c.) as neonates and of their littermates that were not treated with CAP. The CAP-desensitized rats have a severe impairment of heat-defense responses and, consequently, exhibit a much greater increase in Tb. [Modified from Hori T and Tsuzuki S (1981) Thermoregulation in adult rats which have been treated with capsaicin as neonates. Pflugers Arch 390:219–223. Copyright © 1981 Springer Science+Business Media. Used with permission.]
Table 4.
Thermoregulatory responses to systemically or centrally applied CAP or heating in different models of CAP- or RTX-induced desensitization
| Desensitization Model |
Effects |
|||||
|---|---|---|---|---|---|---|
| Age group | Species | TRPV1 Agonist, Dose and Route | Hypothermia Induced by |
Heat-Defense Responses to Whole-Body Heating | ||
| Systemic CAP (0.05–16 mg/kg i.p., s.c., i.v.) | Intracerebral CAP (0.002–0.2 mg/kg) | Brain Heating | ||||
| mg/kg | ||||||
| Adult | Rat | CAP 20–310 i.p., s.c. or RTX 0.2 i.p. | ↓a–d | ↓d,e | ↓d,g | ↓a,b,f–i |
| Mouse | CAP 45 s.c. | — | — | — | ↓j | |
| Guinea pig | CAP 30–40 i.p. | ↓b | — | — | ↓b | |
| Neonate | Rat | CAP 50–450 i.p., s.c. | ↓c,k,l | ↕c,l | ↕g,l | ↓g,h,m |
↑, attenuation; ↕, no change; —, not studied.
Whereas TRPV1 desensitization severely impairs autonomic heat-defense mechanisms, rats treated either with different doses of CAP (50–460 mg/kg s.c.) as neonates or with relatively low doses of CAP (130–160 mg/kg s.c. or i.p.) as adults exhibit no changes in autonomic thermoeffector responses to cold exposure (Jancsó-Gábor et al., 1970a; Szolcsányi and Jancsó-Gábor, 1973; Hori and Tsuzuki, 1981; Yamashita et al., 2008). On the contrary, rats desensitized with high doses of CAP (250–300 mg/kg s.c.) administered in adulthood exhibit attenuated responses to cold (Benedek et al., 1983; Cormarèche-Leydier, 1984); mechanisms of the latter effect are discussed below (section IV.C.5).
4. Pharmacological Desensitization versus Genetic Ablation.
In contrast to the impaired thermoregulation of desensitized animals, genetic ablation of TRPV1 does not cause gross changes in Tb regulation in response to either heat or cold challenges (Szelényi et al., 2004; Iida et al., 2005). There is, however, a report showing that Trpv1 KO mice can defend their deep Tb against severe environmental cooling better than their wild-type counterparts (Motter and Ahern, 2008), but such changes were not seen in a similar study by Iida et al. (2005). Considering that thermoregulatory experiments in mice have multiple pitfalls (Rudaya et al., 2005), more extensive studies of Tb regulation in Trpv1 KO mice may be warranted. It should be further noted that negative results obtained in KO animals are typically inconclusive, because various types of compensatory changes may restore the function of interest, even if the knocked out gene is normally the one responsible for this function. Several new strategies to silence the Trpv1 gene (namely, those involving antisense oligonucleotides, small interfering RNAs, or short hairpin RNAs) have recently been used in pain research (Christoph et al., 2006, 2007, 2008). It would be interesting to study the regulation of Tb in these new models, especially because compensation patterns in different models are likely to differ (Christoph et al., 2008).
Trpv1 KO mice have also been shown to respond to LPS with an attenuated fever in the study by Iida et al. (2005), but this finding contradicts our results (Dogan et al., 2004). Our group has shown that neither pharmacological antagonism of TRPV1 with capsazepine (CPZ) nor localized intra-abdominal TRPV1 desensitization with a low intraperitoneal dose of RTX (for more information, see section IV.E.5) attenuates LPS fever in rats. Even if TRPV1 channels were involved in LPS fever, such an involvement could be in the mechanisms of immune signaling rather than those of thermoregulation per se. Indeed, LPS induces TRPV1 overexpression in rats (Orliac et al., 2007), and several responses to shock-inducing doses of LPS are exaggerated in Trpv1 KO mice (Clark et al., 2007) and in rats treated with CPZ (Wang et al., 2008).
5. Site of Desensitization: The Preoptic Hypothalamus?
To explain the recruitment of multiple thermoeffectors, an involvement of DRG neurons in the thermal afferent pathways has been proposed (Dib, 1983; Donnerer and Lembeck, 1983; Obál et al., 1987; Szallasi and Blumberg, 1990; Benham et al., 2003; Tominaga and Caterina, 2004; Yamashita et al., 2008). Even though the prominent association of TRPV1 channels with polymodal nociceptors (rather than with neurons sensitive to innocuous warming) weakens this proposition, some populations or TRPV1-expressing visceral DRG neurons modulate the activity of autonomic effectors (see section IV.E.5). Desensitization of these visceral neurons may contribute to the early hyperthermia seen in CAP-desensitized animals. However, because acute thermoregulatory effects of TRPV1 agonists can be explained by primarily a central action (on MnPO neurons), it is reasonable to suggest that the same neurons are desensitized after the systemic administration of TRPV1 agonists and that the loss of function of these neurons accounts for thermoregulatory impairments seen in desensitized animals. Indeed, the loss of heat-defense responses (the primary thermoregulatory symptom of desensitized animals) agrees with the loss of function of MnPO neurons within the cutaneous warming pathway (Fig. 1). Confirming an involvement of hypothalamic (rather than peripheral) mechanisms, Jancsó-Gábor et al. (1970b) have found that rats desensitized by intrahypothalamic injections of CAP lose their ability to defend deep Tb against ambient heating (and also show a reduced hypothermic response to subcutaneous CAP). Desensitization of MnPO neurons may also account for the exaggerated fever responses to systemic LPS that have been reported to occur in rats treated with systemic CAP (Székely and Szolcsányi, 1979) or RTX (Dogan et al., 2004). However, some thermoregulatory manifestations occurring in animals treated with high systemic doses of CAP as adults cannot be explained by desensitization of MnPO neurons alone.
For example, Benedek et al. (1983) and Cormarèche-Leydier (1984) found that desensitization with high doses of CAP (250–300 mg/kg s.c.) administered to adult rats resulted in attenuated responses to cold, even though rats treated either with lower or higher doses of CAP (50–460 mg/kg s.c.) as neonates or with lower doses of CAP (130–160 mg/kg s.c. or i.p.) as adults have been repeatedly shown by others to have no deficiency in their autonomic thermoeffector responses to cold (Jancsó-Gábor et al., 1970a; Szolcsányi and Jancsó-Gábor, 1973; Hori and Tsuzuki, 1981; Yamashita et al., 2008). The impairment of cold-defense responses would be consistent with the loss of the MPO neurons (i.e., the first efferent neurons for both thermoregulatory vasoconstriction and BAT thermogenesis) in animals desensitized as adults (Fig. 1). In fact, Cormarèche-Leydier (1984) has pointed to the similarity between the thermoregulatory consequences of TRPV1 desensitization of adult rats with high doses of CAP and the thermoregulatory consequences of POA lesions. It has been well documented that animals with MPO lesions cannot defend their deep Tb autonomically against either cold or heat (Carlisle, 1969; Lipton et al., 1974; Satinoff et al., 1976; Van Zoeren and Stricker, 1976; Schulze et al., 1981; Almeida et al., 2006b).
Another example is that the responses to POA heating are abolished in rats desensitized with higher (120–300 mg/kg) doses of CAP as adults (Jancsó-Gábor et al., 1970b; Obal et al., 1983), even though these responses are normal in rats desensitized with lower (e.g., 50 mg/kg) doses of CAP as adults or with lower (50 mg/kg) or higher (∼735 mg/kg) doses as neonates (Dib, 1983; Obal et al., 1983). These data suggest that high (but not low) doses of systemic CAP affect POA neurons that respond to local warming (i.e., the warm-sensitive MPO cells) (Fig. 1). Indeed, lower doses of systemic CAP (75–100 mg/kg) administered to adult rats failed to cause any degenerative changes in the MPO, at least within the first day after the administration (Ritter and Dinh, 1992), whereas higher doses (170–250 mg/kg) markedly impaired the perikaryal mitochondria in POA (presumably MPO) neurons, and these impairments were present from 2 days to 5 months after the CAP treatment (Szolcsányi et al., 1971). (We were unable to find any morphological studies of POA neurons in adult animals that were treated with CAP as neonates.)
Because MPO neurons do not respond with any postsynaptic changes to direct administration of CAP (Karlsson et al., 2005), we speculate that impairment of these cells in animals treated with high doses of CAP as adults is unlikely to be due to a direct action, but rather might reflect an action on glutamatergic MnPO neurons (section IV.B.3) and consequent secondary changes in MPO cells. It has been established that CAP-induced degeneration can spread transneuronally. For example, CAP can affect DH neurons by acting on DRG neurons (Fitzgerald, 1983). Furthermore, degenerative neuronal changes in the nucleus of the solitary tract caused by systemic CAP do not occur in animals after nodose ganglionectomy, thus suggesting a secondary nature of the observed solitary tract degeneration (Ritter and Dinh, 1988). The proposition that CAP affects MPO neurons secondarily to its effect on MnPO neurons also would account for the fact that MPO neurons are affected only by high doses. High doses of CAP are known to cause irreversible degeneration and neuronal death of TRPV1-expressing neurons (Jancsó et al., 1977; Szallasi and Blumberg, 1999; Szöke et al., 2002), and neuronal death or trauma are well known to cause trans-synaptic (transneuronal) degeneration, sometimes spreading over several subsequent neurons within a neuronal line (Sugimoto and Gobel, 1984; Knyihár-Csillik et al., 1989; Rausell et al., 1992; Sugimoto et al., 1999).
The literature also contains two findings (Dib, 1983; Hajós et al., 1983) that seemingly contradict the proposed scenario, in which CAP acts on TRPV1 channels on MnPO neurons and, at higher doses, also causes secondary degeneration of MPO neurons followed by a permanent loss of their function (if CAP was administered in adulthood) or by partial recovery (if CAP was administered in the neonatal period). Dib (1983) and Hajós et al. (1983) found that rats that received subcutaneous doses of CAP as neonates (either at a lower cumulative dose of 50 mg/kg or at a higher dose of 300–900 mg/kg), showed low or no responses to subcutaneous or intraperitoneal CAP as adults, but still responded with hypothermia and/or tail skin vasodilation to intracerebroventricular or intrapreoptic CAP. These findings are typically interpreted to indicate that effects of systemic CAP are mediated by peripheral TRPV1-positive neurons (perhaps DRG neurons), whereas those of central CAP are mediated by TRPV1-positive POA neurons (perhaps MPO) neurons. However, these findings can still be compatible with our hypothesis that both systemic and central administration of CAP cause hypothermia by acting on the same targets, TRPV1-expressing MnPO neurons. It is likely that not all MnPO neurons are desensitized by the lower doses. It is also possible that at least some neurons desensitized by the higher doses of CAP administered during the neonatal period can partially regenerate or be later replaced by new neurons derived from neuronal progenitor cells, similar to MPO neurons. In either case, at least some MnPO neurons are likely to be responsive to CAP, which makes it reasonable to expect that high local concentrations of CAP (e.g., those after an intrabrain administration) can cause an effect, whereas low local concentrations (e.g., those after a systemic administration) remain ineffective. Hence, intrabrain administration of CAP may be expected to cause hypothermia and skin vasodilation even when subcutaneous CAP does not.
D. Pharmacological Antagonists of the Transient Receptor Potential Vanilloid-1 Channel
Based on experiments in rodent models, including those of cancer and inflammation (Pomonis et al., 2003; Asai et al., 2005; Gavva et al., 2005b; Ghilardi et al., 2005; Honore et al., 2005, 2009), the TRPV1 channel has been explored as a novel target for analgesic therapy (Immke and Gavva, 2006; Szallasi et al., 2007; Gavva, 2008; Holzer, 2008; Gunthorpe and Chizh, 2009; Khairatkar-Joshi and Szallasi, 2009). The possibility of making next-generation pain therapeutics has stimulated immense interest, and many pharmaceutical companies all over the world have entered the race to synthesize and test TRPV1 antagonists. Today, nearly every pharmaceutical company has a TRPV1 program, and a large number of new, highly potent and selective TRPV1 antagonists have been synthesized and reached different phases of development (Broad et al., 2008; Gavva et al., 2008; Gunthorpe and Chizh, 2009). Those antagonists for which effects on Tb have been studied in the rat, the most common laboratory animal, are listed in Tables 5 and 6. Data on the selectivity of new TRPV1 antagonists (and, for comparison, of CPZ and ruthenium red) are listed in Table 7. In the following sections, we analyze what experiments with these TRPV1 antagonists have revealed about the role of the TRPV1 channel in Tb regulation.
Table 5.
TRPV1 antagonists
| Name, Company | IUPAC Name | Structure |
|---|---|---|
| A-425619, Abbott | 1-(5-Isoquinolinyl)-3-(4-(trifluoromethyl)benzyl)urea | 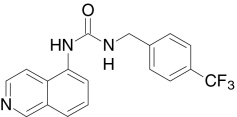 |
| A-889425, Abbott | 1-(3-Methylpyridin-2-yl)-N-(4-trifluoromethyl-sulfonyl)phenyl)-1,2,3,6-tetrahydropyridine-4-carboxamide | 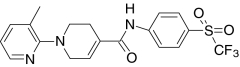 |
| ABT-102, Abbott | (R)-(5-tert-Butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)-urea | 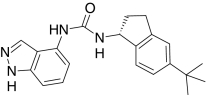 |
| AMG0347, Amgen | (2E)-N-(7-Hydroxy-5,6,7,8-tetrahydro-1-naphthalenyl)-3-(2-(1-piperidinyl)-6-(trifluoro-methyl)-3-pyridinyl)-2-propenamide | 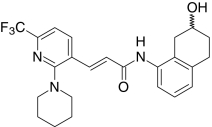 |
| AMG1629, Amgen | 3-Amino-5-((2-((2-methoxyethyl)amino)-6-(4-(trifluoromethyl)-phenyl)-4-pyrimidinyl)-oxy)-2(1H)-quinoxalinone | 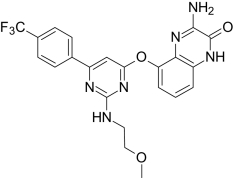 |
| AMG2820, Amgen | 8-((6-(4-(Trifluoromethyl)phenyl)-4-pyrimidinyl)oxy)-3-isoquinolinol | 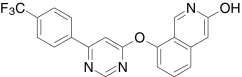 |
| AMG3731, Amgen | N-(4-(((3-(6-((2-Amino-1,3-benzothiazol-4-yl)oxy)-4-pyrimidinyl)-6-(trifluoromethyl)-2-pyridinyl)amino)methyl)-2-fluorophenyl)-methanesulfonamide | 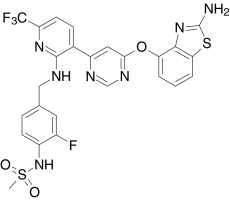 |
| AMG 517, Amgen | N-(4-((6-(4-(Trifluoromethyl)phenyl)-4-pyrimidinyl)oxy)-1,3-benzothiazol-2-yl)-acetamide | 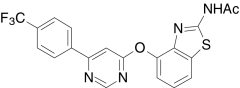 |
| AMG7905, Amgen | N-(6-(2-((Cyclohexylmethyl)amino)-4-(tri-fluoromethyl)phenyl)-4-pyrimidinyl)-1,3-benzothiazol-6-amine | 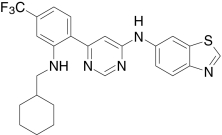 |
| AMG7988, Amgen | 4-((6-(2-((2-(1-Piperidinyl)ethyl)amino)-6-(trifluoromethyl)-3-pyridinyl)-4-pyrimidinyl)-oxy)-1,3-benzothiazol-2-amine | 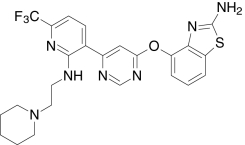 |
| AMG8163, Amgen | tert-Butyl (2-(6-((2-(acetylamino)-1,3-benzothiazol-4-yl)oxy)-4-pyrimidinyl)-5-(trifluoromethyl)phenyl)-carbamate | 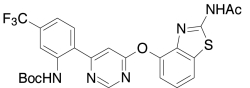 |
| AMG8562, Amgen | (2E)-N-((2R)-2-Hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(1-piperidinyl)-4-(trifluoromethyl)phenyl)-2-propenamide | 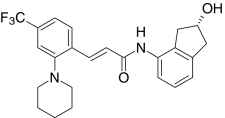 |
| AMG8563, Amgen | (2E)-N-((2S)-2-Hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(1-piperidinyl)-4-(trifluoromethyl)phenyl)-2-propenamide | 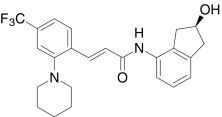 |
| AMG9810, Amgen | (2E)-3-(4-tert-Butylphenyl)-N-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-propenamide | 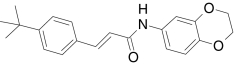 |
| BCTC, Neurogen | N-(4-tert-Butylphenyl)-4-(3-chloro-2-pyridinyl)-1-piperazinecarboxamide | 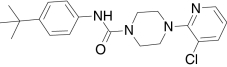 |
| Comp. 41, Johnson & Johnson | 4-(3-(Trifluoromethyl)-2-pyridinyl)-N-(5-(tri-fluoromethyl)-2-pyridinyl)-1-piperazine-carboxamide | 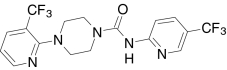 |
| Comp. G, Amgen | 1-(5-Chloro-6-((3R)-3-methyl-4-(6-(trifluoromethyl)-4-(3,4,5-trifluorophenyl)-1H-benzimidazol-2-yl)-1-piperazinyl)-3-pyridinyl)-1,2-ethanediol | 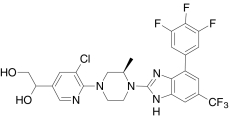 |
| Comp. H, Amgen | 2-((2,6-Dichlorophenyl)-amino)-N-(4-(trifluoro-methyl)phenyl)-1,3-thiazole-4-carboxamide | 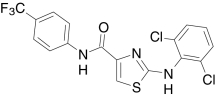 |
| JYL 1421,a Schwarz Pharma | N-(4-((((4-tert-Butylbenzyl)carbamothioyl)-amino)methyl)-2-fluorophenyl)methanesulfon-amide | 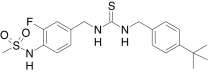 |
This compound was also published as SC0030 by Suh et al. (2003).
Table 6.
TRPV1 antagonists: their potencies at different modes of activation of rat TRPV1 in vitro and their effects upon systemic administration on deep Tb in rats in vivo
| Name | In Vitro: IC50 for Different Activation Modes |
In Vivo: Effect on Deep Tb |
||||
|---|---|---|---|---|---|---|
| CAP (500 nM) | pH (5.0) | Heat (45°C) | Dose | Route | Effect | |
| nM | nM | nM | mg/kg | |||
| A-425619 | 10 ± 2a | 13 ± 2a | 58 ± 4a | 4–100 | p.o. | ↑ab |
| A-889425 | 335c | — | — | 4–13 | p.o. | ↑c |
| 2 | i.v. | ↑c | ||||
| ABT-102 | 1d | 16d | 100d | 1–10 | p.o. | ↑e |
| AMG0347 | 0.7 ± 0.1f | 0.8 ± 0.3f | 0.2 ± 0.1f | 0.01–0.5 | i.v. | ↑f |
| AMG1629 | 0.6 ± 0.4a | 1 ± 0a | 0.2 ± 0.0a | 3 | p.o. | ↑a |
| AMG2820 | 1 ± 0a | >4000a | 23 ± 6a | 3 | i.v. | ↑a |
| AMG3731 | 6 ± 5a | 7 ± 1a | 5 ± 0a | 3–10 | p.o. | ↑a |
| AMG 517 | 1 ± 1g | 0.5 ± 0.2g | 2 ± 1g | 0.1–3 | p.o. | ↑gh |
| 0.9 ± 0.8h | 0.5 ± 0.2h | — | 0.1 | i.v. | ↑i | |
| AMG7988 | 14 ± 2a | >4000a | 360 ± 97a | 3 | i.v. | ↑a |
| AMG8163 | 0.6 ± 0.3aj | 0.6 ± 0.3aj | 0.2 ± 0.1aj | 0.1–10 | p.o. | ↑ag |
| 0.3 | i.v. | ↑j | ||||
| AMG8563 | 3 ± 1j | >4000j | 2 ± 0j | 3 | i.v. | ↑j |
| AMG9810 | 79 ± 9a | 349 ± 66a | 9 ± 1a | 30 | i.p. | ↑a |
| 86 ± 39k | 294 ± 192k | 21 ± 17k | ||||
| BCTC | 0.4 ± 0.3a | 0.5 ± 0.6a | 0.1 ± 0.0a | 3 | i.v. | ↑a |
| 0.5 ± 0.1k | 0.7 ± 0.4k | 0.6 ± 0.2k | ||||
| Comp. 41 | 102 ± 12l | 16 ± 4l | — | 30 | p.o. | ↑l |
| Comp. G | 1 ± 0a | 1 ± 0a | 2 ± 0a | 30 | p.o. | ↑a |
| Comp. H | 18 ± 3a | 70 ± 5a | 16 ± 9a | 30 | p.o. | ↑a |
| AMG7905 | 39 ± 17j | N.A. | N.A. | 0.3–30 | p.o. | ↓j |
| AMG8562 | 2 ± 1j | N.A. | >4000j | 1–30 | p.o. | ↓j |
| 3 | i.v. | ↓j | ||||
| JYL 1421 | 8a | N.A. | >4000j | 3–100 | p.o. | ↕a |
| 37m | — | — | 1–10 | i.p. | ↕m | |
N.A., not applicable (IC50 for the marked activation mode could not have been measured, because potentiation was observed instead of inhibition); —, IC50 for this mode of activation was not determined; ↑, an increase in deep Tb; ↓, a decrease in deep Tb; ↕, no effect.
Gavva et al. (2007b). pH of 5.5 was used in this study of A-425619 in which no TRPV1 inhibition occurred at pH of 5.0.
McGaraughty et al. (2009). CAP concentration used in this study was not specified.
Surowy et al. (2008). CAP concentration of 1 μM, pH of 5.5, and temperature of 50°C were used in this study.
Swanson et al. (2005). CAP concentration of 100 nM and a pH range of 5.8 to 6.0 were used in this study.
Table 7.
Selectivity of TRPV1 antagonists
IC50 values for TRPV1 antagonists against rat TRPV1 are shown for the CAP activation mode; for more information, see Table 6. IC50 values against other TRP channels were obtained by using human TRPV2,c TRPV3,a,c–g,l,o TRPV4,a,c–g,j,l,n,p TRPA1,a,c–g,l TRPM8,a,c–g,i,l rat TRPV2,a,d–g,j,l,m,s TRPV3,f TRPV4,f TRPA1,f TRPM8,f and murine TRPV3,n TRPV4,n,p and TRPM8,h as well as channels from an unspecified species.b
| Name | IC50 Values against TRP Channels |
Additional Targets | |||||
|---|---|---|---|---|---|---|---|
| TRPV1 | TRPV2 | TRPV3 | TRPV4 | TRPA1 | TRPM8 | ||
| nM | |||||||
| A-425619 | 10 | — | >20,000b | >20,000a | >10,000b | 8000b | 0 (of 83 tested)b |
| ABT-102 | 1 | — | >20,000c | >20,000c | >20,000c | >20,000c | 0 (of 75 tested)c |
| AMG0347 | 0.7 | >10,000d | >10,000d | >10,000d | >10,000d | >10,000d | — |
| AMG1629 | 0.6 | >4000e | >4000e | >4000e | >4000e | >4000e | — |
| AMG3731 | 6 | >4000e | >4000e | >4000e | >4000e | >4000e | — |
| AMG 517 | 1 | >20,000f | >20,000f | >20,000f | >20,000f | >20,000f | 3 (of 90 tested)f |
| AMG8163 | 0.6 | >4000g | >4000e | >4000e | >4000e | >4000e | — |
| AMG9810 | 79–86 | — | >4000g | >4000g | >4000g | >4000g | 4 (of 90 tested)g |
| BCTC | 0.4–0.5 | — | >20,000a | 1500a | >20,000a | 143–800h,i | — |
| AMG7905 | 39 | >4000j | >4000j | >4000j | >4000j | >4000j | — |
| AMG8562 | 2 | >20,000j | >20,000j | >20,000j | >20,000j | >20,000j | 0 (of 50 tested)j |
| CPZ | 420–887g,k,l | >10,000l,m | >10,000l–o | >10,000l,n,p | >100,000l | 18,000h,i,l | 3q |
| Ruthenium red | 50–512b,r | 200–2028f,j,m,s | <10,000o | 24–1000f,j,p | 367f,j | — | 3t |
—, no information available.
M. H. Norman, personal communication.
Gavva et al. (2007a). Of 90 targets tested in this study, AMG 517 interacted with three: monoamine oxidase B, AEA transporter, and Na+ channel (site 2).
Gavva et al. (2005b). Of 90 targets tested in this study, AMG9810 interacted with four: Ca2+ channel (L-type: DHP site), Na+ channel (site 2), dopamine transporter, and angiotensin-II.
C.R. Faltynek, P.R. Kym, and R.M. Reilly, personal communication.
M. J. Caterina, personal communication.
CPZ has been reported to interact with hyperpolarization-activated cation channel (Gill et al., 2004), nicotinic-acetylcholine receptor (Liu and Simon, 1997), and voltage-gated calcium channel (Docherty et al., 1997).
Ruthenium red has been reported to interact with TRPV5 (Nilius et al., 2001), TRPV6 (Nilius et al., 2001), voltage-gated calcium channel (Cibulsky and Sather, 1999), and ryanodine receptor (Lukyanenko et al., 2000).
E. Thermoregulatory Effects of Transient Receptor Potential Vanilloid-1 Antagonists
1. Effect on Body Temperature.
Testing new TRPV1 antagonists revealed almost immediately that many of them cause hyperthermia, an unwanted side effect (Table 6). This effect has been reported for compounds of different chemotypes, including ureas (A-425619 and JYL 1421), cinnamides (SB366791 and AMG9810), piperazines (BCTC), and pyrimidines (AMG 517), administered to different animal species, including rats, mice, dogs, and monkeys (Fig. 5). This hyperthermic side effect was also presented in clinical trials of AMG 517 (Gavva et al., 2008) (also see Fig. 5). The observed hyperthermia was pronounced: in one patient, Tb (measured as tympanic temperature) reached ∼40°C (Gavva et al., 2008). Even though some TRPV1 antagonists have been shown to cause hyperthermia at very high doses [e.g., 40 mg/kg i.p. in mice for JNJ-17203212 (Huang et al., 2008)], two compounds, AMG0347 and AMG 517, have been shown to cause hyperthermia in rats at low doses. The minimum intravenous dose that causes maximal effect on Tb (EDmax) was reported to be 50 μg/kg (∼100 nmol/kg) for AMG0347 (Steiner et al., 2007) and 100 μg/kg (∼200 nmol/kg) for AMG 517 (Gavva et al., 2008). For AMG0347, a threshold intravenous dose (i.e., the minimum dose that significantly increases deep Tb) was ∼20 nmol/kg (Steiner et al., 2007). The fact that TRPV1 antagonists of different chemotypes cause the hyperthermic effect at low doses and in different animal species strongly suggests that this is an on-target effect (i.e., mediated by TRPV1). Indeed, the mediation of this effect by TRPV1 channels has been demonstrated in the case of AMG0347: Trpv1 KO mice did not respond to AMG0347 with hyperthermia, whereas their normal counterparts did (Fig. 6). In our recent experiments, another antagonist, AMG 517, increased core Tb in control mice, but the same dose of AMG 517 failed to cause hyperthermia in Trpv1 KO mice (A. Garami, N. R. Gavva, and A. A. Romanovsky, unpublished observations). The fact that TRPV1 antagonism results in hyperthermia agrees with the transient rise in Tb observed in several earlier studies in rats during the first few days after the administration of desensitizing doses of CAP (Jancsó-Gábor et al., 1970a; Szolcsányi and Jancsó-Gábor, 1973; Székely and Szolcsányi, 1979; Szikszay et al., 1982) (Table 3). These findings suggest that TRPV1 channels are tonically active in vivo, thus constantly suppressing deep Tb and keeping it at its normal level; when TRPV1 channels are blocked, this suppression is removed, and hyperthermia occurs.
Fig. 5.
TRPV1 antagonists cause hyperthermia in different species. A, effect of AMG0347 (500 μg/kg i.p.) or its vehicle on abdominal temperature in mice at a neutral Ta of 31°C. B, effect of AMG0347 (500 μg/kg i.v.) or its vehicle on colonic temperature in rats at a neutral Ta of 28°C. C, effect of daily administration of AMG 517 (10 mg p.o.) or placebo over days 0 to 6 on tympanic temperature in humans at room temperature. Note that the arrow in C shows just the first day of drug administration. D, effect of AMG 517 (100 μg/kg i.v.) or its vehicle on colonic temperature in rats at a neutral Ta of 26°C. E, effects of JYL 1421 (30 mg/kg p.o.) or its vehicle on Tb (location not specified) in dogs at room temperature. F, effects of JYL 1421 (30 mg/kg p.o.) or its vehicle on Tb (location not specified) in cynomolgus monkeys at room temperature. [A and B are modified from Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. (2007) Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 27:7459–7468. Copyright © 2007 Society for Neuroscience. C and D are modified from Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, et al. (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136:202–210. Copyright © 2008 International Association for the Study of Pain (IASP). E and F are modified from Gavva NR, Bannon AW, Surapaneni S, Hovland DN Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, et al. (2007) The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci 27:3366–3374. Copyright © 2007 Society for Neuroscience. All images used with permission.].
Fig. 6.
AMG0347 (500 μg/kg i.v.) causes hyperthermia in control mice, but not in Trpv1 KO mice. [Modified from Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. (2007) Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 27:7459–7468. Copyright © 2007 Society for Neuroscience. Used with permission.].
2. Thermoeffector Pattern.
The effector patterns of the hyperthermic responses of rats to TRPV1 antagonists can involve tail skin vasoconstriction, activation of thermogenesis (most likely nonshivering thermogenesis in BAT), or both (Steiner et al., 2007; Gavva et al., 2008). Examples of tail skin vasoconstriction (decrease in the HLI) and metabolic activation (increase in oxygen consumption) occurring just before the AMG0347-induced rise in core Tb are shown in Fig. 7, A and B, respectively. Similar to responses to many other substances [e.g., PGE1 (Crawshaw and Stitt, 1975; Szelényi et al., 1992), cholecystokinin-8 (Szelényi et al., 1992), or LPS (Székely and Szelényi, 1979)], the partial contributions of skin vasoconstriction and thermogenesis to the overall hyperthermic response to AMG0347 differ depending on Ta (Steiner et al., 2007). Unlike the responses to PGE1 (Crawshaw and Stitt, 1975; Marques et al., 1984) and to LPS (Almeida et al., 2006a), the response to AMG0347 does not involve a change in the preferred Ta. When rats were treated with a high dose of AMG0347 (500 μg/kg i.v.) in a thermogradient apparatus, they developed hyperthermia, but their position in the apparatus did not change (Fig. 7C). In other words, they did not move a few inches to warm up from the readily available environmental heat, but used other (autonomic) means of heat production and conservation to mount the hyperthermic response.
Fig. 7.
Recruitment of autonomic, but not behavioral, thermoeffectors in the hyperthermic response of rats to AMG0347. AMG0347 (50 μg/kg i.v.) was administered to loosely restrained rats either at a low neutral Ta of 24°C (A) or at a subneutral Ta of 17°C (B). In either case, the injections were performed through a preimplanted jugular catheter, from outside the environmental chamber, without disturbing the animals (Steiner et al., 2007). Because the procedure of drug administration did not cause stress, injection of a vehicle alone did not affect colonic temperature. At 24°C, the hyperthermic response to AMG0347 occurred, at least in part, because of tail skin vasoconstriction (a decrease in the HLI). At 17°C, the hyperthermic response to AMG0347 occurred, at least in part, because of thermogenesis activation (an increase in oxygen consumption). AMG0347 (500 μg/kg i.v.) also caused hyperthermia in a thermogradient apparatus (C). In this setup, the procedure for drug administration involved handling, and the injection of vehicle caused stress hyperthermia, but the hyperthermic response to AMG0347 was higher and lasted longer than the response to the vehicle. Even though a high dose of AMG0347 was used in this experiment, thermoregulatory behavior was not recruited, and the drug-injected rats preferred the same Ta as the rats injected with the vehicle. [Modified from Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. (2007) Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 27:7459–7468. Copyright © 2007 Society for Neuroscience. Used with permission.].
3. The Mode of Action: Do Transient Receptor Potential Vanilloid-1 Antagonists Cause Hyperthermia by Blocking Thermal Signals?
We have already presented some evidence suggesting that TRPV1 channels on MPO neurons do not serve as thermosensors (section III.B). Can TRPV1 channels at other brain or extra-brain locations mediate those responses to thermal signals that control thermoeffector activity? Even though the TRPV1 channel is activated in vitro only at very high (>42°C) temperatures (Caterina et al., 1997; Tominaga et al., 1998), concomitant activation (or sensitization) by endovanilloids and protons can decrease, via multiple mechanisms (Tominaga et al., 1998; Premkumar and Ahern, 2000; Bhave et al., 2003; Carlton et al., 2004; Van Der Stelt and Di Marzo, 2004; Ahern et al., 2005; Zhang et al., 2008), the activation temperature in vivo. Such sensitization to thermal stimuli could be speculated to bring the activation threshold closer to the physiological values of core Tb. For example, research by Ni et al. (2006) has shown that increasing Tb within the normal or even hypothermic range (perhaps as low as 34.5°C) can directly activate pulmonary sensory neurons and that this effect is likely mediated, at least in part, by TRPV1. Hence, the intriguing possibility that TRPV1 is involved in normal thermoregulation by serving as a thermoreceptor cannot be ruled out.
If normal Tb values somewhere in the body were high enough to activate TRPV1 channels, and if the thermal activation of TRPV1 were involved in normal thermoregulation, blocking the thermal activation by TRPV1 antagonists would be expected to cause a stronger response at a higher Tb. Depending on the location of the TRPV1 channels involved, such stronger responses to TRPV1 antagonists would occur either at a higher core Tb (if the thermoreceptor function is carried out by TRPV1 channels located in the body core) or at a higher shell Tb (if the responsible TRPV1 channels are located at the body surface and are activated by shell temperatures such as Tsk). We studied the hyperthermic response to AMG0347 administered to rats in different thermal environments, when their initial (at the time of drug administration) core (colonic) Tb was between 36.6 and 39.3°C, and their initial tail Tsk ranged from 16.9 to 37.0°C (Fig. 8A). A positive correlation between the magnitude of the hyperthermic response and the initial values of colonic Tb, Tsk, or both would have indicated that the hyperthermic response to AMG0347 was caused by the blockade of the thermal activation of TRPV1 channels located in the core, skin, or both, respectively. However, no positive correlation was found. On the contrary, there was a weak negative correlation between the maximal change in colonic Tb and the initial Tsk (Fig. 8C) and a tendency for a negative correlation between the maximal value of the change in colonic Tb caused by AMG0347 and the initial value of colonic Tb (Fig. 8B), which is in agreement with the fact that the hyperthermic response to a drug often decreases with an increase in Tb, as reported for LPS (Blatteis, 1974; Székely and Szelényi, 1979; Ivanov and Romanovsky, 2002), PGE1 and PGE2 (Malkinson et al., 1988; Feng et al., 1989; Szelényi et al., 1992, 1994), cholecystokinin (Szelényi et al., 1992, 1994), and other substances. Because drugs generally affect elements of the thermoregulatory system that are sensitive to temperature (and not a change in temperature), Tb of a drug-treated organism often balances at a level independent of the initial Tb, thus resulting in a negative correlation between the initial Tb and the drug-induced change in Tb (Feng et al., 1989). It is also plausible that some drugs are metabolized faster at a higher Tb, thus producing a smaller effect (McAllister and Tan, 1980). In the case of AMG0347, the clear lack of a positive correlation between the magnitude of antagonist-induced hyperthermia and the rats' core Tb or Tsk indicates that the normally present tonic suppression of Tb arises from a tonic activation of TRPV1 channels by nonthermal factors and not by temperature. Such nonthermal factors, as yet unidentified, may include low (or high) pH, inorganic cations, or endovanilloids.
Fig. 8.
AMG0347 hyperthermia is independent of either basal colonic temperature or basal tail Tsk. AMG0347 (50 μg/kg i.v.) was injected in rats at Ta of 17, 24, or 28°C (A). At all Ta values tested, the drug induced hyperthermic responses of similar magnitude. When the response magnitude (the maximal increase in colonic temperature) for each rat was plotted against its basal (at the time of drug administration) colonic temperature (B) or against its basal Tsk (C), no positive correlation was found. [Modified from Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. (2007) Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 27:7459–7468. Copyright © 2007 Society for Neuroscience. Used with permission.]
4. Do Transient Receptor Potential Vanilloid-1 Antagonists Cause Hyperthermia by Acting inside or outside the Brain?
We also attempted to determine the location of TRPV1 channels responsible for the hyperthermic effect of AMG0347 (Steiner et al., 2007). The first question was whether AMG0347 crosses the BBB. Rats were injected with the EDmax dose (50 μg/kg i.v.), and their arterial blood and brains were harvested 60 min later, at the time corresponding to the peak of AMG0347-induced hyperthermia. At that time point, the concentration of the drug in the brain (4 μg/g) was only 3.5 times lower than the concentration in the blood plasma (14 μg/g), clearly demonstrating that AMG0347 crosses the BBB. Hence, the hyperthermic effect could have resulted from a drug's action inside or outside the central nervous system.
We then investigated whether AMG0347 can cause hyperthermia by acting inside the brain or spinal cord. If one of these sites were a primary site of the hyperthermic action of AMG0347, the intracerebroventricular or intrathecal administration of AMG0347 would be expected to cause hyperthermia at doses 1 to 2 orders of magnitude lower than the intravenous administration. For example, Hori (1984) reported that intrabrain administration of CAP causes hypothermia at doses that are 25 times lower than the minimal effective intravenous dose found by Donnerer and Lembeck (1983). However, this was not the case with the hyperthermic response to AMG0347, which, when administered intravenously in rats, showed a tendency to increase deep Tb at a dose of 6 μg/kg and had a significant effect at 10 μg/kg (the minimally effective intravenous dose). When we administered AMG0347 intracerebroventricularly (into the lateral ventricle) or intrathecally at a dose as high as 6 μg/kg, the drug still caused no significant changes in Tb (Steiner et al., 2007). Because AMG0347 is no more effective in causing hyperthermia when administered into the brain or spinal cord than when it is administered systemically, we conclude that the drug causes hyperthermia by acting outside the BBB. McGaraughty et al. (2009) have recently conducted a study in rats that involved the intravenous, intrathecal, and intra-POA administration of another TRPV1 antagonist, A-889425. The authors conclude that A-889425 also causes hyperthermia by acting outside the BBB.
There are brain structures, however, that lack the typical BBB and can be readily reached by circulating drugs (McKinley et al., 2003). These structures, collectively named the circumventricular organs, include the organum vasculosum of the lamina terminalis (OVLT), a POA structure that forms the anterior wall of the third cerebral ventricle. It has been hypothesized that TRPV1 antagonists increase core Tb by acting in the OVLT (Gavva et al., 2007b). The OVLT hypothesis agrees with the fact that electrolytic lesions of the anterior wall of the third ventricle cause hyperthermia (Romanovsky et al., 2003). However, TRPV1 antagonists failed to cause marked hyperthermia when administered via either the intracerebroventricular route (which provides good access to the OVLT and other circumventricular structures) or directly into the POA (Steiner et al., 2007; McGaraughty et al., 2009). We conclude that TRPV1 antagonists cause hyperthermia by acting outside the brain, in peripheral tissues.
5. Site of Blockade: The Abdominal Viscera.
Because TRPV1 channels are abundant in primary DRG and nodose neurons that innervate the abdominal viscera, we tested the hypothesis that the antagonists cause hyperthermia by acting on intra-abdominal targets (Steiner et al., 2007). We injected RTX in rats at a low dose of 20 μg/kg i.p. and used a battery of tests (Table 8) to confirm that the desensitization of TRPV1 channels in this model was restricted to the abdominal cavity (Table 9). We then found that rats with such localized, intra-abdominal desensitization do not respond with hyperthermia to intravenous AMG0347 (Fig. 9) or AMG 517 (A. Garami, N. R. Gavva, and A. A. Romanovsky, unpublished observations), each administered at its EDmax. The inability of rats pretreated with RTX (20 μg/kg i.p.) to respond with hyperthermia to another TRPV1 antagonist, A-889425, has also been demonstrated (McGaraughty et al., 2009). Based on these studies, we conclude that the site of the hyperthermic action of TRPV1 antagonists lies within the abdominal cavity.
Table 8.
Selected tests for sensitivity of TRPV1 channels in different bodily compartments
| Compartment | Test | Reference |
|---|---|---|
| Abdominal cavity | Satiety response to intraperitoneal cholecystokinin | Dogan et al. (2004) |
| Writhing response to intraperitoneal RTX | Steiner et al. (2007) | |
| Eyes | Eye-wiping response to topical ammonium hydroxide | Dogan et al. (2004) |
| Eye-wiping response to topical RTX | Steiner et al. (2007) | |
| Skin | Locomotor response to noxious heat (hot-plate test, 55°C) | Steiner et al. (2007) |
| Thoracic cavity | Hypotensive response to RTX into superior vena cava (Bezold-Jarish reflex) | Steiner et al. (2007) |
| Brain | Tail skin vasodilation response to CAP into third ventricle | Steiner et al. (2007) |
Table 9.
Sites of TRPV1 desensitization in RTX- or vehicle-pretreated rats
In the schematics of the desensitization pattern, the desensitized compartments are shown in grey; the nondesensitized compartments are shown in white. Based on data from Dogan et al. (2004) and Steiner et al. (2007).
| Pretreatment | Compartment |
Desensitization Pattern | Desensitization Extent | ||||
|---|---|---|---|---|---|---|---|
| Abdominal Cavity | Eyes | Skin | Thoracic Cavity | Brain | |||
| Vehicle | ○ | ○ | ○ | ○ | ○ | None | |
| RTX 0.2 mg/kg i.p. | × | × | × | × | × | Systemic | |
| RTX 0.02 mg/kg i.p. | × | ○ | ○ | ○ | ○ | Localized intra-abdominal | |
×, desensitized; ○, nondesensitized.
Fig. 9.
Localized intra-abdominal TRPV1 desensitization abolishes the AMG0347-induced hyperthermia in rats. Shown is the effect of AMG0347 (50 μg/kg i.v.) on colonic temperature of rats pretreated with RTX (20 μg/kg i.p.) or its vehicle 10 days before the experiment. [Modified from Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. (2007) Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 27:7459–7468. Copyright © 2007 Society for Neuroscience. Used with permission.].
6. A Hypothetical Neural Pathway.
Because TRPV1-mediated signals from the abdominal viscera affect autonomic thermoregulatory responses but do not affect the selection of preferred Ta, these signals are likely to impinge on the neural pathways for autonomic thermoregulation downstream from the point of separation from the pathways for thermoregulatory behaviors. This may happen in the MPO, because even large POA lesions (that destroy the MPO completely or nearly completely) do not affect thermoregulatory locomotion in a thermogradient apparatus (Almeida et al., 2006b), whereas a more upstream (closer to the afferent input) structure, the MnPO, seems to be involved in at least some behavioral thermoregulatory responses (Konishi et al., 2007). This hypothesis is schematically represented in Fig. 10. By analogy with other autonomic pathways (e.g., with those that convey cutaneous warming and cooling signals to the thermoeffectors), we propose that the second and third sensory neurons of the pathway activated by visceral nonthermal, TRPV1-mediated signals are located in the DH and LPB, respectively, and that both are glutamatergic. Via the proposed pathway, the tonic activation of visceral TRPV1 channels by nonthermal stimuli would be shifting the balance of synaptic inputs to warm-sensitive MPO neurons in the same way as cutaneous warming. TRPV1 antagonists remove the effect of tonic, nonthermal, visceral TRPV1 activation and produce an effect on the synaptic inputs to warm-sensitive MPO neurons equivalent to the removal of cutaneous warming, thereby increasing the activity of the sympathoexcitatory efferent pathways that drive BAT thermogenesis and skin vasoconstriction. Inhibition of warm-sensitive MPO neurons by a systemic administration of A-889425 has been demonstrated (McGaraughty et al., 2009).
Fig. 10.
The proposed pathway for tonic inhibition of BAT thermogenesis and skin vasoconstriction by nonthermal activation of visceral TRPV1 channels and proposed mechanisms of the thermoregulatory effects of TRPV1 agonists, TRPV1 desensitization, and TRPV1 antagonists. The proposed pathway (dotted line) is compared with the thermoregulatory pathways activated by innocuous skin warming and cooling (solid lines). On the efferent side (right portion of the figure), neurons in the brain stem and spinal cord structures that are common to all three pathways are omitted (to avoid repetition of Fig. 1). All omitted synapses are excitatory. Abbreviations and symbols are the same as in Fig. 1. We propose that TRPV1 agonists and antagonists act on two neurons: the glutamatergic MnPO neuron within the pathway activated by innocuous warming of the skin and a glutamatergic polymodal visceral DRG neuron. TRPV1 channels on the MnPO neurons are normally closed and are therefore readily affected by TRPV1 agonists but not by antagonists. Channels on the DRG neurons are tonically activated by nonthermal factors and are therefore more susceptible to the action of antagonists. Because TRPV1 agonists affect the MnPO neurons, these neurons lose their function first during desensitization. Higher doses of TRPV1 agonists induce secondary degeneration of warm-sensitive GABA-ergic MPO neurons, an effect that is subject to partial recovery when the desensitizing dose of the agonist is administered in the neonatal period. For further explanations, see sections IV.E.6 and V.A.
7. Transient Receptor Potential Vanilloid-1 Antagonism without Hyperthermia: Implications for Drug Development.
Several strategies of achieving TRPV1 antagonist-induced analgesia without hyperthermia have been proposed. At least three of these strategies have not produced the desired result, at least not yet. The first strategy was based on a hope that the hyperthermic effect was a chemotype-specific, off-target effect. This, however, seems not to be the case (Swanson et al., 2005; Gavva et al., 2007a,b, 2008; Steiner et al., 2007). The second strategy was to combine a TRPV1 antagonist with an antipyretic (e.g., an inhibitor of PG synthesis) (Gavva et al., 2007a), but the hyperthermic response to TRPV1 antagonists does not seem to be mediated by PGs. Although acetaminophen did block AMG8163-induced hyperthermia in rats, it did so only at a very high, hypothermia-inducing dose of 300 mg/kg (Gavva et al., 2007a), which is analogous to a 21-g bolus dose for a 70-kg human. In a recent clinical trial (Gavva et al., 2008), high hyperthermia (>39°C) that occurred after a molar extraction in a patient treated with AMG 517 persisted for 3 days despite the repeated administration of a reasonably high dose of acetaminophen (Tylenol; 1.3 g). In the third strategy, Steiner et al. (2007) have proposed to explore the possibility of dissociating the analgesic and hyperthermic effects of TRPV1 antagonists based on the fact that the TRPV1 channels responsible for these effects are located in different bodily compartments, but no specific recommendations have been developed so far.
The fourth and the fifth strategies have resulted in some advances. The fourth strategy is aimed at taking advantage of the fact that the hyperthermic effect of some TRPV1 antagonists fades away with repeated administration, whereas the analgesic effect shows no attenuation (Gavva et al., 2007a). Such dissociation has been reported for two of Amgen's antagonists (AMG 517 and AMG8163) studied in rats, dogs, and monkeys (Gavva et al., 2007a). A recent study in rats with Abbott Laboratories' drug ABT-102 (Honore et al., 2009) also has shown that repeated dosing can favorably shift the ratio of the desired effect (analgesia) to the unwanted side effect (hyperthermia). The selective restoration of some TRPV1-mediated functions after the administration of a TRPV1 antagonist can be due to the antagonist-sensitive regulation of the subcellular distribution of TRPV1; via such a mechanism, TRPV1 antagonists can increase the cell surface expression of the TRPV1 protein and, subsequently, the cellular sensitivity to TRPV1 agonists (Johansen et al., 2006). However, when AMG 517 was administered in humans in Amgen's trials, the results seemed less promising: of the three doses tested, only the highest dose (10 mg) caused a hyperthermic response that attenuated with repeated dosing (Fig. 5C); hyperthermic responses to the lower doses (2 and 5 mg) showed no attenuation (Gavva et al., 2008).
The fifth (and arguably the most promising) strategy is based on the fact that the TRPV1 channel has independent gating mechanisms for vanilloids, protons, and heat (Welch et al., 2000). Therefore, a TRPV1 antagonist exhibits different pharmacological characteristics depending on the mode of activation of the TRPV1 channel (McIntyre et al., 2001; Gavva et al., 2005a, 2008; Broad et al., 2008; Lehto et al., 2008). From this point of view, the profile of CPZ, one of the older TRPV1 antagonists, deserves attention. Although CPZ has been used extensively in thermoregulation studies in rats and mice (Dogan et al., 2004; Jakab et al., 2005; Shimizu et al., 2005), it has never been reported to cause hyperthermia. However, CPZ does not block activation of the TRPV1 channel by protons (IC50 > 4000) in the rat or mouse (McIntyre et al., 2001; Savidge et al., 2002; Correll et al., 2004; Gavva et al., 2005a,b), even though it blocks proton activation in the guinea pig (IC50 = 360 nM) (Savidge et al., 2002). Hence, it would be important to look at the thermoregulatory responses to TRPV1 antagonists with different pharmacological profiles. One such study was conducted by Lehto et al. (2008), who used four structurally related TRPV1 antagonists (Table 5) with different profiles (Table 6): AMG8163, AMG8563, AMG8562, and AMG7905. All of the antagonists used inhibited TRPV1 activation by CAP. Those antagonists that either blocked (AMG8163) or partially attenuated (AMG8563) proton activation in vitro also caused hyperthermia in rats, whereas antagonists that potentiated proton activation (AMG8562 and AMG7905) did not cause hyperthermia and caused hypothermia instead (Table 6). AMG8562, a competitive antagonist at the CAP-binding pocket and a positive allosteric modulator for proton activation, had significant efficacy in several rodent models of pain, thus providing an example of TRPV1-antagonist-induced analgesia without hyperthermia in rats. The authors concluded that the hyperthermic effect occurs if an antagonist inhibits CAP activation and also inhibits (fully or partially) proton activation. On the other hand, if an antagonist inhibits CAP activation but potentiates proton activation, it does not cause hyperthermia (and may cause hypothermia instead). If this conclusion is true, the success of AMG8562 in causing hyperthermia-free analgesia in rats cannot be reproduced in humans, because this antagonist is a potent inhibitor of all modes of activation of the human TRPV1 channel (Lehto et al., 2008).
V. Hypotheses, Conclusions, and Future Directions
A. Mechanisms of the Thermoregulatory Effects of Transient Receptor Potential Vanilloid-1 Agonists and Antagonists: A Unifying Hypothesis
Based on the studies reviewed herein, we propose that there are two principal populations of TRPV1-expressing cells that have connections with efferent pathways that control autonomic thermoeffectors. These two populations are the first-order sensory (polymodal) glutamatergic DRG (and possibly nodose) neurons that innervate the abdominal viscera and the higher-order (probably fourth-order) sensory glutamatergic neurons presumably located in the MnPO. We further hypothesize that the entire variety of the thermoregulatory responses to TRPV1 agonists and antagonists can be explained by the actions on TRPV1 channels on these two populations of neurons (Fig. 10). As reviewed above, the responses to TRPV1 agonists and antagonists include 1) the acute, immediate hypothermic responses to systemic TRPV1 agonists, 2) the increased deep Tb during the first few days after systemic administration of TRPV1 agonists at desensitization-inducing doses, 3) the chronic heat-defense deficiency caused by systemic doses of TRPV1 agonists administered to newborn or adult animals, 4) the chronic cold-defense deficiency and decreased central thermosensitivity of adult animals treated with very high systemic doses of TRPV1 agonists, and 5) the short-term hyperthermic effect of TRPV1 antagonists administered systemically. We propose that both agonists and antagonists act on both neuronal populations, but that the effects of agonists are mediated predominantly by MnPO neurons, whereas the effects of antagonists are mediated predominantly by DRG neurons. Such relative selectivity can be explained as follows. Because, as described above, administration of TRPV1 antagonists into the third cerebral ventricle or directly into the POA does not cause hyperthermia (whereas TRPV1 agonists are highly effective in causing hypothermia when administered via the same routes), we conclude that TRPV1 channels on MnPO neurons are not activated under normal conditions. Hence, TRPV1 agonists activate (open) the channels on these neurons and have the consequent effects, whereas TRPV1 antagonists are ineffective. On the other hand, visceral TRPV1 channels are tonically activated, and TRPV1 antagonists block this activation and cause marked effects, whereas additional activation by systemic TRPV1 agonists has little effect. Indeed, recent experiments have shown that the hypothermic response to intravenous RTX is unaffected by localized intra-abdominal desensitization of TRPV1 channels (A. Garami and A. A. Romanovsky, unpublished observation), even though, as discussed above, the same desensitization eliminates hyperthermic responses to the intravenous administration of TRPV1 antagonists, including AMG0347, AMG 517, and A-889425. The inability of TRPV1 to respond to low concentrations of a full agonist (such as RTX) would be expected if the channel were tonically activated by an abundant partial agonist that occupied the binding site competitively with respect to the full agonist (Ross and Kenakin, 2001). An example of a partial agonist is AEA (Ross, 2003). Furthermore, several substances decrease the responsiveness of the TRPV1 channel to RTX and CAP. For instance, adenosine and its analogs interact with TRPV1, inhibit RTX binding, and inhibit CAP-induced inward currents in DRG neurons (Puntambekar et al., 2004), whereas calmodulin binds to the NH2-terminal region of the TRPV1 channel and inhibits gating to reduce the probability of channel opening (Rosenbaum et al., 2004). There is also evidence for a separate regulatory site (that binds several neuroleptic drugs), which can either increase or decrease vanilloid binding and the resulting calcium influx (Acs et al., 1995; Szallasi et al., 1996).
Hence, we propose that the acute hypothermic response to systemic TRPV1 agonists described above is the result of an action on nonactivated TRPV1 channels on MnPO neurons (Fig. 10). The activation is followed by the functional impairment (desensitization) of these neurons, which can lead to hyperthermia even in the absence of heat exposure. This hyperthermia, typically of low grade, lasts for a few days and ceases thereafter, presumably as a result of the development of compensatory mechanisms. The functional loss of the same neurons is responsible for the attenuated heat-defense responses; such attenuation typically lasts for a long time and represents the most prominent symptom of systemic TRPV1 desensitization. When the dose of an agonist is extremely high, secondary degeneration of the next neuron (the MPO neuron) occurs, thus effectively interrupting all pathways to autonomic thermoeffectors. If the agonist is administered in the neonatal period, no permanent loss of function occurs, possibly because of proliferation and differentiation of neuronal progenitor cells and their migration into the MPO. However, if a very high systemic dose of a TRPV1 agonist (>100 mg/kg for CAP) is administered in adulthood, the loss of warm-sensitive MPO neurons is not recovered, at least not completely, and the animals exhibit the loss of hypothalamic thermosensitivity and a deficiency of cold-defense responses. As for the acute hyperthermic response to systemic TRPV1 antagonists, it is probably the result of a blockade of nonthermal tonic stimulation of visceral DRG neurons.
B. The Transient Receptor Potential Vanilloid-1 Channel in Normal Thermoregulation: A Thermosensor It Is Not
1. Transient Receptor Potential Vanilloid-1 Channels on Preoptic Neurons Are Not Activated at Normal Body Temperatures.
The fact that low doses of TRPV1 antagonists do not cause hyperthermia upon central administration (Steiner et al., 2007; McGaraughty et al., 2009), even when administered directly into the POA (McGaraughty et al., 2009), suggests that hypothalamic TRPV1 channels are not activated at normal Tb values. As discussed in section III.B, core Tb rarely approaches the ∼43°C activation threshold of the TRPV1 channel determined in in vitro studies (Caterina et al., 1997; Tominaga et al., 1998). Furthermore, when brain slices containing POA neurons are exposed to different temperature changes, the effects evoked (changes in brief ionic currents of the depolarizing prepotential, but not in the resting membrane potential) seem incompatible with a pivotal role of TRP channels (including TRPV1) in the thermal sensitivity of POA neurons (Boulant, 2006). Moreover, warm-sensitive MPO neurons do not express TRPV1 (I. V. Tabarean, J. Eberwine, B. Conti, and T. Bartfal, personal communication), and TRP antagonism does not reduce the thermosensitivity of warm-sensitive MPO neurons (Unger et al., 2008).
2. Transient Receptor Potential Vanilloid-1 Channels on Visceral Dorsal Root Ganglia Neurons Are Activated, but Not by Temperature.
Studies with TRPV1 antagonists have shown that tonic activation of TRPV1 channels in the abdominal viscera inhibits BAT thermogenesis and skin vasoconstriction, thus having a tonic suppressive effect on core Tb. TRPV1 antagonists cause hyperthermia by removing this tonic activation and consequently by triggering skin vasoconstriction and activating BAT thermogenesis. However, the TRPV1-mediated, visceral, Tb-suppressing signals that are blocked by TRPV1 antagonists are nonthermal, at least as evidenced by the finding that AMG0347 causes the same effect at different deep Tb values and in a wide range of Tsk values (Steiner et al., 2007). Even though TRPV1 channels are involved in the detection of noxious heat stimuli that are important for pain responses, they are not involved in the detection of those innocuous thermal stimuli (shell and core temperatures) that regulate the activity of thermoeffectors under physiological conditions.
3. “Thermoregulatory” Effect: What's in a Name?
It seems clear that TRPV1 channels do not play the role of thermosensors for Tb regulation under normal conditions. However, it is not completely clear how to classify the nonthermal, tonic, inhibitory effect of visceral TRPV1 channels on autonomic cold-defense effectors under physiological conditions. According to the most recent review by Gavva (2008), this effect shows that the TRPV1 channel “regulates Tb,” but whether this conclusion is legitimate depends on the definition of thermoregulation. If by regulation we mean adjusting core Tb (the main control variable) based on its changes via negative feedback or based on changes in Tsk (the auxiliary variable) via negative or positive feedback, then TRPV1 channels are not involved in Tb regulation. They sense neither core Tb nor Tsk; hence, they are uninvolved in the control of the main variable via feedback mechanisms. Consequently, if one subscribes to a traditional, conservative definition of Tb regulation as a feedback control, a proper conclusion would be that TRPV1 channels are uninvolved in normal thermoregulation and instead tonically modulate Tb by modulating thermoeffector activity in response to nonthermal stimuli.
However, thermoregulation involves not only feedback mechanisms using the main control variable core Tb and the auxiliary variable Tsk but also elements of meshed control, often nonthermal in their nature (for explanations and examples, see section II.A.2). Therefore, it may be reasonable to use a broader definition of thermoregulation that includes all processes affecting Tb. If such a definition were adapted, inhibition of BAT thermogenesis and skin vasoconstriction by the nonthermal signals mediated by visceral TRPV1 might possibly be viewed as a thermoregulatory phenomenon.
C. Physiological and Pathological Significance
The biological significance of the recently found tonic inhibition of thermoeffectors by nonthermal signals originating in the abdominal cavity and mediated by visceral TRPV1 channels remains speculative. This inhibition may constitute a connection between the organs supplying the body with chemical energy (GIT) and the organs burning (BAT) and dissipating (skin blood vessels) this energy. If the nonthermal TRPV1-mediated signals indeed originate in the GIT, they may be involved in thermoregulatory adjustments to changes in the feeding status such as diet-induced thermogenesis and postprandial hyperthermia in mammals (Székely, 2000; Gobel et al., 2001), starvation-associated hypometabolism in mammals (Székely, 2000), adaptive daytime hypothermia in nocturnal mammals (Kanizsai et al., 2009), or nutrition-dependent nocturnal hypothermia in birds (Geran and Rashotte, 1997). By activating TRPV1 channels, the luminal pH, which fluctuates through the feeding cycle (McConnell et al., 2008) and decreases during prolonged fasting (Hood and Tannen, 1998), may contribute to the hypothermic response to fasting. Indeed, fasting-induced hypothermia was found to be attenuated in Trpv1 KO mice (Kanizsai et al., 2009). The concentration of endogenous TRPV1 agonists (e.g., OEA) (Fu et al., 2007), in the intestinal wall also fluctuates during the feeding cycle. It is noteworthy that the intestinal wall may be the only place in the body where OEA can reach concentrations comparable with its EC50 (Fu et al., 2007). Indeed, responses to OEA are ablated in CAP-desensitized animals, suggesting that OEA causes at least some of its effects by acting on TRPV1-expressing sensory fibers (Rodríguez de Fonseca et al., 2001). Endovanilloids and low pH can activate TRPV1 channels that are present on the majority (∼80%) of DRG afferents (and many nodose neurons) servicing the stomach and the large intestine (Holzer, 2004; Hwang et al., 2005). DRG (and also nodose) neurons innervating the stomach respond to acid in a manner compatible with TRPV1 mediation (Sugiura et al., 2005), whereas genetic deletion of TRPV1 reduces the responsiveness of sensory neurons in the GIT to low pH (Rong et al., 2004), and the pharmacological blockade of the TRPV1 channel attenuates the visceromotor pain response to acid (Holzer, 2007). Furthermore, TRPV1-expressing DRG neurons play an important role in the acid-induced rise in mucosal blood flow and other responses in the rat stomach and colon (Holzer, 2007).
Another possibility is that the tonic suppression of autonomic thermoeffectors originates not from TRPV1 channels in a specific part of the GIT, but from those distributed diffusely throughout the abdominal viscera. If this is the case, intra-abdominal TRPV1 channels can be speculated to have at least two distinct functions. The first putative function comes into play when Tb in the abdomen reaches extremely high values, thus exceeding the 43°C activation threshold of TRPV1 channels. The abdominal temperature increases more readily than the brain temperature, especially in those species that possess mechanisms for selective brain cooling. For example, an abdominal temperature of >47°C was recorded in a running gazelle (Taylor and Lyman, 1972). Perhaps DRG that express TRPV1 channels and project (polysynaptically) to the neural pathways that control thermoeffectors (Fig. 10) can participate in the recruitment of heat-defense responses at extreme levels of body heating. Exposure of the abdominal viscera of guinea pigs to temperatures of 42–44°C readily causes heat-defense responses, including skin vasodilation (Romanovsky and Blatteis, 1996). However, even for the abdominal location, temperatures that exceed the 43°C activation threshold typically signify pathological conditions. For example, the gazelle observed by Taylor and Lyman (1972) died shortly after the 47°C temperature was recorded, and the intraperitoneal heating used in the study in guinea pigs by Romanovsky and Blatteis (1996), when conducted for more than 40 min, caused cessation of heat-defense responses and death.
The second hypothetic function of intra-abdominal TRPV1 channels may be the detection of visceral acidosis, a nonspecific severity indicator in various diseases, including inflammatory ones (Montgomery et al., 1990; Fiddian-Green, 1993; Chendrasekhar et al., 1996; Nielsen et al., 1996). The body responds to mild inflammation or mild infection with fever (to activate immune defenses), but to severe inflammation with hypothermia (to attenuate the effects of tissue hypoxia by decreasing metabolic requirements) (Romanovsky and Székely, 1998; Romanovsky et al., 2005; Almeida et al., 2006a). The inflammation-associated hypothermia is a brain-mediated (Almeida et al., 2006b) response occurring because of the inhibition of metabolism (Romanovsky et al., 1996). Hence, it is plausible to speculate that visceral acidosis can trigger metabolic inhibition and hypothermia in systemic inflammation. It is noteworthy that peritoneal acidosis is also thought to be protective by limiting the systemic inflammatory response (Hanly et al., 2007). That many inflammatory mediators (e.g., PGs) can coactivate the TRPV1 channel together with protons (Tominaga and Caterina, 2004) makes a TRPV1 involvement in triggering hypothermia in severe disease even more likely. It can also be speculated that TRPV1 channels on POA neurons, although not activated under normal conditions, may react to brain hyperthermia or acidosis.
D. Puzzles Yet to Be Solved
We conclude this review with a partial list of important questions that either have not been addressed experimentally or that have produced unclear or contradictory answers. The first group of such questions relates to hypothalamic TRPV1 channels. Even though it is widely accepted that TRPV1 channels are present on POA neurons, which population(s) of POA cells involved in thermoregulatory pathways (i.e., glutamatergic MnPO cells, GABA-ergic MnPO cells, or GABA-ergic MPO cells) express TRPV1 channels is unknown. The pathways proposed in this review are based largely on the assumption that TRPV1-expressing cells are glutamatergic MnPO neurons. Despite substantial circumstantial support, this hypothesis has not been confirmed in a direct experiment, and a different opinion is expressed by several authors who propose that TRPV1 channels are expressed by warm-sensitive MPO neurons. A related question to be addressed experimentally is how systemic desensitization with TRPV1 agonists affects MnPO and MPO neurons with identified neurochemical chemotypes and thermal sensitivity and with a confirmed involvement in the pathways that control thermoeffectors.
Another neural substrate that awaits identification is the one connecting TRPV1-expressing neurons that service the abdominal viscera with efferent neural pathways that control autonomic thermoeffectors. We hypothesize that the connecting pathways involve LPB neurons and do not involve MnPO neurons, but both propositions have yet to be verified in direct experiments. Of special interest is the level at which the pathways controlling autonomic thermoeffector responses to nonthermal TRPV1-mediated signals from the abdominal viscera converge with the pathways that control responses of the same effectors to cutaneous warming and cooling. It should also be clarified how these two groups of pathways relate to pathways that control the selection of preferred thermal environment in response to innocuous skin warming and cooling.
Of great practical importance are those questions that can be instrumental in separating the analgesic effect of TRPV1 antagonists from their hyperthermic on-target side effect. Because both effects seem to be due to action on primary sensory neurons, it seems rather unlikely that they can be separated based on a different distribution of the TRPV1 channels involved. However, the nature of the visceral signals that TRPV1 antagonists have to block to disinhibit thermogenesis and skin vasoconstriction, and thereby to cause hyperthermia, remains unknown. Identification of these tonically active nonthermal visceral signals is a high-priority task. Depending on their nature, it may be possible to design TRPV1 antagonists that would cause analgesia without causing hyperthermia. It would also make sense to conduct a systematic, quantitative study to establish a correlation between the potency of TRPV1 antagonists to cause hyperthermia and their potency of blocking the activation of the TRPV1 channel by stimuli of different modalities: protons, temperature, and vanilloids. Many of the questions listed above aim at testing hypotheses, putative scenarios, and likely pathways proposed in this review.
Acknowledgments.
Our research summarized in this review has been supported in part by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants R01-NS41233, R01-NS40987] (to A.A.R. and S.F.M., respectively); the Arizona Biomedical Research Commission [Category II Grant 8016] (to A.A.R.); St. Joseph's Foundation (to A.A.R.); and Amgen, Inc. (study agreements with A.A.R.). We thank Dr. Jürgen Werner for reviewing and providing important feedback on the portions of the manuscript that deal with body temperature control.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.001263.
- AEA
- arachidonoylethanolamide
- BAT
- brown adipose tissue
- BBB
- blood-brain barrier
- BCTC
- N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)-tetrahydropyrazine-1(2H)-carboxamide
- 1>CAP
- capsaicin
- CPZ
- capsazepine
- DH
- dorsal horn
- DMH
- dorsomedial hypothalamus
- DRG
- dorsal-root ganglion (ganglia)
- EC50
- 50% effective concentration of an agonist (produces 50% of the maximum possible response)
- EDmax
- maximal effective dose (the dose above which no additional improvement in efficacy is obtained)
- GIT
- gastrointestinal tract
- HLI
- heat loss index
- IC50
- 50% of the maximum inhibitory response)
- JNJ-17203212
- 4-[3-(trifluoromethyl)-2-pyridinyl]-N-[5-(trifluoromethy l)-2-pyridinyl]-1-piperazinecarboxamide
- KO
- knockout
- LC
- locus ceruleus
- LPB
- lateral parabrachial nucleus
- LPS
- lipopolysaccharide
- MnPO
- median preoptic nucleus
- MPO
- medial preoptic area
- NADA
- N-arachidonoyldopamine
- OEA
- oleoylethanolamide
- OLDA
- N-oleoyldopamine
- OVLT
- organum vasculosum of the lamina terminalis
- PG
- prostaglandin
- POA
- preoptic area of the hypothalamus
- rRPa
- rostral raphe pallidus nucleus
- RTX
- resiniferatoxin
- SB 366791
- 4′-chloro-3-methoxycinnamanilide
- Ta
- ambient temperature
- Tb
- body temperature
- Tsk
- skin temperature
- TRP
- transient receptor potential (channel)
- TRPA
- ankyrin TRP
- TRPM
- melastatin TRP
- TRPV
- vanilloid TRP
References
- Acs G, Palkovits M, Blumberg PM. (1995) Trifluoperazine modulates [3H]resiniferatoxin binding by human and rat vanilloid (capsaicin) receptors and affects 45Ca uptake by adult rat dorsal root ganglion neurones. J Pharmacol Exp Ther .274: 1090–1098 [PubMed] [Google Scholar]
- Acs G, Palkovits M, Blumberg PM. (1996) Specific binding of [3H]resiniferatoxin by human and rat preoptic area, locus ceruleus, medial hypothalamus, reticular formation and ventral thalamus membrane preparations. Life Sci .59: 1899–1908 [DOI] [PubMed] [Google Scholar]
- Adams IB, Compton DR, Martin BR. (1998) Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther .284: 1209–1217 [PubMed] [Google Scholar]
- Adelson DW, Wei JY, Kruger L. (1997) Warm-sensitive afferent splanchnic C-fiber units in vitro. J Neurophysiol .77: 2989–3002 [DOI] [PubMed] [Google Scholar]
- Ahern GP. (2003) Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem .278: 30429–30434 [DOI] [PubMed] [Google Scholar]
- Ahern GP, Brooks IM, Miyares RL, Wang XB. (2005) Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci .25: 5109–5116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia J, Rang H, Nagy I. (2002) The putative role of vanilloid receptor-like protein-1 in mediating high threshold noxious heat-sensitivity in rat cultured primary sensory neurons. Eur J Neurosci .16: 1483–1489 [DOI] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA.(2006a)Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci .23: 3359–3367 [DOI] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS ONE. 2006b;1:e1. doi: 10.1371/journal.pone.0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Coimbra NC, Branco LG. (2004) Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus. J Physiol .558: 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U, Otto WR, Facer P, Zebda N, Selmer I, Gunthorpe MJ, Chessell IP, Sinisi M, Birch R, Anand P. (2008) TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett .438: 221–227 [DOI] [PubMed] [Google Scholar]
- Andrew D, Craig AD. (2001) Spinothalamic lamina I neurones selectively responsive to cutaneous warming in cats. J Physiol .537: 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H, Ozaki N, Shinoda M, Nagamine K, Tohnai I, Ueda M, Sugiura Y. (2005) Heat and mechanical hyperalgesia in mice model of cancer pain. Pain .117: 19–29 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature .448: 204–208 [DOI] [PubMed] [Google Scholar]
- Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. (2004) Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol .141: 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Szikszay M, Obál F. (1983) Impaired thermoregulation against cold in capsaicin pretreated rats. Pflugers Arch .399: 243–245 [DOI] [PubMed] [Google Scholar]
- Benham CD, Gunthorpe MJ, Davis JB. (2003) TRPV channels as temperature sensors. Cell Calcium .33: 479–487 [DOI] [PubMed] [Google Scholar]
- Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC. (1992) Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol .107: 544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW.4th (2003) Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci U S A .100: 12480–12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt K, Zhong F, Koerber HR, Davis BM. (2006) Phenotypic characterization of gastric sensory neurons in mice. Am J Physiol Gastrointest Liver Physiol .291:G987–997 [DOI] [PubMed] [Google Scholar]
- Bisogno T, Melck D, Bobrov MYu, Gretskaya NM, Bezuglov VV, De Petrocellis L, Di Marzo V.N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J (2000) 351: 817–824 [PMC free article] [PubMed] [Google Scholar]
- Blatteis CM. (1974) Influence of body weight and temperature on the pyrogenic effect of endotoxin in guinea pigs. Toxicol Appl Pharmacol .29: 249–258 [DOI] [PubMed] [Google Scholar]
- Boulant JA. (2006) Counterpoint: heat-induced membrane depolarization of hypothalamic neurons: an unlikely mechanism of central thermosensitivity. Am J Physiol Regul Integr Comp Physiol .290: R1481–R1484 discussion R1484 [DOI] [PubMed] [Google Scholar]
- Britt RH, Lyons BE, Pounds DW, Prionas SD. (1983) Feasibility of ultrasound hyperthermia in the treatment of malignant brain tumors. Med Instrum .17: 172–177 [PubMed] [Google Scholar]
- Broad LM, Keding SJ, Blanco MJ. (2008) Recent progress in the development of selective TRPV1 antagonists for pain. Curr Top Med Chem .8: 1431–1441 [DOI] [PubMed] [Google Scholar]
- Buck SH, Burks TF. (1986) The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev .38: 179–226 [PubMed] [Google Scholar]
- Cabanac M, Cormareche-Leydier M, Poirier LJ. (1976) The effect of capsaïcin on temperature regulation of the rat. Pflugers Arch .366: 217–221 [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol Rev .84: 277–359 [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. (2003) Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol .460: 303–326 [DOI] [PubMed] [Google Scholar]
- Carlisle HJ. (1969) Effect of preoptic and anterior hypothalamic lesions on behavioral thermoregulation in the cold. J Comp Physiol Psychol .69: 391–402 [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Du J, Hargett GL, Ji G, Coggeshall RE. (2004) Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain .110: 616–627 [DOI] [PubMed] [Google Scholar]
- Caterina MJ. (2007) Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol .292: R64–76 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science .288: 306–313 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature .398: 436–441 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature .389: 816–824 [DOI] [PubMed] [Google Scholar]
- Chendrasekhar A, Pillai S, Fagerli JC, Barringer LS, Dulaney J, Timberlake GA. (1996) Rectal pH measurement in tracking cardiac performance in a hemorrhagic shock model. J Trauma .40: 963–967 [DOI] [PubMed] [Google Scholar]
- Christianson JA, McIlwrath SL, Koerber HR, Davis BM. (2006) Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience .140: 247–257 [DOI] [PubMed] [Google Scholar]
- Christoph T, Bahrenberg G, De Vry J, Englberger W, Erdmann VA, Frech M, Kögel B, Röhl T, Schiene K, Schröder W, et al. (2008) Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Mol Cell Neurosci .37: 579–589 [DOI] [PubMed] [Google Scholar]
- Christoph T, Gillen C, Mika J, Grünweller A, Schäfer MK, Schiene K, Frank R, Jostock R, Bahrenberg G, Weihe E, et al. (2007) Antinociceptive effect of antisense oligonucleotides against the vanilloid receptor VR1/TRPV1. Neurochem Int .50: 281–290 [DOI] [PubMed] [Google Scholar]
- Christoph T, Grünweller A, Mika J, Schäfer MK, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, Kurreck J. (2006) Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun .350: 238–243 [DOI] [PubMed] [Google Scholar]
- Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem (2003) 278: 13633–13639 [DOI] [PubMed] [Google Scholar]
- Cibulsky SM, Sather WA. (1999) Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J Pharmacol Exp Ther .289: 1447–1453 [PubMed] [Google Scholar]
- Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. (2007) The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J .21: 3747–3755 [DOI] [PubMed] [Google Scholar]
- Clark WG, Lipton JM. (1984) Drug-related heatstroke. Pharmacol Ther .26: 345–388 [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. (2007) Attenuated cold sensitivity in TRPM8 null mice. Neuron .54: 379–386 [DOI] [PubMed] [Google Scholar]
- Cormarèche-Leydier M. (1984) The effects of long warm and cold ambient exposures on food intake water intake and body weight in the capsaicin desensitized rat. Pflugers Arch .400: 183–187 [DOI] [PubMed] [Google Scholar]
- Correll CC, Phelps PT, Anthes JC, Umland S, Greenfeder S. (2004) Cloning and pharmacological characterization of mouse TRPV1. Neurosci Lett .370: 55–60 [DOI] [PubMed] [Google Scholar]
- Craig AD, Krout K, Andrew D. (2001) Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol .86: 1459–1480 [DOI] [PubMed] [Google Scholar]
- Cranston WI, Hellon RF, Townsend Y. (1978) Thermal stimulation of intra-abdominal veins in conscious rabbits. J Physiol .277: 49–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL, Robinson JK, Felder CC, Devane WA, Axelrod J. (1993) Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol Biochem Behav .46: 967–972 [DOI] [PubMed] [Google Scholar]
- Crawshaw LI, Stitt JT. (1975) Behavioural and autonomic induction of prostaglandin E-1 fever in squirrel monkeys. J Physiol .244: 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. (2000) Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett .483: 52–56 [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. (2001) The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem .276: 12856–12863 [DOI] [PubMed] [Google Scholar]
- de Vries DJ, Blumberg PM. (1989) Thermoregulatory effects of resiniferatoxin in the mouse: comparison with capsaicin. Life Sci .44: 711–715 [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. (2007) TRPM8 is required for cold sensation in mice. Neuron .54: 371–378 [DOI] [PubMed] [Google Scholar]
- Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Patapoutian A. (2009) TRPV1 is activated by both acidic and basic pH. J Neurosci .29: 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. (2006) Trp ion channels and temperature sensation. Annu Rev Neurosci .29: 135–161 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. (2002) Anandamide receptors. Prostaglandins Leukot Essent Fatty Acids .66: 377–391 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Lastres-Becker I, Bisogno T, De Petrocellis L, Milone A, Davis JB, Fernandez-Ruiz JJ. (2001) Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur J Pharmacol .420: 123–131 [DOI] [PubMed] [Google Scholar]
- Dib B. (1982) Effects of intracerebroventricular capsaicin on thermoregulatory behavior in the rat. Pharmacol Biochem Behav .16: 23–27 [DOI] [PubMed] [Google Scholar]
- Dib B. (1983) Dissociation between peripheral and central heat loss mechanisms induced by neonatal capsaicin. Behav Neurosci .97: 822–825 [DOI] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. (2007) The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol .292: R47–63 [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Piper AS. (1997) Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol .121: 1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan MD, Patel S, Rudaya AY, Steiner AA, Székely M, Romanovsky AA. (2004) Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br J Pharmacol .143: 1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnerer J, Amann R, Schuligoi R, Lembeck F. (1990) Absorption and metabolism of capsaicinoids following intragastric administration in rats. Naunyn Schmiedebergs Arch Pharmacol .342: 357–361 [DOI] [PubMed] [Google Scholar]
- Donnerer J, Lembeck F. (1983) Heat loss reaction to capsaicin through a peripheral site of action. Br J Pharmacol .79: 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin YH, Andresen MC. (2002) Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci .22: 8222–8229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois EF. (1949) Why are fever temperatures over 106 degrees F. rare? Am J Med Sci .217: 361–368 [DOI] [PubMed] [Google Scholar]
- Egan GF, Johnson J, Farrell M, McAllen R, Zamarripa F, McKinley MJ, Lancaster J, Denton D, Fox PT. (2005) Cortical, thalamic, and hypothalamic responses to cooling and warming the skin in awake humans: a positron-emission tomography study. Proc Natl Acad Sci U S A .102:5262–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kouhen R, Surowy CS, Bianchi BR, Neelands TR, McDonald HA, Niforatos W, Gomtsyan A, Lee CH, Honore P, Sullivan JP, et al. (2005) A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel and selective transient receptor potential type V1 receptor antagonist, blocks channel activation by vanilloids, heat, and acid. J Pharmacol Exp Ther .314: 400–409 [DOI] [PubMed] [Google Scholar]
- El Ouazzani T, Mei N. (1982) Electrophysiologic properties and role of the vagal thermoreceptors of lower esophagus and stomach of cat. Gastroenterology .83: 995–1001 [PubMed] [Google Scholar]
- Everaerts W, Sepúlveda MR, Gevaert T, Roskams T, Nilius B, De Ridder D. (2009) Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn Schmiedebergs Arch Pharmacol .379: 421–425 [DOI] [PubMed] [Google Scholar]
- Feng JD, Price M, Cohen J, Satinoff E. (1989) Prostaglandin fevers in rats: regulated change in body temperature or change in regulated body temperature? Am J Physiol .257: R695–699 [DOI] [PubMed] [Google Scholar]
- Fiddian-Green RG. (1993) Associations between intramucosal acidosis in the gut and organ failure. Crit Care Med .21: S103–107 [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. (1983) Capsaicin and sensory neurones—a review. Pain .15: 109–130 [DOI] [PubMed] [Google Scholar]
- Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, Piomelli D. (2007) Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem .282: 1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR. (2008) Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci .29: 550–557 [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Hovland DN, Jr, Lehto SG, Klionsky L, Surapaneni S, Immke DC, Henley C, Arik L, Bak A, et al. (2007a)Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp Ther .323: 128–137 [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, et al. (2007b)The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci .27: 3366–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Klionsky L, Norman MH, Louis JC, Wild KD, Treanor JJ. (2005a)Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1. Mol Pharmacol .68: 1524–1533 [DOI] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, et al. (2005b)AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther .313: 474–484 [DOI] [PubMed] [Google Scholar]
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, et al. (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain .136: 202–210 [DOI] [PubMed] [Google Scholar]
- Geisthövel E, Ludwig O, Simon E. (1986) Capsaicin fails to produce disturbances of autonomic heat and cold defence in an avian species (Anas platyrhynchos). Pflugers Arch .406: 343–350 [DOI] [PubMed] [Google Scholar]
- Geran LC, Rashotte ME . (1997) Participation of gastrointestinal-load volume in “setting” the pigeon's nocturnal body temperature. Naturwissenschaften .84: 350–353 [Google Scholar]
- Ghilardi JR, Röhrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, et al. (2005) Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci .25: 3126–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill CH, Randall A, Bates SA, Hill K, Owen D, Larkman PM, Cairns W, Yusaf SP, Murdock PR, Strijbos PJ, et al. (2004) Characterization of the human HCN1 channel and its inhibition by capsazepine. Br J Pharmacol .143: 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel G, Ember A, Petervari E, Kis A, Szekely M. (2001) Postalimentary hyperthermia: a role for gastrointestinal but not caloric signals. J Therm Biol .26: 519–523 [Google Scholar]
- Gourine AV, Rudolph K, Korsak AS, Kubatko J, Tesfaigzi J, Kozak W, Kluger MJ. (2001) Role of capsaicin-sensitive afferents in fever and cytokine responses during systemic and local inflammation in rats. Neuroimmunomodulation .9: 13–22 [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Chizh BA. (2009) Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today .14: 56–67 [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. (1999) Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci .11: 946–958 [DOI] [PubMed] [Google Scholar]
- Gupta BN, Nier K, Hensel H. (1979) Cold-sensitive afferents from the abdomen. Pflugers Arch .380: 203–204 [DOI] [PubMed] [Google Scholar]
- Hachiya S, Kawabata F, Ohnuki K, Inoue N, Yoneda H, Yazawa S, Fushiki T. (2007) Effects of CH-19 Sweet, a non-pungent cultivar of red pepper, on sympathetic nervous activity, body temperature, heart rate, and blood pressure in humans. Biosci Biotechnol Biochem .71: 671–676 [DOI] [PubMed] [Google Scholar]
- Hajós M, Engberg G, Nissbrandt H, Magnusson T, Carlsson A. (1988) Capsaicin-sensitive vasodilatatory mechanisms in the rat substantia nigra and striatum. J Neural Transm .74: 129–139 [DOI] [PubMed] [Google Scholar]
- Hajós M, Hjorth S, Carlsson A. (1987) Injection of capsaicin into the nucleus raphe dorsalis elicits heat loss in the rat. Neurosci Lett .75: 199–204 [DOI] [PubMed] [Google Scholar]
- Hajós M, Obál F, Jr, Jancsó G, Obál F. (1983) The capsaicin sensitivity of the preoptic region is preserved in adult rats pretreated as neonates, but lost in rats pretreated as adults. Naunyn Schmiedebergs Arch Pharmacol .324: 219–222 [DOI] [PubMed] [Google Scholar]
- Hanly EJ, Aurora AA, Shih SP, Fuentes JM, Marohn MR, De Maio A, Talamini MA. (2007) Peritoneal acidosis mediates immunoprotection in laparoscopic surgery. Surgery .142: 357–364 [DOI] [PubMed] [Google Scholar]
- Hart GR, Anderson RJ, Crumpler CP, Shulkin A, Reed G, Knochel JP. (1982) Epidemic classical heat stroke: clinical characteristics and course of 28 patients. Medicine (Baltimore) .61: 189–197 [PubMed] [Google Scholar]
- Henry CJ, Emery B. (1986) Effect of spiced food on metabolic rate. Hum Nutr Clin Nutr .40: 165–168 [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. (2007) Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci .27: 2435–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. (1991) Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev .43: 143–201 [PubMed] [Google Scholar]
- Holzer P. (2004) TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol .500: 231–241 [DOI] [PubMed] [Google Scholar]
- Holzer P. (2007) Taste receptors in the gastrointestinal tract. V. Acid sensing in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol .292: G699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. (2008) The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol .155: 1145–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, Joshi SK, Ghilardi JR, Sevcik MA, Fryer RM, et al. (2009) Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia. Pain .142: 27–35 [DOI] [PubMed] [Google Scholar]
- Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, Gomtsyan A, El Kouhen R, Lee CH, Marsh K, et al. (2005) A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther .314: 410–421 [DOI] [PubMed] [Google Scholar]
- Hood VL, Tannen RL. (1998) Protection of acid-base balance by pH regulation of acid production. N Engl J Med .339: 819–826 [DOI] [PubMed] [Google Scholar]
- Hori T. (1984) Capsaicin and central control of thermoregulation. Pharmacol Ther .26: 389–416 [DOI] [PubMed] [Google Scholar]
- Hori T, Shinohara K. (1979) Hypothalamic thermo-responsive neurones in the new-born rat. J Physiol .294: 541–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Tsuzuki S. (1981) Thermoregulation in adult rats which have been treated with capsaicin as neonates. Pflugers Arch .390: 219–223 [DOI] [PubMed] [Google Scholar]
- Horn T, Wilkinson MF, Landgraf R, Pittman QJ. (1994) Reduced febrile responses to pyrogens after lesions of the hypothalamic paraventricular nucleus. Am J Physiol .267: R323–328 [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, et al. (2002) Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2 Proc Natl Acad Sci U S A .99:8400–840512060783 [Google Scholar]
- Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ. (2008) Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci .28: 13727–13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Oh JM, Valtschanoff JG. (2005) Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res .1047: 261–266 [DOI] [PubMed] [Google Scholar]
- Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. (2005) Attenuated fever response in mice lacking TRPV1. Neurosci Lett .378: 28–33 [DOI] [PubMed] [Google Scholar]
- Immke DC, Gavva NR. (2006) The TRPV1 receptor and nociception. Semin Cell Dev Biol .17: 582–591 [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. (2002) Fever responses of Zucker rats with and without fatty mutation of the leptin receptor. Am J Physiol Regul Integr Comp Physiol .282: R311–316 [DOI] [PubMed] [Google Scholar]
- Jakab B, Helyes Z, Varga A, Bölcskei K, Szabó A, Sándor K, Elekes K, Börzsei R, Keszthelyi D, Pintér E, et al. (2005) Pharmacological characterization of the TRPV1 receptor antagonist JYL1421 (SC0030) in vitro and in vivo in the rat. Eur J Pharmacol .517: 35–44 [DOI] [PubMed] [Google Scholar]
- Jancsó-Gábor A, Szolcsányi J, Jancsó N. (1970a)Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J Physiol .206: 495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó-Gábor A, Szolcsányi J, Jancsó N. (1970b)Stimulation and desensitization of the hypothalamic heat-sensitive structures by capsaicin in rats. J Physiol .208: 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó G, Király E. (1981) Sensory neurotoxins: chemically induced selective destruction of primary sensory neurons. Brain Res .210: 83–89 [DOI] [PubMed] [Google Scholar]
- Jancsó G, Kiraly E, Jancsó-Gábor A. (1977) Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature .270: 741–743 [DOI] [PubMed] [Google Scholar]
- Jancsó N, Jancsó A. (1949) Desensitization of sensory nerve endings. Kiserl Orvostud .152 Suppl. [Google Scholar]
- Jennings EA, Vaughan CW, Roberts LA, Christie MJ. (2003) The actions of anandamide on rat superficial medullary dorsal horn neurons in vitro. J Physiol .548: 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen C. (1981) Independent clamps of peripheral and central temperatures and their effects on heat production in the goat. J Physiol .311: 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen C. (1985) Thermal afferents in the control of body temperature. Pharmacol Ther .28: 107–134 [DOI] [PubMed] [Google Scholar]
- Johansen ME, Reilly CA, Yost GS. (2006) TRPV1 antagonists elevate cell surface populations of receptor protein and exacerbate TRPV1-mediated toxicities in human lung epithelial cells. Toxicol Sci .89: 278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joó F, Szolcsányi J, Jancsó-Gábor A. (1969) Mitochondrial alterations in the spinal ganglion cells of the rat accompanying the long-lasting sensory disturbance induced by capsaicin. Life Sci .8: 621–626 [DOI] [PubMed] [Google Scholar]
- Jordt SE, Julius D. (2002) Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell .108: 421–430 [DOI] [PubMed] [Google Scholar]
- Kanizsai P, Garami A, Solymár M, Szolcsányi J, Szelényi Z . (2009) Energetics of fasting heterothermia in TRPV1-KO and wild type mice. Physiol Behav .96: 149–154 [DOI] [PubMed] [Google Scholar]
- Kanosue K, Sadato N, Okada T, Yoda T, Nakai S, Yoshida K, Hosono T, Nagashima K, Yagishita T, Inoue O, et al. (2002) Brain activation during whole body cooling in humans studied with functional magnetic resonance imaging. Neurosci Lett .329: 157–160 [DOI] [PubMed] [Google Scholar]
- Kark T, Bagi Z, Lizanecz E, Pásztor ET, Erdei N, Czikora A, Papp Z, Edes I, Pórszász R, Tóth A. (2008) Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol .73: 1405–1412 [DOI] [PubMed] [Google Scholar]
- Karlsson U, Sundgren-Andersson AK, Johansson S, Krupp JJ. (2005) Capsaicin augments synaptic transmission in the rat medial preoptic nucleus. Brain Res .1043: 1–11 [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. (1995) Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res .77: 1222–1228 [DOI] [PubMed] [Google Scholar]
- Khairatkar-Joshi N, Szallasi A. (2009) TRPV1 antagonists: the challenges for therapeutic targeting. Trends Mol Med .15: 14–22 [DOI] [PubMed] [Google Scholar]
- Kim CS, Kawada T, Kim BS, Han IS, Choe SY, Kurata T, Yu R. (2003) Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal .15: 299–306 [DOI] [PubMed] [Google Scholar]
- Kim H, Mittal DP, Iadarola MJ, Dionne RA. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J Med Genet. 2006;43:e40. doi: 10.1136/jmg.2005.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA. (2004) Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain .109: 488–496 [DOI] [PubMed] [Google Scholar]
- Knyihár-Csillik E, Rakic P, Csillik B. (1989) Transneuronal degeneration in the Rolando substance of the primate spinal cord evoked by axotomy-induced transganglionic degenerative atrophy of central primary sensory terminals. Cell Tissue Res .258: 515–525 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Osaka T, Namba Y, Inoue S, Lee TH, Kimura S. (1998) Capsaicin activates heat loss and heat production simultaneously and independently in rats. Am J Physiol .275: R92–98 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. (2005) Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with aδ/c-fibers and colocalization with trk receptors. J Comp Neurol .493: 596–606 [DOI] [PubMed] [Google Scholar]
- Kobayashi S. (1989) Temperature-sensitive neurons in the hypothalamus: a new hypothesis that they act as thermostats, not as transducers. Prog Neurobiol .32: 103–135 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Hori A, Matsumura K, Hosokawa H. (2006) Point: Heat-induced membrane depolarization of hypothalamic neurons: a putative mechanism of central thermosensitivity. Am J Physiol Regul Integr Comp Physiol .290R1479–R1480 discussion R1484 [DOI] [PubMed] [Google Scholar]
- Konishi M, Kanosue K, Kano M, Kobayashi A, Nagashima K. (2007) The median preoptic nucleus is involved in the facilitation of heat-escape/cold-seeking behavior during systemic salt loading in rats. Am J Physiol Regul Integr Comp Physiol .292: R150–159 [DOI] [PubMed] [Google Scholar]
- Laird AS, Carrive P, Waite PM. (2006) Cardiovascular and temperature changes in spinal cord injured rats at rest and during autonomic dysreflexia. J Physiol .577: 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. (2005) Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci .25: 1304–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS. (1954) Physiological gustatory sweating in a warm climate. J Physiol .124: 528–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, Lee D, Louis JC, Magal E, Manning BH, et al. (2008) Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther .326: 218–229 [DOI] [PubMed] [Google Scholar]
- Lipton JM. (1968) Effects of preoptic lesions on heat-escape responding and colonic temperature in the rat. Physiol Behav .3: 165–169 [Google Scholar]
- Lipton JM, Dwyer PE, Fossler DE. (1974) Effects of brainstem lesions on temperature regulation in hot and cold environments. Am J Physiol .226: 1356–1365 [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. (1997) Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neurosci Lett .228: 29–32 [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D. (2005) Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci .62: 708–716 [DOI] [PubMed] [Google Scholar]
- Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. (2001) Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci .21: 4864–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Györke I, Subramanian S, Smirnov A, Wiesner TF, Györke S. (2000) Inhibition of Ca2+ sparks by ruthenium red in permeabilized rat ventricular myocytes. Biophys J .79: 1273–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. (2004) Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol .286: R320–325 [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. (2009) Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol .296: R831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud ME, Shimizu Y, Shiina T, Nikami H, Dosoky RM, Ahmed MM, Takewaki T. (2007) Involvement of a capsaicin-sensitive TRPV1-independent mechanism in lipopolysaccharide-induced fever in chickens. Comp Biochem Physiol A Mol Integr Physiol .148: 578–583 [DOI] [PubMed] [Google Scholar]
- Malkinson TJ, Cooper KE, Veale WL. (1988) Physiological changes during thermoregulation and fever in urethan-anesthetized rats. Am J Physiol .255: R73–81 [DOI] [PubMed] [Google Scholar]
- Marques PR, Spencer RL, Burks TF, McDougal JN. (1984) Behavioral thermoregulation, core temperature, and motor activity: simultaneous quantitative assessment in rats after dopamine and prostaglandin E1. Behav Neurosci .98: 858–867 [DOI] [PubMed] [Google Scholar]
- Mason JR, Maruniak JA. (1983) Behavioral and physiological effects of capsaicin in red-winged blackbirds. Pharmacol Biochem Behav .19: 857–862 [DOI] [PubMed] [Google Scholar]
- Matsumi N, Matsumoto K, Mishima N, Moriyama E, Furuta T, Nishimoto A, Taguchi K. (1994) Thermal damage threshold of brain tissue—histological study of heated normal monkey brains. Neurol Med Chir (Tokyo) .34: 209–215 [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Katakura M, Hara T, Li G, Hashimoto M, Shido O. (2009) Proliferation of neuronal progenitor cells and neuronal differentiation in the hypothalamus are enhanced in heat-acclimated rats. Pflugers Arch .458: 661–673 [DOI] [PubMed] [Google Scholar]
- McAllen RM, Farrell M, Johnson JM, Trevaks D, Cole L, McKinley MJ, Jackson G, Denton DA, Egan GF. (2006) Human medullary responses to cooling and rewarming the skin: a functional MRI study Proc Natl Acad Sci U S A .103:809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister RG, Jr, Tan TG. (1980) Effect of hypothermia on drug metabolism. In vitro studies with propranolol and verapamil. Pharmacology .20: 95–100 [DOI] [PubMed] [Google Scholar]
- McConnell EL, Basit AW, Murdan S. (2008) Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol .60: 63–70 [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Segreti JA, Fryer RM, Brown BS, Faltynek CR, Kym PR. (2009) Antagonism of TRPV1 receptors indirectly modulates activity of thermoregulatory neurons in the medial preoptic area of rats. Brain Res .1268: 58–67 [DOI] [PubMed] [Google Scholar]
- McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, et al. (2001) Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Br J Pharmacol .132: 1084–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. (2003) The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol .172III–XII 1–122, back cover [DOI] [PubMed] [Google Scholar]
- Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. (2000) Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A .97:3655–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Brendel K, Burks TF, Sipes IG. (1983) Interaction of capsaicinoids with drug-metabolizing systems. Relationship to toxicity. Biochem Pharmacol .32: 547–551 [DOI] [PubMed] [Google Scholar]
- Mills C, McMackin M, Jaffe R, Yu J, Zininberg E, Slee D, Gogas K, Bradbury M. (2008) Effects of the transient receptor potential vanilloid 1 antagonist A-425619 on body temperature and thermoregulation in the rat. Neuroscience .156: 165–174 [DOI] [PubMed] [Google Scholar]
- Montgomery A, Almqvist P, Arvidsson D, Lindgren S, Haglund U. (1990) Early detection of gastrointestinal mucosal ischemia in porcine E. coli sepsis. Acta Chir Scand .156: 613–620 [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. (2005) Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science .307: 1468–1472 [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. (2008) Central control of thermogenesis in mammals. Exp Physiol .93: 773–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter AL, Ahern GP. (2008) TRPV1-null mice are protected from diet-induced obesity. FEBS Lett .582: 2257–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahed P, Jönsson BA, Birnir B, Wingstrand JA, Jørgensen TD, Ermund A, Sterner O, Zygmunt PM, Högestätt ED. (2005) Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem .280: 38496–38504 [DOI] [PubMed] [Google Scholar]
- Nagashima K, Nakai S, Tanaka M, Kanosue K. (2000) Neuronal circuitries involved in thermoregulation. Auton Neurosci .85: 18–25 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hübschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogköi Z, König M, Thiel HJ, Gerstberger R, et al. (2004) Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci .24: 5370–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. (2002) The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci .22: 4600–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. (2007a)Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol .292: R127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. (2007b) Identification of lateral parabrachial neurons mediating cutaneous thermoregulatory afferent signals to the preoptic area (Abstract). Soc Neurosci Abstr .33731–13 [Google Scholar]
- Nakamura K, Morrison SF. (2008a)Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol .586: 2611–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. (2008b)A thermosensory pathway that controls body temperature. Nat Neurosci .11: 62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. (2005) Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci .22: 3137–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Morrison SF. (2009) Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience .161: 614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Suzuki M, Ishikawa Y, Nishio A. (1978) Effects of capsaicin on hypothalamic thermo-sensitive neurons in the rat. Neurosci Lett .7: 151–155 [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab .293: E444–E452 [DOI] [PubMed] [Google Scholar]
- Nelson E. (1919) The constitution of capsaicin, the pungent principal of capsicum. J Am Chem Soc .41: 1115–1121 [Google Scholar]
- Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. (2006) Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol .291: R541–R550 [DOI] [PubMed] [Google Scholar]
- Nielsen VG, Tan S, Baird MS, McCammon AT, Parks DA. (1996) Gastric intramucosal pH and multiple organ injury: impact of ischemia-reperfusion and xanthine oxidase. Crit Care Med .24: 1339–1344 [DOI] [PubMed] [Google Scholar]
- Nikami H, Mahmoud ME, Shimizu Y, Shiina T, Hirayama H, Iwami M, Dosoky RM, Ahmed MM, Takewaki T. (2008) Capsaicin pretreatment attenuates LPS-induced hypothermia through TRPV1-independent mechanisms in chicken. Life Sci .82: 1191–1195 [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Vennekens R, Hoenderop JG, Bindels RJ, Droogmans G. (2001) Pharmacological modulation of monovalent cation currents through the epithelial Ca2+ channel ECaC1. Br J Pharmacol .134: 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto S, Shibata M, Iriki M, Riedel W. (2004) Role of afferent pathways of heat and cold in body temperature regulation. Int J Biometeorol .49: 67–85 [DOI] [PubMed] [Google Scholar]
- Obál F, Jr, Bari F, Benedek G, Obál F. (1982) Elevation of thermoregulatory vasodilatation threshold in the rat after capsaicin treatment. Acta Physiol Acad Sci Hung .59: 203–207 [PubMed] [Google Scholar]
- Obál F, Jr, Benedek G, Jancsó-Gábor A, Obál F. (1979) Salivary cooling, escape reaction and heat pain in capsaicin-desensitized rats. Pflugers Arch .382: 249–254 [DOI] [PubMed] [Google Scholar]
- Obál F, Jr, Benedek G, Jancsó-Gábor A, Obál F. (1980) Tail skin vasodilatation and bath test in capsaicin-desensitized rats. Pflugers Arch .387: 183–188 [DOI] [PubMed] [Google Scholar]
- Obál F, Jr, Jancsó G, Hajós M, Obál F. (1987) Differences in the mechanisms of the thermoregulatory impairment induced by capsaicin in newborn and adult rats. Acta Physiol Hung .69: 437–445 [PubMed] [Google Scholar]
- Obal F, Jr, Jancso G, Jancso-Gabor A, Obal F. (1983) Vasodilatation on preoptic heating in capsaicin-treated rats. Experientia .39: 221–223 [DOI] [PubMed] [Google Scholar]
- Ohnuki K, Niwa S, Maeda S, Inoue N, Yazawa S, Fushiki T. (2001) CH-19 sweet, a non-pungent cultivar of red pepper, increased body temperature and oxygen consumption in humans. Biosci Biotechnol Biochem .65: 2033–2036 [DOI] [PubMed] [Google Scholar]
- Okane N, Osaka T, Kobayashi A, Inoue S, Kimura S. (2001) Effects of the capsaicin analogue resiniferatoxin on thermoregulation in anesthetized rats. J Therm Biol .26: 345–349 [Google Scholar]
- Okazawa M, Takao K, Hori A, Shiraki T, Matsumura K, Kobayashi S. (2002) Ionic basis of cold receptors acting as thermostats. J Neurosci .22: 3994–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, McAllen RM. (2005) Interactive drives from two brain stem premotor nuclei are essential to support rat tail sympathetic activity. Am J Physiol Regul Integr Comp Physiol .289: R1107–1115 [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, McAllen RM. (2006) Comparison between two rat sympathetic pathways activated in cold defense. Am J Physiol Regul Integr Comp Physiol .291: R589–595 [DOI] [PubMed] [Google Scholar]
- Orliac ML, Peroni RN, Abramoff T, Neuman I, Podesta EJ, Adler-Graschinsky E. (2007) Increases in vanilloid TRPV1 receptor protein and CGRP content during endotoxemia in rats. Eur J Pharmacol .566: 145–152 [DOI] [PubMed] [Google Scholar]
- Osaka T. (2004) Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am J Physiol Regul Integr Comp Physiol .287: R306–313 [DOI] [PubMed] [Google Scholar]
- Osaka T, Kobayashi A, Inoue S. (2002) Vago-sympathoadrenal reflex in thermogenesis induced by osmotic stimulation of the intestines in the rat. J Physiol .540: 665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka T, Kobayashi A, Namba Y, Ezaki O, Inoue S, Kimura S, Lee TH. (1998) Temperature- and capsaicin-sensitive nerve fibers in brown adipose tissue attenuate thermogenesis in the rat. Pflugers Arch .437: 36–42 [DOI] [PubMed] [Google Scholar]
- Osaka T, Lee TH, Kobayashi A, Inoue S, Kimura S. (2000) Thermogenesis mediated by a capsaicin-sensitive area in the ventrolateral medulla. Neuroreport .11: 2425–2428 [DOI] [PubMed] [Google Scholar]
- Partridge LD. (1982) The good enough calculi of evolving control systems: evolution is not engineering. Am J Physiol .242: R173–177 [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. (2003) ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci .4: 529–539 [DOI] [PubMed] [Google Scholar]
- Perl E. (1992) Function of dorsal root ganglion neurons: an overview, in Sensory Neurons: Diversity, Development, and Plasticity (Scott SA. ed) pp 3–23, Oxford University Press, New York [Google Scholar]
- Pétervári E, Garami A, Pákai E, Székely M. (2005) Effects of perineural capsaicin treatment of the abdominal vagus on endotoxin fever and on a non-febrile thermoregulatory event. J Endotoxin Res .11: 260–266 [DOI] [PubMed] [Google Scholar]
- Pierau FK, Szolcsányi J, Sann H. (1986) The effect of capsaicin on afferent nerves and temperature regulation of mammals and birds. J Therm Biol .11: 95–100 [Google Scholar]
- Pingle SC, Matta JA, Ahern GP. (2007) Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol .179: 155–171 [DOI] [PubMed] [Google Scholar]
- Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K.N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. In vivo characterization in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther (2003) 306: 387–393 [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. (2000) Induction of vanilloid receptor channel activity by protein kinase C. Nature .408: 985–990 [DOI] [PubMed] [Google Scholar]
- Proulx K, Cota D, Castañeda TR, Tschöp MH, D’Alessio DA, Tso P, Woods SC, Seeley RJ. (2005) Mechanisms of oleoylethanolamide-induced changes in feeding behavior and motor activity. Am J Physiol Regul Integr Comp Physiol .289: R729–737 [DOI] [PubMed] [Google Scholar]
- Puntambekar P, Van Buren J, Raisinghani M, Premkumar LS, Ramkumar V. (2004) Direct interaction of adenosine with the TRPV1 channel protein. J Neurosci .24: 3663–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe LS, Buck SH, Moreno L, Burks TF, Dafny N. (1980) Neurophysiological and thermoregulatory effects of capsaicin. Brain Res Bull .5: 755–758 [DOI] [PubMed] [Google Scholar]
- Rathner JA, Madden CJ, Morrison SF. (2008) Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am J Physiol Regul Integr Comp Physiol .295: R343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E, Cusick CG, Taub E, Jones EG. (1992) Chronic deafferentation in monkeys differentially affects nociceptive and nonnociceptive pathways distinguished by specific calcium-binding proteins and down-regulates gammaaminobutyric acid type A receptors at thalamic levels. Proc Natl Acad Sci U S A .89: 2571–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly CA, Ehlhardt WJ, Jackson DA, Kulanthaivel P, Mutlib AE, Espina RJ, Moody DE, Crouch DJ, Yost GS. (2003) Metabolism of capsaicin by cytochrome P450 produces novel dehydrogenated metabolites and decreases cytotoxicity to lung and liver cells. Chem Res Toxicol .16: 336–349 [DOI] [PubMed] [Google Scholar]
- Riedel W. (1976) Warm receptors in the dorsal abdominal wall of the rabbit. Pflugers Arch .361: 205–206 [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT. (1988) Capsaicin-induced neuronal degeneration: silver impregnation of cell bodies, axons, and terminals in the central nervous system of the adult rat. J Comp Neurol .271: 79–90 [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT. (1990) Capsaicin-induced neuronal degeneration in the brain and retina of preweanling rats. J Comp Neurol .296: 447–461 [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT. (1992) Age-related changes in capsaicin-induced degeneration in rat brain. J Comp Neurol .318: 103–116 [DOI] [PubMed] [Google Scholar]
- Roberts LA, Christie MJ, Connor M. (2002) Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br J Pharmacol .137: 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WW. (1988) Differential thermosensor control of thermoregulatory grooming, locomotion, and relaxed postural extension. Ann N Y Acad Sci .525: 363–374 [DOI] [PubMed] [Google Scholar]
- Roberts WW, Martin JR. (1977) Effects of lesions in central thermosensitive areas on thermoregulatory responses in rat. Physiol Behav .19: 503–511 [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Navarro M, Gómez R, Escuredo L, Nava F, Fu J, Murillo-Rodríguez E, Giuffrida A, LoVerme J, Gaetani S, et al. (2001) An anorexic lipid mediator regulated by feeding. Nature .414: 209–212 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. (2004) Do fever and anapyrexia exist? Analysis of set point-based definitions. Am J Physiol Regul Integr Comp Physiol .287: R992–995 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. ( 2007a) Temperature regulation, in Lecture Notes on Human Physiology, 5th ed( Petersen O. ed) pp 603–615, Blackwell, Oxford, UK [Google Scholar]
- Romanovsky AA. (2007b) Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol .292: R37–46 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. (2005) Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci .10: 2193–2216 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Blatteis CM. (1996) Heat stroke: opioid-mediated mechanisms. J Appl Physiol .81: 2565–2570 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. (2002) Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol .92: 2667–2679 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T. (1996) Endotoxin shock: thermoregulatory mechanisms. Am J Physiol .270: R693–703 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Sugimoto N, Simons CT, Hunter WS. (2003) The organum vasculosum laminae terminalis in immune-to-brain febrigenic signaling: a reappraisal of lesion experiments. Am J Physiol Regul Integr Comp Physiol .285: R420–428 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Székely M. (1998) Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses .50: 219–226 [DOI] [PubMed] [Google Scholar]
- Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. (2004) Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol .560: 867–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. (2004) Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol .123: 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E, Kenakin TP. ( 2001) Pharmacodynamics: mechanisms of drug action and the relationship between drug concentration and effect, in Goodman & Gilman's The Pharmacological Basis of Therapeutics ( Hardman JG, Limbird LE, Gilman AG. eds) pp 31–44, McGraw-Hill, New York [Google Scholar]
- Ross RA. (2003) Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol .140: 790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, Di Marzo V, Pertwee RG. (2001) Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol .132: 631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. (2005) Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol .289: R1244–1252 [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Yamazaki M. (1988) Hepatic portal injection of glucose elevates efferent sympathetic discharges of interscapular brown adipose tissue. Exp Neurol .101: 464–469 [DOI] [PubMed] [Google Scholar]
- Sakurada S, Shido O, Fujikake K, Nagasaka T. (1993) Relationship between body core and peripheral temperatures at the onset of thermoregulatory responses in rats. Jpn J Physiol .43: 659–667 [DOI] [PubMed] [Google Scholar]
- Sanchez JF, Krause JE, Cortright DN. (2001) The distribution and regulation of vanilloid receptor VR1 and VR1 5′ splice variant RNA expression in rat. Neuroscience .107: 373–381 [DOI] [PubMed] [Google Scholar]
- Saria A, Skofitsch G, Lembeck F. (1982) Distribution of capsaicin in rat tissues after systemic administration. J Pharm Pharmacol .34: 273–275 [DOI] [PubMed] [Google Scholar]
- Sasamura T, Sasaki M, Tohda C, Kuraishi Y. (1998) Existence of capsaicin-sensitive glutamatergic terminals in rat hypothalamus. Neuroreport .9: 2045–2048 [DOI] [PubMed] [Google Scholar]
- Satinoff E. (1978) Neural organization and evolution of thermal regulation in mammals. Science .201: 16–22 [DOI] [PubMed] [Google Scholar]
- Satinoff E, Rutstein J. (1970) Behavioral thermoregulation in rats with anterior hypothalamic lesions. J Comp Physiol Psychol .71: 77–82 [DOI] [PubMed] [Google Scholar]
- Satinoff E, Valentino D, Teitelbaum P. (1976) Thermoregulatory cold-defense deficits in rats with preoptic/anterior hypothalamic lesions. Brain Res Bull .1: 553–565 [DOI] [PubMed] [Google Scholar]
- Savidge J, Davis C, Shah K, Colley S, Phillips E, Ranasinghe S, Winter J, Kotsonis P, Rang H, McIntyre P. (2002) Cloning and functional characterization of the guinea pig vanilloid receptor 1. Neuropharmacology .43: 450–456 [DOI] [PubMed] [Google Scholar]
- Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. (2004) Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci .19: 1811–1818 [DOI] [PubMed] [Google Scholar]
- Schulze G, Tetzner M, Topolinski H. (1981) Operant thermoregulation of rats with anterior hypothalamic lesions. Naunyn Schmiedebergs Arch Pharmacol .318: 43–48 [DOI] [PubMed] [Google Scholar]
- Sessler DI. (1997) Perioperative thermoregulation and heat balance. Ann N Y Acad Sci .813: 757–777 [DOI] [PubMed] [Google Scholar]
- Shimizu I, Iida T, Horiuchi N, Caterina MJ. (2005) 5-Iodoresiniferatoxin evokes hypothermia in mice and is a partial transient receptor potential vanilloid 1 agonist in vitro. J Pharmacol Exp Ther .314: 1378–1385 [DOI] [PubMed] [Google Scholar]
- Simon HB. (1993) Hyperthermia. N Engl J Med .329: 483–487 [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, et al. (2002) . TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature .418: 186–190 [DOI] [PubMed] [Google Scholar]
- Smith JE, Jansen AS, Gilbey MP, Loewy AD. (1998) CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res .786: 153–164 [DOI] [PubMed] [Google Scholar]
- Starowicz K, Cristino L, Di Marzo V. (2008) TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications. Curr Pharm Des .14: 42–54 [DOI] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. (2007) Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci .27: 7459–7468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DP, Charkoudian N, Benevento JM, Johnson JM, Saumet JL. (2001) The influence of topical capsaicin on the local thermal control of skin blood flow in humans. Am J Physiol Regul Integr Comp Physiol .281: R894–901 [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell .112: 819–829 [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Gobel S. (1984) Dendritic changes in the spinal dorsal horn following transection of a peripheral nerve. Brain Res .321: 199–208 [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Xiao C, Takeyama A, He YF, Takano-Yamamoto T, Ichikawa H. (1999) Apoptotic cascade of neurons in the subcortical sensory relay nuclei following the neonatal infraorbital nerve transection. Brain Res .824: 284–290 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. (2005) Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci .25: 2617–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YG, Lee YS, Min KH, Park OH, Seung HS, Kim HD, Park HG, Choi J, Lee J, Kang SW, et al. (2003) Novel non-vanilloid VR1 antagonist of high analgesic effects and its structural requirement for VR1 antagonistic effects. Bioorg Med Chem Lett .13: 4389–4393 [DOI] [PubMed] [Google Scholar]
- Surowy CS, Neelands TR, Bianchi BR, McGaraughty S, El Kouhen R, Han P, Chu KL, McDonald HA, Vos M, Niforatos W, et al. (2008) (R)-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)-urea (ABT-102) blocks polymodal activation of transient receptor potential vanilloid 1 receptors in vitro and heat-evoked firing of spinal dorsal horn neurons in vivo. J Pharmacol Exp Ther .326: 879–888 [DOI] [PubMed] [Google Scholar]
- Swanson DM, Dubin AE, Shah C, Nasser N, Chang L, Dax SL, Jetter M, Breitenbucher JG, Liu C, Mazur C, et al. (2005) Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl)piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist. J Med Chem .48: 1857–1872 [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. (1989) Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience .30: 515–520 [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. (1990) Resiniferatoxin and its analogs provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor. Life Sci .47: 1399–1408 [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. (1999) Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev .51: 159–212 [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. (2007) The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov .6: 357–372 [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cruz F, Geppetti P. (2006) TRPV1: a therapeutic target for novel analgesic drugs? Trends Mol Med .12: 545–554 [DOI] [PubMed] [Google Scholar]
- Szallasi A, Nilsson S, Blumberg PM, Hökfelt T, Lundberg JM. (1996) Binding of neuroleptic drugs (trifluoperazine and rimcazole) to vanilloid receptors in porcine dorsal horn. Eur J Pharmacol .298: 321–327 [DOI] [PubMed] [Google Scholar]
- Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg PM, Hökfelt T, Lundberg JM. (1995) Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res .703: 175–183 [DOI] [PubMed] [Google Scholar]
- Szekely M. (1986) Capsaicin-induced changes in behavioural thermoregulation of newborn rabbits (Lepus cuniculus). J Therm Biol .11: 101–104 [Google Scholar]
- Székely M. (2000) The vagus nerve in thermoregulation and energy metabolism. Auton Neurosci .85: 26–38 [DOI] [PubMed] [Google Scholar]
- Székely M, Romanovsky AA. Thermoregulatory reactions of capsaicin pretreated rats (Abstract). FASEB J. 1997;11:A528. [Google Scholar]
- Székely M, Szelényi Z. (1979) Endotoxin fever in the rat. Acta Physiol Acad Sci Hung .53: 265–277 [PubMed] [Google Scholar]
- Székely M, Szolcsányi J. (1979) Endotoxin fever in capsaicin treated rats. Acta Physiol Acad Sci Hung .53: 469–477 [PubMed] [Google Scholar]
- Szelényi Z, Barthó L, Székely M, Romanovsky AA. (1994) Cholecystokinin octapeptide (CCK-8) injected into a cerebral ventricle induces a fever-like thermoregulatory response mediated by type B CCK-receptors in the rat. Brain Res .638: 69–77 [DOI] [PubMed] [Google Scholar]
- Szelényi Z, Hummel Z, Szolcsányi J, Davis JB. (2004) Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur J Neurosci .19: 1421–1424 [DOI] [PubMed] [Google Scholar]
- Szelényi Z, Székely M, Romanovskĭ AA. (1992) The central thermoregulatory action of cholecystokinin-8 and prostaglandin E1. Fiziol Zh SSSR Im I M Sechenova .78: 94–101 [PubMed] [Google Scholar]
- Szikszay M, Obál F, Jr, Obál F. (1982) Dose-response relationships in the thermoregulatory effects of capsaicin. Naunyn Schmiedebergs Arch Pharmacol .320: 97–100 [DOI] [PubMed] [Google Scholar]
- Szöke E, Seress L, Szolcsányi J. (2002) Neonatal capsaicin treatment results in prolonged mitochondrial damage and delayed cell death of B cells in the rat trigeminal ganglia. Neuroscience .113: 925–937 [DOI] [PubMed] [Google Scholar]
- Szolcsányi J. (1983) Disturbances of thermoregulation induced by capsaicin. J Therm Biol .8: 207–212 [Google Scholar]
- Szolcsányi J. ( 1990) Capsaicin, irritation, and desensitization: neurophysiological basis and future perspectives, in Chemical Senses ( Green BG, Mason JR, Kare MR. eds) pp 141–169, Marcel Dekker, New York [Google Scholar]
- Szolcsányi J. (2004) Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides .38: 377–384 [DOI] [PubMed] [Google Scholar]
- Szolcsányi J, Jancsó-Gábor A. ( 1973) Capsaicin and other pungent agents as pharmacological tools in studies on thermoregulation, in The Pharmacology of Thermoregulation ( Schonbaum E, Lomax P. eds) pp 395–409, Karger, Basel [Google Scholar]
- Szolcsányi J, Jancsó-Gábor A. ( 1975) Analysis of the role of warmth detectors by means of capsaicin under different conditions, in Temperature Regulation and Drug Action Lomax P, Schonbaum E, Jacob J. eds) pp 331–338, Karger, Basel [Google Scholar]
- Szolcsányi J, Joó F, Jancsó-Gábor A. (1971) Mitochondrial changes in preoptic neurons after capsaicin desensitization of the hypothalamic thermodetectors in rats. Nature .229: 116–117 [DOI] [PubMed] [Google Scholar]
- Szolcsányi J, Sándor Z, Petho G, Varga A, Bölcskei K, Almási R, Riedl Z, Hajos G, Czéh G. (2004) Direct evidence for activation and desensitization of the capsaicin receptor by N-oleoyldopamine on TRPV1-transfected cell, line in gene deleted mice and in the rat. Neurosci Lett .361: 155–158 [DOI] [PubMed] [Google Scholar]
- Szolcsányi J, Sann H, Pierau FK. (1986) Nociception in pigeons is not impaired by capsaicin. Pain .27: 247–260 [DOI] [PubMed] [Google Scholar]
- Tamayo N, Liao H, Stec MM, Wang X, Chakrabarti P, Retz D, Doherty EM, Surapaneni S, Tamir R, Bannon AW, et al. (2008) Design and synthesis of peripherally restricted transient receptor potential vanilloid 1 (TRPV1) antagonists. J Med Chem .51: 2744–2757 [DOI] [PubMed] [Google Scholar]
- Tanaka M, McKinley MJ, McAllen RM. (2009) Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am J Physiol Regul Integr Comp Physiol .296: R1248–1257 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Nagashima K, McAllen RM, Kanosue K. (2002) Role of the medullary raphé in thermoregulatory vasomotor control in rats. J Physiol .540: 657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Ootsuka Y, McKinley MJ, McAllen RM. (2007) Independent vasomotor control of rat tail and proximal hairy skin. J Physiol .582: 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CR, Lyman CP. (1972) Heat storage in running antelopes: independence of brain and body temperatures. Am J Physiol .222: 114–117 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ. (2004) Thermosensation and pain. J Neurobiol .61: 3–12 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron .21: 531–543 [DOI] [PubMed] [Google Scholar]
- Unger NT, Kaple ML, Boulant JA. TRPV blockade by ruthenium red does not suppress thermosensitivity in hypothalamic neurons (Abstract). FASEB J. 2008;22:95615. [Google Scholar]
- Van Der Stelt M, Di Marzo V. (2004) Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem .271: 1827–1834 [DOI] [PubMed] [Google Scholar]
- Van Zoeren JG, Stricker EM. (1976) Thermal homeostasis in rats after intrahypothalamic injections of 6-hyroxydopamine. Am J Physiol .230: 932–939 [DOI] [PubMed] [Google Scholar]
- Vennekens R, Owsianik G, Nilius B. (2008) Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des .14: 18–31 [DOI] [PubMed] [Google Scholar]
- Wang Y, Novotny M, Quaiserová-Mocko V, Swain GM, Wang DH. (2008) TRPV1-mediated protection against endotoxin-induced hypotension and mortality in rats. Am J Physiol Regul Integr Comp Physiol .294: R1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, et al. (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem .277: 13569–13577 [DOI] [PubMed] [Google Scholar]
- Watanabe K, Matsunaga T, Nakamura S, Kimura T, Ho IK, Yoshimura H, Yamamoto I. (1999) Pharmacological effects in mice of anandamide and its related fatty acid ethanolamides, and enhancement of cataleptogenic effect of anandamide by phenylmethylsulfonyl fluoride. Biol Pharm Bull .22: 366–370 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kawada T, Kurosawa M, Sato A, Iwai K. (1988) Adrenal sympathetic efferent nerve and catecholamine secretion excitation caused by capsaicin in rats. Am J Physiol .255: E23–27 [DOI] [PubMed] [Google Scholar]
- Weil A, Moore SE, Waite NJ, Randall A, Gunthorpe MJ. (2005) Conservation of functional and pharmacological properties in the distantly related temperature sensors TRVP1 and TRPM8. Mol Pharmacol .68: 518–527 [DOI] [PubMed] [Google Scholar]
- Welch JM, Simon SA, Reinhart PH. (2000) The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc Natl Acad Sci U S A .97:13889–13894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J. (1979) The concept of regulation for human body temperature. J Therm Biol .5: 75–82 [Google Scholar]
- Werner J. (1988) Functional mechanisms of temperature regulation, adaptation and fever: complementary system theoretical and experimental evidence. Pharmacol Ther .37: 1–23 [DOI] [PubMed] [Google Scholar]
- White MD. (2006) Components and mechanisms of thermal hyperpnea. J Appl Physiol .101: 655–663 [DOI] [PubMed] [Google Scholar]
- Wiley JL, Razdan RK, Martin BR. (2006) Evaluation of the role of the arachidonic acid cascade in anandamide's in vivo effects in mice. Life Sci .80: 24–35 [DOI] [PubMed] [Google Scholar]
- Wood JN, Winter J, James IF, Rang HP, Yeats J, Bevan S. (1988) Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci .8: 3208–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Wang Z, Wang Y, Furuyama T, Kontani Y, Sato Y, Mori N. (2008) Impaired basal thermal homeostasis in rats lacking capsaicin-sensitive peripheral small sensory neurons. J Biochem .143: 385–393 [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, DiMicco JA. (2003) Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett .340: 1–4 [DOI] [PubMed] [Google Scholar]
- Zhang L, Jones S, Brody K, Costa M, Brookes SJ. (2004) Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol .286: G983–991 [DOI] [PubMed] [Google Scholar]
- Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, et al. (2007) Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res .100: 1063–1070 [DOI] [PubMed] [Google Scholar]
- Zhang X, Li L, McNaughton PA. (2008) Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron .59: 450–461 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Boulant JA. (2005) Temperature effects on neuronal membrane potentials and inward currents in rat hypothalamic tissue slices. J Physiol .564: 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]