Abstract
The calcium-sensing receptor (CaSR) controls parathyroid hormone (PTH) secretion, which, in turn, via direct and indirect actions on kidney, bone, and intestine, maintains a normal extracellular ionized calcium concentration (Ca2+o). There is less understanding of the CaSR's homeostatic importance outside of the parathyroid gland. We have employed single and double knockout mouse models, namely mice lacking PTH alone (CaSR+/+ PTH−/−, referred to as C+P−), lacking both CaSR and PTH (CaSR−/− PTH−/−, C−P−) or wild-type (CaSR+/+ PTH+/+, C+P+) mice to study CaSR-specific functions without confounding CaSR-mediated changes in PTH. The mice received three hypercalcemic challenges: an oral Ca2+ load, injection or constant infusion of PTH via osmotic pump, or a phosphate-deficient diet. C−P− mice show increased susceptibility to developing hypercalcemia with all three challenges compared with the other two genotypes, whereas C+P− mice defend against hypercalcemia similarly to C+P+ mice. Reduced renal Ca2+ clearance contributes to the intolerance of the C−P− mice to Ca2+ loads, as they excrete less Ca2+ at any given Ca2+o than the other two genotypes, confirming the CaSR's direct role in regulating renal Ca2+ handling. In addition, C+P+ and C+P−, but not C−P−, mice showed increases in serum calcitonin (CT) levels during hypercalcemia. The level of 1,25(OH)2D3 in C−P− mice, in contrast, was similar to those in C+P− and C+P+ mice during an oral Ca2+ load, indicating that increased 1,25(OH)2D3 production cannot account for the oral Ca2+-induced hypercalcemia in the C−P− mice. Thus, CaSR-stimulated PTH release serves as a “floor” to defend against hypocalcemia. In contrast, high-Ca2+o-induced inhibition of PTH is not required for a robust defense against hypercalcemia, at least in mice, whereas high-Ca2+o-stimulated, CaSR-mediated CT secretion and renal Ca2+ excretion, and perhaps other factors, serve as a “ceiling” to limit hypercalcemia resulting from various types of hypercalcemic challenges.
Keywords: serum calcium, calcitonin, kidney, vitamin D, urinary calcium, diuretic
the extracellular calcium (Ca2+o)-sensing receptor (CaSR) plays key roles in Ca2+o homeostasis (5). A large body of evidence indicates that the CaSR in the parathyroid chief cell, where it is expressed at arguably the highest level in the body, plays crucial roles in Ca2+ homeostasis by inhibiting parathyroid hormone (PTH) secretion (6, 22, 31), PTH gene expression (26), and parathyroid cellular proliferation (22, 45), as well as by upregulating the vitamin D receptor (41). Key pieces of evidence in this regard are the excessive, unregulated PTH secretion and parathyroid cell growth in mice homozygous for knockout (KO) of the CaSR gene (22) as well as in humans homozygous for inactivating mutations of the CaSR as part of the syndrome of neonatal severe hyperparathyroidism (NSHPT) (5, 21, 27). Furthermore, allosteric activators (“calcimimetics”) (31) and inhibitors (“calcilytics”) of the CaSR (18), as expected, inhibit and stimulate parathyroid function, respectively.
In addition to being present in the parathyroid, the CaSR is also expressed in other tissues participating in Ca2+o homeostasis, including the calcitonin (CT)-secreting C-cells of the thyroid gland (15, 17) and several sites along the renal tubule (38, 39). Available data support the CaSR's role in mediating high-Ca2+o-stimulated CT secretion (15, 17, 28), in promoting renal Ca2+ excretion in response to hypercalcemia (1, 2, 11, 13, 14), and in regulating the secretion of parathyroid hormone-related protein (PTHrP) and the transport of calcium in breast epithelial cells (44). Although the CaSR is present in bone cells (8, 23, 29, 47), as well as in epithelial cells of the small and large intestines (10, 16), the predominant cells that express it and its roles in regulating bone turnover and intestinal function, including Ca2+ absorption, are not fully understood. Recent studies, however, using conditional knockout (KO) mouse models have implicated the CaSR as playing key roles in chondrocytes and osteoblasts (9).
The neonatal lethality of the homozygous CaSR KO mice has limited their utility for detailed studies of the CaSR's physiological roles in vivo (22). However, several other mouse models are available for such investigations. Two such models “rescue” the homozygous CaSR KO mice by crossing heterozygous CaSR KO mice with mice in which either the parathyroid gland (42) or the PTH gene (24) has been knocked out. Studies using these two models have established that the CaSR contributes importantly to the fine control of the serum Ca2+ concentration but have not evaluated in any detail the importance of the CaSR in C-cells and kidney and other tissues in maintaining Ca2+o homeostasis not only in the absence, but also in the presence of PTH (e.g., given via infusion). As noted above, mice with conditional KO in various tissues offer an additional approach to studying the CaSR's roles in vivo (9). However, the conditional KO mice lack the advantages that global KO mice offer, i.e., the ability to observe homeostatic responses in the absence of the compensatory role(s) played by CaSR present in nontargeted tissues (see discussion for more details).
In the present studies we have utilized as an experimental model the exon 5 CaSR KO mice rescued by deletion of the PTH gene (CaSR−/− PTH−/−, referred to as C−P− mice), utilizing mice with KO of PTH alone (CaSR+/+ PTH−/−, C+P−) or wild-type mice (CaSR+/+ PTH+/+, C+P+) as controls to dissect the relative roles of the CaSR in parathyroid, kidney and C-cell in maintaining Ca2+o homeostasis. The results indicate that, while CaSR-regulated PTH secretion provides a “floor” that defends against hypocalcemia, it is not needed to mount a robust and effective defense against hypercalcemic challenges, such as an oral calcium load or PTH infusion. The near total lack of Ca2+o-stimulated CT secretion in the C−P− mice as well as their impaired ability to upregulate renal Ca2+ excretion, especially in the presence of PTH, are likely important contributors to their increased susceptibility to hypercalcemia induced by increased Ca2+ ingestion, by PTH injection or PTH infusion by minipump or by phosphate depletion. These results illustrate the CaSR's second key homeostatic role, as a “ceiling” that defends effectively against hypercalcemia even in the absence of CaSR-mediated suppression of PTH.
MATERIALS AND METHODS
Generation of C+P+, C+P−, and C−P− mouse lines.
The two parental strains of CaSR−/− mice and PTH−/− mice were generated by homologous recombination in embryonic stem cells (22, 30). Mice heterozygous for KO of exon 5 of the Casr gene as well as mice heterozygous for the null Pth allele are fertile and were bred to generate offspring heterozygous at both the Casr and Pth loci. The latter were then used to generate C+P+, C+P− and C−P− mice. We maintained lines of C−P−, C+P−, and C+P+ mice that had been extensively back-crossed on a mixed genetic background comprising contributions from C57B6, 129/svJ, and 129/SvEv strains. These three lines were periodically back-crossed to minimize genetic drift, and biochemical parameters in blood and urine were periodically tested to ensure stability of the biochemical phenotypes.
KO mice.
The WT (C+P+), PTH KO (C+P−), and PTH and CaSR double-KO (C−P−) mice were maintained in microisolator cages in a Brigham and Women's Hospital animal facility. We routinely genotyped and screened the mice biochemically to ensure the authenticity and stability of their genetic backgrounds. The PCR primers and genotyping protocols were applied as described previously (22, 30).
In vivo experiments.
Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Harvard Medical School and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Mice were housed in microisolator cages in a pathogen-free facility according to the regulations of the Harvard Medical School Center for Animal Resources and Comparative Medicine (ARCM). As described below (see results), in some experiments, C+P− and C−P− mice were rendered hypocalcemic (owing to their hypoparathyroidism) by feeding them standard mouse chow (with 0.8% Ca2+ wt/wt and 0.4% phosphate; Harlan-Teklad TD99224; Harlan-Teklad, Madison, WI) as well as drinking water with no added calcium (referred to as 0% Ca2+ water). In other experiments, the three genotypes were rendered equivalently normocalcemic by feeding the C−P− and C+P− mice the same chow along with drinking water supplemented with CaCl2 [0.75% (wt/vol) and 1.5% (wt/vol), respectively]. Note that the percentage of calcium expressed as wt/vol refers to the weight of the CaCl2 salt added to the water and not to elemental Ca2+. Mice were subjected to various experimental manipulations as described in results, and blood and urine samples were collected for determination of the analytes described below.
Blood and urine measurements.
Serum and urine analytes were measured using the following kits: serum and urine Ca2+ (Eagle Diagnostics, De Soto, TX), urine creatinine, Creatinine Analyzer 2 (Beckman Coulter, Galway, Ireland), serum creatinine, alkaline phosphatase (ALP) (Stanbio Laboratory, Boerne, TX), 1,25(OH)2D3, RIA (Immunodiagnostics Systems, Fountain Hills, AZ), urine deoxypyridinoline (DPD) crosslinks (Quidel, San Diego, CA), osteocalcin and CT (Alpco Diagnostics, Salem, NH), and genotyping, DNeasy kit and primers (Qiagen).
Osmotic minipumps.
Miniosmotic pumps (model 1002) and human PTH1-34 were purchased from Alzet (Cupertino, CA) and Bachem (Torrance, CA), respectively. Pumps were filled with hPTH1-34 dissolved in PBS with 2% cysteine (adjusted to pH 4.3 with addition of 20 mM acetic acid) and preconditioned by incubation in the same buffer overnight at 37°C. Mice were anesthetized briefly with isoflurane, during which a subcutaneous pocket was prepared on the lower dorsal surface for subsequent implantation of the minipump. After implantation, the incision was clipped, and postoperative analgesia was administered as needed for the first 24 h. The mice were kept in separate cages to avoid one mouse damaging the minipump implanted on another one by chewing. The pump volume, pumping rate, and duration were ∼100 μl, 0.25 μl/h, and 14 days, respectively.
Statistics.
Statistical analyses were carried out by unpaired t-testing when two groups were compared or by ANOVA using Dunnett's t-test for comparisons of three or more group means. Statistical calculations used an n equal to the number of mice in each group; a P value <0.05 was taken to indicate statistical significance. In general, each experiment was performed at least twice, and results were pooled if the experimental design was identical.
RESULTS
Response of mice to oral Ca2+ loading.
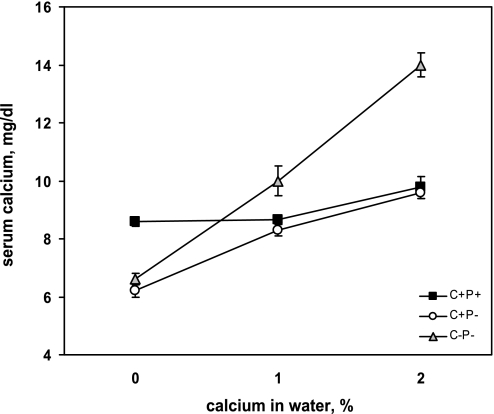
We initially assessed the capacity of the C+P+, C+P−, and C−P− mice to maintain normocalcemia in response to oral Ca2+ loading achieved by increasing the concentration of Ca2+ in the water. Figure 1 shows that, in contrast to the normocalcemic C+P+ mice, the C+P− and C−P− mice were both hypocalcemic to a similar degree (6–7 mg/dl) when maintained on chow with 0.8% Ca2+ and 0% Ca2+ water. Therefore, as noted previously (22, 24, 42), loss of the CaSR did not protect against the development of hypocalcemia in the absence of PTH. When the mice were provided with drinking water containing 1% (wt/vol) CaCl2 (referred to hereafter as 1% Ca2+ water), the C+P− mice exhibited an increase in serum Ca2+ to 8.3 mg/dl, whereas the C−P−mice showed a substantially greater increase in serum Ca2+ under these conditions, to 10–11 mg/dl (P < 0.05). When the Ca2+ content of the water was increased to 2% (referred to as 2% Ca2+ water), the C−P− mice became overtly hypercalcemic (∼14 mg/dl), but the C+P− mice were able to defend effectively against hypercalcemia, maintaining a serum Ca2+ of 9.6 mg/dl. The C+P+ mice remained normocalcemic throughout the study. The mice drank equivalent amounts of water (3.2–3.6 ml/25 g mouse per day), and serum creatinine concentrations after 1 wk on the 2% Ca2+ water did not differ among the three genotypes and were unchanged relative to the corresponding levels when the mice were receiving 0% Ca2+ water.
Fig. 1.
Serum Ca2+ concentration in wild-type (C+P+), parathyroid hormone (PTH) knockout (C+P−), and PTH and calcium-sensing receptor (CaSR) double-knockout (C−P−) mice as a function of Ca2+ in the drinking water. All mice received chow with 0.8% Ca2+ throughout the study. After 1 wk on 0% Ca2+ water, blood and urine samples were obtained, and 1% Ca2+ (as CaCl2) was added to the water. After 1 wk on 1% Ca2+ water, blood and urine samples were obtained, and Ca2+ in the water was changed to 2%. Blood and urine samples were obtained a third time 1 wk later. The serum Ca2+ concentration of the C−P− mice was significantly higher than in the C+P− mice with both 1% and 2% Ca2+ water and significantly higher than that of the C+P+ mice with 2% Ca2+ water (means ± SE, P < 0.01, n = 12–16 in each genotype).
Possible hormonal factors contributing to oral Ca2+-induced hypercalcemia in the C−P− mice.
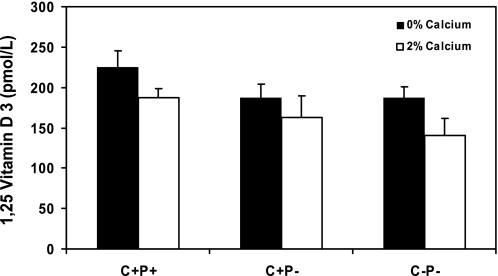
To explore possible hormonal and other factors that could contribute to the exaggerated hypercalcemic response of the C−P− mice to receiving 2% Ca2+ water, we measured serum 1,25(OH)2D3 levels in the three genotypes of mice receiving 0 or 2% Ca2+ water (Fig. 2). The C+P− and C−P− mice had slightly but not significantly lower levels of 1,25(OH)2D3 than the C+P+ mice at baseline, and the C+P− and C−P− mice did not differ in their 1,25(OH)2D3 levels. When mice were receiving 2% Ca2+ water, the 1,25(OH)2D3 levels of all three genotypes remained nearly constant, indicating that a higher level of 1,25(OH)2D3 was not a contributor to the difference in the serum Ca2+ between C+P− and C−P− mice receiving 2% Ca2+ water. The high bioavailability of the Ca2+ from the CaCl2 in the drinking water, with resultant impairment of gastrointestinal phosphate absorption and hypophosphatemia, may have contributed to the lack of suppression of the 1,25(OH)2D3 in the C+P+ mice receiving 2% Ca2+ water. Indeed, serum phosphate in the C+P+, C+P−, and C−P− mice decreased from 7.5 ± 0.36 to 4.1 ± 0.3, from 9.2 ± 0.47 to 4.9 ± 0.43, and from 9.8 ± 0.73 to 3.9 ± 0.27 mg/dl, respectively, when the mice changed from 0 to 2% Ca2+ water.
Fig. 2.
Serum 1,25(OH)2D3 levels in C+P+, C+P−, and C−P− mice receiving 0 or 2% Ca2+ water. Mice were fed normal chow and 0% Ca2+ water for 1 wk, and blood was drawn for measurement of serum levels of 1,25(OH)2D3 as described in materials and methods. Mice then received normal chow and 2% Ca2+ water for 1 wk, and measurements of 1,25(OH)2D3 were repeated. The level of 1,25(OH)2D3 in the C+P+ mice on 0% Ca2+ water was significantly higher than that in either C+P− or C−P− mice. Values of 1,25(OH)2D3 did not differ significantly among the 3 genotypes when receiving 2% Ca2+ in the drinking water. (means ± SE, n = 8–16 in each genotype).
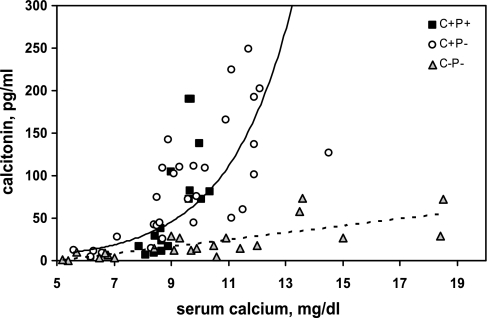
Figure 3 shows the relationship between serum Ca2+ and CT concentrations in C+P+, C+P−, and C−P− mice administered 0, 1, or 2% Ca2+ water. The trend lines fitting the data for the C+P+ and C+P− mice on the one hand, and the C−P− mice on the other, were provided as visual aids to highlight the differences in the responses between the genotypes. Statistical analyses were carried out over specific ranges of serum calcium concentration as described below. In contrast to the C+P+ and C+P− mice, which showed a steep increase in serum CT between ∼9 and 12 mg/dl, the C−P− mice exhibited minimal if any changes in serum CT. The CT values for the three genotypes over a near-physiological range of serum calcium concentrations (8–10 mg/dl) as well as for >10 mg/dl were compared, and the CT values of C−P− mice were significantly lower (P < 0.01) than those of C+P− and C+P+ mice.
Fig. 3.
Serum calcitonin (CT) levels in C+P+, C+P−, and C−P− mice as a function in serum Ca2+ concentration. Mice were maintained on standard chow and 0% Ca2+ water for 1 wk, 1% Ca2+ water for 1 wk and finally, 2% Ca2+ water for a 3rd wk. Serum samples were obtained at the end of each of the 3 wk, and levels of Ca2+ and CT were determined as described in materials and methods. Data are plotted as serum Ca2+ concentration in any given serum sample vs. the CT concentration in that sample. Trend lines represent C+P− and C+P+ (solid) and C−P− (dotted).
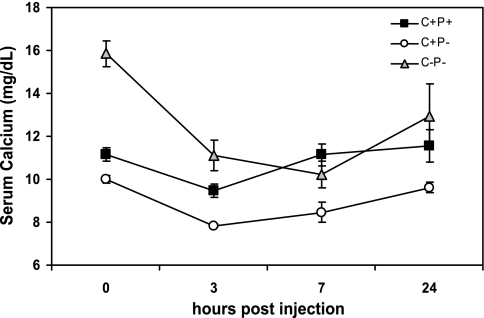
To assess responsiveness of the mice to CT, they were maintained on 2% Ca2+ water for 1 wk and then injected with a single dose of 150 ng/g body wt salmon CT (Fig. 4). The CT injection produced a substantial fall in the serum Ca2+ concentration of the C−P− mice. Regardless of whether it was expressed as absolute decrement (4.7 mg/dl for C−P− vs. 2.1 mg/dl for C+P− and 1.7 mg/dl for C+P+) or as percentage decrease (29.8% for C−P− vs. 21.3% for C+P− and 15.0% for C+P+), the drop in serum calcium concentration for the C−P− mice was as great or greater than that in the C+P+ and C+P− mice. This result documented that impaired responsiveness to endogenous CT could not account for the hypercalcemia in the C−P− mice administered 2% Ca2+ water. CT produced only a small decrement in serum Ca2+ in the C+P+ mice, presumably because they were able to mount a sufficient PTH response to prevent hypocalcemia.
Fig. 4.
Serum Ca2+ concentrations in C+P+, C+P−, and C−P− mice receiving 2% Ca2+ water as a function of time after receiving an injection of CT. Mice were maintained on normal chow and 2% Ca2+ water for 1 wk. They then received an SC injection of salmon CT (150 ng/g body wt), and serum samples were obtained for determination of serum Ca2+ concentration as before at times shown. The maximal decrement in the absolute level of serum Ca2+ concentration after the CT injection in C−P− mice was greater than in C+P+ or C+P− mice (P < 0.05).
Renal contribution to oral Ca2+-induced hypercalcemia in the C−P− mice.
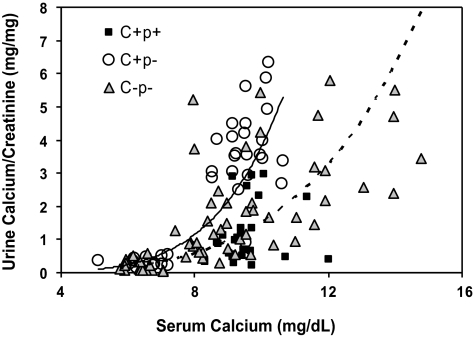
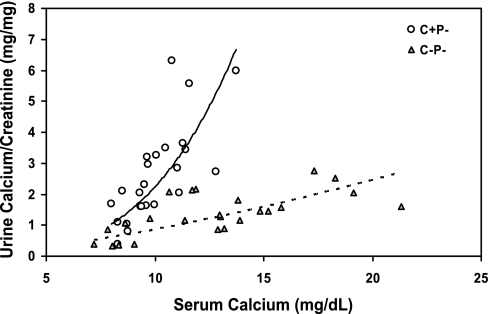
Available data in humans suggest that a key homeostatic action of the CaSR is to regulate renal tubular reabsorption of calcium, most likely through actions in the thick ascending limb (TAL) (1, 2, 12, 14). We studied, therefore, the impact of loss of the CaSR on renal calcium handling in the C−P− mice. Figure 5 shows the relationship between serum and urine Ca2+ concentrations in the three genotypes of mice when they were receiving 0, 1, or 2% Ca2+ water. The urinary Ca2+ data are expressed as milligrams of Ca2+ per milligram of creatinine, because it was difficult to obtain full 24-h collections. There was a steep rise in the urinary Ca2+-to-creatinine ratio when serum Ca2+ increased to ∼10 mg/dl in both the C+P+ and C+P− mice. The similar responses of these two genotypes illustrates that suppression of PTH was not required to observe the increase in urinary Ca2+ excretion accompanying elevations in serum Ca2+, even within the normal range. For the C−P− mice, many of the mice required Ca2+ concentrations of 12–15 mg/dl to achieve levels of urinary Ca2+ excretion equivalent to those of the C+P− and C+P+ mice when their serum Ca2+ levels rose to ∼10 mg/dl. At serum Ca2+ concentrations between 8 and 10 mg/dl, corresponding to a physiological range of serum Ca2+ concentrations, the urinary Ca2+ concentrations of the C+P+, C+P−, and C−P− mice were 1.2 ± 0.18, 3.6 ± 0.27, and 1.6 ± 0.25 mg/mg creatinine, respectively (P < 0.01 for C−P− vs. C+P−). The urinary Ca2+ concentrations of the C+P− and C−P− mice also differed significantly even when they were hypocalcemic on 0% Ca2+ water (0.33 ± 0.038 and 0.17 ± 0.03, respectively, P < 0.001).
Fig. 5.
Urine Ca2+-to-creatinine ratio as a function of serum Ca2+ concentration in C+P+, C+P−, and C−P− mice maintained on 0, 1, and then 2% Ca2+ water. Mice were initially maintained on normal chow and 0% Ca2+ water for 1 wk. Blood and spot urine specimens were collected on the 7th day. The drinking water was then changed to 1% Ca2+ for 7 days, and blood and urine specimens again obtained on day 7. Finally, the water was changed to 2% Ca2+ for 7 days, and urine and blood collections were again obtained on day 7. Serum Ca2+ and urine Ca2+ and creatinine were measured as in materials and methods, and results are plotted as serum Ca2+ concentration vs. corresponding Ca2+-to-creatinine ratio. Urine Ca2+ levels in C−P− mice receiving 0% Ca2+ water were significantly lower than those in C+P− mice under the same conditions (0.33 ± 0.038 and 0.17 ± 0.03, respectively, P < 0.001). The urine Ca2+ concentration in C+P− and C−P− mice with serum Ca2+ concentrations between 8 and 10 mg/dl also differed significantly (3.6 ± 0.27 vs. 1.6 ± 0.25 mg/dl, respectively, P < 0.01). It was not possible to compare urinary Ca2+ concentration in C+P+ and C+P−genotypes at higher Ca2+ concentrations because of the difficulty in raising serum Ca2+ concentration above 10 mg/dl in C+P− (and C+P+) mice (means ± SE, n = 8–12 in each genotype). Trend lines represent C+P− (solid) and C−P− (dotted).
Responses of bone turnover markers to oral Ca2+ loading.
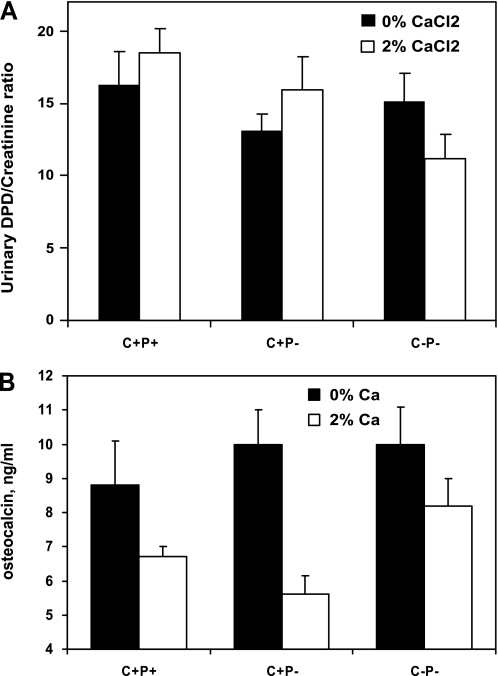
Figure 6 shows that the levels of urinary deoxypyridinoline (DPD) in the C+P− and C−P− mice were lower than those of the C+P+ mice when receiving 0% Ca2+ water and that the two KO genotypes had nearly equivalent levels of DPD. When they received 2% Ca2+ water, the levels of DPD in the two KO genotypes did not change, suggesting that a difference in bone resorption between these two genotypes could not account for the greater hypercalcemic response of the C−P− mice to an oral Ca2+ load. Serum osteocalcin (OC) concentrations in the three genotypes were similar when receiving 0% Ca2+ water. When they received 2% Ca2+ water, however, there were significant reductions in OC in the C+P+ and C+P− mice but not in the C−P− mice, suggesting a role for the CaSR in regulating osteoblast function as assessed by circulating levels of OC. In addition, the results suggest that impaired bone formation did not contribute to the hypercalcemia of the C−P− mice receiving 2% Ca2+ water.
Fig. 6.
Urinary deoxypyridinolines (DPD; A) and serum osteocalcin (OC) concentrations (B) in C+P+, C+P−, and C−P− mice receiving 0 or 2% Ca2+ water. Mice were maintained for 1 wk on each concentration of Ca2+ in the water, and serum and urine specimens were obtained on the last day of each of the 2 wk. Urinary DPD and creatinine as well as serum OC were measured as in materials and methods. There were no significant differences in DPD levels of C−P− and C+P− mice on 0 or 2% Ca2+ water, and changes in DPD within genotypes also did not change significantly when calcium in the drinking water was increased from 0 to 2%. Serum OC levels were not significantly different among the 3 genotypes when receiving 0% Ca2+ water. OC levels for C+P+ and C+P− mice dropped significantly (P < 0.05) when mice received 2% Ca2+ water; however, OC levels for C−P− mice showed no significant decline when mice received 2% Ca2+ water (P > 0.5) (means ± SE, n = 8–12 in each genotype for both DPD and OC).
Effect of PTH administration on C+P+, C+P−, and C−P− mice.
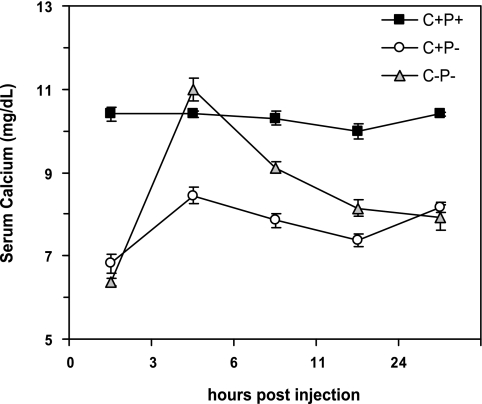
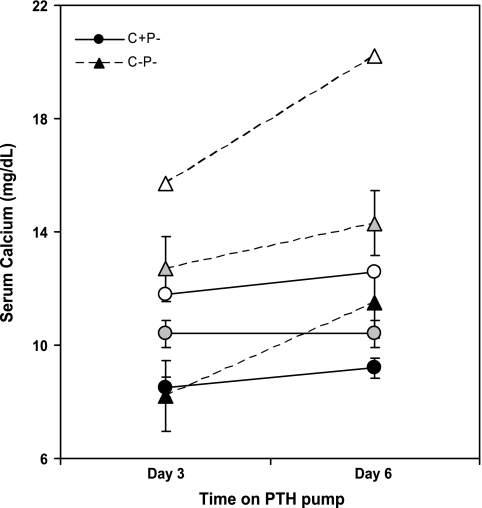
Figure 7 shows that injecting a single dose of PTH (500 ng/g body wt) had essentially no effect on the C+P+ mice but increased serum Ca2+ substantially in the C+P− and C−P− mice when receiving 0% Ca2+ water. The increase in serum Ca2+ in the C−P− mice, however, was twice that in the C+P− mice and produced frank hypercalcemia (P < 0.01), again showing the enhanced sensitivity of the C−P− mice to agents inducing hypercalcemia. The action of the PTH was, as expected, of short duration. Therefore, we investigated the effect of infusion with PTH1-34 by osmotic minipump on the two KO genotypes by collecting samples at days 3 and 6 after the initiation of the infusion. We did not include the C+P+ mice in these studies because of the confounding effect of changes in their endogenous PTH secretion that might result from infusion of PTH1-34 and resultant increases in serum Ca2+. Figure 8 shows that the C−P− mice had substantially greater hypercalcemic responses to infused PTH at any given dose, reaching levels of serum Ca2+ as high as 20 mg/dl at the highest dose tested (0.08 mg·kg−1·day−1). The levels of serum Ca2+ were significantly higher in the C−P− than in the C+P− mice receiving 0.04 mg·kg−1·day−1 at 3 and 6 days after initiating the infusion (P < 0.05), documenting the increased propensity of the C−P− mice to PTH-induced hypercalcemia.
Fig. 7.
Effect of injection with 500 ng/g body wt of hPTH1-34 on serum Ca2+ of C+P+, C+P−, and C−P− mice. Mice received 0% Ca2+ water for 7 days prior to the experiment. Serum was obtained just before PTH injection and then 3, 6, 11, and 24 h later for measurement of serum Ca2+ concentration. Serum Ca2+ was unchanged in C+P+ mice. It increased significantly in both C+P− and C−P− mice, but the increment was greater in C−P− mice at 3 (P < 0.1), 6 (P < 0.1), and 11 h (P < 0.5) after injection (means ± SE, n = 4 for each genotype).
Fig. 8.
Effect of infusion with hPTH1-34 via minipump on serum Ca2+ in C+P− (circles) and C−P− (triangles) mice. Mice were maintained on normal chow and 0% Ca2+ water for 1 wk. Osmotic minipumps were then implanted sc, and hPTH1-34 was infused at 0.02, 0.04, or 0.08 mg·kg−1·day−1 as denoted by solid, shaded, and open symbols, respectively. Serum Ca2+ concentrations were determined on days 3 and 6 postimplantation (n = 5 for mice receiving 0.02 and 0.04 mg·kg−1·day−1 and n = 2 for mice receiving 0.08 mg·kg−1·day−1). Mean serum Ca2+ for noninfused mice (n = 13–16) after 1 wk on normal chow and 0% Ca2+ water, as determined on an independent set of mice, were 6.8 for C+P− mice and 6.3 for C−P− mice. Serum Ca2+ was significantly higher in C−P− than in C+P− mice infused with 0.04 mg·kg−1·day−1 for either 3 (P < 0.05) or 6 days (P < 0.02). Although serum Ca2+ was markedly higher in C−P− mice than in C+P− mice infused with 0.08 mg·kg−1·day−1, the small number of animals studied precluded statistical analysis.
Figure 9 shows the relationship between serum and urine Ca2+ during the PTH1-34 infusion in the C−P− and C+P− mice when the results were expressed as urinary Ca2+-to-creatinine ratios as a function of serum Ca2+ obtained on the same day as the urine for both the 3- and 6-day time points. There was a marked flattening of the slope of the relationship between serum and urine Ca2+ in C−P− compared with C+P− mice, reflecting the very modest increase in urine Ca2+ in the C−P− mice with escalating doses of PTH1-34, despite the accompanying increases in serum Ca2+ to as high as 20 mg/dl. Five mice per genotype for the doses of 0.02 and 0.04 mg·kg−1·day−1 and two mice per genotype for the dose of 0.08 mg·kg−1·day−1 were implanted with pumps. Note that there are four points each for urinary calcium concentration for the C+P− and C−P− mice receiving 0.08 mg·kg−1·day−1 PTH1-34 because each mouse was studied on 2 days (days 3 and 6 of the infusion). Despite their higher level of serum Ca2+ when receiving 0.04 mg·kg−1·day−1 of PTH, the urinary Ca2+ concentration of the C−P− mice was lower than that in the C+P− mice. Moreover, the urinary Ca2+ concentrations for the PTH-infused mice at serum calcium concentrations corresponding to 8–10 mg/dl were 0.903 ± 0.250 and 0.382 ± 0.144 for C+P− and C−P−, respectively. The difference in the response between the two KO genotypes is highly significant (P <0.006).
Fig. 9.
Effect of PTH infusion on urinary Ca2+ concentration as a function of serum Ca2+ in C−P− and C+P− mice. Two independent urine samples were obtained on the same days as the serum samples in the experiment shown in Fig. 8, and urine Ca2+-to-creatinine ratios are plotted as a function of serum Ca2+ concentration. Urine Ca2+ values were significantly lower in C−P− mice than in C+P− mice receiving 0.04 mg·kg−1·day−1 PTH, despite significantly higher serum Ca2+ concentrations of C−P− mice, demonstrating their relative hypocalciuria. C−P− mice exhibited only a modest increase in urinary Ca2+ concentration with increasing rates of PTH infusion, in contrast to the marked increase in urinary Ca2+ concentration in C+P− mice, as serum Ca2+ concentration rose in response to PTH infusion. There were significant differences in urinary Ca2+ concentration (P < 0.05) between C+P− and C−P− mice at the 0.02, 0.04, and 0.08 mg·kg−1·day−1 infusion rates when data from days 3 and 6 were pooled. Trend lines represent C+P− (solid) and C−P− (dotted).
Effect of phosphate depletion on serum Ca2+ concentration in C+P+, C+P−, and C−P− mice.
Figure 10 shows the effect of administering a phosphate-deficient diet (nominally zero phosphate in the food and water) on serum Ca2+ and phosphate in mice of the three genotypes. There was a markedly greater increase in serum Ca2+ (and decrease in serum phosphate) in the C−P− compared with the C+P− mice (increments in serum Ca2+ of ∼3 and 10 mg/dl, respectively, above the level observed with 0% Ca2+ water; P < 0.01), whereas the C+P+ mice only showed modest increases in serum Ca2+ (∼1–2 mg/dl above the baseline shown in Fig. 1). Serum 1,25(OH)2D3 levels rose to comparable extents in all three genotypes (1,193 ± 124, 1,076 ± 116, and 1,152 ± 201 pmol/l, respectively, in the C+P+, C+P−, and C−P− mice).
Fig. 10.
Effect of phosphate-deficient diet on serum Ca2+ and phosphate concentrations in C+P+, C+P−, and C−P− mice. Mice were maintained on a nominally phosphate-free diet with 0% Ca2+ water for 6 days. Serum was then obtained for measurements of Ca2+ and phosphate as described in materials and methods. Serum Ca2+ levels differed significantly among all genotypes (P < 0.01) (means SEM, n = 8 in each genotype).
DISCUSSION
The goal of the present study was to examine the role of the CaSR in maintaining Ca2+o homeostasis in the absence of PTH and, consequently, CaSR-regulated PTH secretion. Several points are germane in this regard. We utilized mice with KO of exon 5 of the CaSR, which, in the homozygous state when their parathyroid glands are intact, die of severe hypercalcemia and hyperparathyroidism within the first few weeks of life (22). This result supports the conclusion that this model represents a complete or nearly complete KO of the CaSR in the parathyroid and, perhaps, other tissues involved in Ca2+o homeostasis. However, subsequently it has become clear that a splice variant of the CaSR arising from splicing out of exon 5 of the Casr gene can be formed in some tissues under normal circumstances as well as in mice homozygous for KO of exon 5 of the Casr (33, 34). It has not been possible to express high levels of this truncated receptor on the cell surface of heterologous cell systems, preventing direct documentation that it actually has biological activity. At the same time, it appears that the chondrocytes from exon 5 CaSR KO mice still respond to Ca2+o, providing indirect evidence that the CaSR lacking exon 5 may have biological activity in some tissues (40). Recent studies strongly suggest that the CaSR lacking exon 5 in the global exon 5 CaSR KO rescues the otherwise severe cartilage and bone phenotypes that results from tissue-selective KO of the CaSR in chondrocytes and osteoblasts (7, 9). However, as shown in our results, and discussed further below, mice with global KO of exon 5 of the Casr gene have marked abnormalities in the control of CT and renal Ca2+ handling in response to hypercalcemia (22), suggesting that the full-length CaSR, which is lost in the exon 5 KO mice, is a key mediator of these two biological actions of high Ca2+ as well as of Ca2+o-regulated PTH secretion and parathyroid cellular proliferation.
Our results are relevant to the CaSR's roles in defending against not only hypercalcemia but also hypocalcemia. As shown earlier, loss of the CaSR in the setting of loss of either PTH or the parathyroid glands does not “rescue” the double-KO mice from hypoparathyroidism (24, 42), and, as observed here, the C+P− and C−P− mice have similar degrees of hypocalcemia (6–7 mg/dl). This result has the important and well-recognized implication that hypocalcemia-induced, CaSR-mediated secretion of PTH provides a key, powerful “floor” defending against the development of either acute or prolonged hypocalcemia. Indeed, with adequate levels of vitamin D and normal renal function, only marked, prolonged Ca2+ deficiency, as occurs in certain regions of Africa (36), can cause persistent hypocalcemia and, in turn, the associated skeletal lesions of osteomalacia and/or rickets.
An interesting and unanticipated result of our study is that hypercalcemia-induced, CaSR-mediated suppression of PTH is not needed for a robust defense against various hypercalcemic challenges (e.g., increased oral intake of Ca2+, PTH injection/infusion, or phosphate depletion). That is, the C−P− mice effectively lack a “ceiling” above which it is difficult to elevate Ca2+o through a hypercalcemic challenge, and, instead, they exhibit marked hypercalcemic responses, particularly those arising from PTH infusion via minipump and phosphate depletion. The serum Ca2+ of the C+P− mice, in contrast, increases to a level very similar to that of the C+P+ mice receiving the same challenge, and it is difficult to elevate their serum Ca2+ concentration above 10–11 mg/dl, i.e., the upper limit of normal.
What is the importance of various tissues in the CaSR-mediated defense against hypercalcemia in the C+P+ and C+P− mice that is lacking in the C−P− mice? The kidney likely plays a key role in this regard. C+P− and C+P+ mice showed marked and similar elevations in urinary Ca2+ excretion with increases in Ca2+ intake, which appears to provide an important mechanism for maintaining normocalcemia during oral Ca2+ loading. Since the responses of these two genotypes to an oral Ca2+ load were similar, it appears, at least in mice, that a direct action of Ca2+o on renal tubular handling of Ca2+ is a dominant mechanism underlying this effect and that suppression of PTH as serum Ca2+ rises is not an absolute requirement for upregulating Ca2+ excretion. In contrast, there was a right shift in the relationship between serum and urine Ca2+ during oral Ca2+ loading in C−P− mice, which was even more marked during PTH infusion, consistent with available evidence that PTH upregulates the molecular machinery mediating renal Ca2+ reabsorption in the distal convoluted tubule (DCT) (43). It is possible that the reduction in serum phosphate observed in the C−P− mice during oral Ca2+ loading partially overcame the CaSR's hypocalciuric action, as phosphate depletion causes hypercalciuria in CaSR-intact animals and people (4, 25). Thus, the failure of the kidney to upregulate Ca2+ excretion in the face of hypercalcemia in the C−P− mice is likely an important contributor to their intolerance to oral Ca2+ loading and to hypercalcemia induced by PTH infusion.
Where is the most likely site of action of the CaSR in the kidney that is perturbed in the C−P− mice? There is a clear precedent for a key role of the cTAL (cortical thick ascending limb) in producing absolute or relative hypocalciuria in patients with familial hypocalciuric hypercalcemia (FHH) and NSHPT, who are heterozygous and homozygous, respectively, for inactivating CaSR mutations (1). Even in patients with FHH/NSHPT who were aparathyroid, there was persistent, excessively avid renal tubular reabsorption of Ca2+, which was substantially reduced by the loop diuretic ethacrynic acid (1). Further investigations, beyond the scope of this study, will be needed to perform similar studies in the mouse models employed here. The importance of other segments also requires further investigation. The CaSR, like the PTH receptor, is also expressed in the DCT, a site where thiazide diuretics are known to modulate renal tubular calcium reabsorption in a PTH-dependent (3, 20, 32) and -independent manner (35, 37), but we did not examine the CaSR's role in this nephron segment.
We did not directly assess gastrointestinal Ca2+ absorption. However, the similar levels of 1,25(OH)2D3 in the C+P− and C−P− mice, when receiving both 0% and 2% Ca2+ water, suggests that abnormalities in 1,25(OH)2D3 metabolism and, presumably, in its action on the intestine were not important causes of the intolerance of the C−P− mice to oral Ca2+ loading. In fact, if anything, the level of 1,25(OH)2D3 in the C−P− mice was lower than that in the C+P− mice when both genotypes were receiving 2% Ca2+ in the drinking water, although this difference was not statistically significant.
In a similar manner, the levels of 1,25(OH2)D3 rose equivalently in C+P+, C+P−, and C−P− mice fed a nominally phosphate-free diet despite the markedly higher level of serum Ca2+ that developed in the C−P− mice under these conditions. Therefore, a difference in 1,25(OH)2D3 levels also cannot account for the marked hypercalcemia in the phosphate-depleted C−P− mice. We were initially surprised at the failure of 1,25(OH2)D3 levels to fall in mice maintained on 2% Ca2+ water, at least in the C+P+ and C+P− genotypes, since hypercalcemia generally reduces 1,25(OH)2D3 levels indirectly by suppressing PTH secretion and directly by inhibiting 1α-hydroxylation in the proximal tubule (4, 46).
There was a near-total abolition of the stimulation of CT by hypercalcemia in the C−P− mice, in marked contrast to the C+P+ and C+P− mice, both of which showed robust increases in serum CT during oral Ca2+ loading. The C−P− mice exhibited a responsiveness to the hypocalcemic action of exogenous CT that was similar to or greater than that of the C+P− mice. The lack of a hypocalcemic action of CT on the C+P+ mice presumably resulted from their capacity to mount a sufficient PTH response to forestall the development of hypocalcemia, at least with this dose of CT. In any event, the failure of the C−P− mice to mount a CT response to hypercalcemia, rather than impaired responsiveness to their admittedly low endogenous CT levels, likely contributes, along with altered renal Ca2+ handling, to their development of hypercalcemia during oral Ca2+ loading. In contrast, in the C+P+ and C+P− mice, hypercalcemia-induced CT secretion and upregulation of renal Ca2+ excretion both appear to be important components of their homeostatic ceiling defending against hypercalcemia. From our data, however, we cannot quantify the relative importance of these two mechanisms to the defense against hypercalcemia in mice or, for that matter, in humans.
Changes in urinary DPD and serum osteocalcin levels could not explain the differential responses of the C+P− and C−P− mice to hypercalcemic challenges. That is, there was neither a higher level of a bone resorption marker (i.e., DPD) nor a reduced level of a bone formation marker (osteocalcin) in the hypercalcemic C−P− mice compared with the normocalcemic C+P− mice ingesting 2% Ca2+ water. This result must be interpreted with caution, however, given the available data suggesting that the CaSR lacking exon 5 in the CaSR KO model utilized here may be hypomorphic with respect to CaSR-mediated actions in cartilage and bone (9). It is of interest that both the C+P+ and C+P− mice exhibited a fall in serum OC when changed from 0% to 2% Ca2+ drinking water whereas serum OC did not change in the C−P− mice, suggesting a role for the CaSR in OC production/secretion. Of note in this regard, serum OC levels nearly doubled in normal human subjects during induction of hypocalcemia by infusion of EDTA (19), but the relevance of this acute action of hypocalcemia to the more chronic effects of oral Ca2+ loading in our studies is unclear.
In summary, the CaSR contributes to the defense against both hypo- and hypercalcemia. The ability of the parathyroid gland, in a CaSR-dependent manner, to increase PTH secretion, PTH gene expression, and parathyroid cellular proliferation in response to hypocalcemia provides a powerful homeostatic defense against hypocalcemia that couples enhanced PTH secretion to its actions on kidney and bone and indirectly, via its stimulation of 1,25(OH)2D3 synthesis, on intestine. The defense against hypercalcemia, in contrast, at least in mice, does not require CaSR-induced suppression of parathyroid function but instead utilizes CaSR-mediated upregulation of urinary Ca2+ excretion and CT secretion and, perhaps, additional mechanisms in bone, intestine, and additional tissues not defined here.
GRANTS
This work was supported by National Institutes of Health Grant 1RO1 DK-078331 (to E. M. Brown).
ACKNOWLEDGMENTS
We thank Prof. Joseph Bonventre and Eileen O'Leary for allowing us access to use the creatinine analyzer in their laboratory.
REFERENCES
- 1.Attie MF, Gill J, Jr, Stock JL, Spiegel AM, Downs RW, Jr, Levine MA, Marx SJ. Urinary calcium excretion in familial hypocalciuric hypercalcemia. Persistence of relative hypocalciuria after induction of hypoparathyroidism. J Clin Invest 72: 667–676, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ba J, Friedman PA. Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium 35: 229–237, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Breslau N, Moses AM, Weiner IM. The role of volume contraction in the hypocalciuric action of chlorothiazide. Kidney Int 10: 164–170, 1976 [DOI] [PubMed] [Google Scholar]
- 4.Bringhurst FR, Demay MB, Kronenberg HM. Hormones, and disorders of mineral metabolism. In: Williams Textbook of Endocrinology (9th ed.), edited by Wilson JD, Foster DW, Kronenberg HM, Larsen PR. Philadelphia, PA: WB Saunders, 1998, p. 1155–1209 [Google Scholar]
- 5.Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab 3: 122–133, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Brown EM, Lian JB. New insights in bone biology: unmasking skeletal effects of the extracellular calcium-sensing receptor. Sci Signal 1: pe40, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Chang W, Tu C, Chen TH, Komuves L, Oda Y, Pratt S, Miller S, Shoback D. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 140: 5883–5893, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal 1: ra1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Identification and localization of extracellular Ca2+-sensing receptor in rat intestine. Am J Physiol Gastrointest Liver Physiol 274: G122–G130, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Davies M, Adams PH, Lumb GA, Berry JL, Loveridge N. Familial hypocalciuric hypercalcaemia: evidence for continued enhanced renal tubular reabsorption of calcium following total parathyroidectomy. Acta Endocrinol (Copenh) 106: 499–504, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Desfleurs E, Wittner M, Pajaud S, Nitschke R, Rajerison RM, Di Stefano A. The C Ca2+-sensing receptor in the rabbit cortical thick ascending limb (CTAL) is functionally not coupled to phospholipase C. Pflügers Arch 437: 716–723, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Desfleurs E, Wittner M, Simeone S, Pajaud S, Moine G, Rajerison R, Di Stefano A. Calcium-sensing receptor: regulation of electrolyte transport in the thick ascending limb of Henle's loop. Kidney Blood Press Res 21: 401–412, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Di Stefano A, Desfleurs E, Simeone S, Nitschke R, Wittner M. Ca2+ and Mg2+ sensor in the thick ascending limb of the loop of Henle. Kidney Blood Press Res 20: 190–193, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Freichel M, Zink-Lorenz A, Holloschi A, Hafner M, Flockerzi V, Raue F. Expression of a calcium-sensing receptor in a human medullary thyroid carcinoma cell line and its contribution to calcitonin secretion. Endocrinology 137: 3842–3848, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Gama L, Baxendale-Cox LM, Breitwieser GE. Ca2+-sensing receptors in intestinal epithelium. Am J Physiol Cell Physiol 273: C1168–C1175, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Garrett JE, Tamir H, Kifor O, Simin RT, Rogers KV, Mithal A, Gagel RF, Brown EM. Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology 136: 5202–5211, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Gowen M, Stroup GB, Dodds RA, James IE, Votta BJ, Smith BR, Bhatnagar PK, Lago AM, Callahan JF, DelMar EG, Miller MA, Nemeth EF, Fox J. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest 105: 1595–1604, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gundberg CM, Grant FD, Conlin PR, Chen CJ, Brown EM, Johnson PJ, LeBoff MS. Acute changes in serum osteocalcin during induced hypocalcemia in humans. J Clin Endocrinol Metab 72: 438–443, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Hanson RC, White JB, Gomoll AW. Acute and chronic diuretic-induced alterations in urinary sodium and calcium excretion in the conscious rat. Miner Electrolyte Metab 8: 314–324, 1982 [PubMed] [Google Scholar]
- 21.Heath DA. Familial hypocalciuric hypercalcemia. In: The Parathyroids, edited by Bilezikian JP, Marcus R, Levine MA. New York: Raven, 1994, p. 699–710 [Google Scholar]
- 22.Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, Brown EM, Seidman JG, Seidman CE. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism [see comments]. Nat Genet 11: 389–394, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Kameda T, Mano H, Yamada Y, Takai H, Amizuka N, Kobori M, Izumi N, Kawashima H, Ozawa H, Ikeda K, Kameda A, Hakeda Y, Kumegawa M. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem Biophys Res Commun 245: 419–422, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, Guise TA, Pollak MR. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest 111: 1021–1028, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau K, Agus ZS, Goldberg M, Goldfarb S. Renal tubular sites of altered calcium transport in phosphate-depleted rats. J Clin Invest 64: 1681–1687, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi R, Ben-Dov IZ, Lavi-Moshayoff V, Dinur M, Martin D, Naveh-Many T, Silver J. Increased parathyroid hormone gene expression in secondary hyperparathyroidism of experimental uremia is reversed by calcimimetics: correlation with posttranslational modification of the trans acting factor AUF1. J Am Soc Nephrol 17: 107–112, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Marx SJ, Fraser D, Rapoport A. Familial hypocalciuric hypercalcemia. Mild expression of the gene in heterozygotes and severe expression in homozygotes. Am J Med 78: 15–22, 1985 [DOI] [PubMed] [Google Scholar]
- 28.McGehee DS, Aldersberg M, Liu KP, Hsuing S, Heath MJ, Tamir H. Mechanism of extracellular Ca2+ receptor-stimulated hormone release from sheep thyroid parafollicular cells. J Physiol (Lond) 502: 31–44, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mentaverri R, Yano S, Chattopadhyay N, Petit L, Kifor O, Kamel S, Terwilliger EF, Brazier M, Brown EM. The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J 20: 2562–2564, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest 109: 1173–1182, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA 95: 4040–4045, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nijenhuis T, Hoenderop JG, Loffing J, van der Kemp AW, van Os CH, Bindels RJ. Thiazide-induced hypocalciuria is accompanied by a decreased expression of Ca2+ transport proteins in kidney. Kidney Int 64: 555–564, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Oda Y, Tu CL, Chang W, Crumrine D, Komuves L, Mauro T, Elias PM, Bikle DD. The calcium sensing receptor and its alternatively spliced form in murine epidermal differentiation. J Biol Chem 275: 1183–1190., 2000 [DOI] [PubMed] [Google Scholar]
- 34.Oda Y, Tu CL, Pillai S, Bikle DD. The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J Biol Chem 273: 23344–23352, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Parfitt AM. The interactions of thiazide diuretics with parathyroid hormone and vitamin D. Studies in patients with hypoparathyroidism. J Clin Invest 51: 1879–1888, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettifor JM. Vitamin D &/or calcium deficiency rickets in infants & children: a global perspective. Indian J Med Res 127: 245–249, 2008 [PubMed] [Google Scholar]
- 37.Popovtzer MM, Subryan VL, Alfrey AC, Reeve EB, Schrier RW. The acute effect of chlorothiazide on serum-ionized calcium. Evidence for a parathyroid hormone-dependent mechanism. J Clin Invest 55: 1295–1302, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol 274: F611–F622, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Riccardi D, Lee WS, Lee K, Segre GV, Brown EM, Hebert SC. Localization of the extracellular Ca2+-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F951–F956, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez L, Tu C, Cheng Z, Chen TH, Bikle D, Shoback D, Chang W. Expression and functional assessment of an alternatively spliced extracellular Ca2+-sensing receptor in growth plate chondrocytes. Endocrinology 146: 5294–5303, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez ME, Almaden Y, Canadillas S, Canalejo A, Siendones E, Lopez I, Aguilera-Tejero E, Martin D, Rodriguez M. The calcimimetic R-568 increases vitamin D receptor expression in rat parathyroid glands. Am J Physiol Renal Physiol 292: F1390–F1395, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest 111: 1029–1037, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Abel M, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ. Coordinated control of renal Ca2+ transport proteins by parathyroid hormone. Kidney Int 68: 1708–1721, 2005 [DOI] [PubMed] [Google Scholar]
- 44.VanHouten J, Dann P, McGeoch G, Brown EM, Krapcho K, Neville M, Wysolmerski JJ. The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. J Clin Invest 113: 598–608, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wada M, Furuya Y, Sakiyama Ji Kobayashi N, Miyata S, Ishii H, Hagano N. The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. J Clin Invest 100: 2977–2983, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weisinger JR, Favus MJ, Langman CB, Bushinsky D. Regulation of 1,25-dihydroxyvitamin D3 by calcium in the parathyroidectomized, parathyroid hormone-replete rat. J Bone Miner Res 4: 929–935, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi T, Chattopadhyay N, Kifor O, Ye C, Vassilev PM, Sanders JL, Brown EM. Expression of extracellular calcium-sensing receptor in human osteoblastic MG-63 cell line. Am J Physiol Cell Physiol 280: C382–C393, 2001 [DOI] [PubMed] [Google Scholar]