Abstract
It remains to be determined whether systemic insulin replacement normalizes synthesis rates of different plasma proteins and whether there are differential effects on various plasma proteins. We tested a hypothesis that insulin deprivation differentially affects individual plasma protein synthesis and that systemic insulin treatment may not normalize synthesis of all plasma proteins. We measured synthesis rates of 41 plasma proteins in seven each of type 1 diabetic (T1DM) and nondiabetic participants (ND) using [ring-13C6]phenylalanine as a tracer. T1DM were studied while on chronic insulin treatment and during 8 h insulin deprivation. Insulin treatment normalized glucose levels, but plasma insulin levels were higher during insulin treatment than during insulin deprivation in T1DM and ND. Individual plasma proteins were purified by affinity chromatography and two-dimensional gel electrophoresis. Only 41 protein gel spots from over 300 were chosen based on their protein homogeneity. Insulin deprivation and hyperglycemia either significantly increased (n = 12) or decreased (n = 12) synthesis rates of 24 of 41 plasma proteins in T1DM compared with ND. Insulin treatment normalized synthesis rates of 13 of these 24 proteins, which were altered during insulin deprivation. However, insulin treatment significantly altered the synthesis of 14 additional proteins. In conclusion, acute insulin deprivation caused both a decrease and increase in synthesis rates of many plasma proteins with various functions. Moreover, chronic systemic insulin treatment not only did not normalize synthesis of all plasma proteins but also altered synthesis of several additional proteins that were unaltered during insulin deprivation.
Keywords: type 1 diabetes, stable isotope, protein synthesis, two-dimensional gel electrophoresis, fractional synthesis rate
insulin treatment has been shown to correct the catabolic state that exists in people with type 1 diabetes (T1DM) (13, 23, 29). Studies have also shown that insulin deprivation and associated metabolic changes in T1DM result in an overall increase in protein synthesis in splanchnic bed despite the concurrent catabolic state in muscle (23). Currently, no information is available on the specific nature of these proteins, although differential effects on synthesis of fibrinogen and albumin have been observed during insulin deprivation in T1DM (7). It remains to be determined whether insulin deficiency has similar differential effects on other liver proteins. Metabolic and hormonal derangements that occur during insulin deprivation and poor glycemic control may not be fully corrected during systemic insulin treatment. For example, systemic insulin treatment that achieves euglycemia in T1DM is also known to result in a portal-to-peripheral insulin concentration gradient of 1, which is different from the physiological gradient of 2.0–2.4 in nondiabetic (ND) people (4, 15, 20). We hypothesized that this difference in systemic vs. hepatic insulin levels during insulin treatment may alter hepatic protein synthesis. Studies done in partially depancreatized and streptozotocin-induced “type 1 diabetic” dogs have shown that glycemic control achieved by systemic vs. by portal insulin infusion have differential effects on liver protein synthesis and energy metabolism (11). It has also been observed that tight glycemic control in type 2 diabetic participants using insulin altered the transcript levels of several genes in skeletal muscle (28), suggesting that high levels of circulating insulin alter gene expression.
It is likely that alterations of plasma proteins, which are mostly synthesized in liver, are likely to have substantial impact on many bodily functions and may play an important role in the pathophysiology of chronic complications in diabetes. Indeed, several plasma proteins, including fibrinogen, lipoproteins, and acute-phase (inflammatory) proteins such as C-reactive proteins, are related to macrovascular complications (14). We tested a hypothesis that insulin deprivation will result in altered synthesis of various plasma proteins compared with ND participants and that intensive insulin therapy may normalize plasma protein synthesis of some but not all plasma proteins. In addition, we also hypothesized that change in insulin gradient between systemic and portal circulation and possible systemic hyperinsulinemia may alter synthesis of several other plasma proteins of liver origin. Identification of such proteins may pave the way for future studies to identify potential biomarkers of diabetic complications (30, 31).
In the current report, we extend our methodology to measure fractional synthesis rates (FSR) of multiple individual proteins (16, 17) to investigate the impact of insulin deprivation and treatment on plasma protein synthesis in type 1 diabetes. Because insulin deprivation has been shown to have profound impact on splanchnic protein synthesis (23) and most of the plasma proteins are of hepatic origin, we opted to study plasma proteins. We determined the FSR of several plasma proteins in T1DM participants during insulin deficiency and treatment and compared them with ND people.
RESEARCH DESIGN AND METHODS
Study participants.
Seven T1DM participants with average diabetes duration of 18.7 (range 7–35) years were compared with seven well-matched ND healthy participants (Table 1). All study participants were screened with a detailed history, physical examination, hematological, and biochemical profile. Exclusion criteria included renal insufficiency, coronary artery disease, other vascular disease, neuropathy, poor wound healing, and use of β-blockers, tricyclic antidepressants, or anticoagulants. The clinical characteristics of study participants are summarized in Table 1.
Table 1.
Study subject characteristics, plasma glucose, and hormones
| Variables | ND | T1DM | |
|---|---|---|---|
| Age, yr | 29.7±3 | 30±3 | |
| Weight, kg | 81±6.4 | 78.2±5 | |
| BMI, kg/m2 | 25±1.1 | 26.2±1.3 | |
| Fat mass, % | 33.2±4 | 31.6±4.1 | |
| HbA1c, % | 5.0±0.05 | 7.2±0.5* | |
| Duration of type 1 diabetes, yr | 18.7±4 | ||
| T1DM (I−) | T1DM (I+) | ||
| Glucose, mmol/l | 4.9±0.1 | 17.0±0.6"*† | 5.2±0.2 |
| Glucagon, ng/l | 99.0±19.0 | 82.6±17.3 | 50.3±6.8* |
| Insulin, pmol/l | 23.4±4.52 | 3.9±1.36*† | 69.8±17.8* |
Data are means ± SE for 7 participants (3 women and 4 men)/group. ND, nondiabetic study participants; T1DM, type 1 diabetic participants; I−, insulin-deprived state; I+, insulin-treated state.
P < 0.05 vs. ND (*) and
vs. T1DM (I+) (†).
Study protocol and sample collection.
The study protocol was approved by the Institutional Review Board of Mayo Clinic and Foundation, and all participants provided their informed consent before entering the study. T1DM participants were studied on two separate occasions (the insulin-treated study followed by the insulin-deprived study) separated by 1–2 wk. A schematic representation of the study day procedures is given in Fig. 1. All participants, including the ND participants, were placed on a weight-maintaining diet (energy content as carbohydrate-protein-fat 55:15:30%) provided from the Mayo Clinical and Translational Science Activities Clinical Research Unit (CRU) for three consecutive days as outpatients before each inpatient study period. The insulin regimen of T1DM participants changed during this time. Long-acting insulin was discontinued, and those participants on a multiple daily injection regimen (n = 4 of the 7 participants) were instructed to use ultra rapid-acting insulin (aspart or lispro, recombinant insulins) before each meal and bedtime based on blood glucose. Instructions were given to use short-acting insulin as needed to keep blood glucose concentration within the goal range of 4.4–6.6 mmol/l. Those participants on insulin pump (n = 3) using ultra rapid acting insulin continued their regimen and achieved a target glucose range of 4.4–6.6 mmol/l.
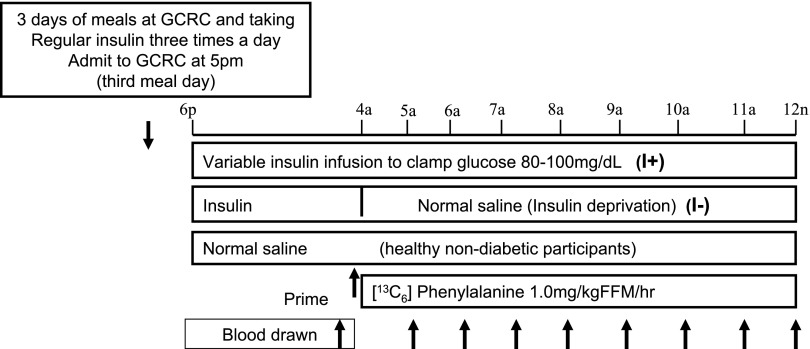
Fig. 1.
Schematic representation of study day procedures. I+, with insulin; I−, without insulin; FFM, fat-free mass.
On the evening before each study, participants were admitted to the CRU at 5:00 P.M. and stayed overnight until 12:00 noon the next day. After a standard dinner at 6:00 P.M., no food was ingested, except for water, until the end of the study at 12:00 noon the next day. A retrograde catheter was inserted in a dorsal hand vein for sample collection, and the hand was kept in a heating pad. A second intravenous catheter was placed in the contralateral forearm for infusions. In the morning, the hand with the retrograde catheter was kept in a “hotbox” at 60°C to obtain arterialized venous blood (6). On the insulin-treated day, an intravenous insulin infusion using regular human insulin was started and the plasma glucose was maintained between 4.44 and 5.56 mmol/l overnight until 12:00 noon the next day. Plasma glucose was measured every 30–60 min, and the insulin dose was adjusted every 30 min until midnight and every 15 min from midnight until the end of the study day. At 4:00 A.M., baseline venous samples for isotopic measurement were taken, and a priming dose of [ring-13C6]phenylalanine [1.00 mg/kg fat-free mass (FFM)] was given and continued until 12:00 noon at a rate of 1.00 mg·kg FFM−1·h−1. On the insulin-deprived day, the insulin infusion was discontinued from 4:00 A.M. to 12:00 noon and replaced with normal saline. ND participants were studied only with normal saline infusion during the entire period. Blood samples were drawn at every hour in the anticoagulant tubes, and plasma was collected and stored at −80°C until analysis.
Hormones and substrates.
Plasma glucose, insulin, and glucagon levels were measured as previously described (19).
Purification and identification of individual plasma proteins.
Plasma samples were first fractionated by depleting six high-abundant proteins through immunoaffinity chromatography (Multiple Affinity Removal Column Hu-6; Agilent Technologies). An enriched pool of low-abundant proteins and high abundant proteins was collected from the column flow through and eluate. Separation of individual plasma proteins was achieved by two-dimensional (2-D) gel electrophoresis (2-DGE) for the low abundant proteins and single-dimension (1-D) electrophoresis for the high abundant proteins (Fig. 2). Protein amounts of 300 and 20 μg were used for 2DGE and 1D electrophoresis, respectively. For 2DGE we used pH 4–7, 24-cm immobilized pH gradient (IPG) strips for the first dimension and 24 × 20 cm large SDS-PAGE for the second dimension. The proteins were visualized by silver staining. Protein gel spots were excised, destained, and subjected to in-gel trypsin digestion. The extracted peptides were analyzed for protein identification by nanoflow liquid chromatography electrospray tandem mass spectrometry (nanoLC-ESI-MS/MS) using a ThermoFinnigan LTQ Orbitrap Hybrid Mass Spectrometer (ThermoElectron, Bremen, Germany) coupled to an Eksigent nanoLC-2D HPLC system (Eksigent, Dublin, CA). Tandem mass spectra were extracted by BioWorks version 3.2 and the database search was done using Mascot (version 2.2.04; Matrix Science, London, UK), Sequest (version 27, rev. 12; ThermoFinnigan, San Jose, CA), and X! Tandem (version 2006.09.15.3; www.thegpm.org). All were set up to search the Swissprot database. Scaffold (version Scaffold_2_00_06; Proteome Software, Portland, OR) was used to validate MS/MS-based peptide and protein identifications. A more detailed description of protein identification is given in Supplemental Table S1 (Supplemental material for this article can be found on the American Journal of Physiology: Endocrinology and Metabolism website.).
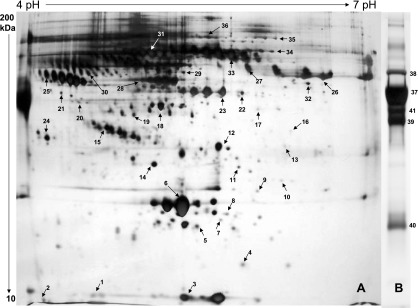
Fig. 2.
Electrophoresis separation and purification of individual plasma proteins. Plasma proteins were first fractionated by immunoaffinity chromatography to low abundant and high abundant proteins. Low abundant proteins were separated by 2-dimensional gel electrophoresis (A), and the high abundant proteins were separated by single-dimension gel electrophoresis (B). The gels were stained with silver to visualize protein gel spots/bands. The numbers displayed are gel spots/bands used for isotopic enrichment analysis, and the numbers match the protein serial numbers in Table 2 and Table S1.
Isotopic enrichment of individual plasma proteins and plasma free phenylalanine.
Plasma samples obtained at 2, 4, and 6 h were used for analysis of isotopic enrichment of individual plasma proteins. Figure 2 displays protein gel spots/bands used for isotopic enrichment analysis. We previously described the basic methodology used here for measuring isotopic enrichment of individual proteins purified and isolated from 2DGE (17). Briefly, plasma protein gel spots/bands were excised and hydrolyzed using 6 M HCl overnight at 110°C. The amino acids were isolated using Bio-Rad AG-50W x8 cation exchange resin and excess solvent evaporated to dryness. The amino acid residues were derivatized to their N-heptafluorobutyryl methyl esters and analyzed by tandem mass spectrometry using a ThermoFinnigan TSQ 7000 GC/MS/MS under negative ion chemical ionization conditions using isobutane as reactant gas as described previously (17).
Free amino acids were extracted from plasma samples using acetic acid as described (8) and analyzed as their t-butyldimethylsilyl ester derivative (27). The molar percent excess of [13C6]phenylalanine was calculated above background as previously described (8).
Fractional synthesis rate of individual plasma proteins.
As has been done previously (12), we used the slope of isotopic enrichment of [13C6]phenylalanine (Ie) for proteins that showed a clear linear increment (e.g., proteins that are synthesized at slow rate), whereas in case of proteins that started to plateau (e.g., proteins that are synthesized at fast rate) we have used the difference in isotopic enrichment between two time points (Ie) during the linear phase (e.g., 2 and 4 h). The integrated values for plasma [13C6]phenylalanine isotopic enrichment of hourly time points (2–6 h) were used as the precursor enrichment (Pe). The calculation of protein FSR has been done as previously (24, 25) described: FSR (%/h) = [Ie/Pe × t]100, where t represents time. The FSR calculation is based on an assumption that phenylalanine isotopic enrichment in liver and in plasma is altered in a similar direction during insulin replacement. Our previous studies that sampled from hepatic vein and artery showed that the ratios of stable isotopic enrichment of phenylalanine, leucine, and ketoisocaproate in hepatic vein to artery were not different during saline vs. insulin infusion (2, 22, 23).
Statistical analyses.
All statistical analyses were conducted using SAS software (version 9.1; SAS, Cary, NC). Data are presented as means ± SE. The FSR data were log transformed before analysis to produce symmetric-shaped distributions, and these measurements were analyzed using mixed-effects ANOVA. The model specification for these models included parameters to estimate the treatment main effect (nondiabetic, insulin treated, insulin deprived) on the mean response. In addition, the model included random effects that represented the between- and within-subject error terms to account for the subject matching and repeated-measures design. The ANOVA model parameters were estimated based on the principles of restricted maximum likelihood, with the variance-covariance structure estimated in the compound symmetry form. For all analyses, pair-wise comparisons between the means were constructed to test our a priori hypotheses. Fisher's Restricted Least Significant Differences criterion was used to maintain the a priori type I error rate at 0.05.
RESULTS
Clinical and metabolic characteristics.
The only significant difference between T1DM and ND was the higher hemoglobin A1c (HbA1c) levels in T1DM (Table 1). During insulin deprivation, plasma glucose levels were significantly higher and insulin levels were lower in T1DM compared with the insulin-treated state and also with ND. Plasma glucagon levels were significantly lower in T1DM during insulin treatment than in ND, with similar plasma glucose levels.
Purification of individual plasma proteins.
Figure 2 shows electrophoretic separation of individual plasma proteins and the selected 41 gel spots representing individual proteins. These proteins were chosen based on their reproducibility of appearance in 2DGE in multiple gel preparations, intensity and resolution of the spot, as well as uniqueness of single protein identification with high confidence. Out of more than 300 plus gel spots, we choose only 41 gel spots corresponding to 41 plasma proteins, because our measurements require protein homogeneity in these individual gel spots. The list of selected proteins is given in Table 2 with their corresponding gene symbol, accession number, name, protein identification probability, number of unique peptides, and percentage sequence coverage. The numbers indicated by arrows in 2DGE (Fig. 2) represent the serial number of proteins in Table 2.
Table 2.
List of plasma proteins that were chosen for isotopic enrichment analyses
| No. | Gene Symbol | Accession No. | Protein Name | Identification Probability, % | Unique Peptides | Sequence Coverage, % |
|---|---|---|---|---|---|---|
| 1 | APOA2_HUMAN | P02652 | Apolipoprotein A-II precursor | 99.90 | 3 | 21.00 |
| 2 | VTNC_HUMAN | P04004 | Vitronectin precursor | 100.00 | 9 | 18.80 |
| 3 | TTHY_HUMAN | P02766 | Transthyretin precursor | 100.00 | 25 | 85.70 |
| 4 | HPT_HUMAN | P00738 | Haptoglobin precursor | 100.00 | 18 | 36.70 |
| 5 | TETN_HUMAN | P05452 | Tetranectin precursor | 100.00 | 11 | 50.00 |
| 6 | APOA1_HUMAN | P02647 | Apolipoprotein A-I precursor | 100.00 | 26 | 73.80 |
| 7 | PRDX2_HUMAN | P32119 | Peroxiredoxin-2 | 100.00 | 12 | 54.50 |
| 8 | GPX3_HUMAN | P22352 | Glutathione peroxidase 3 precursor | 100.00 | 8 | 35.40 |
| 9 | SAMP_HUMAN | P02743 | Serum amyloid P-component precursor | 100.00 | 8 | 40.40 |
| 10 | FHR2_HUMAN | P36980 | Complement factor H-related protein 2 precursor | 100.00 | 10 | 41.10 |
| 11 | CERU_HUMAN | P00450 | Ceruloplasmin precursor | 100.00 | 11 | 11.10 |
| 12 | APOE_HUMAN | P02649 | Apolipoprotein E precursor | 100.00 | 19 | 62.80 |
| 13 | FCN3_HUMAN | O75636 | Ficolin-3 precursor | 100.00 | 9 | 34.80 |
| 14 | AMBP_HUMAN | P02760 | AMBP protein precursor | 100.00 | 10 | 33.50 |
| 15 | CLUS_HUMAN | P10909 | Clusterin precursor | 100.00 | 13 | 29.40 |
| 16 | HPTR_HUMAN | P00739 | Haptoglobin-related protein precursor | 99.90 | 8 | 23.90 |
| 17 | CO4A_HUMAN | P0C0L4 | Complement C4-A precursor | 100.00 | 16 | 9.92 |
| 18 | APOA4_HUMAN | P06727 | Apolipoprotein A-IV precursor | 100.00 | 28 | 63.40 |
| 19 | ZA2G_HUMAN | P25311 | Zinc-α2-glycoprotein precursor | 100.00 | 13 | 39.70 |
| 20 | PON1_HUMAN | P27169 | Serum paraoxonase/arylesterase 1 | 100.00 | 8 | 27.60 |
| 21 | A2GL_HUMAN | P02750 | Leucine-rich α2-glycoprotein precursor | 100.00 | 11 | 27.10 |
| 22 | PEDF_HUMAN | P36955 | Pigment epithelium-derived factor precursor | 100.00 | 14 | 37.60 |
| 23 | FIBG_HUMAN | P02679 | Fibrinogen γ-chain precursor | 100.00 | 34 | 75.30 |
| 24 | FETUA_HUMAN | P02765 | α2-HS-glycoprotein precursor | 100.00 | 20 | 54.80 |
| 25 | A2MG_HUMAN | P01023 | α2-Macroglobulin precursor | 100.00 | 12 | 10.30 |
| 26 | FIBB_HUMAN | P02675 | Fibrinogen β-chain precursor | 100.00 | 33 | 72.30 |
| 27 | APOH_HUMAN | P02749 | α2-Glycoprotein I precursor | 100.00 | 21 | 55.10 |
| 28 | VTDB_HUMAN | P02774 | Vitamin D-binding protein precursor | 100.00 | 26 | 52.10 |
| 29 | ANGT_HUMAN | P01019 | Angiotensinogen precursor | 100.00 | 18 | 33.20 |
| 30 | KNG1_HUMAN | P01042 | Kininogen-1 precursor | 100.00 | 14 | 23.40 |
| 31 | CO4B_HUMAN | P0C0L5 | Complement C4-B precursor | 100.00 | 15 | 9.63 |
| 32 | HEMO_HUMAN | P02790 | Hemopexin precursor | 100.00 | 24 | 57.60 |
| 33 | FIBA_HUMAN | P02671 | Fibrinogen α-chain precursor | 100.00 | 19 | 25.30 |
| 34 | CO3_HUMAN | P01024 | Complement C3 precursor | 100.00 | 21 | 16.80 |
| 35 | GELS_HUMAN | P06396 | Gelsolin precursor | 100.00 | 24 | 32.70 |
| 36 | C1R_HUMAN | P00736 | Complement C1r subcomponent precursor | 100.00 | 19 | 38.40 |
| 37 | ALBU_HUMAN | P02768 | Serum albumin precursor | 100.00 | 60 | 77.30 |
| 38 | TRFE_HUMAN | P02787 | Serotransferrin precursor | 100.00 | 23 | 44.00 |
| 39 | IGHG1_HUMAN | P01857 | Ig γ1-chain C region | 100.00 | 11 | 36.40 |
| 40 | KAC_HUMAN | P01834 | Ig κ-chain C region | 100.00 | 10 | 80.20 |
| 41 | A1AT_HUMAN | P01009 | α1-Antitrypsin precursor | 100.00 | 15 | 42.80 |
Plasma proteins were purified by 2-dimensional gel electrophoreses and SDS-PAGE and identified by tandem mass spectrometry. The serial number corresponds to the protein gel spot number given in Fig. 2.
FSR of individual plasma proteins.
When the FSR of proteins in T1DM were compared with ND, 24 proteins were altered during insulin deprivation (Figs. 3 and 4), and 14 proteins were altered only during insulin treatment (Fig. 5). Among the 24 proteins that were altered during insulin deprivation, 12 showed significantly lower FSR (Fig. 3) and another 12 showed significantly higher FSR (Fig. 4) when compared with ND. Figures 3A and 4B show 11 proteins that show no difference in their FSR between the insulin-deprived and insulin-treated state, whereas Figs. 3B and 4A show that the FSR of the other 13 proteins were similar between the ND and insulin-treated state in T1DM. Among the 14 proteins that were altered only during insulin treatment in TIDM, FSR of five proteins was significantly lower (Fig. 5A), and FSR of nine proteins was significantly higher (Fig. 5B) when compared with ND. Supplemental Table S1 shows the function of various proteins that showed altered synthesis in T1DM.
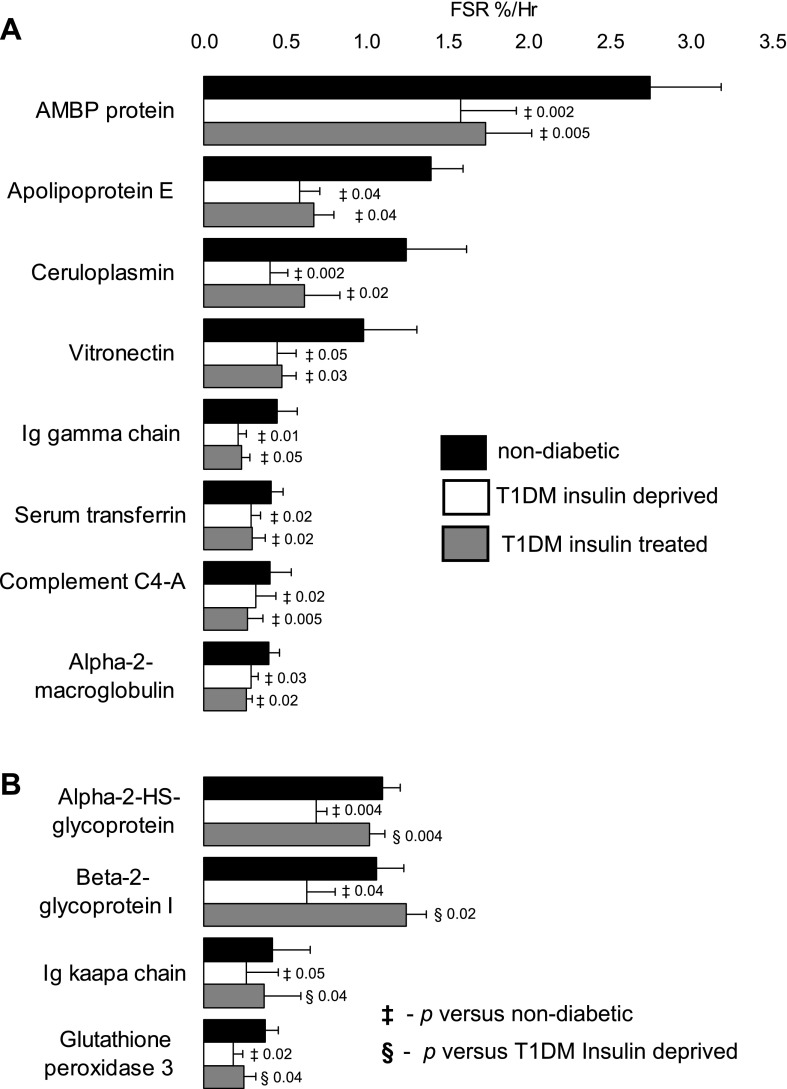
Fig. 3.
Decline in plasma protein synthesis during insulin deprivation in type 1 diabetes mellitus (T1DM). The fractional synthesis rate (FSR) of 12 plasma proteins decreased during insulin deprivation in T1DM compared with nondiabetic participants. Of the above 12 proteins, the FSR of 8 proteins did not differ between the insulin-treated and insulin-deprived state (A), but the FSR of 4 proteins were increased (B) by insulin treatment compared with insulin deprivation in T1DM. AMBP, α1-microglobulin/bikunin precursor. Data are represented as means ± SE (n = 7 subjects). ‡Significant difference vs. nondiabetic participants. §Significant difference vs. T1DM insulin-deprived state.
fig. 4.
Elevation in plasma protein synthesis during insulin deprivation in T1DM. FSR of 12 plasma proteins increased during insulin deprivation in T1DM compared with nondiabetic participants. Of the above 12 proteins, the FSR of 9 proteins were decreased (A) by insulin treatment compared with insulin deprivation in T1DM, but the FSR of 3 proteins (B) did not differ between the insulin-treated and insulin-deprived state. Data are represented as means ± SE (n = 7). ‡Significant difference vs. nondiabetic participants. §Significant difference vs. T1DM insulin-deprived state.
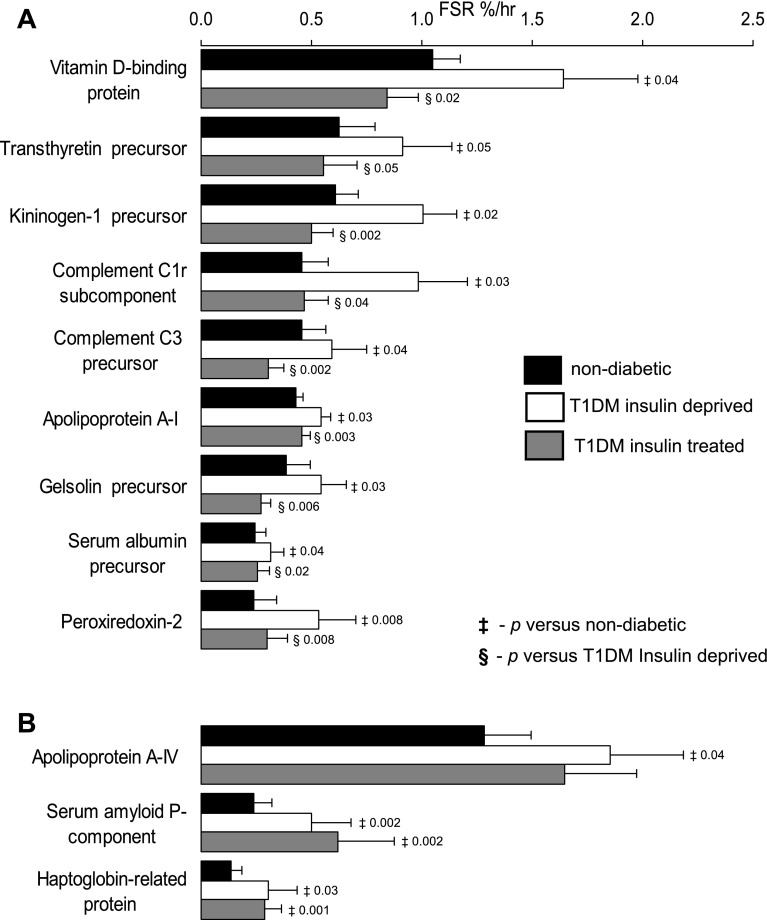
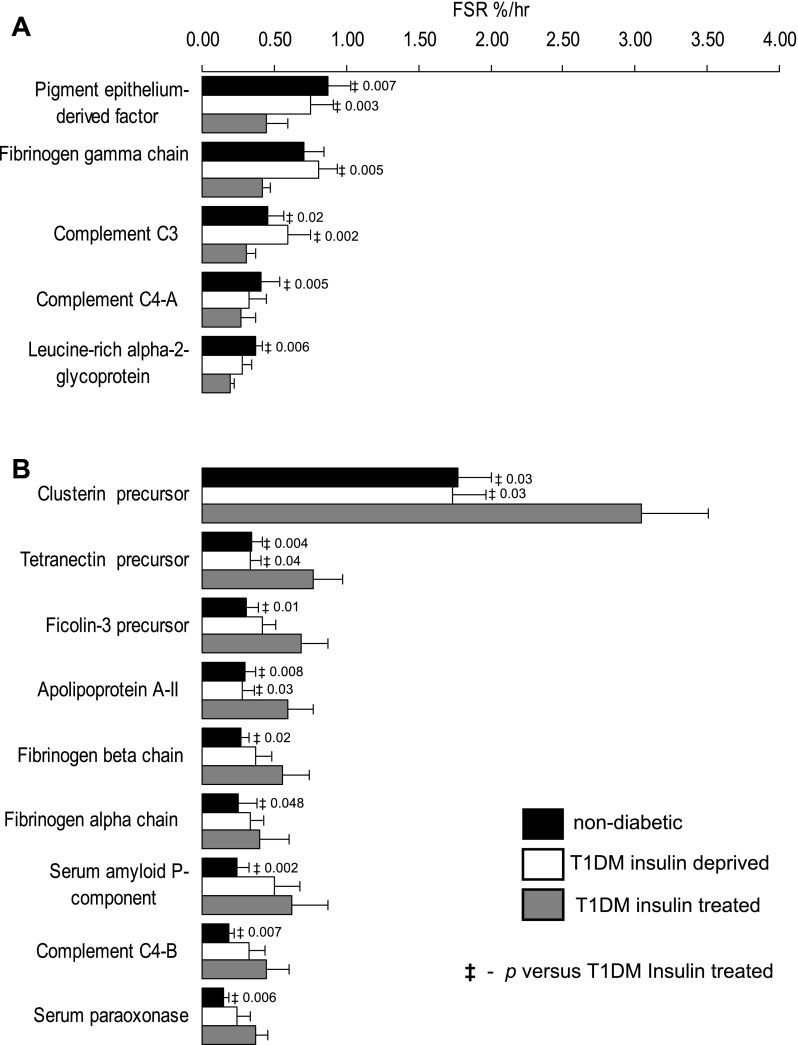
Fig. 5.
Impact of insulin treatment and alteration of plasma protein synthesis in T1DM. Fifteen plasma proteins are altered in T1DM during insulin treatment compared with nondiabetic participants. FSR of 5 proteins were lower (A) and FSR of 9 proteins were higher (B). Data are represented as means ± SE (n = 7). ‡Significant difference vs. T1DM insulin treated state.
DISCUSSION
The current study demonstrates that a relatively short period of insulin deprivation and the associated elevation of blood glucose resulted in either an increase (n = 12) or decrease (n = 12) in the FSR of 24 plasma proteins in T1DM. Of these 24 proteins, 13 had similar FSR in ND and during insulin treatment, suggesting that treatment normalized the alterations that occurred with insulin deficiency. However, synthesis of 11 of 24 proteins remains different between the insulin-treated state in T1DM and ND, suggesting that glycemic control by systemic insulin treatment may not normalize synthesis of all plasma proteins. Moreover, insulin treatment, despite normal glycemia, was associated with either an increase (n = 9) or decrease (n = 5) in the FSR of 14 additional proteins that were unaltered during insulin deprivation. The plasma insulin levels in T1DM during insulin deprivation were very low, and glucose levels were high compared with ND (Table 1). However, insulin levels during the insulin-treated state were substantially higher and glucose levels similar in T1DM and ND.
We studied a limited number of proteins; however, this is an unprecedented large-scale approach to measure protein synthesis. The measurement of fractional synthesis rates of these proteins may not be directly translated to an increase in concentrations of these proteins within the study period because of the time required to increase the concentrations of these proteins sufficiently high to be quantified by many quantitative proteomic approaches. There is also a possibility that degradation rates of these proteins also may be altered by insulin deprivation and systemic insulin treatment. However, the current approach provides important information that may not be obtained through measurement of protein concentration. First, protein concentration represents the net changes in the rate of synthesis compared with changes in degradation rate and does not provide mechanistic information on its regulation. To illustrate, an increase in the concentration of a protein might be a result of elevated synthesis or diminished degradation rate. Normally, when rate of degradation of a protein is lower, the plasma concentration of that protein will increase until a new steady-state condition occurs where synthesis and degradation are equal. However, during insulin withdrawal, it is likely that one process (e.g., degradation in the case of some proteins is higher than synthesis or vice versa) or both processes may alter and there is no chance of reaching a new steady state within our study period. The detectable changes in concentration of proteins with slow turnover occur only over a long period; thus, the changes in concentrations of these proteins are not readily identified during short-term intervention studies. Moreover, the fast turnover rates of many low abundant proteins result in small changes in concentration that require highly sensitive large-scale protein quantification approaches, which are not currently available. We attempted to measure relative protein concentrations by tagging proteins with isotopes (21) in the current study, but no measurable differences could be detected (Johnson KL, Mason CJ, Eckel Passow JE, Charlesworh CM, Nair KS, Bergen HR 3rd, unpublished observation).
The effect of insulin deprivation on protein synthesis in the current study represents the acute withdrawal of insulin in T1DM. However, the insulin treatment was uninterrupted, thus showing the chronic effect of insulin treatment. We discontinued long-acting insulin and treated T1DM with multiple daily injections of short-acting insulin or continued their subcutaneous insulin for 3 days before the study. To maintain a steady state during the isotopic infusion, we maintained their glucose at postabsorptive levels by intravenous infusion. The insulin requirement was lower during intravenous infusion compared with their routine insulin treatment, but plasma insulin levels were higher than ND. It is acknowledged that the results from the current study are applicable only in the fasted state, and chronic insulin deficiency and variable degrees of glycemic control may have a different effect on individual protein synthesis. The current results allow us to identify proteins that may change on chronic poor glycemic control and during tight glycemic control while T1DM people are on systemic insulin treatment.
The results support a hypothesis that the present approach of treating T1DM with peripheral administration of insulin, although achieving glycemic control may alter the synthesis rates of several plasma proteins. Insulin deficiency and associated hyperglycemia caused an alteration in the synthesis of several proteins with specific functions (Supplemental Table S1). Insulin replacement by the systemic route did normalize synthesis of some proteins, and, in addition, insulin treatment altered the synthesis rates of several additional proteins. The main comparison of T1DM insulin treated and insulin deprived was done with ND who have normal plasma glucose levels. These ND participants are likely to have twofold higher insulin levels in liver than in the systemic circulation (26), unlike the T1DM receiving insulin treatment who have similar hepatic and peripheral insulin levels. The results are in support of a study performed in a diabetic dog model that showed altered synthesis of liver proteins, albumin, and fibrinogen when insulin was delivered peripherally compared with portal administration that achieved similar glucose levels (11). Other metabolic alterations such as changes in amino acid (9) and urea (10) metabolism related to the route of insulin administration have also been reported.
The potential pathophysiological importance of altered synthesis rate of these plasma proteins remains to be fully understood. However, it is well known that several plasma proteins are involved in cardiovascular disease (3, 33). Interestingly, during the insulin-treated state, T1DM participants had altered synthesis of several subunits of fibrinogen and apolipoproteins, both of which are involved in macrovascular disease (5, 32). In addition, we also noted that several proteins involved in inflammation such as complement factors that may contribute to vascular complications were also altered in T1DM participants while on insulin treatment. This supports a hypothesis that peripheral insulin treatment normalizes glucose, but may contribute to macrovascular complications. Insulin treatment also failed to normalize complement factor H-related proteins as well as many immunoglobulins. Synthesis of several proteins that increased during insulin deprivation included transport proteins (transthyretin, vitamin D-binding protein, and albumin), kininogen complement C3, and several other proteins that may affect lipoprotein metabolism.
There are several proteins whose synthesis rates were altered by insulin deprivation but corrected by insulin treatment. The synthesis rates of proteins that were reduced by insulin deprivation include proteins involved in complement pathways (e.g., component C4A) and those involved in reducing hydrogen peroxide, lipid peroxide, and glutathione that may offer protection against oxidative damage of DNA, proteins, and tissues. The decline in synthesis rate of vitronectin may affect high-density lipoprotein metabolism, since this protein inhibits a membrane-damaging effect, and ceruloplasmin, which has copper-binding properties and ferroxidase activity in association with lipids. Other proteins may prevent activation of the blood coagulation cascade (e.g., β2-glucoproteins).
The current study involving large-scale measurement of FSR of plasma proteins supports results from a previous study that demonstrated that insulin deprivation and treatment in T1DM have differential effects on albumin and fibrinogen synthesis (7). Specifically, the FSR of some of the plasma proteins were increased by insulin treatment while others decreased. The regulation of insulin on protein synthesis at various levels of the synthetic machinery has not been studied in the current report. However, it is known that insulin promotes translation of gene transcripts to proteins (18), although it is unclear whether this effect is selective. It is more likely that the effect of insulin on gene transcript levels is more selective, with certain gene transcripts increasing while others decrease as noted during insulin administration in type 2 (1) and type 1 (19) diabetic people. It is possible that the effects that we have observed here are not primarily due to insulin deficiency or treatment because insulin deficiency not only results in hyperglycemia but also in many other metabolic/hormonal changes, including alterations in glucagon, insulin-like growth factor-I, amino acids, fatty acids, and ketones, all of which may affect gene transcription and translation (protein synthesis). It is therefore possible that the alterations in protein synthesis reported here are not directly related to the increase in systemic insulin concentrations or insulin deficiency in our T1DM participants.
In summary, the current study demonstrated that 1) insulin deprivation and associated metabolic and hormonal changes caused both upregulation and downregulation in the synthesis of several plasma proteins; and 2) systemic insulin treatment corrected glucose levels and normalized some of the alterations in protein synthesis but resulted in alterations in synthesis of several proteins that were unaltered during insulin deficiency, suggesting that systemic insulin treatment that alters the peripheral-to-hepatic insulin ratio may alter synthesis of liver proteins. The proteins with altered synthesis are involved in inflammation, immunity, lipoprotein metabolism, and other cellular functions, but long-term studies measuring the concentration of these proteins are needed to fully understand the potential clinical significance of our observation.
GRANTS
K. S. Nair is supported by National Institutes of Health (NIH) Grants R33 DK-70179, R01 DK-41973 and the David Murdock Dole Professorship. B. A. Irving is supported by NIH Grant 1 KL2 RR-024151.
Supplementary Material
ACKNOWLEDGMENTS
The study was performed at Mayo Clinical and Translational Science Activities (CTSA) (UL1 RR024150-01) facilities, including the Clinical Research Unit and Metabolomics Core Laboratory. We also gratefully acknowledge our study participants, Maureen Bigelow for coordinating the study, Melissa Aakre for secretarial assistance, and the nursing and technical staff of Mayo CTSA.
REFERENCES
- 1. Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow M, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 55: 3309–3319, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Barazzoni R, Meek SE, Ekberg K, Wahren J, Nair KS. Arterial KIC as marker of liver and muscle intracellular leucine pools in healthy and type 1 diabetic humans. Am J Physiol Endocrinol Metab 277: E238–E244, 1999. [DOI] [PubMed] [Google Scholar]
- 3. Bjorkbacka H. Atherosclerosis: cell biology and lipoproteins. Curr Opin Lipidology 19: 215–217, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Carpentier A, Patterson BW, Uffelman KD, Giacca A, Vranic M, Cattral MS, Lewis GF. The effect of systemic versus portal insulin delivery in pancreas transplantation on insulin action and VLDL metabolism. Diabetes 50: 1402–1413, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am 35: 491–510, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Copeland KC, Kenney FA, Nair KS. Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am J Physiol Endocrinol Metab 263: E1010–E1014, 1992. [DOI] [PubMed] [Google Scholar]
- 7. De Feo P, Gan Gaisano M, Haymond MW. Differential effects of insulin deficiency on albumin and fibrinogen synthesis in humans. J Clin Invest 88: 833–840, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford GC, Cheng KN, Halliday D. Analysis of [1-C-13]leucine and [c-13]KIC in plasma by capillary gas chromatography/mass spectrometry in protein turnover studies. Biomed Mass Spectrom 12: 432–436, 1985. [DOI] [PubMed] [Google Scholar]
- 9. Freyse EJ, Fischer U, Albrecht G, Marx S, Keilacker H. The effect of prehepatic insulin administration on alanine flux rates in diabetic dogs. Diabetologia 30: 402–408, 1987. [DOI] [PubMed] [Google Scholar]
- 10. Freyse EJ, Rebrin K, Schneider T, Petrzika M, Fischer U. Increased urea synthesis in insulin-dependent diabetic dogs maintained normoglycemic. Diabetes 45: 667–674, 1996. [DOI] [PubMed] [Google Scholar]
- 11. Freyse EJ, Fischer U, Knospe S, Ford GC, Nair KS. Differences in protein and energy metabolism following portal versus systemic administration of insulin in diabetic dogs. Diabetologia 49: 543–51, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Fu A, Nair KS. Age effect on fibrinogen and albumin synthesis in humans. Am J Physiol Endocrinol Metab 275: E1023–E1030, 1998. [DOI] [PubMed] [Google Scholar]
- 13. Geyelin HR, Du Bois EF. A case of diabetes of maximum severity with marked improvement: a study of blood,urine and respiratory metabolism. J Am Med Assoc 66: 1532–1534, 1916. [Google Scholar]
- 14. Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. J Am Med Assoc 290: 932–940, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Hother-Nielsen O, Schmitz O, Bak J, Beck-Nielsen H. Enhanced hepatic insulin sensitivity, but peripheral insulin resistance in patients with type 1 (insulin-dependent) diabetes. Diabetologia 30: 834–840, 1987. [DOI] [PubMed] [Google Scholar]
- 16. Jaleel A, Nehra V, Persson XM, Boirie Y, Bigelow M, Nair KS. In vivo measurement of synthesis rate of multiple plasma proteins in humans. Am J Physiol Endocrinol Metab 291: E190–E197, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Jaleel A, Short KR, Asmann YW, Klaus KA, Morse DM, Ford GC, Nair KS. In vivo measurement of synthesis rate of individual skeletal muscle mitochondrial proteins. Am J Physiol Endocrinol Metab 295: E1255–E1268, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jefferson LS, Koehler JO, Morgan HE. Effect of insulin on protein synthesis in skeletal muscle of an isolated perfused preparation of rat hemicorpus. Proc Natl Acad Sci USA 69: 816–820, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karakelides H, Asmann YW, Bigelow ML, Short KR, Dhatariya K, Coenen-Schimke J, Kahl J, Mukhopadhyay D, Nair KS. Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes 56: 2683–2689, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Lewis GF, Steiner G, Polonsky KS, Weller B, Zinman B. A new method for comparing portal and peripheral venous insulin delivery in humans: tolbutamide versus insulin infusion. J Clin Endocrinol Metab 78: 66–70, 1994. [DOI] [PubMed] [Google Scholar]
- 21. Mason CJ, Therneau TM, Eckel-Passow JE, Johnson KL, Oberg AL, Olson JE, Nair KS, Muddiman DC, Bergen HR 3rd. A method for automatically interpreting mass spectra of 18O-labeled isotopic clusters. Mol Cell Proteomics 6: 305–318, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Meek SE, Persson M, Ford GC, Nair KS. Differential regulation of amino acid exchange and protein dynamics across splanchnic and skeletal muscle beds by insulin in healthy human subjects. Diabetes 47: 1824–1835, 1998. [DOI] [PubMed] [Google Scholar]
- 23. Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splachnic and leg tissues in type I diabetic patients. J Clin Invest 95: 2926–2937, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol Endocrinol Metab 254: E208–E213, 1988. [DOI] [PubMed] [Google Scholar]
- 25. Nair KS, Welle SL, Halliday D, Campbell RG. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J Clin Invest 82: 198–205, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polonsky KS. Lilly Lecture 1994. The beta-cell in diabetes: from molecular genetics to clinical research. Diabetes 44: 705–717, 1995. [DOI] [PubMed] [Google Scholar]
- 27. Schwenk WF, Berg PJ, Beaufrère B, Miles JM, Haymond MW. Use of t-butyldimethylsilylation in the gas chromatographic/mass spectrometric analysis of physiologic compounds found in plasma using electron-impact ionization. Anal Biochem 141: 101–109, 1984. [DOI] [PubMed] [Google Scholar]
- 28. Sreekumar R, Halvatsiotis P, Schimke JC, Nair KS. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 51: 1913–1920, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Tessari P, Nosadini R, Trevisan R, De Kreutzemberg SV, Inchiostro S, Duner E, Biolo G, Marescotti MC, Tiengo A, Crepaldi G. Defective suppression by insulin of leucine-carbon appearance and oxidationin Type 1, insulin-dependent diabetes mellitus. J Clin Invest 77: 1797–1804, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The Diabetes Control, and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993. [DOI] [PubMed] [Google Scholar]
- 31. UK Prospestive Diabetes Study (U.KPDS) Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38 UK Prospective Diabetes Study Group. Br Med J 317: 703–13, 1999. [PMC free article] [PubMed] [Google Scholar]
- 32. Welsh P, Woodward M, Rumley A, Lowe G. Associations of plasma pro-inflammatory cytokines, fibrinogen, viscosity and C-reactive protein with cardiovascular risk factors and social deprivation: the fourth Glasgow MONICA study. Br J Haematol 141: 852–61, 2008. [DOI] [PubMed] [Google Scholar]
- 33. Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 359: 1897–908, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.