Abstract
Monosaccharides enter cells by slow translipid bilayer diffusion by rapid, protein-mediated, cation-dependent cotransport and by rapid, protein-mediated equilibrative transport. This review addresses protein-mediated, equilibrative glucose transport catalyzed by GLUT1, the first equilibrative glucose transporter to be identified, purified, and cloned. GLUT1 is a polytopic, membrane-spanning protein that is one of 13 members of the human equilibrative glucose transport protein family. We review GLUT1 catalytic and ligand-binding properties and interpret these behaviors in the context of several putative mechanisms for protein-mediated transport. We conclude that no single model satisfactorily explains GLUT1 behavior. We then review GLUT1 topology, subunit architecture, and oligomeric structure and examine a new model for sugar transport that combines structural and kinetic analyses to satisfactorily reproduce GLUT1 behavior in human erythrocytes. We next review GLUT1 cell biology and the transcriptional and posttranscriptional regulation of GLUT1 expression in the context of development and in response to glucose perturbations and hypoxia in blood-tissue barriers. Emphasis is placed on transgenic GLUT1 overexpression and null mutant model systems, the latter serving as surrogates for the human GLUT1 deficiency syndrome. Finally, we review the role of GLUT1 in the absence or deficiency of a related isoform, GLUT3, toward establishing the physiological significance of coordination between these two isoforms.
Keywords: glucose transport, facilitated diffusion, major facilitator superfamily protein, blood-brain barrier, placenta, diabetes, glucose transporter 1 deficiency syndrome, development
most cells transport sugars rapidly down the prevailing concentration gradient into or out of the cell. This equilibrative transport process is mediated by a family of sugar transporters called GLUTs. GLUT1 was the first glucose transporter isoform to be identified, purified (66, 132), and cloned (93) and is one of 13 proteins that comprise the human equilibrative glucose transporter family (63). GLUT1 is a membrane-spanning glycoprotein containing 12 transmembrane domains with a single N-glycosylation site, and its gene is located on chromosome 1 (1p35-31.3) (93). GLUT1 is expressed at the highest levels in the plasma membranes of proliferating cells forming the early developing embryo, in cells forming the blood-tissue barriers, in human erythrocytes and astrocytes, and in cardiac muscle (86). Having a catalytic turnover of ∼1,200/s (115), glucose transporter 1 (GLUT1) provides an efficient pathway for cellular import and export of glucose. In addition, GLUT1 transports galactose and ascorbic acid (81, 107). This review examines the catalytic properties, structure, molecular regulation, and physiology of GLUT1.
GLUT1 CATALYTIC PROPERTIES
Facilitated Diffusion
The cytoplasm of most cells equilibrates rapidly with nonmetabolizable extracellular sugars. This process is mediated by sugar transport proteins that catalyze unidirectional sugar uptake and exit, resulting in a net sugar movement down a concentration gradient from high to low sugar concentration. These sugar transport proteins are members of a family of integral membrane proteins called GLUTs that display strong specificity for d-stereoisomers of pentose and hexose monosaccharides adopting the chair configuration of the pyranose ring [e.g., d-glucose, d-galactose (7)], although some members of the protein family prefer fructose, a sugar that adopts the furanose ring form (87). This equilibrative sugar transport is often called “facilitated diffusion” because it is several orders of magnitude faster than glucose diffusion across artificial lipid bilayers, and the equilibrium transmembrane distribution of sugar is identical to that produced by transbilayer diffusion. Equilibrative sugar transport contrasts with that mediated by “active sugar transporters,” or sodium glucose-linked transporters, which exploit the free energy available in transmembrane cation gradients to transport sugars against a concentration gradient (128, 129).
GLUT1-Mediated Sugar Transport in Red Blood Cells
GLUT1 comprises 10–20% of the integral membrane protein content of human red cells, where it is quantitatively the only significant isoform of expressed GLUT (38). The availability of human red cells, their high GLUT1 content, and the relative uniformity of red cell size and surface area has resulted in more than 60 years of sophisticated kinetic analysis of erythrocyte sugar transport.
Steady-State Kinetics of Transport
Accurate analysis of the concentration dependence of sugar transport requires the measurement of “steady-state” transport rates, which are obtained only when the concentrations of GLUT1 sugar intermediates involved in transport are unchanged during the transport assay. In practical terms, this means that transport measurements are made at very early time points, where the amount of sugar in the cell increases (or decreases) linearly with time (the observed rate of transport is independent of time). This exposes the Achilles heel of sugar transport measurements in human red blood cells. GLUT1 is expressed so abundantly in human erythrocytes that it becomes necessary to either limit transport measurements to <1 s at 37°C or lower temperature to 4°C to satisfy this requirement. Despite this complexity, glucose transport in human red blood cells has been characterized extensively and has resulted in the development of a number of revealing experimental conditions that permit full characterization of any passive transport system (79).
GLUT1 Substrate Specificity
Competitive inhibition studies by Barnett et al. (7) suggest that the hydroxyl (OH) groups at C1 and C3 of d-glucose serve as hydrogen bond acceptors when d-glucose is seated in the GLUT1 sugar uptake site. C4 may form a hydrogen bond with GLUT1 because the C4 epimer of d-glucose, d-galactose, has 10-fold lower affinity for GLUT1 than d-glucose. However, an alternative explanation for this is that the nongluco configuration of the sugar hydroxyl group sterically hinders transport. The OH group at C6 seems not to hydrogen bond with GLUT1, and bulky substitutions at this position are tolerated. However, bulky substitutions at C1 are not tolerated. The reverse appears to be true for the sugar exit site. Bulky substitutions at C1 are tolerated, whereas substitutions at C6 are not. A similar pattern is observed for sugar interaction with GLUT4, although the impact of nongluco configurations of hydroxyls is less marked (106). A remaining challenge is to understand the binding requirements at the C1 position of d-glucose. Studies with fluoro analogs of d-glucose suggest that the β-configuration of the C1 hydroxyl (the OH group is above the ring or positioned cis to the C5-CH2OH group) is preferred (8), whereas studies with α- and β-d-glucose indicate mixed results (for review, see Ref. 74). More recent studies demonstrate that α- and β-d-glucose are transported with equal avidity by GLUT1 (74). Answers to these questions must await crystallization of the GLUT1-d-glucose complex.
Transport Kinetic Asymmetry
Sugar transport is termed “asymmetric” when maximal velocity (Vmax) and Km for sugar exit into sugar-free medium (zero-trans exit) are not identical to Km and Vmax for sugar entry into sugar-free cells (zero-trans entry). Although more complex than anticipated, this behavior does not violate the passive nature of transport. When [sugar] ([S]) is much lower than Km, the Michaelis-Menten expression for transport may be simplified to uptake, vout to in = Vmax(entry) [S]out/Km(entry), and because sugar uptake and exit must be identical when extracellular [S] = intracellular [S] uptake, vout to in = Vmax(entry) [S]out/Km(entry) = exit = vin to out = Vmax(exit) [S]in/Km(exit). Thus Vmax(entry)/Km(entry) must equal Vmax(exit)/Km(exit), and within experimental error this is satisfied.
Characteristics of asymmetry.
GLUT1-mediated sugar transport in human red cells is very asymmetric at low temperatures (Vmax for exit is 10-fold greater than Vmax for entry at 4°C), but because Vmax and Km for entry increase more rapidly with temperature than do the exit parameters, asymmetry falls with increasing temperature (82). When studying red cell “ghosts” (cytoplasm is replaced with saline by reversible hypotonic hemolysis), transport asymmetry is greatly diminished [Vmax and Km for uptake increase and approach Vmax and Km for exit (18)]. This is caused by the loss of cytoplasmic ATP, which allosterically modifies the catalytic properties of GLUT1 by binding reversibly to a GLUT1 ATP-binding site (10, 16).
Physiological significance of asymmetry.
Simulations of transport reveal important insights into transport function. Using standard transport equations that are independent of presumed transport mechanism (15), we can examine the consequences of asymmetry on net sugar import by red cells initially lacking intracellular sugar. Setting Vmax for zero-trans sugar entry as a constant, we ask what happens as we vary Vmax for zero-trans sugar exit from 10-fold less than Vmax for entry through 10-fold greater than Vmax for entry. The results show that the rate constant for equilibration of cytoplasmic water with extracellular sugar increases in a saturable manner with increasing asymmetry (Vmax exit/Vmax entry). Asymmetry of the kind observed in red cells allows cells to equilibrate much more rapidly with extracellular sugar. Thus, glucose-depleted red cells emerging from glucose-consuming organs such as the brain or placenta are more readily refilled upon reentering glucose-rich circulation.
GLUT1 Displays Accelerated Exchange
Accelerated exchange transport describes the stimulating effect that the presence of sugar at the opposite or “trans” side of the membrane exerts on the rate of unidirectional sugar flux from the cis to the trans side. It is for this reason that accelerated exchange is also called “trans-acceleration.” In an accelerated exchange uptake experiment, cells are first loaded with various concentrations of unlabeled sugar, and the rate of unidirectional, radiolabeled sugar uptake is measured at a fixed extracellular sugar concentration. At 20°C and lower, where transport can be measured accurately, unidirectional sugar uptake is accelerated severalfold by loading cells with unlabeled sugar, and exit is accelerated by extracellular sugar (73).
Characteristics of accelerated exchange.
Two types of accelerated exchange or trans-acceleration experiments have been described (78, 96). In “equilibrium exchange” studies, intracellular [sugar] = extracellular [sugar], and unidirectional sugar uptake or exit is measured by using radiotracer sugars. In infinite trans experiments the concentration of unlabeled sugar at the opposite, trans side of the membrane is saturating, and the concentration of sugar (plus radiotracer sugar) at the cis side is varied. Unidirectional radiotracer sugar flux is then measured in the direction cis to trans. Data obtained from these experiments have revealed much about the GLUT1 transport mechanism.
Vmax and Km for equilibrium exchange are 50-fold greater than Vmax and Km for zero-trans sugar uptake and five- to 10-fold greater than Vmax and Km for zero-trans sugar exit at 4°C (24, 82); for review, see Ref. 80. As temperature increases, the difference between exchange and net transport parameters decreases (82). GLUT1-mediated sugar transport in dolphin erythrocytes displays remarkably similar exchange transport properties (26). The availability of cytoplasmic ATP exaggerates trans-acceleration in human erythrocytes by suppressing the maximum rate of zero-trans sugar uptake and by decreasing Km for equilibrium exchange (23).
Physiological significance of exchange transport.
How does accelerated exchange impact net sugar export from red cells initially containing 5 mM d-glucose into serum containing 2 mM sugar? Assuming that transport is asymmetric (Vmax exit = 10 Vmax entry) and varying Vmax for exchange from 50-fold greater than Vmax for zero-trans entry to Vmax for exchange ≈ Vmax for zero-trans entry, our simulations show that the half-time for loss of intracellular sugar to serum increases as Vmax for exchange transport falls. Thus red cells deliver their intracellular glucose content to serum more rapidly when Vmax for exchange transport is greater than Vmax for zero-trans entry. This lends support to the hypothesis that human and dolphin red cell sugar transport have evolved to deliver intracellular glucose to glucose-dependent tissues such as the brain and placenta (26).
GLUT1 Cooperativity
Two fundamentally different models have been suggested for protein-mediated sugar transport (the simple carrier and fixed-site transporter models; see Equilibrium Ligand Binding below). Multiple independent analyses of human red cell sugar transport steady-state kinetics have demonstrated persistent deviations of sugar transport behavior from the behavior expected of these models (5, 24, 36, 40, 44). Some studies have suggested that the behavior of the red cell transport system is compatible with these models (82, 126). In combination, these studies suggest either that transport is more complicated than anticipated or that previous transport measurements are technically flawed. Recent published reports confirm the former interpretation (9, 74).
Characteristics of cooperativity.
Measurements of Vmax and Km for zero-trans and equilibrium exchange sugar transport permit computation of a predicted Km for infinite trans exit [unidirectional sugar exit from red cells into solutions containing saturating sugar concentrations (79)]. The experimental Km for infinite trans exit is consistently five- to 10-fold lower than that predicted by standard models for transport (5, 24, 36, 40, 44). Therefore, it appears that saturation of the external sugar-binding site increases the affinity of the internal sugar-binding site(s) for sugar.
Other interesting trans effects are also observed. Low concentrations of cytochalasin B and forskolin (inhibitors of glucose transport that bind at or close to the sugar export site) increase the affinity of the external site for transported sugars (25). Extracellular maltose, which binds to the sugar uptake site but is too large to translocate through the transporter, stimulates sugar uptake at extremely low maltose concentrations and inhibits transport as its concentration is raised (41). Thus endofacial and exofacial inhibitors accelerate sugar import at subsaturating inhibitor concentrations. These observations suggest that multiple ligand binding sites exist on the glucose transporter that modulates the affinity of adjacent cis or trans sites for transported substrate.
Physiological significance of cooperativity.
The net impact of the high-affinity exit site is best illustrated when we compare simulated net sugar uptake with measurements of sugar uptake. Simulated net uptake at 20 mM extracellular sugar proceeds relatively linearly with time because the internal sugar exit site is predicted to have low affinity for sugar. Measured net sugar uptake slows from the predicted rate at relatively low intracellular sugar levels due to the high-affinity sugar exit site. Therefore, net uptake is inhibited significantly at low intracellular sugar levels due to the high-affinity sugar exit site. This may serve to inhibit further glucose uptake when sufficient intracellular glucose is available to saturate hexokinase.
Equilibrium Ligand Binding
GLUT1 ligand-binding studies have significantly extended our understanding of the sugar transport mechanism by permitting the direct quantitation of transporter sugar-binding sites and by allowing for experimental approaches to questions such as “Can sugars bind simultaneously at sugar import and export sites, or are binding sites only alternately accessible?”
GLUT1 ligand-binding sites.
Two experimental approaches can assess whether GLUT1 exposes sugar import and sugar export sites simultaneously or alternately. The first makes direct measurements of ligand binding to GLUT1 and examines the effects of exo- and endofacial site substrates on ligand binding. The second examines the effects of the simultaneous presence of endo- and exofacial inhibitors on glucose transport.
The textbook model for glucose transport [variously termed the simple carrier, the alternating conformer carrier, the mobile carrier, or the iso-uni-uni model for glucose transport (60, 79, 127)] proposes that the transporter exposes one sugar-binding site (an import site or an exit site) at any instant and that conversion of the import to the export site through a substrate-binding-induced conformational change is the central catalytic step mediating substrate translocation. Although the various models listed above may represent different physical mechanisms, their King-Altman representations describing the key transport intermediates and their interconversions are identical. Alternatively, the fixed-site or two-site transport mechanism describes a transporter that presents sugar import and sugar export sites simultaneously. Sugars bind at these sites and then dissociate into a central cavity that is sufficiently large as to allow sugars to bypass each other en route to the trans-binding site. If the fixed-site model is correct, then a ligand such as cytochalasin B or forskolin that binds close to the endofacial sugar-binding site should not eliminate the exofacial sugar-binding site unless occupancy of the endofacial site by cytochalasin B greatly reduces the affinity of the exofacial site for sugar. If the simple carrier mechanism is correct, a ligand such as cytochalasin B or forskolin that binds close to the endofacial sugar-binding site should eliminate the exofacial sugar-binding site because both sites cannot exist simultaneously. In this case, the binding of cytochalasin B and exofacial ligands should be competitive. The available data suggest that GLUT1 ligand binding is compatible with the fixed-site transport mechanism, although simple carrier behavior is observed under special circumstances.
Characteristics of ligand binding.
The early purifications of human erythrocyte GLUT1 also demonstrated that cytochalasin B and exofacial inhibitor binding to GLUT1 are mutually exclusive (6, 37, 118). This behavior is compatible with the simple carrier mechanism for sugar transport. This approach was quickly followed up by ingenious studies (71) of sugar transport in human red cells that asked the question, “Is the inhibition of sugar transport produced by pairs of inhibitors (one acting at the import site and one acting at the export site) consistent with a model in which inhibitor binding sites are mutually exclusive (the simple carrier) or coexist (the fixed-site transporter)?” The answer appeared to be that the simple carrier model is upheld with the proviso that, should fixed-site transporter import and export sites show negative cooperativity (i.e., binding of inhibitor to 1 site reduces the affinity of the remaining trans site for ligand), this conclusion would be negated. Therefore, the outcome was ambiguous.
Later studies using purified GLUT1, red cell membranes, and intact red cells (13, 17, 52) supported a fixed-site transport mechanism with interacting (negatively cooperative) import and export sites. The type of cooperativity between exo- and endofacial binding sites is strongly dependent on the exofacial ligand. For example, the exofacial ligand phloretin exerts a very strongly negative cooperative effect on Kd for cytochalasin B binding to the endofacial site by increasing Kd for cytochalasin B binding 40-fold (52). At low concentrations (<1 mM), maltose and ethylidene glucose enhance cytochalasin B binding to GLUT1 (positive cooperativity). At higher concentrations these ligands have a negative cooperative effect on binding (25, 52). Exofacial d-glucose and endofacial cytochalasin B binding are independent (17).
These different behaviors are determined by the redox state of the transporter. GLUT1 purified in the presence of reductant appears to behave as a simple carrier, whereas the transporter obtained in the absence of reductant or studied in situ appears to function as a fixed-site transporter (48, 49, 133). We shall return to this observation when we consider glucose transporter quaternary structure.
Transient Kinetics
Steady-state kinetic studies allow the observer to build models that describe the intermediates (e.g., GLUT1-sugar complexes) involved in sugar transport. Transient kinetic studies provide a more critical test of the proposed models by monitoring the rate of transition of one conformational state to another.
Four important studies of GLUT1 transient kinetics have been reported. Appleman and Lienhard (3) studied substrate-induced changes in GLUT1 conformational states by monitoring the intrinsic tryptophan fluorescence of the purified transporter. Their studies employed reduced GLUT1 and support the hypothesis that exofacial ligands can trap GLUT1 in one conformational state that subsequently relaxes to a second state upon dilution of exofacial ligand. These findings are compatible with both simple carrier and fixed-site transporter models for transport. Similar studies with nonreduced GLUT1 (120) demonstrate that micromolar levels of exofacial ligands promote one conformational state, whereas higher micromolar levels promote a second, inhibited state. These findings are compatible with only the fixed-site transporter model for transport. Rapid quench transport measurements in red cells (83) showing transient acceleration of glucose uptake upon dilution of extracellular maltose are inconclusive, being consistent with the findings of Appleman and Lienhard (3), with fixed-site transporter predictions (14, 97, 98) and may be explained by the stimulatory effect of maltose on transport (41).
More recent studies demonstrate three phases of sugar uptake by red cells (9). The first, the rapid (but quantitatively smallest) phase, describes sugar association with GLUT1 (1 mol sugar:1 mol GLUT1). The second, the fast phase, describes sugar import into cytosol and accounts for two-thirds of total d-glucose uptake by red cells. The third and slowest phase describes a slowing of transport as endofacial sugar-binding sites become saturated (74). The potential significance of these results will be discussed below.
GLUT1 STRUCTURE
Human GLUT1 is a strongly hydrophobic protein comprising 492 amino acids. Hydropathy analysis suggests 12 hydrophobic, membrane-spanning α-helices [TMs (93)]. This conclusion is supported by scanning-glycosylation mutagenesis (55), mass spectrometry analysis of GLUT1 proteolytic cleavage sites (11), circular dichroism and fourier transform infrared spectroscopy analysis (2, 21), and analysis of the crystal structures of distantly related bacterial transporters [major facilitator superfamily (MFS) proteins] (1, 56, 109). GLUT1 presents cytoplasmic NH2 and COOH termini, a long cytoplasmic loop connecting TMs 6 and 7, a long COOH terminus, and a glycosylated extracellular loop between TMs 1 and 2. Despite its hydrophobicity, GLUT1 is readily accessible to solvent water (2, 64). Indeed, eight of the 12 GLUT1 putative TMs are amphipathic (93), suggesting that GLUT1 presents a water-filled channel for sugar translocation. Two TMs (1 and 8) are poised at the limits of bilayer solubility [TM1 is released by trypsin treatment of GLUT1, and TM 8 is released by addition of endofacial ligand to trypsinized GLUT1 (11)]. GLUT1 undergoes significant conformational change upon binding substrates (22), inhibitors (2), and ATP (10). Interactions between the GLUT1 COOH terminus and middle loop may control transport activity and are regulated by ATP (10).
GLUT1 Topology and Architecture
Our understanding of GLUT1 architecture has been shaped by three types of study: 1) biochemical analyses, 2) molecular biology/mutagenesis approaches, and 3) homology-modeling GLUT1 structure using the crystal structures of distantly related proteins from the MFS. The results of these studies have transformed our understanding of GLUT1 architecture and have revealed new insights into determinants of stereospecificity. However, much remains to be achieved before a detailed understanding of the transport mechanism emerges.
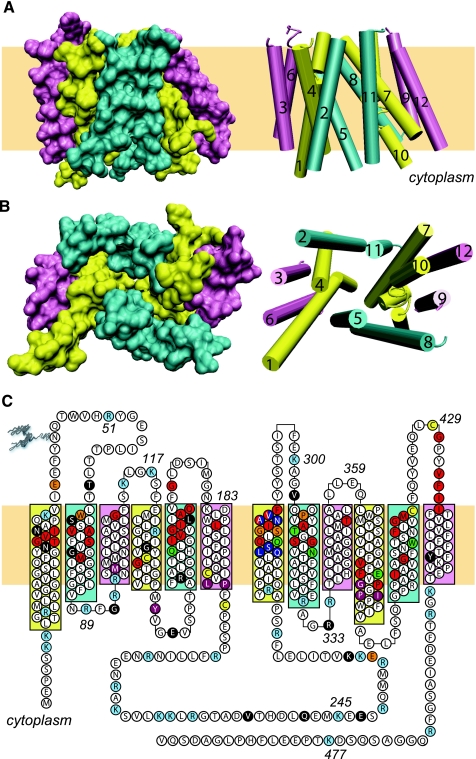
GlpT and LacY were the first MFS proteins to yield high-resolution crystal structures upon X-ray diffraction analysis (1, 56). Both structures comprise NH2-and COOH-terminal halves each containing six transmembrane α-helices (TMs). The NH2- and COOH-terminal domains are connected by a long cytoplasmic loop and, when reviewed in isolation, are almost structurally superimposable (Fig. 1A). In the crystal structure, the COOH-terminal domain is rotated 180° around the central axis normal to the plane of the bilayer (Fig. 1B). Each domain contains two strongly hydrophobic TMs (3 and 6 in the NH2-terminal domain and 9 and 12 in the COOH-terminal domain) that anchor four more amphipathic TMs (1, 2, 4, and 5 in the NH2-terminal domain and 7, 8, 10, and 11 in the COOH-terminal half). The subunit structure approaches that of a “Mayan temple with a flat rectangular top and bottom” (56). The homology-modeled GLUT1 structure (109) closely resembles GlpT and LacY structures.
Fig. 1.
Glucose transporter 1 (GLUT1) structure. A and B: GLUT1 structure as modeled in Ref. 10 and drawn using VMD (version 1.8.5). The 12 transmembrane helices are shown in 2 forms, as a surface representation (left) or as a cartoon representation (right). Extracellular and cytoplasmic structures are omitted. The cartoon representations include the transmembrane helix numbers. A: transmembrane α-helices (TMs) are shown parallel to the membrane. B: the TMs are viewed along the membrane normal from the cytoplasmic side. C: GLUT1 sequence and putative topology. Amino acids are shown using the 1 letter code. The 12 TMs are colored as in A and B. Some amino acids are numbered. Individual amino acids that are not colored white: cyan, sites of trypsin cleavage or lysine modification by N-hydroxy-succinimide esters (11); red, amino acids, which when mutagenized to cysteine are reactive with PCMBS in the external solvent (94); green, amino acids, which when mutagenized to cysteine are reactive with p-chloromercuribenzylsulfonic (PCMBS) in the external solvent in a substrate-protected manner (94); yellow, cysteine residues that are accessible to iodoacetamide (11); purple, amino acids, which when mutagenized to cysteine result in ≥90% inhibition of GLUT1 (94); orange, putative substrate-binding sites predicted by docking studies (27, 109); blue, amino acids implicated in substrate discrimination (87); black, sites at which mutations cause GLUT1 deficiency syndrome (68, 124). Some amino acids fall into multiple categories.
The MFS structure presents an open cavity to cytoplasm and appears to be sealed to the interstitium. This structure is visually consistent with theoretical models for carrier-mediated sugar transport in which GLUT1 isomerizes between import and export states and may represent the export (also called the e1) state of GLUT1. No MFS protein structures representing the import (or e2) state have been described to date; hence, it is possible that the Mayan temple structure represents a more fixed or rigid architecture and that the flexible domains associated with transport conformations are more deeply buried within the temple's core.
Blodgett et al. (11) have compared the proposed topology of GLUT1 (109) with the trypsin and N-hydroxy-succinimide (NHS)-ester accessibility of GLUT1. Making the assumption that trypsin cleavage, lysine modification, and cysteine modification do not radically alter the architecture of GLUT1, only minor adjustments in the threaded topology of GLUT1 are necessary. These include adjustments to TM5, loops 5 and 6, and TM6 and TM12. Figure 1C summarizes the proposed modified GLUT1 topology and highlights seven different categories of amino acids: 1) residues that are accessible to trypsin, NHS-LC-biotin, and iodoacetamide (11), 2) residues that when mutagenized to cysteine allow sugar transport inhibition by extracellular p-chloromercuribenzylsulfonic acid (PCMBS) (94), 3) amino acids that when mutagenized to cysteine afford sugar-dependent protection against transport inhibition by PCMBS (94), 4) residues whose substitution by cysteine results in transport inhibition (94), 5) amino acids whose mutagenesis results in GLUT1 deficiency syndrome (68, 124), 6) amino acids whose positions are inferred glucose-binding sites in docking studies with Glpt-threaded GLUT1 structures (27, 109), and 7) residues thought to discriminate between glucose and fructose (87).
Oligomeric Structure
The crystal structure of the MFS protein LacY, which contains a bound substrate, suggests that the catalytic unit of the transporter (and that of its structurally similar, relative GlpT) is the protein monomer. Studies with purified GLUT1 indicate that reduced GLUT1 binds 1 mol cytochalasin B/mol GLUT1, whereas nonreduced GLUT1 binds 0.5 mol cytochalasin B/mol GLUT1 (49, 117, 132). This suggests that each reduced GLUT1 molecule provides one fully functional sugar export site, and since exofacial ligands competitively displace cytochalasin B, each subunit also presents a sugar import site. Therefore, it is probable that each GLUT1 molecule represents a fully functional catalytic unit. Freeze-fracture electron microscopy suggests that reduced GLUT1 is a dimer (40, 117) and that nonreduced GLUT1 is a tetramer. Hydrodynamic size analysis of detergent-solubilized GLUT1 and chemical cross-linking studies support this conclusion (40, 49, 133), although some detergents disrupt GLUT1 oligomeric structure (40, 43).
Coimmunoprecipitation studies using GLUT1–GLUT4 chimeras also confirm that GLUT1 forms oligomeric complexes (104) but that GLUT heterocomplexes are not found. Unpublished findings using GLUT1–GLUT3 chimeras demonstrate that GLUT1 TM9 is essential for GLUT1 dimerization (Levine KB, DeZutter J, and Carruthers A, unpublished observations). The reductant sensitivity of tetrameric GLUT1 has been ascribed to a specific transporter fold deriving from an intramolecular disulfide bridge or from two mixed disulfides (involving Cys347 and Cys421) present in each subunit (49, 133).
Functional coexpression studies in Xenopus oocytes suggest that mutant forms of GLUT2 and GLUT3 do not suppress the activity of wild-type GLUT (12). Heterologous expression of mutant GLUT1 in mammalian cells demonstrates either unaltered parental GLUT1 activity (59, 101) or modified parental GLUT1 activity (76, 77). We have proposed that dimeric GLUT1 (observed with reduced GLUT1 or GLUT1 expressed in Xenopus oocytes) comprises two structurally associated but functionally independent GLUT1 subunits (133). Tetrameric (nonreduced) GLUT1 comprises a dimer of GLUT1 dimers in which subunits interact cooperatively. The net result is a complex simultaneously presenting at least two exofacial and two endofacial ligand-binding sites (25, 41, 133).
Unifying Model for Structure and Transport
Recent docking studies have suggested that GLUT1 presents two exofacial and endofacial glucose-binding sites connected by a cavity sufficiently large to contain a glucose molecule (27, 109). It should be noted, however, that homology modeling is not immune to overinterpretation. Homology-modeled LacY structure using GlpT as a template approximates transporter topology and architecture but less successfully reproduces the spatial arrangement of amino residues involved in substrate binding to LacY (75). Naftalin has suggested that the simple carrier hypothesis is flawed on thermodynamic grounds (99). This dogma-challenging hypothesis reasons that the high-affinity exofacial form of GLUT1 (e2) must reorient to a high-affinity endofacial form before relaxing to the low-affinity endofacial form (e1). Similarly, the low-affinity endofacial form e1 must isomerize to a low-affinity exofacial form before relaxing to the high-affinity exofacial state e2.
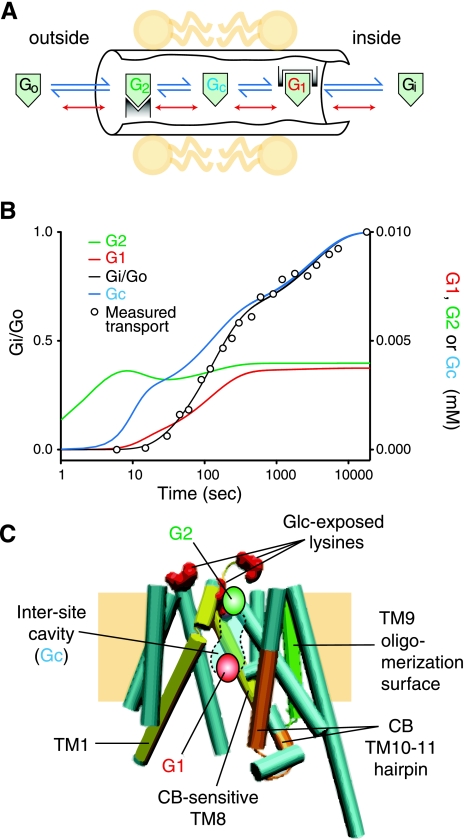
Previous studies have demonstrated that the complexities of erythrocyte sugar transport are not explained by classical simple carrier or fixed-site transporter models (see above) (Fig. 2). Naftalin proposes a variant of the fixed-site transporter in which sugar can bind to coexistent endo- and exofacial sugar-binding sites and exchanges between these sites via a connecting central cavity. Exchange between binding sites and cavity is accelerated when binding sites are occupied by sugar via a process termed “geminate exchange”. This model not only explains the stereospecificity and complexity of steady-state sugar transport (99) but also accounts for multiphasic sugar transport kinetics (74), the ability of GLUT1 to trap or occlude a sugar molecule within a central cavity (9), and cooperative, multisite ligand binding (41, 71). The enhanced cooperativity of tetrameric GLUT1 may result from additional subunit interface interactions that are present in the tetramer. Future structural, mutagenesis, and ligand-binding studies will resolve this question by demonstrating coexistent or mutually exclusive import and export sites.
Fig. 2.
Model for GLUT1-mediated sugar transport. A: schematic representation of the catalytic center of the transporter. Extracellular sugar (Go) and intracellular sugar (Gi) react with exo- and endofacial sites, respectively, to form G2 and G1, respectively. Sugar dissociates from these sites into the intersite cavity to form Gc. When G2 and G1 are occupied, dissociation to Gc and reassociation are accelerated (red dissociation steps; adapted from Ref. 99). B: simulation of biphasic exchange transport by this carrier mechanism (adapted from Ref. 74). C: The GLUT1 model (adapted from Fig. 1B, with TMs 2 and 11 removed for clarity) showing Gi complexed to the G1 site, a putative G2 site, the small intersite cavity (109), the TM9 oligomerization surface (Levine KB, DeZutter J, and Carruthers A, unpublished observations), the 2 conformationally dynamic TMs (1 and 8) that are released into the aqueous solvent when the GLUT1 backbone is cleaved by trypsin (11), the 3 exofacial lysine residues exposed by d-glucose (11), and the putative cytochalasin B-binding hairpin (11, 109).
Cell Biology of GLUT1
Approximately 20 to 40% of total cellular GLUT1 is expressed at the cell surface of cardiomyocytes (84), blood-brain barrier endothelial cells (116), adipocytes (54), and astrocytes (85). Intracellular GLUT1 is located within the endoplasmic reticulum, the Golgi, and endosomes (84). Endosomal GLUT1 cycles between the plasma membrane and endosomal compartments in cardiomyocytes in an AMP kinase- and phosphatidylinositol 3-kinase-dependent manner (84), but this recycling compartment is distinct from that of the insulin-responsive glucose transporter isoform GLUT4 in adipose, heart, and muscle (89). It has been suggested that acute regulation of cell surface GLUT1 levels could provide an effective mechanism for controlling sugar transport across the blood-brain barrier and in astrocytes (116), but supporting evidence is unavailable.
Early Implantation and Embryo Development
GLUT1 plays an important role in mediating implantation of the embryo. This is a highly synchronized process that occurs between an activated blastocyst and the receiving endometrium. Investigators have employed endometrial stromal cells in both mouse and human and observed that a fine balance has to be struck between the two sex steroids, namely progesterone and estrogen. Although estrogen primes the endometrium, it is progesterone that transforms the endometrium to provide a receptive environment for the embryo. Progesterone increases GLUT1 and GLUT1-dependent glucose uptake in endometrial stromal cells. However, estrogen demonstrates no effect on its own, but when introduced in the presence of progesterone, estrogen brings the progesterone-induced increased GLUT1 expression and glucose uptake back to normal untreated concentrations. Furthermore, in the presence of a progesterone receptor antagonist (RU-486), progesterone effect on GLUT1 and glucose uptake is abolished. Thus it appears that the progesterone-induced receptivity by the endometrium is reliant on GLUT1-mediated glucose uptake by the endometrial stromal cells (33).
In preimplantation embryos, GLUT1 is expressed on the basolateral surface of the polarized trophectodermal cells and the inner cell mass. Maternal diabetes with hyperglycemia causes a suppression of GLUT1 expression in the preimplantation embryos at 48 and 96 h after conception (92). This suppression interferes with glucose uptake, resulting in upregulation of Bax and DNA fragmentation consistent with apoptosis (91). Maternal diabetes in Bax−/− genotypic mice resulted in suppression of GLUT1 expression and glucose uptake without an increase in apoptosis, confirming a role for Bax in mediating apoptosis due to GLUT1 reduction (20). More importantly, during the late-gestation stages of fetal development, GLUT1 is expressed by most tissues; specifically at e10, it is noted in the neural tube, gut, heart, and optic vesicle (90). As the fetus matures into late gestation, most tissues demonstrate GLUT1 expression.
Regulation of GLUT1 Expression
Postnatally, through the suckling phase of life, various tissues such as brain (30, 67, 108), skeletal muscle (108, 112), and myocardium (112) reveal high concentrations of GLUT1 expression. In the late-gestation ovine fetus, insulin and glucocorticoids regulate skeletal muscle GLUT1 expression by increasing the concentrations (39, 47). After weaning during the postsuckling phase and beyond into adult stages, there is a decline in GLUT1 expression in most tissues except the brain (30, 67, 108, 112). This decline is replaced by the emergence of tissue-specific isoforms such as the insulin-responsive GLUT4 in skeletal muscle and myocardium (108, 112). GLUT1 is expressed in white adipocytes even in the adult, suggesting a mechanism of basal glucose uptake by these cells, which provides a storage function for the body (65).
In addition to the developmental regulation noted with GLUT1 expression in various tissues, perturbations in circulating glucose concentrations demonstrate an ability to regulate GLUT1 expression (114). Furthermore, hypoxic conditions as encountered in cancerous transformation or otherwise result in enhanced GLUT1 expression (19). Regulation of GLUT1 expression occurs at two levels; the first is transcriptional and the second is posttranscriptional. Transcriptional regulation involves transactivation by Sp1 and repression by Sp3. The ratio of these two nuclear factors determines the ultimate expression levels of GLUT1 mRNA and protein (57, 110). Developmental regulation consisting of a postsuckling decline in myocardial GLUT1 expression is mediated by myoD that inhibits Sp1-induced transactivation of the GLUT1 promoter (123).
In addition, specific RNA-binding proteins are known to bind a consensus sequence at the 3′ end of the GLUT1 gene-mediating mRNA stabilization of the GLUT1 gene. Studies employing the bovine GLUT1 gene revealed nucleotides 2,181–2,190 to bind cytosolic proteins and provide mRNA stability (35). Glucose deprivation and hypoxia-induced increase in GLUT1 expression are mediated by this cis-acting regulatory element in the 3′-untranslated region (UTR) of the GLUT1 gene (35, 42). Furthermore, RNA-binding proteins hnRNP A2 and hnRNP L, which complex with each other and independently bind the AU-rich response element in the GLUT1 3′-UTR, regulate expression by translational repression and mRNA instability (42). Hypoxia and hypoglycemia both selectively decreased polysomal hnRNP A2 and L protein concentrations, thereby contributing to increased GLUT1 mRNA stability and translation efficiency with resultant increased GLUT1 expression (42). Other investigations have demonstrated binding of the RNA-binding protein that belongs to the Hu/ELAV family (HuR) with the nucleocytoplasmic shuttling sequence that functions as an adaptor protein in the nuclear export of mRNAs that contain adenylate/uridylate-rich elements in the 3′-UTR, thus contributing while in the cytoplasm toward mRNA stability and translational efficiency (35). In terminally differentiated adipocytes alone, HuR complexes with EBPβ and translocates to the cytosol, where HuR binds the GLUT1 3′-UTR, thereby leading to increased expression of GLUT1. In contrast, in undifferentiated preadipocytes, HuR was observed within the nucleus alone (35).
Placental Expression of GLUT1
GLUT1 is considered the major glucose transporter isoform of the syncytiotrophoblastic cells of the human placenta (58). Increased concentrations are observed on the microvillous surface of the trophoblastic layer, which is the maternal surface, as opposed to the basal layer facing the fetus (58). Investigations in the human diabetic placenta have revealed an increase in GLUT1 concentrations that correlates with a large infant (58). In contrast, the presence of intrauterine growth restriction revealed a decrease in placental GLUT1 concentrations. Although GLUT3, a related isoform also responsible for basal glucose transport, is expressed in cytotrophoblasts during early gestation, its expression during late gestation is limited to the fetal vascular endothelium (70). Various investigations in mice/rats reveal that, unlike the human placenta, GLUT1 coexists with a related isoform, GLUT3, in syncytiotrophoblast layers of the placental labrynthine region throughout gestation. GLUT1 is noted on the maternal and fetal-facing surfaces of syncytiotrophoblast layers I and II in rats (113). Maternal diabetes in the streptozotocin-treated pregnant rat and the nonobese diabetic mouse failed to alter placental GLUT1 expression (28, 29). In contrast, intrauterine growth restriction in rats due to uterine artery ligation led to a 50% decrease in GLUT1 concentrations (28). In contrast, more recent experiments in high-fat-fed pregnant mice demonstrated increased transplacental glucose transport mediated by increased placental GLUT1 concentrations during late gestation with resulting large fetuses (62). In chronic experiments of hyperglycemia in the sheep fetus, an initial increase in placental GLUT1 over 48 h was observed, followed ultimately by a decline (28). In contrast, insulin-induced chronic maternal hypoglycemia caused a decline in placental GLUT1 concentrations (29). Thus it appears that placental GLUT1 plays a vital role in mediating materno-placental glucose transport, which in turn regulates the placento-fetal transport of glucose via the coordinate expression of GLUT3 (113).
Blood-Brain Barrier GLUT1
The endothelial cells of the brain microvasculature, the astrocytes, and the choroid plexus also express GLUT1. In the endothelial cells, GLUT1 plays a vital role in brain glucose uptake. Circulating glucose concentrations regulate the endothelial GLUT1 protein concentrations (30). Whereas chronic hypoglycemia upregulates GLUT1 concentrations, hyperglycemia has no effect (114). The presence of GLUT1 on astrocytes suggests that glucose taken up by these cells is converted into lactate and released into the interstitium as an alternate substrate to be utilized by neurons, particularly in the absence of an adequate supply of glucose (103). Although controversial (115), this concept supports astrocytes as local energy storage sites necessary to fuel neurons, providing credence to the astrocyte-neuron shuttle theory (103). Further provision of an n-3 polyunsaturated fatty acid-deficient diet (docosahexaenoic and eicosapentaenoic acids) in rats led to a decrease in microvascular GLUT1 expression, whereas an n-3 polyunsaturated fatty acid-rich supplemented diet increased this protein (105).
Blood-Retinal and Blood-Neural Barrier GLUT1
The blood-retinal barrier consists of the retinal pigmented epithelial cells that are rich in GLUT1 (88). In addition to expression in the retinal pigmented epithelial cells, the choroidal, iridial, and pars planus, the retinal Mueller cells, the lens fiber cells, the iridial microvascular endothelial cells, and to a lesser extent the outer segments of the photoreceptor cells of the adult human eye expressed GLUT1. This distribution pattern was conserved throughout development and was evident as early as 8 wk gestation in the human embryo (88). In addition, the endothelial cells of vitreous hyaloid vessels expressed GLUT1 at 8 wk gestation (88). Diabetes mellitus decreases GLUT1 expression in the vascular endothelium (4), whereas it increases GLUT1 in the blood-retinal barrier (72), thereby mediating increased glucose transport across the blood-retinal barrier. Further vascular endothelial growth factor mediates this increase in GLUT1 of the vascular endothelial cells (119). The presence of a polymorphism located in the GLUT1 gene was associated with diabetic nephropathy but not with diabetic retinopathy, with GLUT1 being expressed primarily in the mesangial cells of the kidney (53).
Similarly, the perineurial cells that form the blood-nerve barrier also demonstrate GLUT1 expression. Peripheral nerves are ensheathed by layers of flattened fibroblast-like cells that form the perineurium. The perineurium and the vascular endothelium together form the blood-nerve barrier, and both are rich in GLUT1. This isoform mediates transport of glucose to the peripheral nerves (122). Thus, under conditions of hyperglycemia related to diabetes, increased glucose transport across this barrier may mediate peripheral neuropathy. However, in vitro cultures of the sciatic nerve in the presence of 25 mM glucose demonstrated a downregulation of GLUT1 expression (95). Whether similar changes occur in vivo remains to be established.
GLUT1 Overexpression Investigations
Transgenic overexpression of human GLUT1 in skeletal muscle led to a large increase in basal glucose transport and metabolism but impaired stimulation of insulin-induced glucose transport, resulting in reduction in fasting and fed circulating glucose concentrations with enhanced glucose clearance. However, overexpression of GLUT1 in skeletal muscle (extensor digitorum longus) did not interfere with the insulin-responsive translocation of GLUT4 but rather dissociated the intrinsic activity of GLUT4 from the plasma membrane concentrations of the protein (32, 45). In contrast, when transgenic overexpression of GLUT1 was accomplished in myocardium, a remodeling of fatty acid and ketone metabolic pathways was observed to accommodate the chronic increase in intracellular glucose concentrations targeted at maintaining cardiac energy and function. However, when exposed to a high-fat diet, the GLUT1 transgenic mice suffered from increased oxidative stress and contractile dysfunction (130).
GLUT1 Null Mutations
Various groups have created mutations in the GLUT1 gene to assess its functional role during development. Initially, GLUT1-deficient transgenic mice were produced using antisense-GLUT1 constructs. In preimplantation embryos obtained from these mice, glucose uptake was reduced compared with wild-type mice (51). In addition, homozygous GLUT1 mutant mouse embryos obtained from the antisense-GLUT1 transgenic mouse mating revealed severely impaired organogenesis consisting of caudal regression, agenesis of kidneys, stunted growth, absent head, microphthalmia, and other malformations reminiscent of the human infant of a hyperglycemic diabetic mother (51). The rate of still births was higher than that seen in heterozygous mating of the antisense-GLUT1 and wild-type mice. The offspring of this mating were stunted in growth but achieved adulthood with no differences in circulating blood glucose concentrations, but they demonstrated the antisense-GLUT1 transcript in the heart, spleen, lung, skeletal muscle, liver, kidney, and adipose tissue (51). In these heterozygous mice, nitric oxide-dependent endothelial relaxation in aorta was reduced, supporting an important role for GLUT1-dependent glucose metabolism in regulating endothelial function (102).
Using morpholinos and creating loss of GLUT1 function in the Xenopus (frog) embryo resulted in microcephaly and an axis elongation error. Furthermore, these morpholinos against GLUT1 suppressed cell movement in dorsal marginal zones, supporting an important role for GLUT1 during gastrulation cell movement (121). Using morpholinos targeted against GLUT1 in a Danio rerio (zebra fish) embryonic model, a decline in embryonic growth along with disruption of the developing brain structure was evident secondary to apoptosis (61). This GLUT1-deficient apoptosis was Bax dependent (61). In separate investigations, the study of murine embryonic stem cells (GLUT1+/−) that demonstrate GLUT1 deficiency revealed a reduction of glucose uptake but enhanced apoptosis. Furthermore, inhibition of oxidative phosphorylation led to impaired upregulation of GLUT1 protein in GLUT1+/− embryonic stem cells, which was evident in wild-type cells (50).
Two different groups created GLUT1-null mutant mice, disrupting exon 1 of the gene (100, 125). The homozygous null mutation demonstrated embryonic lethality at e10 through e14. These embryos at e13 revealed morphological abnormalities consisting of a small size, lack of eyes, diminutive rostral embryonic pole, and overall developmental delay vs. the wild-type littermates. No live birth occurred in any of them. In contrast, the heterozygous null mutation led to no decrease in body weight but a decrease in brain weight and hypoglycorrhachia in the absence of hypoglycemia. Furthermore, abnormal motor performance was seen in the rotorod test that emerged early in adult life, only to worsen with advancing age. Beam walking revealed abnormalities in balance and motor coordination, and the footprint test showed no difference in stride length or hindpaw base during gait (125). Further spontaneous seizures were observed along with a decline in brain glucose uptake, as observed with positron emission tomography. These findings in mice paralleled that observed in human infants with the GLUT1 deficiency syndrome (G1DS) (31, 68).
G1DS
G1DS is an autosomal dominant disorder where differing mutations have been described, resulting in haploinsufficiency of GLUT1. This interferes with red blood cell glucose transport and produces hypoglycorrhachia with unperturbed lactic acid in the cerebrospinal fluid, the presence of seizures, abnormal gait, and developmental delays. Introduction of ketogenic diet early during infancy through teenage years ameliorates seizures, although resurgence can be encountered despite the ketogenic diet requiring additional anticonvulsant therapy. However, ketogenic diet does not reverse the neurobehavioral findings or developmental delays (31, 68).
Further investigation of GLUT1-haplodeficient mice revealed that the peak decline in brain GLUT1 occurred at P14, with a compensatory increase in Mct1 (moncarboxylate transporter mediates ketone, lactate, and pyruvate transport) seen at P0. However, in the adult brain, a compensatory increase in GLUT1 expression occurred, reaching levels seen in wild-type mice (100). The functional implications of these developmentally sensitive observations remain to be determined. Fifty percent of adult patients with G1DS are able to function well within a social context, but the rest are greatly disadvantaged due to neurodevelopmental impairments. In various experiments, phenobarbital has been observed to reduce GLUT1 protein concentrations. These observations have led to an avoidance of this anticonvulsant in infants suspected of being afflicted by G1DS. Furthermore, other classes of drugs such as methyl xanthines, caffeine, and tricyclic antidepressants known to interfere with GLUT1 function are avoided (31, 68). Hence, the mouse models of GLUT1 deficiency have provided valuable insights in understanding this syndrome that affects humans.
GLUT1 Functions Coordinately With GLUT3 in Trophectoderm/Placenta and Brain
Although it is clear that GLUT1 is essential for normal embryonic development, it is incapable of salvaging an embryo in the absence of a closely related isoform, namely GLUT3 (Km 0.8 mM). Thus, in the presence of a homozygous null mutation of GLUT3, despite the presence of intact GLUT1, there is murine embryonic loss at e8.5. Thus neither GLUT1 nor GLUT3 alone is sufficient for the well being of the early embryo (34). In contrast, in another related isoform, namely GLUT8 when mutated, the homozygous null mutant mice are born normally and progress to adulthood (111). More importantly, in heterozygous null mutation of GLUT3 in the presence of intact GLUT1 in the placenta, a decrease in transplacental glucose transport to the fetus is observed. Again, this observation attests to the importance of coordinated functionality of GLUT1 with GLUT3 in mediating transplacental glucose transport (34). Furthermore, recent studies in the heterozygous GLUT3 mutant mouse revealed that there was a compensatory increase in brain GLUT1 expression resulting in preserving glucose transport across the blood-brain barrier (131). However, since GLUT1 was expressed only in astrocytes and not neurons, despite this compensation by GLUT1 in brain, electroencephalographic seizures and cognitive deficits were observed (131). Therefore, it appears that both GLUT1 and GLUT3 are necessary for normal brain development and function (125, 131). Thus there appears to be an interdependency of GLUT1 and GLUT3 that is coordinately expressed in certain tissues toward adequately mediating the glucose transport function necessary to meet the tissue requirements or that of other cells/tissues present beyond the barrier.
GRANTS
S. U. Devaskar is supported by National Institute of Child Health and Human Development Grants HD-33997, HD-46979, HD-41230, and HD-25024. A. Carruthers is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-36081 and DK-44888.
REFERENCES
- 1.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science 301: 610–615, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez J, Lee DC, Baldwin SA, Chapman D. Fourier transform infrared spectroscopic study of the structure and conformational changes of the human erythrocyte glucose transporter. J Biol Chem 262: 3502–3509, 1987 [PubMed] [Google Scholar]
- 3.Appleman JR, Lienhard GE. Kinetics of the purified glucose transporter. Direct measurement of the rates of interconversion of transporter conformers. Biochemistry 28: 8221–8227, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Badr GA, Tang J, Ismail-Beigi F, Kern TS. Diabetes downregulates Glut1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes 49: 1016–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Baker GF, Naftalin RJ. Evidence of multiple operational affinities for d-glucose inside the human erythrocyte membrane. Biochim Biophys Acta 550: 474–484, 1979 [DOI] [PubMed] [Google Scholar]
- 6.Baldwin SA, Baldwin JM, Gorga FR, Lienhard GE. Purification of the cytochalasin B binding component of the human erythrocyte monosaccharide transport system. Biochim Biophys Acta 552: 183–188, 1979 [DOI] [PubMed] [Google Scholar]
- 7.Barnett JE, Holman GD, Chalkley RA, Munday KA. Evidence for two asymmetric conformational states in the human erythrocyte sugar-transport system. Biochem J 145: 417–429, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett JE, Holman GD, Munday KA. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem J 131: 211–221, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blodgett DM, Carruthers A. Quench-flow analysis reveals multiple phases of GluT1-mediated sugar transport. Biochemistry 44: 2650–2660, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Blodgett DM, De Zutter JK, Levine KB, Karim P, Carruthers A. Structural basis of GLUT1 inhibition by cytoplasmic ATP. J Gen Physiol 130: 157–168, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blodgett DM, Graybill C, Carruthers A. Analysis of glucose transporter topology and structural dynamics. J Biol Chem 283: 36416–36424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burant CF, Bell GI. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry 31: 10414–10420, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Carruthers A. ATP regulation of the human red cell sugar transporter. J Biol Chem 261: 11028–11037, 1986 [PubMed] [Google Scholar]
- 14.Carruthers A. The glucose transporter reconsidered. Trends Biochem Sci 13: 426–428, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Carruthers A. Mechanisms for the facilitated diffusion of substrates across cell membranes. Biochemistry 30: 3898–3906, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Carruthers A, Helgerson AL. The human erythrocyte sugar transporter is also a nucleotide binding protein. Biochemistry 28: 8337–8346, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Carruthers A, Helgerson AL. Inhibitions of sugar transport produced by ligands binding at opposite sides of the membrane. Evidence for simultaneous occupation of the carrier by maltose and cytochalasin B. Biochemistry 30: 3907–3915, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Carruthers A, Melchior DL. Asymmetric or symmetric? Cytosolic modulation of human erythrocyte hexose transfer. Biochim Biophys Acta 728: 254–266, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem 276: 9519–9525, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem 275: 41052–41057, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Chin JJ, Jung EK, Chen V, Jung CY. Structural basis of human erythrocyte glucose transporter function in proteoliposome vesicles: circular dichroism measurements. Proc Natl Acad Sci USA 84: 4113–4116, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin JJ, Jung EK, Jung CY. Structural basis of human erythrocyte glucose transporter function in reconstituted vesicles. J Biol Chem 261: 7101–7104, 1986 [PubMed] [Google Scholar]
- 23.Cloherty EK, Diamond DL, Heard KS, Carruthers A. Regulation of GLUT1-mediated sugar transport by an antiport/uniport switch mechanism. Biochemistry 35: 13231–13239, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Cloherty EK, Heard KS, Carruthers A. Human erythrocyte sugar transport is incompatible with available carrier models. Biochemistry 35: 10411–10421, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Cloherty EK, Levine KB, Carruthers A. The red blood cell glucose transporter presents multiple, nucleotide-sensitive sugar exit sites. Biochemistry 40: 15549–15561, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Craik JD, Young JD, Cheeseman CI. GLUT-1 mediation of rapid glucose transport in dolphin (Tursiops truncatus) red blood cells. Am J Physiol Regul Integr Comp Physiol 274: R112–R119, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Cunningham P, Afzal-Ahmed I, Naftalin RJ. Docking studies show that d-glucose and quercetin slide through the transporter GLUT1. J Biol Chem 281: 5797–5803, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Das UG, Sadiq HF, Soares MJ, Hay WW, Jr, Devaskar SU. Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am J Physiol Regul Integr Comp Physiol 274: R339–R347, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Devaskar SU, Devaskar UP, Schroeder RE, deMello D, Fiedorek FT, Jr, Mueckler M. Expression of genes involved in placental glucose uptake and transport in the nonobese diabetic mouse pregnancy. Am J Obstet Gynecol 171: 1316–1323, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Devaskar S, Zahm DS, Holtzclaw L, Chundu K, Wadzinski BE. Developmental regulation of the distribution of rat brain insulin-insensitive (Glut1) glucose transporter. Endocrinology 129: 1530–1540, 1991 [DOI] [PubMed] [Google Scholar]
- 31.De Vivo DC, Leary L, Wang D. Glucose transporter 1 deficiency syndrome and other glycolytic defects. J Child Neurol 17, Suppl 3: 3S15–3S23, 2002 [PubMed] [Google Scholar]
- 32.Etgen GJ, Zavadoski WJ, Holman GD, Gibbs EM. Insulin-sensitive regulation of glucose transport and GLUT4 translocation in skeletal muscle of GLUT1 transgenic mice. Biochem J 337: 51–57, 1999 [PMC free article] [PubMed] [Google Scholar]
- 33.Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology 150: 1512–1520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganguly A, McKnight RA, Raychaudhuri S, Shin BC, Ma Z, Moley K, Devaskar SU. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab 292: E1241–E1255, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Gantt KR, Cherry J, Richardson M, Karschner V, Atasoy U, Pekala PH. The regulation of glucose transporter (GLUT1) expression by the RNA binding protein HuR. J Cell Biochem 99: 565–574, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Ginsburg H, Stein WD. Zero-trans and infinite-cis uptake of galactose in human erythrocytes. Biochim Biophys Acta 382: 353–368, 1975 [DOI] [PubMed] [Google Scholar]
- 37.Gorga FR, Lienhard GE. Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry 20: 5108–5113, 1981 [DOI] [PubMed] [Google Scholar]
- 38.Gorga FR, Lienhard GE. Changes in the intrinsic fluorescence of the human erythrocyte monosaccharide transporter upon ligand binding. Biochemistry 21: 1905–1908, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Gray S, Stonestreet BS, Thamotharan S, Sadowska GB, Daood M, Watchko J, Devaskar SU. Skeletal muscle glucose transporter protein responses to antenatal glucocorticoids in the ovine fetus. J Endocrinol 189: 219–229, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Graybill C, van Hoek AN, Desai D, Carruthers AM, Carruthers A. Ultrastructure of human erythrocyte GLUT1. Biochemistry 45: 8096–8107, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Hamill S, Cloherty EK, Carruthers A. The human erythrocyte sugar transporter presents two sugar import sites. Biochemistry 38: 16974–16983, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Hamilton BJ, Nichols RC, Tsukamoto H, Boado RJ, Pardridge WM, Rigby WF. hnRNP A2 and hnRNP L bind the 3′′UTR of glucose transporter 1 mRNA and exist as a complex in vivo. Biochem Biophys Res Commun 261: 646–651, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Haneskog L, Andersson L, Brekkan E, Englund AK, Kameyama K, Liljas L, Greijer E, Fischbarg J, Lundahl P. Monomeric human red cell glucose transporter (Glut1) in non-ionic detergent solution and a semi-elliptical torus model for detergent binding to membrane proteins. Biochim Biophys Acta 1282: 39–47, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Hankin BL, Lieb WR, Stein WD. Rejection criteria for the asymmetric carrier and their application to glucose transport in the human red blood cell. Biochim Biophys Acta 288: 114–126, 1972 [DOI] [PubMed] [Google Scholar]
- 45.Hansen PA, Wang W, Marshall BA, Holloszy JO, Mueckler M. Dissociation of GLUT4 translocation and insulin induced glucose transport in transgenic mice overexpressing Glut1 in skeletal muscle. J Biol Chem 273: 18173–18179, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Harris EJ. An analytical study of the kinetics of glucose movement in human erythrocytes. J Physiol 173: 344–353, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He J, Thamotharan M, Devaskar SU. Insulin-induced translocation of facilitative glucose transporters in fetal/neonatal rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 284: R1138–R1146, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Hebert DN, Carruthers A. Cholate-solubilized erythrocyte glucose transporters exist as a mixture of homodimers and homotetramers. Biochemistry 30: 4654–4658, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Hebert DN, Carruthers A. Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1. J Biol Chem 267: 23829–23838, 1992 [PubMed] [Google Scholar]
- 50.Heilig C, Brosius F, Siu B, Concepcion L, Mortensen R, Heilig K, Zhu M, Weldon R, Wu G, Conner D. Implications of glucose transporter protein type 1 (GLUT1)-haplodeficiency in embryonic stem cells for their survival in response to hypoxic stress. Am J Pathol 163: 1873–1885, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heilig CW, Saunders T, Brosius FC 3rd, Moley K, Heilig K, Baggs R, Guo L, Conner D. Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc Natl Acad Sci USA 100: 15613–15618, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helgerson AL, Carruthers A. Equilibrium ligand binding to the human erythrocyte sugar transporter. Evidence for two sugar-binding sites per carrier. J Biol Chem 262: 5464–5475, 1987 [PubMed] [Google Scholar]
- 53.Hodgkinson AD, Millward BA, Demaine AG. Polymorphisms of the glucose transporter (GLUT1) gene are associated with diabetic nephropathy. Kidney Int 59: 985–989, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Holman GD, Kozka IJ, Clark AE, Flower CJ, Saltis J, Habberfield AD, Simpson IA, Cushman SW. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J Biol Chem 265: 18172–18179, 1990 [PubMed] [Google Scholar]
- 55.Hresko RC, Kruse M, Strube M, Mueckler M. Topology of the Glut 1 glucose transporter deduced from glycosylation scanning mutagenesis. J Biol Chem 269: 20482–20488, 1994 [PubMed] [Google Scholar]
- 56.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 301: 616–620, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Hwang DY, Ismail-Beigi F. Control of Glut1 promoter activity under basal conditions and in response to hyperosmolarity: role of Sp1. Am J Physiol Cell Physiol 290: C337–C344, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Illsley NP. Glucose transporters in the human placenta. Placenta 21: 14–22, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Ishihara H, Asano T, Katagiri H, Lin JL, Tsukuda K, Shibasaki Y, Yazaki Y, Oka Y. The glucose transport activity of GLUT1 is markedly decreased by substitution of a single amino acid with a different charge at residue 415. Biochem Biophys Res Commun 176: 922–930, 1991 [DOI] [PubMed] [Google Scholar]
- 60.Jardetzky O. Simple allosteric model for membrane pumps. Nature 211: 969–970, 1966 [DOI] [PubMed] [Google Scholar]
- 61.Jensen PJ, Gitlin JD, Carayannopoulos MO. GLUT1 deficiency links nutrient availability and apoptosis during embryonic development. J Biol Chem 281: 13382–13387, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 23: 271–278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schürmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 282: E974–E976, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Jung EK, Chin JJ, Jung CY. Structural basis of human erythrocyte glucose transporter function in reconstituted system. Hydrogen exchange. J Biol Chem 261: 9155–9160, 1986 [PubMed] [Google Scholar]
- 65.Kamel AF, Norgren S, Strigard K, Thorne A, Fakhrai-Rad H, Galli J, Marcus C. Age-dependent regulation of lipogenesis in human and rat adipocytes. J Clin Endocrinol Metab 89: 4601–4606, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Kasahara M, Hinkle PC. Reconstitution and purification of the d-glucose transporter from human erythrocytes. J Biol Chem 253: 7384–7390, 1977 [PubMed] [Google Scholar]
- 67.Khan JY, Rajakumar RA, McKnight RA, Devaskar UP, Devaskar SU. Developmental regulation of genes mediating murine brain glucose uptake. Am J Physiol Regul Integr Comp Physiol 276: R892–R900, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Klepper J, Leiendecker B. Glut1 deficiency syndrome—2007 update. Dev Med Child Neurol 49: 707–716, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Korgun ET, Celik-Ozenci C, Seval Y, Desoye G, Demir R. Do glucose transporters have other roles in addition to placental glucose transport during early pregnancy? Histochem Cell Biol 123: 621–629, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Krupka RM, Devés R. An experimental test for cyclic versus linear transport models. The mechanisms of glucose and choline transport in erythrocytes. J Biol Chem 256: 5410–5416, 1981 [PubMed] [Google Scholar]
- 72.Kumagai AK, Vinores SA, Pardridge WM. Pathological upregulation of inner blood-retinal barrier Glut1 glucose transporter. Brain Res 706: 313–317, 1996 [DOI] [PubMed] [Google Scholar]
- 73.Lacko L, Wittke B, Geck P. The temperature dependence of the exchange transport of glucose in human erythrocytes. J Cell Physiol 82: 213–218, 1973 [DOI] [PubMed] [Google Scholar]
- 74.Leitch JM, Carruthers A. αα- and ββ-Monosaccharide transport in human erythrocytes. Am J Physiol Cell Physiol 296: C151–C161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lemieux MJ. Eukaryotic major facilitator superfamily transporter modeling based on the prokaryotic GlpT crystal structure. Mol Membr Biol 24: 333–341, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Levine KB, Hamill S, Cloherty EK, Carruthers A. Alanine scanning mutagenesis of the human erythrocyte glucose transporter putative ATP binding domain. Blood Cells Mol Dis 27: 139–142, 2001 [DOI] [PubMed] [Google Scholar]
- 77.Levine KB, Robichaud TK, Hamill S, Sultzman LA, Carruthers A. Properties of the human erythrocyte glucose transport protein are determined by cellular context. Biochemistry 44: 5606–5616, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Lieb WR, Stein WD. Testing and characterizing the simpler pore. Biochim Biophys Acta 373: 165–177, 1974 [DOI] [PubMed] [Google Scholar]
- 79.Lieb WR, Stein WD. Testing and characterizing the simple carrier. Biochim Biophys Acta 373: 178–196, 1974 [DOI] [PubMed] [Google Scholar]
- 80.Lieb WR, Stein WD. Simple diffusion across the membrane bilayer. In: Transport and Diffusion Across Cell Membranes, edited by Stein WD. New York: Academic, 1986, p 69–112 [Google Scholar]
- 81.Liu Q, Vera JC, Peng H, Golde DW. The predicted ATP-binding domains in the hexose transporter GLUT1 critically affect transporter activity. Biochemistry 40: 7874–7881, 2001 [DOI] [PubMed] [Google Scholar]
- 82.Lowe AG, Walmsley AR. The kinetics of glucose transport in human red blood cells. Biochim Biophys Acta 857: 146–154, 1986 [DOI] [PubMed] [Google Scholar]
- 83.Lowe AG, Walmsley AR. A single half-turnover of the glucose carrier of the human erythrocyte. Biochim Biophys Acta 903: 547–550, 1987 [DOI] [PubMed] [Google Scholar]
- 84.Luiken JJ, Coort SL, Koonen DP, van der Horst DJ, Bonen A, Zorzano A, Glatz JF. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch 448: 1–15, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J 8: 1003–1011, 1994 [DOI] [PubMed] [Google Scholar]
- 86.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83: 183–252, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Manolescu AR, Augustin R, Moley K, Cheeseman C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol 24: 455–463, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Mantych GJ, Hagemann GS, Devaskar SU. Characterization of glucose transporter isoforms in the adult and developing human eye. Endocrinology 133: 600–607, 1993 [DOI] [PubMed] [Google Scholar]
- 89.Martin OJ, Lee A, McGraw TE. GLUT4 distribution between the plasma membrane and the intracellular compartments is maintained by an insulin-modulated bipartite dynamic mechanism. J Biol Chem 281: 484–490, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Matsumoto K, Akazawa S, Ishibashi M, Trocino RA, Matsuo H, Yamasaki H, Yamaguchi Y, Nagamatsu S, Nagataki S. Abundant expression of GLUT1 and GLUT3 in rat embryo during the early organogenesis period. Biochem Biophys Res Commun 209: 95–102, 1995 [DOI] [PubMed] [Google Scholar]
- 91.Moley KH, Chi MM, Knudson CM, Korsmeyer SJ, Mueckler MM. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat Med 4: 1421–1424, 1998 [DOI] [PubMed] [Google Scholar]
- 92.Moley KH, Chi MM, Mueckler MM. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol Endocrinol Metab 275: E38–E47, 1998 [DOI] [PubMed] [Google Scholar]
- 93.Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science 229: 941–945, 1985 [DOI] [PubMed] [Google Scholar]
- 94.Mueckler M, Makepeace C. Model of the exofacial substrate-binding site and helical folding of the human GLUT1 glucose transporter based on scanning mutagenesis. Biochemistry 48: 5934–5942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muona P, Sollberg S, Peltonen J, Uitto J. Glucose transporters of rat peripheral nerve, differential expression of Glut1 gene by Schwann cells and perineural cells in vivo and in vitro. Diabetes 41: 1587–1596, 1992 [DOI] [PubMed] [Google Scholar]
- 96.Naftalin RJ, Holman GD. Transport of sugars in human red cells. In: Membrane Transport in Red Cells, edited by Ellory JC, Lew VL. New York: Academic, 1977, p 257–300 [Google Scholar]
- 97.Naftalin RJ. Pre-steady-state uptake of d-glucose is inconsistent with the mobile carrier model. Trends Biochem Sci 13: 425–426, 1988 [DOI] [PubMed] [Google Scholar]
- 98.Naftalin RJ. Pre-steady-state uptake of d-glucose is inconsistent with a circulating carrier mechanism. Biochim Biophys Acta 946: 431–438, 1988 [DOI] [PubMed] [Google Scholar]
- 99.Naftalin RJ. Alternating carrier models of asymmetric glucose transport violate the energy conservation laws. Biophys J 95: 4300–4314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohtsuki S, Kikkawa T, Hori S, Terasaki T. Modulation and compensation of the mRNA expression of energy related transporters in the brain of glucose transporter 1 deficient mice. Biol Pharm Bull 29: 1587–1591, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Oka Y, Asano T, Shibasaki Y, Lin JL, Tsukuda K, Katagiri H, Akanuma Y, Takaku F. C-terminal truncated glucose transporter is locked into an inward-facing form without transport activity. Nature 345: 550–553, 1990 [DOI] [PubMed] [Google Scholar]
- 102.Park JL, Heilig CW, Brosius FC., 3rd GLUT1-deficient mice exhibit impaired endothelium-dependent vascular relaxation. Eur J Pharmacol 496: 213–214, 2004 [DOI] [PubMed] [Google Scholar]
- 103.Pellerin L. Brain energetics (thought needs food). Curr Opin Clin Nutr Metab Care 11: 701–705, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Pessino A, Hebert DN, Woon CW, Harrison SA, Clancy BM, Buxton JM, Carruthers A, Czech MP. Evidence that functional erythrocyte-type glucose transporters are oligomers. J Biol Chem 266: 20213–20217, 1991 [PubMed] [Google Scholar]
- 105.Pifferi F, Jouin M, Alessandri JM, Haedke U, Roux F, Perrière N, Denis I, Lavialle M, Guesnet P. n-3 Fatty acids modulate brain glucose transport in endothelial cells of the blood-brain barrier. Prostaglandins Leukot Essent Fatty Acids 77: 279–286, 2007 [DOI] [PubMed] [Google Scholar]
- 106.Rees WD, Holman GD. Hydrogen bonding requirements for the insulin-sensitive sugar transport system of rat adipocytes. Biochim Biophys Acta 646: 251–260, 1981 [DOI] [PubMed] [Google Scholar]
- 107.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem 272: 18982–18989, 1997 [DOI] [PubMed] [Google Scholar]
- 108.Sadiq HF, Das UG, Tracy TF, Devaskar SU. Intra-uterine growth restriction differentially regulates perinatal brain and skeletal muscle glucose transporters. Brain Res 823: 96–103, 1999 [DOI] [PubMed] [Google Scholar]
- 109.Salas-Burgos A, Iserovich P, Zuniga F, Vera JC, Fischbarg J. Predicting the three-dimensional structure of the human facilitative glucose transporter glut1 by a novel evolutionary homology strategy: insights on the molecular mechanism of substrate migration, and binding sites for glucose and inhibitory molecules. Biophys J 87: 2990–2999, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santalucia T, Boheler KR, Brand NJ, Sahye U, Fandos C, Vinals F, Ferre J, Testar X, Palacin M, Zorzano A. Factors involved in GLUT1 glucose transporter gene transcription in cardiac muscle. J Biol Chem 274: 17626–17634, 1999 [DOI] [PubMed] [Google Scholar]
- 111.Schmidt S, Gawlik V, Hölter SM, Augustin R, Scheepers A, Behrens M, Wurst W, Gailus-Durner V, Fuchs H, Hrabé de Angelis M, Kluge R, Joost HG, Schürmann A. Deletion of glucose transporter GLUT8 in mice increases locomotor activity. Behav Genet 38: 396–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schroeder RE, Doria-Medina CL, Das UG, Sivitz WI, Devaskar SU. Effect of maternal diabetes upon fetal rat myocardial and skeletal muscle glucose transporters. Pediatr Res 41: 11–19, 1997 [DOI] [PubMed] [Google Scholar]
- 113.Shin BC, Fujikura K, Suzuki T, Tanaka S, Takata K. Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology 138: 3997–4004, 1997 [DOI] [PubMed] [Google Scholar]
- 114.Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem 72: 238–247, 1999 [DOI] [PubMed] [Google Scholar]
- 115.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27: 1766–1791, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simpson IA, Vannucci SJ, DeJoseph MR, Hawkins RA. Glucose transporter asymmetries in the bovine blood-brain barrier. J Biol Chem 276: 12725–12729, 2001 [DOI] [PubMed] [Google Scholar]
- 117.Sogin DC, Hinkle PC. Characterization of the glucose transporter from human erythrocytes. J Supramol Struct 8: 447–453, 1978 [DOI] [PubMed] [Google Scholar]
- 118.Sogin DC, Hinkle PC. Binding of cytochalasin B to human erythrocyte glucose transport. Biochemistry 19: 5417–5420, 1980 [DOI] [PubMed] [Google Scholar]
- 119.Sone H, Deo BK, Kumagai AK. Enhancement of glucose transport by vascular endothelial growth factor in retinal endothelial cells. Invest Ophthalmol Vis Sci 41: 1876–1884, 2000 [PubMed] [Google Scholar]
- 120.Sultzman LA, Carruthers A. Stop-flow analysis of cooperative interactions between GLUT1 sugar import and export sites. Biochemistry 38: 6640–6650, 1999 [DOI] [PubMed] [Google Scholar]
- 121.Suzawa K, Yukita A, Hayata T, Goto T, Danno H, Michiue T, Cho KW, Asashima M. Xenopus glucose transporter 1 (xGLUT1) is required for gastrulation movement in Xenopus laevis. Int J Dev Biol 51: 183–190, 2007 [DOI] [PubMed] [Google Scholar]
- 122.Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. Histochemical demonstration of a monocarboxylate transporter in the mouse perineurium with special reference to GLUT1. Biomed Res 29: 297–306, 2008 [DOI] [PubMed] [Google Scholar]
- 123.Viñals F, Fandos C, Santalucia T, Ferré J, Testar X, Palacín M, Zorzano A. Myogenesis and MyoD down-regulate Sp1. A mechanism for the repression of GLUT1 during muscle cell differentiation. J Biol Chem 272: 12913–12921, 1997 [DOI] [PubMed] [Google Scholar]
- 124.Wang D, Pascual JM, Yang H, Engelstad K, Jhung S, Sun RP, De Vivo DC. GLUT-1 deficiency syndrome: clinical, genetic, and therapeutic aspects. Ann Neurol 57: 111–118, 2005 [DOI] [PubMed] [Google Scholar]
- 125.Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model of Glut1-haplodeficiency. Hum Mol Genet 15: 1169–1179, 2006 [DOI] [PubMed] [Google Scholar]
- 126.Wheeler TJ, Whelan JD. Infinite-Cis kinetics support the carrier model for erythrocyte glucose transport. Biochemistry 27: 1441–1446, 1988 [DOI] [PubMed] [Google Scholar]
- 127.Widdas WF. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J Physiol 118: 23–39, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wright EM, Hirayama B, Hazama A, Loo DD, Supplisson S, Turk E, Hager KM. The sodium/glucose cotransporter (SGLT1). Soc Gen Physiol Ser 48: 229–241, 1993 [PubMed] [Google Scholar]
- 129.Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch 447: 510–518, 2004 [DOI] [PubMed] [Google Scholar]
- 130.Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation 119: 2818–2828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao Y, Fung C, Shin D, Shin BC, Thamotharan S, Sankar R, Ehninger D, Silva A, Devaskar SU. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zoccoli MA, Baldwin SA, Lienhard GE. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J Biol Chem 253: 6923–6930, 1978 [PubMed] [Google Scholar]
- 133.Zottola RJ, Cloherty EK, Coderre PE, Hansen A, Hebert DN, Carruthers A. Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry 34: 9734–9747, 1995 [DOI] [PubMed] [Google Scholar]