Abstract
Little is known about how lactating women accommodate for their increased glucose demands during fasting to avoid maternal hypoglycemia. The objective of this study was to determine whether lactating women conserve plasma glucose by reducing maternal glucose utilization by increasing utilization of FFA and ketone bodies and/or increasing gluconeogenesis and mammary gland hexoneogenesis. Six healthy exclusively breastfeeding women and six nonlactating controls were studied during 42 h of fasting and 6 h of refeeding. Glucose and protein kinetic parameters were measured using stable isotopes and GCMS and energy expenditure and substrate oxidation using indirect calorimetry. After 42 h of fasting, milk production decreased by 16% but remained within normal range. Glucose, insulin, and C-peptide concentrations decreased with the duration of fasting in both groups but were lower (P < 0.05) in lactating women. Glucagon, FFA, and β-hydroxybutyrate concentrations increased with fasting time (P < 0.001) and were higher (P < 0.0001) in lactating women during both fasting and refeeding. During 42 h of fasting, gluconeogenesis was higher in lactating women compared with nonlactating controls (7.7 ± 0.4 vs. 6.5 ± 0.2 μmol·kg−1·min−1, P < 0.05), whereas glycogenolysis was suppressed to similar values (0.4 ± 0.1 vs. 0.9 ± 0.2 μmol·kg−1·min−1, respectively). Mammary hexoneogenesis did not increase with the duration of fasting. Carbohydrate oxidation was lower and fat and protein oxidations higher (P < 0.05) in lactating women. In summary, lactating women are at risk for hypoglycemia if fasting is extended beyond 30 h. The extra glucose demands of extended fasting during lactation appear to be compensated by increasing gluconeogenesis associated with ketosis, decreasing carbohydrate oxidation, and increasing protein and FFA oxidations.
Keywords: stable isotopes, gluconeogenesis, lactation, milk, production
using new techniques to measure gluconeogenesis in vivo, we (4, 12, 18, 30) and others (13, 15) demonstrated that gluconeogenesis accounts for ∼50% (ranging from 35 to 75%) of glucose production following an overnight fast. During carbohydrate (CHO) absorption, plasma glucose and insulin concentrations increase simultaneously, but plasma glucose concentrations remain in a very narrow range in normal individuals. This fine control is mediated by glucose-induced increase in plasma insulin, leading to increasing peripheral glucose utilization and glycogen synthesis, whereas hepatic glucose production is decreasing as a result of inhibited glycogenolysis (22). In contrast, during fasting, plasma glucose and insulin concentrations decrease and plasma FFA, ketone bodies, and glucagon concentrations increase (22), resulting in decreased glucose utilization and/or potentially increased glucose production to maintain euglycemia.
During a 24-h fast, glucose production in lactating women was ∼35% higher than in nonlactating women, presumably to meet the substrate needs for lactose synthesis (30). The increase in glucose production was the result of increased glycogenolysis since gluconeogenesis was essentially identical to that of the nonlactating controls. Over the course of the 24-h fast, rates of lipolysis and β-oxidation of fatty acids, as reflected by glycerol flux and ketonemia, were similar to those of nonlactating controls, suggesting a lack of “mechanism(s)” to reduce glucose utilization in lactating women. However, the contribution of plasma glucose to lactose production decreased from 80 to 60% as a result of an increase in hexoneogenesis within the breast (28).
Humans have limited hepatic glycogen storage capacity. After 40–42 h of fasting in men, the rate of glycogenolysis contributed only ∼8% of total glucose production rate (13, 15). In our previous study, we did not see a difference in glucose storage rate between lactating and nonlactating women in response to feeding (30). By adding the extra demands imposed by lactation, we assume that, beyond a 24-h fast, hepatic glycogen stores would be depleted more rapidly in the lactating than in the nonlactating women.
Despite these new insights, we still do not know whether or how lactating women adapt to their increased glucose demands during extended fasting to avoid maternal hypoglycemia. Therefore, we undertook the present studies to determine whether increasing the duration of fasting from 24 to 42 h in lactating women would result in 1) increased glucose production via an increase in gluconeogenesis together with an increase in hexoneogenesis, 2) decreased glucose utilization by reducing milk production and/or maternal glucose utilization, and 3) increased utilization of FFA and ketone bodies.
MATERIALS AND METHODS
Tracers
Sterile and pyrogen-free [1-13C]glucose (99 atom%), [15N2]urea, [6,6-2H2]glucose, [1-13C]leucine, and 2H2O were purchased from Cambridge Isotope Laboratories (Andover, MA). 2H2O was administered orally without additional preparation. The other isotopes except for the 2H2O were tested again for sterility and pyrogenicity in the investigation pharmacy of Texas Children's Hospital. The isotopes were dissolved in isotonic saline and the solutions filtered through a 0.2-μm filter (Millipore, Bedford, MA) into sterile syringes. The sterile solutions were prepared <48 h before the study and maintained at 4°C until just prior to their use, as described previously (30).
Subjects
Following thorough review and approval by the Institutional Review Board for Human Subjects and the General Clinical Research Center (GCRC) Advisory Committee at Baylor College of Medicine, written consent was obtained from each of the subjects. All subjects were determined healthy if they had a normal physical examination, fasting blood glucose, hemoglobin (Hb), Hb A1c, and liver function tests. None of the women had a history of gestational diabetes or had children, parents, siblings, or grandparents with diabetes. Except for routine postpartum vitamins and mineral supplements prescribed by their physician, none was on any medications, including birth control pills. All women studied had a negative pregnancy test at the time of study. To exclude any pregnancy-related changes in body composition and hormones and to ensure a stable physiological model, all lactating women were studied between 6 and 12 wk postpartum. No attempt was made to study the nonlactating women at a specific time in their menstrual cycle.
No attempt was made to select subjects on the basis of ethnicity. As a result, six healthy lactating women [age 26.8 ± 1.2 yr, body mass index (BMI) 22.7 ± 0.9 kg/m2, height 159.8 ± 2.8 cm, weight 58.8 ± 3.2 kg, and lean body mass (LBM) 38.8 ± 2.1 kg; 4 Caucasians, 2 Hispanic-Americans, and their healthy infants] and six healthy, nonpregnant, nonlactating controls (age 27.9 ± 2.1 yr, BMI 21.8 ± 0.7 kg/m2, height 162.4 ± 1.4 cm, weight 56.6 ± 1.9 kg, and LBM 39.0 ± 1.4 kg; 2 Caucasians, 2 African-Americans, 1 American-Hispanic, and 1 Asian-American) were recruited for the study. The infants of the lactating women weighed 5.69 ± 0.36 kg and were 11.0 ± 0.5 wk of age at the time of study.
Study Design
Women were instructed to consume a diet providing ∼50% CHO, 15% protein, and 35% fat for the week prior to admission. Lactating women were asked to bring 6–10 oz of breast milk to supplement their infants' feeding should they have technical difficulty with breastfeeding and/or decreased milk production. Each woman and her infant were admitted to the GCRC before 4 PM on the afternoon prior to study (day 1). At 5 PM, one intravenous (iv) catheter was introduced in the woman's antecubital fossa or forearm vein under Emla cream (Astra Pharmaceuticals, Wayne, PA) analgesia for blood sampling. Subjects were fed a supper meal of 10 kcal/kg at 6 PM and were subsequently fasted except for water ad libitum until the refeeding portion of the protocol. Blood and milk samples were obtained at 6 PM and subsequently every 6 h until midnight on the last day of the study (day 3). Glucose concentrations were measured immediately at each blood draw. If the glucose decreased to <60 mg/dl, an investigator was contacted and the frequency of glucose monitoring increased. In addition, the mothers were carefully observed, and at any signs and/or symptoms consistent with hypoglycemia, plasma glucose was measured immediately. If the glucose concentration decreased to <40 mg/dl on a quantitative laboratory measurement, the fast was terminated. The mothers were requested to breastfeed their infants every 3 h. At each feeding, the infants were fed from both breasts. Infants were weighed before and after each breastfeeding to determine the volume of milk consumed (2, 19). Following each feeding the mothers emptied their breasts completely using a standard electric breast pump (Playtex Embrace, Dover, DE). The weight of the resulting milk was measured and an aliquot frozen for subsequent analysis. This volume of milk was added to that consumed by the infant at each breastfeeding to determine total milk production (2).
At 8 PM on day 1, a second iv was established in the opposite arm under Emla cream (Astra Pharmaceuticals) analgesia for infusions. Between 8 PM and 9 AM, a primed constant-rate iv infusion of [15N2]urea (8 μmol/kg, 0.13 μmol·kg−1·min−1) was administered to measure urea rate of appearance. Between 6 and 9 AM, the subjects received a primed constant-rate iv infusion of [1-13C]leucine (4 μmol/kg, 0.06 μmol·kg−1·min−1) to measure leucine rate of appearance. Between 6 AM and 1 PM, the subjects received a primed constant-rate iv infusion of [1-13C]glucose (20 μmol/kg and 0.33 μmol·kg−1·min−1) to measure glucose rates of appearance and production and the fractions of milk lactose sugars derived from plasma and mammary gland synthesis (25, 29). Blood samples (2.5 ml each) were obtained every 10 min during the last half-hour of the initial 3-h glucose tracer infusion period (t = −30 to 0 min). At 9 AM, following the 0-min blood sample, an iv bolus of glucose, 0.30 g/kg, containing 10% [6,6-2H2]glucose [stable-label iv glucose tolerance test (SLIVGTT)], was administered over 90–120 s, and blood samples (2.5 ml each) were obtained at +2, 3, 4, 5, 8, 10, 18, 20, 28, 32, 40, 60, 120, 180, and 240 min. Indirect calorimetry was performed three times between 6 and 9 AM (0 time for the SLIVGTT). Following completion of the SLIVGTT, one of the venous access sites was removed, and the second one was maintained for the duration of the study.
Beginning at 11 PM on the 2nd day of admission (at 28 h of fasting), the women consumed four doses of 2H2O at 2-h intervals, corresponding to a total dose of 3 g/kg. At 4 AM, two additional iv catheters were introduced in the mother's antecubital fossa or forearm vein under Emla cream analgesia for isotope infusion and a backup line for blood sampling if needed. At 6 AM, the subjects received primed constant-rate infusions of [6,6-2H2]glucose (9 μmol/kg, 0.14 μmol·kg−1·min−1), [15N2]urea (8 μmol/kg, 0.13 μmol·kg−1·min−1), and [1-13C]leucine (4 μmol/kg, 0.06 μmol·kg−1·min−1). Blood samples (6–10 ml) were collected at 60-min intervals beginning at 6 AM until 6 PM. Milk samples were collected every 3 h from 6 AM through 6 PM. From noon until 6 PM, subjects were refed an ∼70-kcal (∼70 ml) non-galactose-containing nutrient drink, Boost (Mead Johnson; formally Sustacal), every 30 min. This provided ∼840 kcal or ∼50% of their daily requirements over the 6 h of feeding (assuming 12 h of feeding and 12 h of fasting/day; thus, the adult 60-kg subject received ∼1,700 kcal/day). The purpose of this feeding strategy was to evaluate the subject's acute response to refeeding, not to provide full caloric requirements. To avoid problems in data interpretation, both lactating and control subjects received the same caloric load of Boost. CO2 production and O2 consumption were measured every 3 h from 6 AM through 6 PM using indirect calorimetry for calculation of substrate oxidations. Twelve-hour urine collections were obtained during the first 36 h of the study, followed by 6-h collections during the last 12 h. During collection, the urine was kept refrigerated and then frozen at −80°C until nitrogen analyses and pH measurements were performed. Nonlactating women were studied using an identical protocol, except that no breast milk samples were obviously obtained.
Substrate and Hormone Determinations
Plasma glucose and lactate concentrations were determined using enzyme-specific methods (YSI Glucose Analyzer; YSI, Yellow Springs, OH). Plasma insulin and C-peptide concentrations were measured using commercially available radioimmunoassay kits (Linco Research, St. Charles, MO). Plasma FFA and β-hydroxybutyrate concentrations were determined by microfluorometric enzyme analyses using Cobas Fara II Analyzer (Roche Diagnostic Systems, Montclair, NJ). Blood urea nitrogen (BUN) concentration was measured calorimetrically using the Vitros analyzer (Ortho-Clinical Diagnostics, Rochester, NY). Plasma α-ketoisocaproic acid (α-KIC) concentrations were determined by GC-MS and reverse isotope dilution, using [2H7]KIC as an internal standard (27). Lactose was hydrolyzed to glucose and galactose enzymatically and the resultant sugars analyzed individually as described previously (25, 28). Milk lactose concentration was determined using an enzyme-specific method (YSI Glucose Analyzer). The concentration of protein was determined using BCA protein assay kit (Novagen, Madison, WI) (14, 18). Urine pH was measured using a pH meter (Accumet Basic pH meter; Fisher Scientific).
Determination of Isotope Enrichments
[1-13C]glucose and [6, 6-2H2]glucose were converted to the pentaacetate derivative, and the enrichments of 13C and 2H2 were measured using GC-combustion-isotope ratio mass spectrometry and GC-MS, respectively (29). The [15N2]urea enrichment was determined using the 2-pyrimidinol-tert-butyldimethylsilyl derivative as described previously (11, 27). Plasma α-[1-13C]KIC enrichment was measured after derivatization to the oxime-tert-butyldimethylsilyl derivative of α-KIC, as described previously (27). During the iv infusion of compounds labeled with stable isotopes (36–48 h), the fraction of glucose derived from gluconeogenesis was measured as described previously (4). Briefly, 2H2O enrichment was measured using isotope ratio mass spectrometry (4). The 2H enrichment of glucose, excluding C-2, was determined using the recently described method of Chacko et al. (4).
Indirect Calorimetry
Indirect calorimetry was performed using a MedGraphic Model Indirect Calorimeter (MedGraphics, Minneapolis, MN). Substrate oxidation rates for protein, glucose, lipid, and resting energy expenditure were calculated using the gaseous exchange equations described previously (7). The substrate oxidation rates were calculated as
;where V̇co2 and V̇o2 are the l/min of gaseous exchange obtained from the calorimeter and N represents total nitrogen excretion (g/min) estimated from the urea rate of appearance (Ra).
Calculations
Total glucose entry and glucose production.
The total Ra of glucose into the systemic circulation was calculated under near-steady-state condition using the standard isotope dilution equation
,where Ei and Ep are the infusate and plasma enrichments, respectively, of the labeled glucose ([1-13C] following 14-h fasting and [6, 6-2H2] following 42-h fasting and refeeding) and I is the rate of infusion of the labeled glucose.
In the fasting state, glucose production rate (GPR) was calculated by subtracting the amount of infused labeled glucose from the glucose Ra:
.
GPR is the sum of the rates of gluconeogenesis (GNG) and glycogenolysis:
.
The fraction of glucose derived from GNG was determined using 2H2O and the average 2H enrichments of carbons 1, 3, 4, 5, and 6 of glucose as described recently using the following formula (4):
,where (M + 1)2H (m/z 169) is the M + 1 enrichment of 2H glucose measured using the mass-to-charge ratio (m/z) 170/169 fragment of glucose, 6 is the number of 2H labeling sites on the m/z 169 fragment of glucose, and 2H2O is the enrichment of deuterium in body water (4).
The rate of gluconeogenesis is
.
The rate of glycogenolysis in the postabsorptive state was calculated as the difference between glucose production and gluconeogenesis:
.
During meal ingestion, the Ra of dietary glucose into the plasma pool (Ra meal) was estimated from the CHO content in the Boost drink. We assumed that ∼85% of orally ingested glucose entered the systemic circulation in the normal volunteer adults on the basis of our previous studies (i.e., ∼14 g × 0.85/100 ml of drink consumed) (30).
The rate of glycogenolysis during meal absorption was estimated as
.
The fraction of glucose and galactose (product pool) in milk lactose that was derived from plasma glucose (precursor pool) and the fraction derived from de novo synthesis were calculated as described previously (25, 30).
Urea production rate.
The Ra of urea into the systemic circulation was calculated under near-steady-state condition using the standard isotope dilution equation
,where Ei and Ep are the infusate and plasma enrichments, respectively, of the labeled urea and I is the rate of infusion of the labeled urea (27).
Rates of protein oxidation.
Based on urea Ra, protein oxidation was calculated using the following equation:
,where Urea Ra is expressed in g·kg−1·day−1, 0.47 is the fraction of urea that is composed of nitrogen, and 6.25 is the inverse of the fraction of nitrogen in protein.
Rate of proteolysis.
Leucine appearance rate (Ra Leu) was used as an indicator of proteolysis and was calculated using the reciprocal pool model using the [1-13C]KIC enrichment according to the following equation
,where Ei and Ep are the enrichments of the leucine infusate and plasma KIC, respectively, and I is the rate of infusion of the labeled leucine. The rate of proteolysis (g·kg−1·day−1) was calculated using the following equation, as described previously (27):
,where Ra Leu is leucine Ra (μmol·kg−1·min−1), 131 is its molecular weight, 1,440 is to convert minutes to day, 10−6 is to convert micrograms to grams, and 12.5 is the inverse of leucine content in body protein (∼8%).
During fasting, proteolysis is derived solely from endogenous sources, whereas during feeding it is the sum of the rate of entry of leucine from both endogenous and exogenous sources. Endogenous leucine entry can be calculated by subtracting exogenous leucine entry from total leucine Ra. The exogenous leucine entry was calculated on the basis of the content of protein in the Sustacal drink as provided by the manufacturer (27).
The rate of protein synthesis was calculated using the following equation:
Insulin sensitivity.
Insulin sensitivity in the fasting condition was calculated using the averaged baseline insulin and glucose values using the homeostasis model assessment resistance (HOMAR) and quantitative insulin-sensitivity check index (QUICKI) methods (5) according to the following formulae:
.
Insulin sensitivity and the first- and second-phase insulin secretory indices were calculated from the SLIVGTT, as described previously (29).
Statistical Analysis
Values obtained during the steady-state periods were averaged for each subject and are presented as means ± SE. Data between groups were compared using nonpaired Student's t-test, whereas data within the same group were compared using paired Student's t-test. Changes in substrate and hormone concentrations and urine pH over time within and between groups were assessed using repeated-measures analysis of variance (ANOVA). Software programs SPSS (version 16; SPSS, Chicago, IL) and GraphPad Prism (version 4; GraphPad Software) were used for all statistical analyses. Significance was defined as P < 0.05.
RESULTS
Changes in Substrate and Hormone Concentrations Over Time
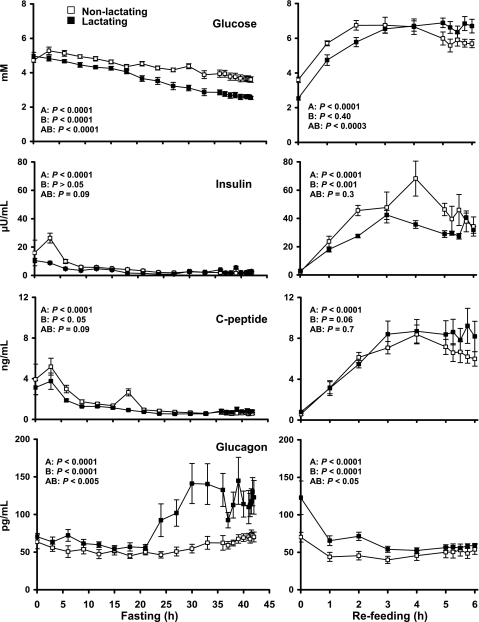
Statistics were carried out using repeated-measures ANOVA to demonstrate differences over time, differences between groups (nonlactating vs. lactating), and differences due to interaction of group and time (Figs. 1–2). Repeated-measures ANOVA revealed a progressive decrease in the plasma glucose concentration in both groups (P < 0.001) with fasting time. The decrease was greater (P < 0.0001) in the lactating compared with the nonlactating women (Fig. 1). During refeeding, plasma glucose rose in both groups (P < 0.001) but was not different between the two groups (P = 0.09).
Fig. 1.
Mean ± SE concentrations of plasma glucose, insulin, C-peptide, and glucagon in the nonlactating (□; n = 6) and lactating (▪; n = 6) women during the fasting (left) and refeeding (right) states. Statistics were carried out using repeated-measures ANOVA denoting differences over time (A), differences between groups (B), and difference due to interaction of group and time (AB).
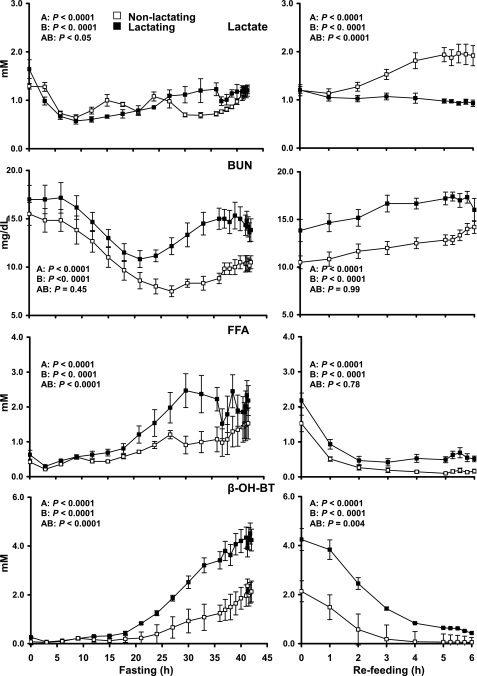
Fig. 2.
Mean ± SE plasma concentrations of lactate in the nonlactating (□; n = 6) and lactating (▪; n = 6) women during fasting and refeeding. Statistics were carried out using repeated-measures ANOVA denoting differences over time (A), differences between groups (B), and difference due to interaction of group and time (AB). BUN, blood urea nitrogen; FFA, free fatty acids; β-OH-BT, β-hydroxybutyrate.
Insulin and C-peptide concentrations followed the same pattern as glucose, i.e., decreasing with the duration of the fasting and increasing (P < 0.0001) in the refeeding period. However, only insulin was lower in the lactating (P < 0.01) compared with the nonlactating women during refeeding (but not during fasting). In contrast, plasma glucagon concentrations increased with fasting (P < 0.0001) and were higher (P < 0.0001) in the lactating compared with the nonlactating women. Refeeding lowered glucagon concentration (P < 0.0001), but the lactating women continued to have higher values (P < 0.001) than the nonlactating controls (Fig. 1).
Lactate concentration fell with time (P < 0.0001) in both nonlactating controls and lactating women (Fig. 2). During refeeding, the plasma lactate concentration increased (P < 0.01) in the nonlactating women, whereas it decreased (P < 0.01) in the lactating women. BUN concentrations were higher (P < 0.0001) in the lactating group during both fasting and refeeding. FFA concentrations and β-hydroxybutyrate were higher (P < 0.0001) in the lactating group over the time course of fasting and refeeding (P < 0.0001) (Fig. 2).
Plasma Substrate and Hormone Concentrations at Specific Time Points
Plasma substrate concentrations.
After 14 h of fasting, the plasma glucose concentrations were slightly lower in the lactating compared with the nonlactating women (4.3 ± 0.1 vs. 4.6 ± 0.1, P = 0.04). Plasma lactate concentrations were also lower in the lactating group, whereas FFA, β-hydroxybutyrate, KIC, and BUN were not different (Table 1).
Table 1.
Average substrate and hormone concentrations during near-steady states
| 14-h Fast |
42-h Fast |
Refeeding |
||||
|---|---|---|---|---|---|---|
| Nonlactating | Lactating | Nonlactating | Lactating | Nonlactating | Lactating | |
| Glucose, mM | 4.6±0.1 | 4.3±0.1* | 3.6±0.2 | 2.6±0.1† | 5.8±0.3 | 6.7±0.3 |
| Insulin, μU/ml | 5.0±0.6 | 3.8±0.7 | 2.0±0.3 | 2.3±0.5 | 40.8±6.1 | 31.7±4.8 |
| C-peptide, ng /ml | 1.4±0.1 | 1.1±0.1 | 0.6±0.1 | 0.8±0.1 | 6.5±0.7 | 8.4±1.3 |
| Glucagon, pg/ml | 47.7±4.3 | 53.9±4.3 | 70.7±3.3 | 118.6±18.5* | 50.6±7.2 | 57.4±2.7 |
| Lactate, mM | 1.0±0.1 | 0.7±0.0* | 1.1±0.1 | 1.2±0.1 | 1.9±0.2 | 1.0±0.0† |
| β-OH-BT, mM | 0.1±0.1 | 0.3±0.1 | 2.0±0.4 | 4.3±0.4† | 0.1±0.0 | 0.5±0.3 |
| FFA, mM | 0.5±0.1 | 0.7±0.1 | 1.5±0.3 | 2.0±0.2* | 0.2±0.0 | 0.6±0.1† |
| BUN, mg/dl | 11.0±1.0 | 13.0±1.1 | 10.4±0.7 | 14.3±1.1* | 13.2±0.5 | 16.8±0.6† |
| α-KIC, μM | 30.7±1.6 | 33.2±3.1 | 50.9±3.7 | 46.3±7.3 | 47.9±3.1 | 58.5±7.9 |
Values are means ± SE; n = 6 subjects in each group. β-OH-BT, β-hydroxybutyrate; FFA, free fatty acids; BUN, blood urea nitrogen; α-KIC, α-ketoisocaproic acid.
*P (nonpaired) < 0.05;
After 42 h of fasting, plasma glucose concentrations in lactating women remained lower than in nonlactating women (2.6 ± 0.1 vs. 3.6 ± 0.2 mM, respectively, P = 0.0002). No difference in plasma lactate or KIC concentrations was observed between the two groups, whereas both plasma β-hydroxybutyrate (4.3 ± 0.4 vs. 2.0 ± 0.4 mM, P = 0.007) and FFA (2.0 ± 0.2 vs. 1.5 ± 0.3, P = 0.04) were higher in lactating women. BUN concentrations were also higher in the lactating group (14.3 ± 1.1 vs. 10.4 ± 0.7 mg/dl, P < 0.05; Table 1).
After 6 h of refeeding, plasma concentrations of glucose were not different between lactating and nonlactating women, whereas plasma lactate concentrations were nearly twice as high (P < 0.01) in the nonlactating compared with the lactating women. Although plasma β-hydroxybutyrate and FFA concentrations decreased after refeeding, FFA concentrations were three times higher in the lactating group (0.6 ± 0.1 vs. 0.2 ± 0.0 mM, P = 0.005) by the end of the study. Plasma KIC concentrations were not different, but BUN concentrations were higher in the lactating group (16.8 ± 0.6 vs. 13.2 ± 0.5 mg/dl, P < 0.01; Table 1).
Hormone concentrations and insulin sensitivity.
After the overnight fast, plasma insulin, C-peptide, and glucagon were not different between the two groups (Table 1). After 42 h of fasting, insulin and C-peptide concentrations were not different between lactating and nonlactating groups. However, the plasma glucagon concentration was higher in the lactating women (119 ± 19 vs. 71 ± 3 pg/ml, P < 0.05). After 6 h of refeeding, plasma concentrations of insulin and C-peptide and glucagon concentrations were not different between the two groups.
After the overnight fast, insulin sensitivity as measured by HOMA (1.04 ± 0.13 vs. 0.73 ± 0.15, P = 0.16) and QUICKI (0.38 ± 0.06 vs. 0.42 ± 0.1, P = 0.12) were not different between the nonlactating and lactating groups, respectively. Using SLIVGTT, insulin sensitivity (min−1)/(μU × ml−1) (5.5 ± 1.1 × 10−4 vs. 8.9 ± 1.6 × 10−4) did not differ (P = 0.10 2 sided, P = 0.05 1 sided) between the nonlactating and the lactating groups. First-phase (φ1 × 10−9; 335 ± 54 vs. 356 ± 74, P = 0.80) and second-phase (φ2 × 10−9; 13.9 ± 1.7 vs. 10.9 ± 1.8, P = 0.26) insulin secretory indexes also were not different between the lactating and nonlactating groups.
Glucose kinetics.
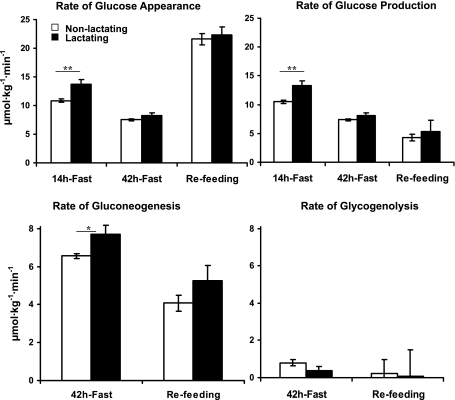
After the overnight fast, GPR was higher in the lactating compared with the nonlactating women, (13.3 ± 0.8 vs. 10.5 ± 0.3 μmol·kg−1·min−1, respectively, P = 0.002; Fig. 3). After 42 h of fasting, GPR decreased (P < 0.01) to 8.1 ± 0.5 and 7.4 ± 0.2 μmol·kg−1·min−1 in the lactating and nonlactating groups, respectively, but was no longer different between the two groups. The rates of gluconeogenesis were higher in lactating than nonlactating women (7.7 ± 0.5 vs. 6.6 ± 0.1 μmol·kg−1·min−1, P = 0.04). However, rates of glycogenolysis were not different between the two groups (0.4 ± 0.2 vs. 0.8 ± 0.2 μmol·kg−1·min−1, P = 0.06). During refeeding, rates of total glucose appearance, glucose production, gluconeogenesis, and glycogenolysis were not different between the two groups.
Fig. 3.
Rates of glucose appearance, glucose production, gluconeogenesis, and glycogenolysis in the nonlactating (□) and the lactating (▪) women following 14 and 42 h of fasting and 6 h of refeeding. Values are means ± SE. All parameters are expressed as μmol·kg−1·min−1. All calculations are based on total body weight (kg). *P (nonpaired) < 0.05; **P (nonpaired) < 0.01, nonlactating (n = 6) vs. lactating (n = 6) women.
Protein kinetics.
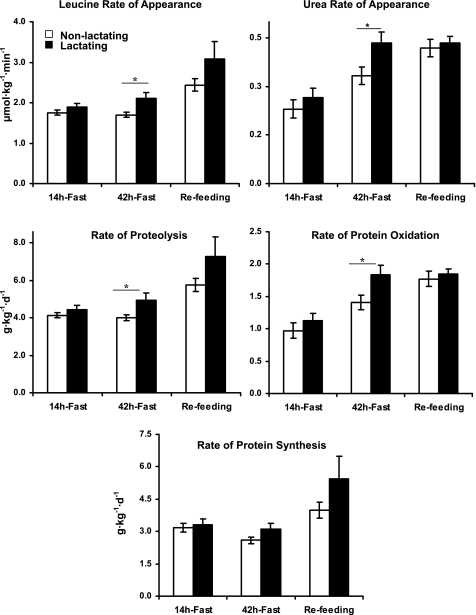
After an overnight fast, leucine Ra and, accordingly, rates of proteolysis were not different between the two groups. Similarly, urea Ra and thus protein oxidation rates were not different between the nonlactating and the lactating women (Fig. 4).
Fig. 4.
Rates of appearance of leucine and urea and rates of proteolysis, protein oxidation, and synthesis in the nonlactating (□) and the lactating (▪) women following 14 and 42 h of the fasting and 6 h of refeeding. Values are means ± SE. All calculations are based on total body weight (kg). *P (nonpaired) < 0.05; **P (nonpaired) < 0.01, controls (n = 6) vs. lactating (n = 6) women.
After 42 h of fasting, rate of leucine appearance was higher in the lactating group (2.1 ± 0.2 vs. 1.7 ± 0.1 μmol·kg−1·min−1, P < 0.05), and accordingly, the corresponding rate of proteolysis was higher in the lactating group (5.0 ± 0.4 vs. 4.0 ± 0.1 g·kg−1·day−1, P < 0.05). Similarly, urea Ra (0.44 ± 0.02 vs. 0.33 ± 0.03 mg·kg−1·min−1, P < 0.05) and thus rate of protein oxidation (1.9 ± 0.1 vs. 1.3 ± 0.1 g·kg−1·day−1, P < 0.05) were higher in the lactating group (Fig. 4). During refeeding, proteolysis, protein oxidation, and synthesis were not different between the two groups. During the refeeding period, proteolysis and protein synthesis rates increased (P < 0.05) compared with the 42-h fasting in both the lactating and nonlactating groups, whereas protein oxidation increased (P < 0.05) in the nonlactating group only (Fig. 4).
Energy expenditure and substrate oxidations.
After an overnight fast, there was no difference in energy expenditure between the nonlactating and the lactating women. However, respiratory quotient (RQ) and CHO oxidation were lower and fat oxidation was higher in the lactating group (Table 2).
Table 2.
Energy expenditure and substrate oxidation rates during fasting and refeeding
| 14 h-Fast |
42 h-Fast |
Refeeding |
||||
|---|---|---|---|---|---|---|
| Nonlactating | Lactating | Nonlactating | Lactating | Nonlactating | Lactating | |
| RQ | 0.85±0.0 | 0.78±0.0† | 0.79±0.0 | 0.73±0.1† | 0.81±0.0 | 0.75±0.0* |
| EE | 21±1 | 21±1 | 22±1 | 24±2 | 26±1 | 27±2 |
| Substrate oxidation rates, g·kg−1·day−1 | ||||||
| Fat | 0.8±0.1 | 1.4±0.1† | 1.2±0.2 | 1.8±0.2* | 1.2±0.2 | 1.9±0.24* |
| CHO | 2.3±0.1 | 1.1±0.3† | 1.4±0.5 | −0.2±0.2† | 1.8±0.3 | 0.3±0.5* |
| Protein | 1.1±0.1 | 1.20±0.1 | 1.1±0.1 | 1.9±0.2* | 1.9±0.1 | 1.8±0.1 |
Values are means ± SE. RQ, respiratory quotient; EE, energy expenditure, expressed as kcal·kg−1·day−1; CHO, carbohydrate.
*P (nonpaired) < 0.05;
†P (nonpaired) < 0.01, nonlactating (n = 5) vs. lactating (n = 5) women.
After 42 h of fasting, energy expenditure was not different between the lactating and the nonlactating women. However, RQ was lower (0.73 ± 0.06 vs. 0.79 ± 0.02, P < 0.01) and fat oxidation higher (1.8 ± 0.2 vs. 1.2 ± 0.2 g·kg−1·day−1, P < 0.05), whereas that of CHO was lower (−0.2 ± 0.2 vs. 1.4 ± 0.5 g·kg−1·day−1, P < 0.01) in the lactating compared with the nonlactating group. During the refeeding, energy expenditure was also not different between the two groups, but fat oxidation remained higher (P < 0.05) and CHO oxidation remained lower (P < 0.05) in the lactating women.
Urine pH.
Repeated-measures ANOVA revealed differences over time (P < 0.001), differences between groups (nonlactating vs. lactating; P < 0.001), and differences due to interaction of group and time (P < 0.001) (Table 3). Using post hoc comparisons, the pH of the urine collected during the first 24 h of fasting was not different between nonlactating and lactating women. However, following 24 h of fasting, the pH was consistently lower in the lactating compared with the nonlactating (P < 0.01) women. From 12 to 42 h of fasting, the pH decreased (6.5 ± 0.2 vs. 4.7 ± 0.1, P < 0.01) in lactating women, whereas it remained unchanged in the nonlactating women (6.7 ± 0.2 vs. 6.1 ± 0.3, P > 0.05). After refeeding, the pH did not increase in either the lactating or the nonlactating women. However, it remained lower (P < 0.01) in lactating women.
Table 3.
Urine pH in nonlactating and lactating women during fasting and refeeding
| Nonlactating | Lactating | |
|---|---|---|
| Fasting time, h | ||
| 0–12 | 6.7±0.2a | 6.5±0.2a |
| 12–24 | 6.5±0.1a | 5.9±0.3a |
| 24–36 | 6.4±0.3a | 5.3±0.2b |
| 36–42 | 6.1±0.3a | 4.7±0.1c |
| Refeeding | 6.3±0.2a | 4.7±0.1c |
Values are means ± SE. Repeated-measures ANOVA revealed differences over time (P < 0.001), differences between groups [nonlactating (n = 4) vs. lactating (n = 5); P < 0.001], and differences due to interaction of group and time (P < 0.001). Different superscripted letters (based on post hoc comparisons) indicate significant difference (P < 0.05).
Milk production and composition.
After an overnight fast, milk volume was 99 ± 7 ml/feeding (feeding represents 3 h). The average milk production was reduced (P < 0.05) by 14% (86 ± 8 ml/feeding) following 42-h fasting and was not increased after 6 h of refeeding (88 ± 8 ml/feeding) (Table 4). Milk protein and lactose concentrations were not different between fasting and refeeding. The percentage of lactose hexoses derived from mammary gland synthesis after 14 and 42 h of fasting were not different (45 ± 3 and 46 ± 5%, respectively, P > 0.05). During refeeding, hexoneogenesis of lactose moieties was entirely abolished.
Table 4.
Milk production, composition, and lactose hexoses derived from hexoneogenesis and plasma glucose during fasting and refeeding
| 14-h Fast | 42-h Fast | Refeeding | |
|---|---|---|---|
| Milk volume, ml/feed | 99±7a | 86±8b | 88±8a,b |
| ml/day* | 793±55a | 684±68b | 704±68a,b |
| Protein concentration, g/dl | 1.7±0.2 | 1.9±0.1 | 1.7±0.3 |
| Lactose concentration, g/dl | 7.4±0.3 | 6.5±0.2 | 6.3±0.2 |
| Milk glucose from hexoneogenesis, % | 41±4a | 40±8a | −16±5 |
| Milk galactose from hexoneogenesis, % | 50±3a | 52±7a | −1±3b |
| Milk lactose from hexoneogenesis, % | 45±3a | 46±8a | −8±3b |
| Milk lactose from plasma glucose, % | 55±3a | 54±8a | 109±3b |
| Milk lactose from hexoneogenesis, μmol·kg−1·min−1† | 1.7±0.1a | 1.4±0.1b | −0.2±0.1c |
| Milk lactose from plasma glucose, μmol·kg−1·min−1‡ | 2.1±0.1a | 1.6±0.1b | 3.2±0.2c |
Values are means ± SE; n = 6. Paired t-test was used. Different superscripted letters within the same row indicate difference. Significance was set as P (paired) < 0.05.
*Calculated by multiplying the milk volume (ml/feed) × 8 feedings/day.
†Based on lactose derived from hexoneogenesis (%), milk volume, and lactose concentration.
‡Based on lactose derived from plasma glucose (%), milk volume, and lactose concentration.
DISCUSSION
In our previous studies of 14- to 24-h fasting lactating women, the plasma substrate and hormone concentrations were similar in lactating and nonlactating women despite a 30% increase in glucose demands in the lactating subjects. The increased glucose demands were met by increased rates of glycogenolysis (12, 30). In the present study, we demonstrated that if the fast was merely extended by 18 h, the lactating women developed relative hypoglycemia, hyperketonemia, and hyper free fatty acidemia compared with the nonlactating controls.
Plasma insulin and C-peptide followed the same pattern as glucose (i.e., decreased with the duration of fasting) in both groups, whereas plasma glucagon concentrations increased progressively, with the time of fasting plateauing after 30 h at twice the basal concentrations in the lactating women. In the nonlactating women in the present study as well as in previously studied 3-day fasted women (9), plasma glucagon increased by only ∼50% from 14 to 42 h of the fast. It is interesting to speculate, as has been done previously (9, 16), that the low plasma insulin and elevated glucagon may be the primary hormonal triggers to orchestrate the controlled mobilization of substrates, regulation of hepatic glucose production, and substrate oxidation.
In the present study, glucose production rate was 30% higher in the lactating compared with the nonlactating women after 14 h of fasting, results similar to those obtained following an overnight fast (12) and 24 h of fasting (30). Extension of fasting to 42 h decreased glucose production rate by 30 and 40% in the nonlactating and lactating women, respectively, and was no longer different between the two groups. However, rates of gluconeogenesis were higher at 42 h of fasting in the lactating women contributing to 96 ± 1% of the glucose production compared with 87 ± 3% in the nonlactating women. Thus, glycogenolysis accounted for a very minor part of the glucose production rate after 42 h fasting, with no difference between nonlactating and lactating women. Our findings agree with those reported by Katz and Tayek (13), who reported that fractional gluconeogenesis increased from 41 to 92% between 12 and 40 h in fasting men. Similar results were reported by Landau et al. (15). These observations suggest that after 42 h of fasting, hepatic glycogen stores are essentially depleted. This also would suggest that the higher rates of glycogenolysis in the lactating women resulted in earlier depletion of hepatic glycogen stores than in the nonlactating women. Therefore, after 42 h of fasting, lactating women were relying nearly exclusively on gluconeogenesis for their glucose requirements.
One potential mechanism to conserve maternal glucose during fasting is to increase the production of lactose from other substrates within the mammary gland. However, at 42 h of fasting, rates of mammary hexoneogenesis were not different from those observed following 14 h of fasting in the present study or 14–24 h of fasting in our previous studies (18, 28, 30). Calculating the minimum amount of lactose derived directly from plasma glucose, i.e., the amount that could not be contributed from hexoneogenesis (1.6 μmol·kg−1·min−1; Table 4), we can calculate the approximate endogenous rate of glucose utilization in the lactating women. Thus, we estimated that the rate of glucose utilization by maternal body tissues was lower in the lactating women (6.4 ± 0.4 vs. 7.4 ± 0.2 μmol·kg−1·min−1, P < 0.05). Even after 42 h of fasting, milk volume remained within the normal range reported previously (600–800 ml/day) (20), as did the composition of the aqueous macronutrients constituents. Therefore, even during extended fasting, priority may be given to the delivery of nutrients to the mammary gland to support milk synthesis.
Concurrent to the increased gluconeogenesis in lactating women, following the 42 h of fasting, β-hydroxybutyrate and FFA concentrations were higher than those of the nonlactating women, reflecting higher rates of lipolysis and fat oxidation. Both the lactating and nonlactating women developed metabolic acidosis based on their ketosis. However, the lactating women were more acidotic, as indicated by their higher plasma ketone body and FFA concentrations. This supposition is supported by the lower urine pH values in the lactating women. Metabolic acidosis increases renal ammoniagenesis as a mechanism to correct the metabolic acidosis (3, 6). Glutamine is the primary substrate for renal ammonia production, whereas the carbon skeleton is incorporated into renal glucose production (6, 8, 21, 23). With extended fasting, the rate of proteolysis increased, and it is reasonable to speculate that the increased gluconeogenesis in the lactating compared with the nonlactating women may be attributed to the active contribution of renal gluconeogenesis. However, the increase in urea production in the lactating women might also indicate an increase in hepatic gluconeogenesis. Presumably, both glycerol (a product of lipolysis) (24, 26) and amino acids (8, 17) provide the carbon needed for the hepatic gluconeogenesis. However, it is impossible to determine the veracity of this argument without considerably more invasive studies. Furthermore, indirect calorimetry data revealed that the lactating women had lower CHO and higher fat oxidation rates following both 42 h of fasting and refeeding compared with the nonlactating women. Thus, the increased rates of gluconeogenesis and decreased rates of glucose utilization and oxidation by maternal tissues, presumably by the higher availability of FFA and ketone bodies, conserved maternal glucose for milk lactose synthesis.
After refeeding, glucose concentrations increased and glucose production rates decreased in both groups, with no difference between lactating and nonlactating women. Glucose production was almost entirely represented by gluconeogenesis, which was apparently decreased and corresponded to the 24-h fasting values previously reported in lactating and nonlactating women (30). FFA and β-hydroxybutyrate decreased with refeeding, but their concentrations remained higher in the lactating group. Despite refeeding for 6 h (a total of 50% of daily requirement), sources of energy utilization did not return to normal (i.e., after 14-h fast). The nonlactating women recovered more rapidly and shifted to CHO oxidation, whereas lactating women continued to rely on both fat and protein as energy sources. Thus, all dietary CHO was most likely delivered to the mammary gland for milk production.
With the ingestion of high-protein Boost during the refeeding period, the Ra of leucine (and thus the estimate of proteolysis) increased in both the lactating and nonlactating women. This most likely does not reflect an increased rate of endogenous proteolysis since in our previous studies endogenous proteolysis decreases with feeding (1, 10, 27). The increase in urea production rate (and thus protein oxidation) could be the result of oxidation of the protein consumed since 25% of the calories are provided as protein and/or this could be due to the incomplete equilibration of the urea tracer over the 6-h period of the study. In the nonlactating women, the increase in protein oxidation rate was consistent with an increase in the rate of proteolysis of the dietary protein. However, the increase in proteolysis in the lactating women during refeeding was not associated with a further increase in protein oxidation, suggesting that the dietary amino acids might be more readily utilized for protein synthesis, e.g., body tissue and/or milk proteins.
In summary, both the nonlactating and lactating women adapted to a 42-h fast by reducing endogenous glucose needs by similar mechanisms. However, due to the added demands imposed by lactation, the metabolic stress was greater in the lactating women, as indicated by lowered glucose concentration, increased glucagon concentrations, decreased utilization of CHO, higher plasma concentrations of FFA and ketone bodies (presumed greater metabolic acidosis), and higher plasma concentrations and rates of urea production, which presumably reflect increased rates of amino acid deamination and availability of carbon to support the increased rate of gluconeogenesis.
The present study concluded that the lactating women compared with the nonlactating women are at risk for fasting hypoglycemia if fasting is extended. Although milk production and composition were preserved, fasted lactating women were more acidotic and subjected to protein loss, as reflected by increased proteolysis and protein oxidation and fat loss as indicated by increased fat mobilization and oxidation.
ACKNOWLEDGMENTS
We acknowledge and thank our laboratory staff (Susan Sharma, Lauren Loyal, Daniel Donaldson, and Karen Jones) and the staff at the GCRC who greatly facilitated the execution of these studies.
GRANTS
This project was supported by National Institutes of Health Grants RO1-DK-55478 and HD-37857 and Baylor GCRC Grant MO1-RR-00188-34.
DISCLOSURES
This work is a publication of the US Department of Agriculture/Agricultural Research Service Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine (Houston, TX). The contents of this publication do not necessarily reflect the views of policies of the US Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement from the US Government.
REFERENCES
- 1.Beaufrere B, Horber FF, Schwenk WF, Marsh HM, Matthews D, Gerich JE, Haymond MW. Glucocorticosteroids increase leucine oxidation and impair leucine balance in humans. Am J Physiol Endocrinol Metab 257: E712–E721, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Borschel MW, Kirksey A, Hannemann RE. Evaluation of test-weighing for the assessment of milk volume intake of formula-fed infants and its application to breast-fed infants. Am J Clin Nutr 43: 367–373, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Brosnan JT, McPhee P, Hall B, Parry DM. Renal glutamine metabolism in rats fed high-protein diets. 235: Am J Physiol E261–E265, 1978 [DOI] [PubMed] [Google Scholar]
- 4.Chacko SK, Sunehag AL, Sharma S, Sauer PJ, Haymond MW. Measurement of gluconeogenesis using glucose fragments and mass spectrometry after ingestion of deuterium oxide. J Appl Physiol 104: 944–951, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 27: 314–319, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Fine A. The effects of chronic metabolic acidosis on liver and muscle glutamine metabolism in the dog in vivo. Biochem J 202: 271–273, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983 [DOI] [PubMed] [Google Scholar]
- 8.Hankard RG, Haymond MW, Darmaun D. Role of glutamine as a glucose precursor in fasting humans. Diabetes 46: 1535–1541, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Haymond MW, Karl IE, Clarke WL, Pagliara AS, Santiago JV. Differences in circulating gluconeogenic substrates during short-term fasting in men, women, and children. Metabolism 31: 33–42, 1982 [PubMed] [Google Scholar]
- 10.Horber FF, Haymond MW. Human growth hormone prevents the protein catabolic side effects of prednisone in humans. J Clin Invest 86: 265–272, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalhan SC. Rates of urea synthesis in the human newborn: effect of maternal diabetes and small size for gestational age. Pediatr Res 34: 801–804, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Kaplan W, Sunehag AL, Dao H, Haymond MW. Short-term effects of recombinant human growth hormone and feeding on gluconeogenesis in humans. Metabolism 57: 725–732, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz J, Tayek JA. Gluconeogenesis and the Cori cycle in 12-, 20-, and 40-h-fasted humans. Am J Physiol Endocrinol Metab 275: E537–E542, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Keller RP, Neville MC. Determination of total protein in human milk: comparison of methods. Clin Chem 32: 120–123, 1986 [PubMed] [Google Scholar]
- 15.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 98: 378–385, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marliss EB, Aoki TT, Unger RH, Soeldner JS, Cahill GF., Jr Glucagon levels and metabolic effects in fasting man. J Clin Invest 49: 2256–2270, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer C, Stumvoll M, Dostou J, Welle S, Haymond M, Gerich J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab 282: E428–E434, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Mohammad MA, Sunehag AL, Haymond MW. Effect of dietary macronutrient composition under moderate hypocaloric intake on maternal adaptation during lactation. Am J Clin Nutr 89: 1821–1827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motil KJ, Kertz B, Thotathuchery M. Lactational performance of adolescent mothers shows preliminary differences from that of adult women. J Adolesc Health 20: 442–449, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, Allen J, Archer P. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr 48: 1375–1386, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Pagliara AS, Goodman AD. Relation of renal cortical gluconeogenesis, glutamate content, and production of ammonia. J Clin Invest 49: 1967–1974, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Studer RK, Snowdowne KW, Borle AB. Regulation of hepatic glycogenolysis by glucagon in male and female rats. Role of cAMP and Ca2+ and interactions between epinephrine and glucagon. J Biol Chem 259: 3596–3604, 1984 [PubMed] [Google Scholar]
- 23.Stumvoll M, Meyer C, Perriello G, Kreider M, Welle S, Gerich J. Human kidney and liver gluconeogenesis: evidence for organ substrate selectivity. Am J Physiol Endocrinol Metab 274: E817–E826, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Sunehag A, Gustafsson J, Ewald U. Glycerol carbon contributes to hepatic glucose production during the first eight hours in healthy term infants. Acta Paediatr 85: 1339–1343, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Sunehag A, Tigas S, Haymond MW. Contribution of plasma galactose and glucose to milk lactose synthesis during galactose ingestion. J Clin Endocrinol Metab 88: 225–229, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Sunehag AL. Parenteral glycerol enhances gluconeogenesis in very premature infants. Pediatr Res 53: 635–641, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Sunehag AL, Haymond MW. Maternal protein homeostasis and milk protein synthesis during feeding and fasting in humans. Am J Physiol Endocrinol Metab 285: E420–E426, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Sunehag AL, Louie K, Bier JL, Tigas S, Haymond MW. Hexoneogenesis in the human breast during lactation. J Clin Endocrinol Metab 87: 297–301, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Sunehag AL, Treuth MS, Toffolo G, Butte NF, Cobelli C, Bier DM, Haymond MW. Glucose production, gluconeogenesis, and insulin sensitivity in children and adolescents: an evaluation of their reproducibility. Pediatr Res 50: 115–123, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Tigas S, Sunehag A, Haymond MW. Metabolic adaptation to feeding and fasting during lactation in humans. J Clin Endocrinol Metab 87: 302–307, 2002 [DOI] [PubMed] [Google Scholar]