Abstract
This article addresses two topics. We provide an overview of the National Institutes of Health Mouse Metabolic Phenotyping Center (MMPC) Program. We then discuss some observations we have made during the first eight years of the Vanderbilt MMPC regarding common phenotyping practices. We include specific recommendations to improve phenotyping practices for tests of glucose tolerance and insulin action. We recommend that methods for experiments in vivo be described in manuscripts. We make specific recommendations for data presentation, interpretation, and experimental design for each test. To facilitate and maximize the exchange of scientific information, we suggest that guidelines be developed for methods used to assess glucose tolerance and insulin action in vivo.
Keywords: insulin resistance, insulin clamp, diabetes
this article addresses two general topics. First, we provide an overview of the National Institutes of Health (NIH) Mouse Metabolic Phenotyping Center (MMPC) Program. Second, we discuss some observations we have made during the first eight years of the Vanderbilt MMPC regarding common phenotyping practices and include specific recommendations to improve them.
The MMPC Program
The MMPC (www.mmpc.org) was established in 2001 as a result of a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) initiative developed to address the need to characterize the growing number of mouse models of chronic metabolic diseases, particularly diabetes and obesity. In its first year, the MMPC consortium, consisting of four centers in conjunction with external advisors, defined as its mission to “advance medical and biological research by providing the scientific community with standardized, high quality metabolic and physiologic phenotyping services for mouse models of diabetes, diabetic complications, obesity and related disorders.” The National Heart, Lung, and Blood Institute (NHLBI) became a project participant in 2006. In the same year, the MMPC program was expanded to six centers and a Coordinating and Bioinformatics Unit was added. The Centers provide experimental and analytical services on a fee-for-service basis.
In addition to providing investigator-initiated phenotyping services, the MMPC program sponsors a Pilot and Feasibility Program, contributes to the standardization of methodology, and conducts an educational program. A goal of MMPC operations is to be responsive to the needs of the scientific community. Finally, the results of the MMPC phenotyping tests are archived on a database that can be accessed from the internet.
Operations and services.
MMPC phenotyping services are offered at Case Western Reserve University (CWRU), University of Cincinnati Medical Center (UC), University of Texas Southwestern Medical Center (UTSW), University of Washington (UW), Vanderbilt University School of Medicine (VU) and Yale University School of Medicine (YU). The Coordinating and Bioinformatics Unit at the Medical College of Georgia manages the web site, database, and Pilot and Feasibility Program. Except for a few areas of necessary overlap, each MMPC provides unique services to the scientific community. CWRU and UTSW provide measurements and analyses of metabolic pathway fluxes by use of mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy, respectively. UC provides comprehensive analysis of lipid metabolism, energy balance, feeding behavior, and cardiovascular parameters. UW provides tests for diabetic complications, energy balance, and feeding behavior. VU provides analysis of glucose metabolism, energy balance, and cardiovascular function in vivo, and YU provides analysis of glucose metabolism in vivo. Most of the centers have, in addition, analytical cores for measurements of metabolites, hormones, lipids, and other compounds. The complete list of services provided by each center can be found at www.mmpc.org.
Specific tests can be requested and inquiries can be made from links at www.mmpc.org. The investigator will be contacted by a center, receive an estimate for the service(s), and be given information for shipping of mice or samples. Data are sent directly to the investigator. Investigators may also contact centers directly using the contact information listed at www.mmpc.org.
Mice sent from an approved vendor such as Jackson Laboratories or Charles River can be admitted directly to an MMPC. Mice from other sources enter a quarantined area upon transfer to an MMPC institution. Arrangements can be made to accommodate specific mouse diets, pharmacological interventions, or nutritional supplements.
Pilot and feasibility grant.
The MMPC Pilot and Feasibility Program provides funds (generally $60,000 per year) for development of new techniques and tests for the characterization of mice. Funding is competitively awarded to proposals that 1) develop new technologies or miniaturize existing technologies for use in mice, 2) develop applications of existing technologies for use in mice, 3) provide new tests to meet identifiable, outstanding needs necessary to phenotype mouse models of metabolic disease, or 4) establish new types of mathematical models, informatics, databases, or products that augment the mission of the center (http://www.mmpc.org/shared/fundingPrograms.aspx).
Database and data ownership.
A material transfer form (http://www.mmpc.org/MTA.pdf) is in place to protect the interests of investigators. All data and intellectual property that are generated by the MMPC belong to the investigator who has submitted the request for services. NIH requests that investigators allow data generated by an MMPC to be placed in the public MMPC database after one of the following two conditions has been met: 1) the data have been published and are therefore in the public domain, or 2) two years have passed since completion of services by the MMPC. Investigators may request that specific data be withheld from the public database for an additional period of time.
The capacity to make multiple measurements in a single mouse allows for more precise analysis of physiological and pathophysiological responses. Considering the expense and time involved in shipping or breeding, it is cost effective and efficient to obtain as much data from a single mouse as possible. Several of the tests utilized by the MMPCs are noninvasive or minimally invasive, thus allowing application of multiple assessments to a single mouse. Some of those tests include tail cuff blood pressure monitoring, echocardiography, urine analysis, exercise tolerance, body composition, food intake, energy expenditure, and activity monitoring. Data obtained from a single mouse are further maximized in terminal procedures by the excising of tissue that can be immediately processed or suitably archived for further analysis. Genetically modified mice can develop unexpected phenotypes, which can be detected with these tests while not compromising the primary research questions.
Standardization of procedures, education, and outreach.
Standardization of procedures and characterization of inbred strains are important goals of the Vanderbilt MMPC (4, 7, 16, 21, 23). The goals of standardization are to optimize experimental designs and allow for direct comparison between different laboratories that have common tests. Achieving standardization has been challenging because techniques and approaches vary considerably from one laboratory to another. Moreover, as techniques used in large animals and humans were transferred to the mouse, compromises to accommodate the small size of the mouse and some serious conceptual errors were made. This problem has been compounded because thorough presentation of methodology has not generally been required for publication. The lack of accurate and complete descriptions of methodology has constrained advancement in some fields. The MMPC, through education and outreach, serves as a resource to open dialogues on standardization of procedures to study the mouse. For many procedures the approaches are relatively easy to learn and can be implemented by any laboratory. Others are more complex and require more advanced skills, expertise, and/or equipment.
The Vanderbilt MMPC receives a large number of requests for training on how to perform glucose clamps in the conscious mouse. In an attempt to train multiple investigators in a time-efficient manner, the Vanderbilt MMPC offers a group training program entitled “Glucose Clamping the Conscious Mouse: A Laboratory Course.” This course is designed to address two major needs of the scientific community. The first is to aid those laboratories needing to perform glucose clamps on a regular basis in the use of this challenging technique. The second is to make the mouse clamp technology more transparent so that scientists wishing to make sense of the growing literature better understand the factors involved in performing clamps in the conscious mouse. Details of the course are described at www.mc.vanderbilt.edu/MMPC/. The first course was offered in the fall of 2005, and it has been offered annually since then. This five-day-long course consists of eight hours of lectures, six hours of demonstrations, and more than 21 hours of supervised “hands on” time, including performing surgeries and clamps. “Glucose Clamping the Conscious Mouse” is limited to 10 participants due to the laboratory nature of the course.
In 2007 and 2009, a course entitled “Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis” was sponsored by the Donald W. Reynolds Institute on Aging, the MMPC, NIDDK, and NHBLI. It was a week-long course in the theory and practice of isotopic tracers (stable and radioactive) for the study of metabolism in humans and animals by means of mass spectrometry and NMR. The course also emphasized isotopomer analysis for metabolic flux rates and metabolic regulation. This course, as well as other more specialized courses, will be offered in the future (www.mmpc.org/shared/courses.aspx).
Evaluating Glucose Homeostasis in the Mouse
The interpretation of data is dependent on issues of experimental design. In this section, we address some observations regarding commonly used tools to test glucose tolerance and insulin sensitivity in the mouse. Many phenotyping tests are impossible to do free of assumptions. The goal of this section is to evaluate experimental designs and assumptions that are the most reasonable and most conducive to unbiased interpretation.
A primary screen to evaluate whether a genetic manipulation alters glucose homoeostasis is the measurement of fed and fasting circulating glucose and insulin. One of the difficulties inherent in this is that the glucose concentration changes throughout the day. For the mouse, the additional stress associated with restraint or anesthesia during blood sampling can add to the variability. In larger species, animals are typically overnight fasted, or even 42 hour fasted in some dog studies, to obtain a more consistent baseline glucose concentration. This approach has also been taken by many investigators using mice as their animal model, and it is recommended as standard operating procedure for phenotyping mice by the Eumorphia Consortium (1) (www.eumorphia.org). However, the impact of such a long fast on metabolism in the mouse is profound, making extrapolation of those data to observations in the “free-living” state difficult. Since mice eat the majority of their food at night and have a very high metabolic rate, overnight fasting a mouse is a major metabolic stress. A lean mouse loses ∼15% of its lean body mass after an overnight fast (4). Some Institutional Animal Care and Use Committees do not allow overnight fasting without additional justification. A further complication is that typically mice are housed at ∼23°C, which is well below their thermoneutral zone (∼30°C), forcing animals to increase their food intake and energy expenditure to maintain body temperature (14). Housing animals at temperatures below their thermoneutral zone can modify metabolic phenotypes (12, 20). Although 23°C is an adequate temperature for breeding and growth, it can result in torpor in individually housed mice when food is restricted (13, 25). Torpor is characterized by hypothermia, hypotension, bradycardia, and decreased metabolic rate. Fasting a mouse for a shorter interval is more physiological and results in reduced metabolic stress. A five- to six-hour fast is often used in the mouse to determine whether fasting glucose levels are normal. Since they tend to nibble throughout the day, mice rarely go into a “true” fast. Therefore, measuring glucose concentrations in the fed state may be informative as well. In both the fasted and fed states, obtaining reliable and reproducible glucose and insulin data requires that careful attention be paid to feeding patterns and time of day. Glycosylated hemoglobin (Hb) is a good marker of long-term differences in glucose concentration in humans and is less sensitive to conditional variations in blood glucose. Hb is also a good marker of blood glucose in mice, and Hb A1c is a strong reflection of total Hb in most mouse strains (16).
Is Glucose Intolerance in Obese Mice an Inevitable Conclusion of the Glucose Tolerance Test?
If the glucose or insulin concentrations suggest that glucose intolerance or insulin insensitivity exist, a glucose tolerance test (GTT) is often performed. Generally glucose is given as an intraperitoneal injection (1–2 g/kg). In some cases, glucose is given orally or by an intravenous injection. While the oral route is more physiological, increased variability associated with inconsistent rates of gastric emptying can complicate data interpretation. Glucose tolerance following oral glucose loading is influenced by intestinal derived factors that can alter insulin secretion and/or insulin action (5, 24). This incretin effect is absent with intraperitoneal injections. Not surprisingly, the glucose and insulin excursions after an oral GTT are less than that seen with an intraperitoneal route (2). In many studies, the animals are overnight fasted prior to the test. Some of the caveats with using an overnight fast have already been discussed. However, there is an additional caveat. As opposed to humans and other species where insulin action worsens with prolonged fasting (9) insulin action in mice is enhanced by a prolonged fast (2, 4, 15, 22). Moreover prolonged fasting tends to provoke a peripheral (muscle) phenotype; peripheral and muscle glucose uptake is higher during a hyperinsulinemic euglycemic clamp in prolonged-fasted mice (15, 17, 22). Given its effects on glucose metabolism and insulin action, overnight fasting prior to a GTT is an unnecessary complication, unless the focus of the investigation is on the effect of a prolonged fast itself. It was concluded in a recent paper that impaired glucose tolerance in obese mice is most sensitively detected after a six-hour fast (2).
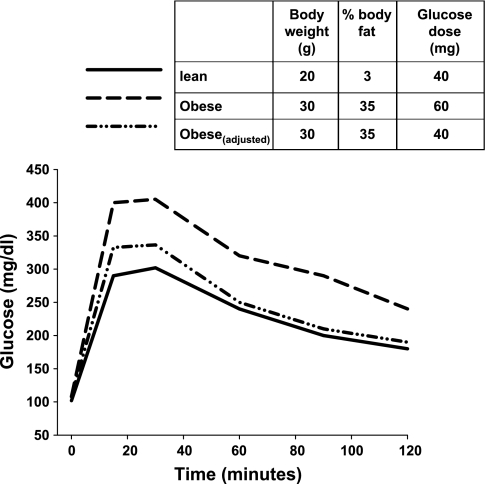
A major variable that receives inadequate consideration in the design of a GTT is the dose of glucose. The primary phenotype in mouse models of diabetes is often a difference in body weight. If one is comparing mice of differing body weight or composition by administering a glucose dose based on body weight, results may be biased so that an impaired glucose tolerance is inevitable. Obesity is primarily caused by an increase in fat mass. Depending on the manipulation (dietary or genetic), fat mass can increase to between 30 and 60% of total body weight (3, 6). However, lean body mass (muscle, liver, brain) is the main site of glucose disposal. Administering glucose on a per body weight basis will therefore increase the glucose dose disproportionately in relation to the major glucose-utilizing tissue. For example, a 10-week-old mouse on a chow diet may weigh 20 g, while an obese mouse may weigh 30 g. If the glucose dose is calculated on a per body weight basis, the obese mouse will receive a 50% larger glucose dose! (Fig. 1) That same mouse, however, may carry little if any additional lean tissue. If lean mass is gained, it generally is not in proportion to the overall weight gain. In the clinic, a fixed amount of glucose is usually administered instead of being normalized on a body weight basis. Although this approach also has limitations, it does provide a clear definition of glucose intolerance (Fig. 1). It may be more precise to normalize the glucose dose on the basis of lean body mass (or fat-free mass) and not total body weight. A recent study assessed the impact of obesity on glucose tolerance in C57BL6 mice. The investigators compared oral glucose tolerance when the glucose load was given as a fixed dose to a dose based on body weight. The mice were moderately obese (7% increase in body weight; body composition was not measured). The glucose intolerance observed in obese mice was markedly less pronounced when a fixed dose of glucose was administered compared with when the glucose load was based on body weight (2). Moreover, in contrast to chow-fed mice, the glucose excursion in obese mice is very sensitive to the dose of glucose administered, especially when given via the oral route (2). Thus, it is imperative that investigators consider changes in body composition when designing protocols to evaluate glucose tolerance.
Fig. 1.
Hypothetical profile of a glucose tolerance test in a lean and an obese mouse. Glucose dosage was either given on a per body weight basis or adjusted for differences in body composition (obeseadjusted). When the dose is adjusted, both lean and obese animals are receiving the equivalent total amount of glucose and have similar glucose tolerance.
If body weight and/or fat pad weights differ between groups, the investigator should ask whether the conclusion is influenced by the body composition. If glucose tolerance is impaired but fat mass expressed as a fraction of body weight is decreased, then repeating the GTT with a fixed dose of glucose may not be needed. In contrast, if the investigator suspects that fat mass is increased and glucose tolerance is impaired (with the glucose load based on body weight), then the investigator should consider determining whether glucose tolerance is also impaired when a fixed glucose dose is used.
The prevailing incoming blood glucose can also complicate the interpretation of a GTT. Several approaches are used to compare groups with differing baseline glucose. They include calculating data as change from baseline, area under the curve, or percent change from baseline. An inherent assumption in all of these calculations is that the absolute glucose concentration is not an important determinant of glucose tolerance. In fact, hyperglycemia, even in the absence of an accompanying rise in insulin secretion, can suppress hepatic glucose production and augment glucose disposal (i.e., glucose effectiveness) (8). Glucose tolerance is influenced by the prevailing glucose level and is reflective of the dynamic relationship between rates of glucose entry and the capacity of the body to rapidly augment glucose disposal. As such, presentation of data should reflect this dynamic relationship. The time course of the glucose concentration during a GTT is informative and should be reported. In addition, the fasting status, glucose dosage, route of glucose delivery, and site of blood sampling must be reported to interpret data from a GTT. See Table 1 for summary of the authors' recommendations.
Table 1.
Glucose tolerance test
| Methods Checklist | Considerations in Experimental Design | Recommendations for Data Presentation |
|---|---|---|
| Body weight and, if possible, body composition | Fast duration | Present time course of absolute blood or plasma glucose profile and give values for glucose AUC |
| Sex, age, and specify whether littermates were used | Group differences in body weight and body composition when glucose dose is calculated | Present plasma insulin concentrations, if possible |
| Glucose dose | Hematocrit changes when hand-held blood glucose meters are used | |
| Route of glucose administration | ||
| Blood sampling site | ||
| Indicate if restraint device or anesthesia was used | ||
| Indicate whether donor blood was used | ||
| Diet and fasting status | ||
| Alterations in environment (temperature, light-dark cycle) |
Considerations and recommendations are those specified by the authors.
Is Insulin Resistance in the Hyperglycemic Mouse an Inevitable Conclusion of the Insulin Tolerance Test?
The insulin tolerance test (ITT) was developed as a simple way to evaluate insulin action in vivo in humans. Overnight-fasted humans are given an intravenous bolus of insulin (0.1–0.5 U/kg), and the rate of fall of glucose is assessed every 2 min over a 15-min period(26). In mice, this test was modified, where a larger bolus of insulin (0.5–2 U/kg) is given after a fast of variable duration and the glucose concentration is monitored for a much longer duration (∼60–90 min). For these tests, the fall in blood glucose is used as a reflection of insulin action. In wild-type mice on a chow diet, glucose concentration falls by ∼50%; the depth and rate of the fall can vary depending on the insulin dose. One of the confounding issues with this test is that the prevailing glucose level will influence the rate and magnitude of its fall. In addition, as glucose concentration falls during the ITT, it can activate insulin counterregulation. The glucose threshold for increased counterregulatory hormone release in the mouse is ∼80 mg/dl (18). This threshold will likely vary depending on a number of factors (e.g., genetic background, nutrition, genetic mutation). A defect in counterregulatory hormone release could be misinterpreted during an ITT as an improvement in insulin action (19).
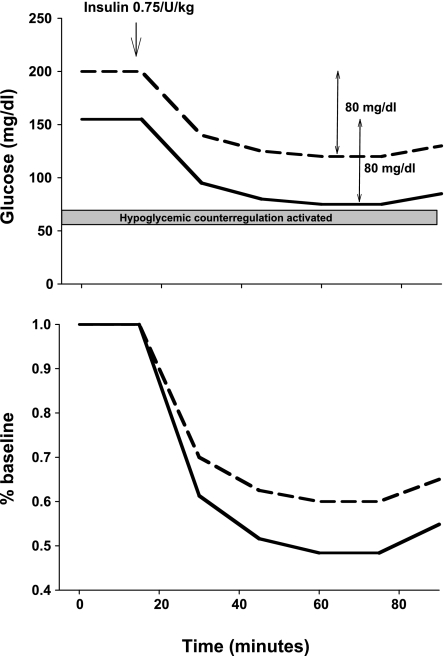
The presence of hyperglycemia at the onset of an ITT could lead to an incorrect “diagnosis” of insulin resistance, depending on how the data are presented. In humans, insulin action is estimated by calculating the rate of fall of the log-transformed glucose. In contrast, in mice, the fall in glucose concentration during an ITT is often presented as a percentage of basal glucose concentration. This is problematic if mice have different circulating glucose concentrations. A hyperglycemic mouse will have a smaller percent fall in blood glucose for the same absolute fall. For example, mice with incoming glucoses of 200 and 150 mg/dl both experience equivalent glucose decrements of 80 mg/dl in response to a bolus of insulin (Fig. 2). The readout as a percentage of basal glucose would suggest that insulin resistance in the hyperglycemic mouse as the decrement in blood glucose would be only 48% of basal compared with 60% in the mouse with the lower blood glucose. Thus, the approach of using percent basal to quantify decrements in glucose will underestimate insulin sensitivity in hyperglycemic mice and will inevitably lead to a diagnosis of insulin resistance that may be erroneous.
Fig. 2.
Hypothetical insulin tolerance test is administered to two animals with differing baseline glucose concentrations. Glucose concentration is expressed either as absolute glucose concentration (top) or as percent baseline (bottom). Top: absolute decrement in glucose was equivalent (80 mg/dl). In one animal, the glucose concentration decreased to near the threshold for glucose counterregulation. Bottom: percent fall in glucose concentration was greater in the animal with the lower incoming glucose.
The problem of normalizing glucose dose to body weight during a GTT was discussed in detail earlier. This same issue creates problems in selecting insulin doses for an ITT. A heavier mouse will receive a higher total insulin dose, although the amount of lean tissue may be the same. As with the GTT, differences in body composition must be considered in determining the appropriate dose of insulin for an ITT. Although the insulin dose is typically given on the basis of the total body weight, normalization to lean body mass may be a more accurate alternative. Another issue that requires attention is the duration of the ITT. Insulin t1/2 is very fast (∼10 min) (10). Thus, the majority of the injected insulin would normally be cleared 60 min after the insulin injection. As a consequence, differences in glucose concentration observed after the initial fall during an ITT may not relate to insulin action. If one is to rely on the latter time frame (>30 min after the onset) of an ITT, measurement of insulin concentrations would be essential to interpretation (see Table 2 for summary of the authors' recommendations).
Table 2.
Insulin tolerance test
| Methods Checklist | Considerations in Experimental Design | Recommendations for Data Presentation |
|---|---|---|
| Body weight and body composition, if possible | Fast duration | Present entire time course of absolute glucose profile |
| Sex, age, and littermates if used | Group differences in body weight and body composition when insulin dose is calculated | Do not normalize glucose data on basis of differing basal glucose concentration |
| Insulin dose and type of insulin | Hematocrit changes if hand-held blood glucose meters are used | Present plasma insulin concentration |
| Route of insulin administration | ||
| Blood sampling site | ||
| Indicate if restraint or anesthesia was used | ||
| Diet and fasting status | ||
| Alterations in environment (temperature, light-dark cycle) |
Considerations and recommendations are those specified by the authors.
What is the Status of the Glucose Clamp Technique in Application to the Mouse?
The hyperinsulinemic euglycemic clamp technique was first developed for human studies (11) and was subsequently adapted for other species. Performing a clamp in mice is very challenging due to their small size and limited blood volume. Despite these challenges, a number of laboratories have established this procedure. Regrettably, standards for presenting protocols and data in published studies using humans and larger species have not been adopted in mice. We have recently discussed this deficit in some detail (27). Needless to say, the experimental design can impact the outcome and interpretation of a clamp study (4). The lack of methodological documentation is complicated by the many variations in the protocol that have evolved. As with a GTT and ITT, basic information regarding methodology, including fasting status, light-dark cycle, diet, tracer and insulin delivery profiles, sampling sites, times of sample collection, and clamp duration need to be reported (Table 3).
Table 3.
Euglycemic hyperinsulinemic clamp
| Methods Checklist | Considerations in Experimental Design | Recommendations for Data Presentation |
|---|---|---|
| Body composition and, if possible, body composition | Fast duration | Present entire time course of glucose concentrations and glucose infusion rates |
| Sex, age, and littermates, if used | Hematocrit changes if hand-held blood glucose meters are used | Present absolute rates of endogenous glucose production |
| Complete protocol for insulin and tracer infusion, including any priming boluses that are used | Group differences in body weight and body composition when insulin dose is calculated | Present actual basal and clamp plasma insulin concentrations |
| Flux calculations when appropriate | Predicted volumes of distribution and fractional turnover rates of insulin and glucose and predicted steady-state values when priming doses are calculated | |
| Route of glucose, insulin and tracer administration | ||
| Blood sampling sites and frequency of collection | ||
| Indicate if restraint or anesthesia was used | ||
| Diet and fasting status | ||
| Alterations in environment (temperature, light-dark cycle) |
Considerations and recommendations are those specified by the authors. Adapted from Wasserman et al. (27), with permission.
An important step in evaluating clamp data is determining how successful the investigator was in clamping the glucose. The “Datum Approach,” which is the reduction of a 2-h clamp experiment to a single point, is common in the mouse literature. The Datum Approach makes the existence of a clamp impossible to assess (27). In addition, many investigators wish to determine whether suppression of endogenous glucose production by insulin is altered. In many cases, data are presented as percent baseline with no reference to the absolute baseline glucose production rates. Percent suppression from baseline is informative only if absolute rates of glucose production are reported as well. Certain basic technical information, which is often omitted, is needed to demonstrate the adequacy of the hyperinsulinemic euglycemic clamp and to interpret the results it yields.
Summary
We believe that applying standards for evaluating glucose tolerance and insulin sensitivity is important in the use of genetically manipulated mice as models of disease. The Eumorphia Consortium (1) recognized that procedures needed to be standardized and adopted (www.eumorphia.org) standards for assessing physiological systems in mice. It has developed such standards for many phenotyping tests. Such standards should be established to define the mouse metabolic phenotype in relation to insulin resistance, diabetes, and obesity. We have made some observations and provided some recommendations based on our experiences, but we recognize that the issues involved are complex. Perhaps our recommendations will serve as an initial iteration from which consensus and standards will form. We welcome comments and hope this article will initiate a discussion of mouse phenotyping practices.
ACKNOWLEDGMENTS
The Vanderbilt Mouse Metabolic Phenotyping Center is supported by NIDDK Grant DK-59637.
REFERENCES
- 1.Anonymous. EMPReSS: standardized phenotype screens for functional annotation of the mouse genome. Nat Genet 37: 1105–1155, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab 295: E1323–E1332, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson RD, Coenen KR, Plummer MR, Gruen ML, Hasty AH. Macrophage-derived apolipoprotein E ameliorates dyslipidemia and atherosclerosis in obese apolipoprotein E-deficient mice. Am J Physiol Endocrinol Metab 294: E284–E290, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Ayala JE, Bracy D, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 150: 1155–1164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56: 1025–1033, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, Jewell MM, Powers AC, Wasserman DH. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 57: 1790–1799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 19: 1018–1030, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Bjorkman O, Eriksson LS. Influence of a 60-hour fast on insulin-mediated splanchnic and peripheral glucose metabolism in humans. J Clin Invest 76: 87–92, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cresto JC, Lavine RL, Buchly ML, Penhos JC, Bhathena SJ, Recant L. Half life of injected 125I-insulin in control and ob/ob mice. Acta Physiol Lat Am 27: 7–15, 1977 [PubMed] [Google Scholar]
- 11.Defronzo RA, Tobin J, Andres R. Glucose clamp technique a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E218, 1979 [DOI] [PubMed] [Google Scholar]
- 12.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 Ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metabolism 9: 203–209, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66: 239–274, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Golozoubova V, Gullberg H, Matthias A, Cannon B, Vennstrom B, Nedergaard J. Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol Endocrinol 18: 384–401, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Halseth AE, Bracy DP, Wasserman DH. Overexpression of hexokinase II increases insulin and exercise-stimulated muscle glucose uptake in vivo. Am J Physiol Endocrinol Metab 276: E70–E77, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Han BG, Hao CM, Tchekneva EE, Wang YY, Lee CA, Ebrahim B, Harris RC, Kern TS, Wasserman DH, Breyer MD, Qi Z. Markers of glycemic control in the mouse: comparisons of 6-h- and overnight-fasted blood glucoses to Hb A1c. Am J Physiol Endocrinol Metab 295: E981–E986, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heijboer AC, Donga E, Voshol PJ, Dang ZC, Havekes LM, Romijn JA, Corssmit EP. Sixteen hours of fasting differentially affects hepatic and muscle insulin sensitivity in mice. J Lipid Res 46: 582–588, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Jacobson L, Ansari T, McGuinness OP. Counterregulatory deficits occur within 24 h of a single hypoglycemic episode in conscious, unrestrained, chronically cannulated mice. Am J Physiol Endocrinol Metab 290: E678–E684, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson L, Ansari T, Potts J, McGuinness OP. Glucocorticoid-deficient corticotropin-releasing hormone knockout mice maintain glucose requirements but not autonomic responses during repeated hypoglycemia. Am J Physiol Endocrinol Metab 291: E15–E22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodhi IJ, Semenkovich CF. Why we should put clothes on mice. Cell Metab 9: 111–112, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Ren JM, Marshall BA, Mueckler MM, McCaleb M, Amatruda JM, Shulman GI. Overexpression of Glut4 protein in muscle increases basal and insulin-stimulated whole body glucose disposal in conscious mice. J Clin Invest 95: 429–432, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rottman JN, Ni G, Brown M. Echocardiographic evaluation of ventricular function in mice. Echocardiography 24: 83–89, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Salehi M, Aulinger BA, D'Alessio DA. Targeting (beta)-cell mass in type 2 diabetes: promise and limitations of new drugs based on incretins 10.1210/er2007–0031. Endocr Rev 29: 367–379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. The full expression of fasting-induced torpor requires (beta)3-adrenergic receptor signaling. J Neurosci 26: 241–245, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace T, Matthews DR. The assessment of insulin resistance in man. Diabet Med 19: 527–534, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Wasserman DH, Ayala JE, McGuinness OP. Lost in translation. Diabetes 58: 1947–1950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]