Abstract
We all have memories that we prefer not to think about. The ability to suppress retrieval of unwanted memories has been documented in behavioral and neuroimaging research using the Think/No-Think (TNT) paradigm with adults. Attempts to stop memory retrieval are associated with increased activation of lateral prefrontal cortex (PFC) and concomitant reduced activation in medial temporal lobe (MTL) structures. However, the extent to which children have the ability to actively suppress their memories is unknown. This study investigated memory suppression in middle childhood using the TNT paradigm. Forty children aged 8–12 and 30 young adults were instructed either to remember (Think) or suppress (No-Think) the memory of the second word of previously studied word-pairs, when presented with the first member as a reminder. They then performed two different cued recall tasks, testing their memory for the second word in each pair after the TNT phase using the same first studied word within the pair as a cue (intra-list cue) and also an independent cue (extra-list cue). Children exhibited age-related improvements in memory suppression from age 8 to 12 in both memory tests, against a backdrop of overall improvements in declarative memory over this age range. These findings suggest that memory suppression is an active process that develops during late childhood, likely due to an age-related refinement in the ability to engage PFC to down-regulate activity in areas involved in episodic retrieval.
Keywords: memory suppression, inhibition, episodic retrieval, prefrontal cortex, medial temporal lobe, childhood, cognitive development
Introduction
Can individuals intentionally suppress unwanted memories? This question has attracted the interest of psychologists and clinicians for decades, because the ability to exert control over one's memories has important implications for cognitive functioning and psychological well-being (Walker et al., 2003). Several studies have demonstrated that adults can intentionally suppress memory of neutral (e.g., Anderson and Green, 2001; Levy and Anderson, 2008; Hanslmayr et al., 2009; but see Bulevich et al., 2006) and emotional stimuli (e.g., Joormann et al., 2005; Depue et al., 2006, 2007), with efforts to limit awareness of unwanted memories leading to impaired retention. Intentional retrieval suppression is associated with concomitant increases in activation of lateral PFC and reduced activity of memory-related structures in the MTL, including the hippocampus (Anderson et al., 2004; Depue et al., 2007; see also Hanslmayr et al., 2009). These results suggest that control processes modulate medial-temporal activity to control declarative memory (see Levy and Anderson, 2008; Anderson and Levy, 2009for reviews).
The question motivating our research concerns the development of this memory suppression capacity during childhood. Despite extensive behavioral research examining how memory control processes at encoding or retrieval may mediate the developing ability to remember (Bjorklund and Douglas, 1997; Schneider and Pressley, 1999), little is known about how these processes may mediate the complementary ability to prevent successful memory. Addressing this question is thus important for a comprehensive theory of memory and its development, as well as to draw interesting connections with other domains of psychology, such as coping and emotion regulation. A growing body of research documents that with development children acquire increasingly sophisticated notions about mind control (Flavell, 1999), and about the cognitive strategies that can be used to cope with stressors. For instance, the frequency with which participants report using coping strategies capitalizing on cognitive distraction, such as diversionary thinking or attempts to forget a stressor, increases from middle childhood to adolescence (e.g., Altshuler and Ruble, 1989; Skinner and Zimmer-Gembeck, 2007). These observations suggest that the ability to exert control over memory retrieval develops during middle childhood years. However, the developmental trajectory of the capacity to actively prevent unwanted information from entering consciousness, and its effects on long-term memory are unknown. Thus, the present research offers a window into the developmental progression of the ability to regulate memory retrieval and into further examinations of its neurodevelopmental underpinnings.
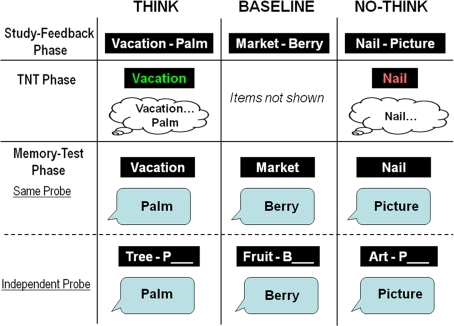
To examine the development of intentional memory suppression, we used a child-friendly version of the TNT paradigm (Anderson and Green, 2001), which has been employed successfully in studies of adult memory suppression, but has not been used to examine memory suppression in children (see Figure 1). Participants first learned a list of word-pairs (e.g., Vacation-Palm), and were then presented with the first member of two thirds of the word-pairs (e.g., Vacation), and were asked either to remember the associated word (e.g., Palm; Think condition), or to keep the memory for the associated word out of mind (No-Think condition). The remaining third of the initial word-pairs was not presented during this TNT phase (Baseline condition). After this phase, participants’ memory for the second word of all initially presented word-pairs was measured with two separate tests. In the Same-probe (SP) test, participants were tested with intra-pair cues; they received the first member of a word-pair (e.g., Vacation), and were asked to retrieve the second member (e.g., Palm). In the Independent-probe (IP) test, participants were tested with extra-pair cues. They received the first letter of the second word in a pair, as well as a word describing the category membership of this word (e.g., Tree-P), and were asked to complete the second member (e.g., Palm).
Figure 1.
Depiction of the three main phases of the TNT procedure: Study-feedback phase, TNT phase, and memory test phase (SP and IP).
Repeated attempts to suppress the No-Think items are associated with lower recall relative to the Think and Baseline items (e.g., Anderson and Green, 2001; Levy and Anderson, 2008). Lower memory for the No-Think items is best assessed with respect to the Baseline items, which are not presented during the TNT phase. These findings provide strong support for the idea that the exertion of intentional inhibitory control over memory retrieval hinders the accessibility of No-Think items.
Using a different procedure, known as directed forgetting, previous studies showed developmental improvements in “intentional” forgetting during middle childhood. In directed-forgetting paradigms, no initial learning of the materials up to a criterion is required. Participants are directly presented with items or blocks of items and cued, usually once, to either remember or forget these items. Then, memory is tested by means of recall and/or recognition. Findings from developmental studies using these procedures showed that children exhibit decreases in the recall of the to-be-forgotten items relative to the to-be-remembered ones (i.e., directed-forgetting effects) as early as third grade in some cases; but they usually do not reach functional levels in this ability until the fifth grade, around age 10–11 (Bray and Ferguson, 1976; Lehman and Bovasso, 1993; Harnishfeger and Pope, 1996; Wilson and Kipp, 1998). Although these findings are consistent with the possibility that the ability to inhibit unwanted memories develops during that period, there are other plausible accounts of these findings. One possibility is that older participants engage in greater selective encoding and/or rehearsal of to-be-remembered than to-be-forgotten items (e.g., Wilson and Kipp, 1998). Another possibility is that participants associate the to-be-forgotten blocks with a different context than the to-be-remembered blocks, thereby making it more difficult to access the contents of these blocks at test (e.g., Sahakyan and Kelley, 2002). Thus, the emergence of an active memory suppression mechanism is not necessary to account for developmental changes observed in directed-forgetting paradigms. However, these findings provide clues as to the developmental trajectory of mnemonic control processes.
The TNT paradigm has several advantages over directed forgetting procedures as a tool for examining active memory suppression. First, it measures online attempts to prevent a previously encoded memory from entering consciousness, at the time when a specific and effective cue is presented. Second, the TNT paradigm incorporates a Baseline condition that serves as a control of non-selectively rehearsed items (i.e., Think and No-Think items should be selectively rehearsed and non-rehearsed respectively) and of the passage of time between the encoding and the final memory tests. Third, recall in the TNT paradigm is also tested with extra-list cues – i.e., independent probes – that allow us to isolate the role of retrieval inhibition from the influence of other possible interference mechanisms (Anderson and Green, 2001), such as associative blocking or retrieval competition between memory traces (e.g., diversionary thoughts that become associated to the original cue). Thus, the TNT paradigm allows us to examine how memory suppression develops under the conditions in which suppression matters the most (i.e., when a cue reminds the child of the to-be-forgotten material), making it possible to rule out a number of alternative explanations for results suggestive of intentional forgetting obtained with other paradigms, such as selective encoding and rehearsal or interference mechanisms.
In the current study, we used the TNT paradigm to test whether children aged 8–12 could actively suppress memories. Although extant research does not provide firm grounds to make clear predictions concerning the age at which active memory suppression should be detected, we hypothesized based on the directed-forgetting findings reviewed above (Wilson and Kipp, 1998) that this ability emerges late in childhood, around age 10–12. More generally, a large behavioral literature demonstrates marked improvements in control over thoughts and actions over ages 8–12 (e.g., Kail, 2002; Davidson et al., 2006), with additional but smaller gains observed during adolescence (e.g., Luna et al., 2004). Indeed, the ability to form and recall memories improves throughout childhood, which could make memory suppression increasingly more challenging with age. To test the hypothesis that memory suppression emerges in late childhood, we measured age differences in memory suppression continuously from age 8 to 12, and also compared performance between children aged 8–9, children aged 10–12, and young adults.
Materials and Methods
Participants
Seventy native English-speakers participated, forty 8- to 12-year-old children (M = 10.30 years; SD = 1.36 years; range = 8.4–12.8 years; 52% females) and thirty young adults (M = 20.59 years; SD = 1.88 years; range = 17.8–24.8 years; 67% females). Four additional children were excluded (one scored on the clinical range of the Child-Behavior-Checklist and three failed to learn at least 50% of the experimental word-pairs after three study repetitions). Participants received either monetary compensation or course credit for their participation.
Stimuli
A total of 56 word-pairs were developed for the present study according to procedures used in prior TNT studies (e.g., Anderson et al., 2004). Of those pairs, 20 were used as practice trials. The remaining 36 pairs were divided into three stimulus sets that were rotated through Think, No-Think, and Baseline conditions across participants. All word stimuli were classified as typically acquired by age seven, and were matched across sets for frequency and concreteness. Additionally, the cue (e.g., Market) and response (e.g., Berry) of each pair were designed to be weakly semantically related, with none of the association strength values above 0.025 (Nelson et al., 2004). Each target word (i.e., right-hand pair member) belonged to a unique semantic category, and the association between targets and category cues was equated to that of the word-pairs.
Procedure
The experiment consisted of three phases: Study-feedback, TNT, and memory test (see Figure 1). During the study-feedback phase, participants were first instructed to learn all 56 word-pairs, and then their memory was tested with correct feedback provided. Each pair was presented visually for 5 s, and the study-feedback procedure was repeated up to three times until the participant remembered at least 50% of the pairs (Children's Average = 63.97%; Adults’ Average = 70.64%).
After training, participants received instructions for the TNT task, and practiced on filler pairs for 7 min. During the 24-min TNT phase, participants were given 15 repetitions of blocks of 24 items (i.e., 12 Think trials, 12 No-Think trials). On each trial, a cue from one of the pairs appeared for 2.7 s in green (Think) or in red (No-Think), followed by a 300 ms inter-trial interval. For Think trials, participants were instructed to recall aloud the second member of the word-pair. For No-Think trials, participants were instructed to keep the target word out of mind while still focusing on the cue word. Think and No-Think stimuli were intermixed.
Finally, during the memory-test phase, participants’ memory for the target words was tested with the SP test (i.e., intra-list cue) and an IP test (i.e., extra-list cue). The order of these tests was counterbalanced. In the SP test, participants were given the previously studied cue word (e.g., Vacation) and were asked to recall the target word aloud (e.g., Palm). In the IP test, participants were given a category cue and the first letter of the target word (e.g., Tree-P__).
To ensure that participants did not restrain themselves from reporting No-Think items during this phase, we encouraged them to recall each and every target, regardless of prior instructions. Moreover, we rewarded participants with 10 ¢ for each correct response in both the SP and IP tests. Individual cues were presented for 4 s, and the memory phase lasted about 10 min.
Results
We conducted analyses of variance (ANOVAs) dividing the children into two groups, 8- to 9-year olds and 10- to12-year olds. We conducted two 3 (Age: 8- to 9-year olds, 10- to 12-year olds, adults) × 3 (Condition: No-Think, Think, Baseline) mixed-model ANOVAs, separately for the proportion of recall in the SP and IP tests.
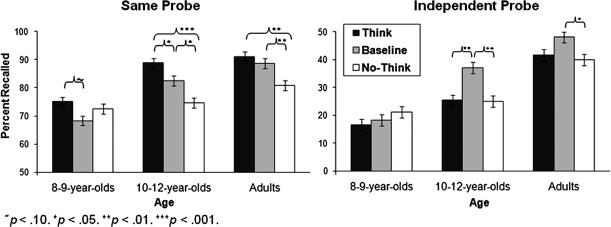
The main effects of age were significant for both the SP, F(1, 67) = 14.02, p < 0.001, , and IP tests, F(1, 67) = 40.68, p < 0.001, . Similarly, the main effects of condition were significant for both the SP, F(2, 134) = 11.66, p < 0.001, , and IP tests, F(2, 134) = 6.03, p < 0.01, . There was also a significant Age × Condition interaction for the SP test, F(4, 134) = 2.66, p < 0.05, , and a marginal interaction for the IP test, F(4, 134) = 2.29, p = 0.06, (see Figure 2).
Figure 2.
Percent recalled for the SP and IP memory tests as a function of age and condition.
Simple-effects analyses indicated that the Age × Condition interaction resulted from age-related improvements in memory suppression. As in prior studies, we found that adults recalled significantly fewer No-Think than Baseline items for both the SP, p < 0.01, and IP tests, p < 0.05. Children aged 10–12 also showed a significant memory suppression effect (SP test, p < 0.05; IP test, p < .01), but 8- to 9-year olds did not in each case, p ≥ 0.33. These results show that memory deficits arising from active memory suppression are not observed consistently until age 10–12. An analogous pattern of results emerged when the analyses were restricted to the word-pair trials for which we had direct evidence of participants’ learning (i.e., word-pairs recollected correctly in the last study-feedback run), ensuring that our findings hold when age-related differences in encoding ability are accounted for.
On the SP test, children tended to recall more Think than Baseline items (10- to 12-year olds, p < 0.05; 8- to 9-year olds, p = 0.08); for adults, this difference was not significant, p = 0.36. Memory enhancement effects on the SP test were also observed for Think relative to No-Think items for 10- to 12-year olds, p < 0.001, and adults, p < 0.01, but not 8- to 9-year olds, p = 0.46. These effects for older children and adults can be explained by selective rehearsal of Think items, given that participants had 15 additional retrieval opportunities for the Think items during the TNT phase.
For the IP test, memory enhancement effects were not observed, consistent with prior data from 687 adults (Levy and Anderson, 2008). In fact, the 10- to 12-year olds exhibited better memory for Baseline than Think items, p < 0.001. Numerically, adults also exhibited a higher proportion of recall for Baseline than Think items, but this effect was not significant (p = 0.15). For additional analyses and a further discussion of these findings, please see the Supplementary Material.
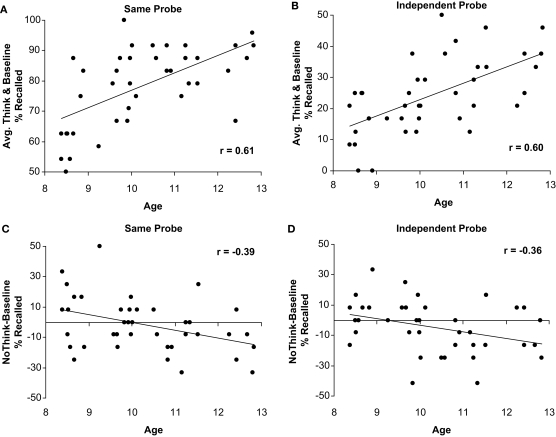
To examine the relationship between age and memory suppression continuously from age 8 to 12, we examined the correlation between age and a memory suppression index (i.e., No-Think mean recall minus Baseline mean recall). As predicted, we observed more effective suppression of memory for No-Think items relative to Baseline items as a function of age, for both the SP, r(40) = −0.39, p < 0.01, and the IP tests, r(40) = −0.36, p < 0.01. These negative correlations between memory impairment for No-Think items and age are shown in Figures 3A,B.
Figure 3.
Correlations between children's age and performance. Negative correlation between children's age and memory for the to-be-suppressed minus baseline items in the SP (A) and IP (B) tests; Positive association between children's age and percent of recall for Think and Baseline conditions averaged together in the SP (C) and IP (D) tests.
These age-related improvements in memory suppression were evident against a backdrop of robust age-related increases in the ability to recall to-be remembered material (i.e., both Think and Baseline items), which should make the suppression task all the more challenging for older children. We found a strong positive association between age and the percent of word-pairs recalled for both Think (SP test, r(40) = 0.59, p < 0.001; IP test, r(40) = 0.48, p < 0.01), and Baseline items (SP test, r(40) = 0.49, p < 0.01; IP test, r(40) = 0.56, p < 0.001). Positive correlations between memory and age for Think and Baseline items averaged together are shown in Figures 3C,D. Thus, children improve at suppressing to-be-forgotten information over the 8–12 age range, despite concomitant improvements in memory for to-be-remembered information.
Discussion
These results constitute the strongest evidence thus far for the development of an active intentional inhibitory-control mechanism that enables suppression of unwanted memories. This improvement in memory suppression across late childhood is observed against a backdrop of overall improvements in declarative memory during this period (see also Brainerd et al., 1990; Ghetti and Angelini, 2008). Just as improvements in memory over childhood are thought to be due to increased ability to use memory encoding and retrieval strategies (e.g., Bjorklund and Douglas, 1997), these data strongly suggest that improvements in memory suppression are due to increased ability to use an active memory suppression mechanism.
In adults, memory suppression in the TNT paradigm is associated with increased activation of lateral PFC, a region involved in the goal-directed control of behavior and that may be part of a general neurocognitive control function for overriding unwanted covert and overt prepotent acts (Levy and Anderson, 2002), and concomitant deactivation of memory-related structures in MTL (Anderson et al., 2004; Depue et al., 2007; see also Hanslmayr et al., 2009). These prior findings are consistent with the idea that PFC suppresses memories by down-regulating the activity of the MTL.
Future research should examine the neural changes that underlie the development of the ability to engage in memory suppression. Cortical gray matter thickness, which reflects neuronal density and the number of connections between neurons, peaks in PFC at around age 10–12, and subsequently declines (Sowell et al., 2004; Shaw et al., 2008), consistent with marked structural changes during the period in which memory suppression emerges. The MTL, long assumed to be structurally mature at an early age, also exhibits changes in cortical thickness throughout childhood and adolescence (Gogtay et al., 2006). Long-range white matter tracts throughout the brain strengthen over development (Lebel et al., 2008), which should make it easier for control-related brain regions to influence activity in memory-related regions. Finally, fMRI studies reveal changes in late childhood in both PFC and MTL activation during performance of cognitive control and memory tasks (Bunge et al., 2002; Hare and Casey, 2005; Ofen et al., 2007; Paz-Alonso et al., 2008). We are currently testing the hypothesis that developmental improvements in memory suppression are due to strengthened prefrontal control over mnemonic representations in the MTL.
One unexpected finding emerged from the present data – namely, the observation that recall performance for Think items was worse than recall for Baseline items on the IP test, especially for 10- to 12-year olds and adults. Thus, repeatedly thinking about the response when prompted with the originally encoded cue (i.e., Think items) hindered later recall when it was tested with an entirely novel cue (i.e., IP test). Although this result may seem surprising at first, it, and other features of the data, are highly consistent with the encoding specificity principle. A large body of literature indicates that changing the cue for an item between encoding and test reduces recall probability, presumably because the initial encoding process biases the meaning of the item towards the original cue (e.g., Thomson and Tulving, 1970). Moreover, the detrimental effect of shifting cues between encoding and test is known to be larger, the more strongly associated an item is to its original cue (e.g., Murphy and Wallace, 1974). All of these effects of cue-specificity can be seen in the present data, with IP cues yielding consistently inferior recall than SP cues across all conditions, more so, when the original cues are more powerfully encoded or strengthened (i.e., Think items).
Importantly, this interpretation predicts that the detrimental effect of shifting cues between study and test should decline with age, consistent with developmental literature that directly addresses this point (e.g., Ackerman, 1982). As can be seen in Supplementary Figure 1, these age-related declines in cue-specificity are extremely robust, and consistent with this prior work (see Supplementary Material for additional discussion). Thus, our results provide additional support for prior work indicating that children's memory retrieval depends more heavily on the match between encoding and retrieval conditions than that of adults.
The ability to suppress unwanted memories has implications for children's use of coping strategies in an effort to avoid thinking about disturbing memories. With age, children show an increased tendency to use coping strategies that capitalize on diversion and avoidance (Skinner and Zimmer-Gembeck, 2007); the present study demonstrates a corresponding improvement in ability to effectively suppress unwanted memories. Of particular relevance to legal hearings involving child testimony usually in regard to negative experiences such as child abuse or witnessing of a crime, children may fail to retrieve relevant information either because they are not provided with effective retrieval cues, or because they have actively suppressed unwanted memories. Developmental changes in memory and mnemonic control should be explored further through the use of emotionally-laden materials (see Depue et al., 2006, 2007). Although much more remains to be learned, the present study provides the first clear demonstration that the basic cognitive mechanism that underlies active memory suppression emerges late in childhood.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/humanneuroscience/paper/10.3389/neuro.09/024.2009/
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by a grant from the Spanish Ministry of Education and Science (Pedro M. Paz-Alonso), and NSF grants 0648564 (Simona Ghetti) and 0448844 (Silvia A. Bunge). We thank Natalie Repin for data collection, Art Shimamura for helpful comments, and M. Souza, J. Lee, S. Wright, C. Knifsend for assistance with the study.
References
- Ackerman B. P. (1982). Retrieval variability: the inefficient use of retrieval cues by young children. J. Exp. Child Psychol. 33, 413–428 10.1016/0022-0965(82)90056-X [DOI] [Google Scholar]
- Altshuler J. L., Ruble D. N. (1989). Developmental changes in children's awareness of strategies for coping with uncontrollable stress. Child Dev. 60, 1337–1349 10.2307/1130925 [DOI] [PubMed] [Google Scholar]
- Anderson M. C., Green C. (2001). Suppressing unwanted memories by executive control. Nature 410, 366–369 10.1038/35066572 [DOI] [PubMed] [Google Scholar]
- Anderson M. C., Levy B. J. (2009). Suppressing unwanted memories. Curr. Dir. Psychol. Sci. 18, 189–194 10.1111/j.1467-8721.2009.01634.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. C., Ochsner K. N., Kuhl B., Cooper J., Robertson E., Gabrieli S. W., Glover G. H., Gabrieli J. D. (2004). Neural systems underlying the suppression of unwanted memories. Science 303, 232–235 10.1126/science.1089504 [DOI] [PubMed] [Google Scholar]
- Bjorklund D. F., Douglas R. N. (1997). The development of memory strategies. In The Development of Memory in Childhood, Cowan N., ed. (Hove, England, Psychology Press; ), pp. 201–246 [Google Scholar]
- Brainerd C. J., Reyna V. F., Howe M. L., Kingma J. (1990). The development of forgetting and reminiscence. Monogr. Soc. Res. Child Dev. 55, v–93. 10.2307/1166106 [DOI] [PubMed] [Google Scholar]
- Bray N. W., Ferguson R. P. (1976). Memory strategies used by young normal and retarded children in a directed forgetting paradigm. J. Exp. Child Psychol. 22, 200–215 10.1016/0022-0965(76)90002-3 [DOI] [PubMed] [Google Scholar]
- Bulevich J. B., Roediger H. L., Balota D. A., Butler A. C. (2006). Failures to find suppression of episodic memories in the think/no-think paradigm. Mem. Cognit. 34, 1569–1577 [DOI] [PubMed] [Google Scholar]
- Bunge S. A., Dudukovic N. M., Thomason M. E., Vaidya C. J., Gabrieli J. D. E. (2002). Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron 33, 301–311 10.1016/S0896-6273(01)00583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. C., Amso D., Anderson L. C., Diamond A. (2006). Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 44, 2037–2078 10.1016/j.neuropsychologia.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue B. E., Banich M. T., Curran T. (2006). Suppression of emotional and non-emotional content in memory: effects of repetition on cognitive control. Psychol. Sci. 17, 441–447 10.1111/j.1467-9280.2006.01725.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue B. E., Curran T., Banich M. T. (2007). Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science 37, 215–219 10.1126/science.1139560 [DOI] [PubMed] [Google Scholar]
- Flavell J. H. (1999). Cognitive development: children's knowledge about the mind. Annu. Rev. Psychol. 50, 21–45 10.1146/annurev.psych.50.1.21 [DOI] [PubMed] [Google Scholar]
- Ghetti S., Angelini L. (2008). The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Dev. 79, 339–358 10.1111/j.1467-8624.2007.01129.x [DOI] [PubMed] [Google Scholar]
- Gogtay N., Nugent T. F., Herman D. H., Ordonez A., Greenstein D., Hayashi K. M., Clasen L., Toga A. W., Giedd J. N., Rapoport J. L., Thompson P. M. (2006). Dynamic mapping of normal human hippocampal development. Hippocampus 16, 664–672 10.1002/hipo.20193 [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Leipod P., Pastötter B., Bäuml K. H. (2009). Anticipatory signatures of voluntary memory suppression. J. Neurosci. 29, 42–47 10.1523/JNEUROSCI.4703-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T. A., Casey B. J. (2005). The neurobiology and development of cognitive control and affective control. Cogn. Brain Behav. 9, 273–286 [Google Scholar]
- Harnishfeger K. K., Pope R. S. (1996). Intending to forget: the development of cognitive inhibition in directed forgetting. J. Exp. Child Psychol. 62, 292–315 10.1006/jecp.1996.0032 [DOI] [PubMed] [Google Scholar]
- Joormann J., Hertel P. T., Brozovich F., Gotlib I. H. (2005). Remembering the good, forgetting the bad: intentional forgetting of emotional material in depression. J. Abnorm. Psychol. 114, 640–648 10.1037/0021-843X.114.4.640 [DOI] [PubMed] [Google Scholar]
- Kail R. (2002). Developmental change in proactive interference. Child Dev.73, 1703–1714 10.1111/1467-8624.00500 [DOI] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulie C. (2008). Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40, 1044–1055 10.1016/j.neuroimage.2007.12.053 [DOI] [PubMed] [Google Scholar]
- Lehman E. B., Bovasso M. (1993). Development of intentional forgetting in children. In Emerging Things in Cognitive Development, Vol I, Foundations, Howe M. L., Pasnak R., eds (New York, Springer-Verlag; ), pp. 214–233 [Google Scholar]
- Levy B. J., Anderson M. C. (2002). Inhibitory processes and the control of memory retrieval. Trends Cogn. Sci. 6, 299–305 10.1016/S1364-6613(02)01923-X [DOI] [PubMed] [Google Scholar]
- Levy B. J., Anderson M. C. (2008). Individual differences in the suppression of unwanted memories: the executive deficit hypothesis. Acta Psychol. 127, 623–635 10.1016/j.actpsy.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K. E., Urban T. A., Lazar N. A., Sweeney J. A. (2004). Maturation of cognitive processes from late childhood to adulthood. Child Dev. 75, 1357–1372 10.1111/j.1467-8624.2004.00745.x [DOI] [PubMed] [Google Scholar]
- Murphy M. D., Wallace W. P. (1974). Encoding specificity: semantic change between storage and retrieval cues. J. Exp. Psychol. 103, 768–774 10.1037/h0037176 [DOI] [Google Scholar]
- Nelson D. L., McEvoy C. L., Schreiber T. A. (2004). The University of South Florida free association, rhyme, and word fragment norms. Behav. Res. Methods Instrum. Comput. 36, 402–407 [DOI] [PubMed] [Google Scholar]
- Ofen N., Kao Y.-C., Sokol-Hessner P., Kim H., Whitfield-Gabrieli S., Gabrieli J. D. E. (2007). Development of the declarative memory system in the human brain. Nat. Neurosci. 10, 1198–1205 10.1038/nn1950 [DOI] [PubMed] [Google Scholar]
- Paz-Alonso P. M., Ghetti S., Donohue S., Goodman G. S., Bunge S. A. (2008). Neurodevelopmental correlates of true and false recognition. Cereb. Cortex 19, 2208–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakyan L., Kelley C. M. (2002). A contextual change account of the directed forgetting effect. J. Exp. Psychol. Learn. Mem. Cogn. 28, 1064–1072 10.1037/0278-7393.28.6.1064 [DOI] [PubMed] [Google Scholar]
- Schneider W., Pressley M. (1999). Memory Development between Two and Twenty. Mahwah, NJ, Lawrence Erlbaum Associates [Google Scholar]
- Shaw P., Kabani N. J., Lerch J. P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J. L., Giedd J. N., Wise S. P. (2008). Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 28, 3586–3594 10.1523/JNEUROSCI.5309-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner E. A., Zimmer-Gembeck M. J. (2007). The development of coping. Annu. Rev. Psychol. 58, 119–144 10.1146/annurev.psych.58.110405.085705 [DOI] [PubMed] [Google Scholar]
- Sowell E. R., Thompson P. M., Leonard C. M., Welcome S. E., Kan E., Toga A. W. (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 24, 8223–8231 10.1523/JNEUROSCI.1798-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. M., Tulving E. (1970). Associative encoding and retrieval: weak and strong cues. J. Exp. Psychol. 86, 255–262 10.1037/h0029997 [DOI] [Google Scholar]
- Walker W. R., Skowronski J. J., Thompson C. P. (2003). Life is pleasant – and memory helps to keep it that way. Rev. Gen. Psychol. 7, 203–210 10.1037/1089-2680.7.2.203 [DOI] [Google Scholar]
- Wilson S. P., Kipp K. (1998). The development of efficient inhibition: evidence from directed-forgetting tasks. Dev. Rev. 18, 86–123 10.1006/drev.1997.0445 [DOI] [Google Scholar]